TO THE EDITOR:
Stroke is one of the leading causes of mortality and permanent disability in the world. Ischemic stroke occurs when a thrombus stops blood flow to the brain resulting in neurological impairment. Despite significant advances in stroke prevention and therapy, the prevalence of stroke is predicted to increase even further.1 Mounting evidence demonstrates inflammation contributes to the development of stroke. This inflammatory response can continue for days to weeks after the initial stroke and thereby lead to further neurological deficits. Controlling the inflammatory response in stroke has therefore been put forward as a potential therapeutic strategy.2 However, although blocking inflammation has been very effective in decreasing brain injury in animal models, translation to the clinic has been challenging. A common critique of preclinical stroke models is the use of young and healthy animals, whereas patients with stroke often are older and have multiple comorbidities.3,4
Type 1 interferons (IFNs) have been shown to protect rodents from ischemic stroke brain injury through reducing the infiltration of leukocytes to the ischemic brain.5-7 However, these studies were exclusively done in young, healthy rodents. In humans, treatment with IFN-ß is approved by the US Food and Drug Administration for patients with relapsing remitting multiple sclerosis (MS) and is currently being pursued for patients with ischemic stroke (NCT00097318). However, IFN-ß treatment is associated with a 1.8-fold increased risk of ischemic stroke in patients with MS.8 Similarly, IFN-α treatment in patients with cancer has been reported to have both antiinflammatory effects9 as well as prothrombotic side effects.10,11 Furthermore, inhibition of type I IFN signaling was recently shown to suppress tissue factor release from macrophages,12 supporting a prothrombotic role for type 1 IFNs. Increasing evidence supports an age-dependent response to type 1 IFNs.13,14 We hypothesized that age could impact the effect type I IFNs have on stroke outcomes and could potentially explain some of the discrepancies between preclinical and human data.
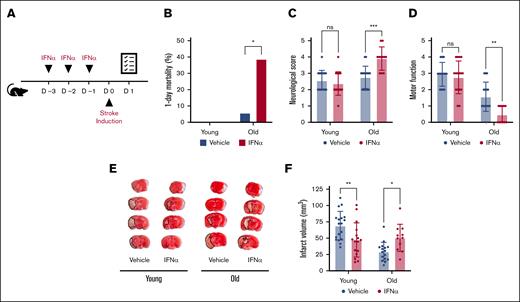
We investigated the impact of type 1 IFNs on ischemic stroke outcomes in both male and female as well as young (aged 4-6 months) and old (>24 months) C57Bl6/J mice subjected to a model of 1-hour transient middle cerebral artery occlusion.15 Mice were randomized and IFN-α (25 000 units/mouse) or vehicle was administered intraperitoneally for 3 consecutive days before stroke onset (Figure 1A). We have previously shown this IFN-α treatment regimen induces a robust type 1 IFN response in mice.16 Stroke outcomes including mortality, neurological behavior, motor function, and ischemic brain injury were measured by an operator blinded to treatment.15 Twenty-four hours after stroke onset, substantial mortality and neurological impairment were observed in old mice treated with IFN-α. IFN-α treatment increased mortality over sixfold in old mice (38.8% vs 5.8%, respectively; Figure 1B). Moreover, in surviving old mice, IFN-α treatment was associated with significantly worse neurological behavior and motor function (Figure 1C-D). This neurological impairment was also evident when brain infarct size was determined (50.6 mm3 ± 21 mm3 vs 29.4 mm3 ± 14.4 mm3; Figure 1E-F). Combined, our results indicate a detrimental role of type 1 IFN signaling in old mice subjected to ischemic stroke. In contrast with old mice, IFN-α treatment significantly reduced ischemic stroke brain injury in young mice (47.3 mm3 ± 26.2 mm3 vs 69.1 mm3 ± 21.9 mm3; Figure 1E-F). However, this reduction in brain injury was mild and not associated with significantly improved neurological or motor outcomes (Figure 1C-D).
IFN-α treatment differentially impacts ischemic stroke outcomes in young and old mice. (A) Mice were treated for 3 days with either vehicle or IFN-α. Twenty-four hours after the induction of transient middle cerebral artery occlusion in young (age, 4-6 months) and old (age >24 months) mice, survival was monitored (B), and neurological (a higher score is worse) (C) and motor function (a lower score is worse) (D) were assessed. (E) Brain tissue was stained with TTC. Infarcted tissue (white) is outlined with a black dotted line. (F) Ischemic stroke brain damage was quantified by planimetric analysis of TTC stained brain slides. All data are represented as mean ± standard deviation. TTC, triphenyltetrazolium chloride.
IFN-α treatment differentially impacts ischemic stroke outcomes in young and old mice. (A) Mice were treated for 3 days with either vehicle or IFN-α. Twenty-four hours after the induction of transient middle cerebral artery occlusion in young (age, 4-6 months) and old (age >24 months) mice, survival was monitored (B), and neurological (a higher score is worse) (C) and motor function (a lower score is worse) (D) were assessed. (E) Brain tissue was stained with TTC. Infarcted tissue (white) is outlined with a black dotted line. (F) Ischemic stroke brain damage was quantified by planimetric analysis of TTC stained brain slides. All data are represented as mean ± standard deviation. TTC, triphenyltetrazolium chloride.
The protective effects of type 1 IFNs in ischemic stroke have been attributed to its antiinflammatory properties. Specifically, type 1 IFNs reduce the expression of MMP9,17 thereby limiting leukocyte infiltration in ischemic brain trissue5 and dampening pathological inflammation.7 Here, we measured plasma markers of inflammation, C-reactive protein (CRP), and interleukin-6 (IL-6), and we quantified neutrophil infiltration in the brain via histology 24 hours after stroke onset. In young mice, IFN-α treatment resulted in a nearly 60% reduction of the amount of neutrophil recruited to the brain (Figure 2A). In old mice, IFN-α treatment also significantly reduced neutrophil recruitment (Figure 2A). However, the number of neutrophils observed in the brain tissue of IFN-α–treated old mice closely resembled those in vehicle-treated young mice. Similarly, CRP plasma levels were significantly reduced in young mice subjected to stroke treated with IFN-α, whereas CRP levels were not affected by IFN-α treatment in old mice (Figure 2B). Lastly, IL-6 plasma levels were only increased in old mice subjected to stroke, and IFN-α treatment increased this even further (Figure 2C). These results indicate IFN-α treatment is unable to block detrimental inflammation contributing to ischemic brain injury in old mice. Furthermore, our data indicate a distinct inflammatory response to ischemic stroke between young and old mice. CRP levels were higher in young mice subjected to stroke than in old mice, whereas IL-6 levels were higher in old mice after stroke. Of note, 3 days of IFN-α treatment affected neither CRP nor IL-6 levels in healthy young or old mice (baseline; Figure 2B-C).
IFN-α treatment is prothrombotic in old mice subjected to stroke. Mice were treated for 3 days with either vehicle or IFN-α. Twenty-four hours after the induction of transient middle cerebral artery occlusion in young (age, 4-6 months) and old (age >24 months) mice, brain tissue and plasma were collected. (A) Quantification of neutrophil recruitment in all groups in the ipsilesional hemisphere, that is, where the ischemic injury occurred. (B) Plasma CRP levels and (C) plasma IL-6 levels were determined by ELISA. (D) Brain tissue was stained for platelets, and platelet deposition in ipsilesional brain tissue from young and old mice was quantified. (E) Brain tissue was stained for fibrin(ogen), and fibrin(ogen) deposition in ipsilesional brain tissue from young and old mice was quantified. (F) Plasma VWF levels and (G) plasma TAT levels were determined by ELISA. All data are represented as mean ± standard deviation. ELISA, enzyme-linked immunosorbent assay.
IFN-α treatment is prothrombotic in old mice subjected to stroke. Mice were treated for 3 days with either vehicle or IFN-α. Twenty-four hours after the induction of transient middle cerebral artery occlusion in young (age, 4-6 months) and old (age >24 months) mice, brain tissue and plasma were collected. (A) Quantification of neutrophil recruitment in all groups in the ipsilesional hemisphere, that is, where the ischemic injury occurred. (B) Plasma CRP levels and (C) plasma IL-6 levels were determined by ELISA. (D) Brain tissue was stained for platelets, and platelet deposition in ipsilesional brain tissue from young and old mice was quantified. (E) Brain tissue was stained for fibrin(ogen), and fibrin(ogen) deposition in ipsilesional brain tissue from young and old mice was quantified. (F) Plasma VWF levels and (G) plasma TAT levels were determined by ELISA. All data are represented as mean ± standard deviation. ELISA, enzyme-linked immunosorbent assay.
Next, we investigated brain tissue and plasma for thrombotic biomarkers, as type 1 IFNs have been linked to increased thrombosis. In young mice, IFN-α treatment had no effect on platelet or fibrin(ogen) deposition in ischemic stroke brain tissue (Figure 2D-E). In old mice, however, IFN-α increased both platelet and fibrin(ogen) deposition in the brain (Figure 2D-E). In agreement with these results, plasma von Willebrand factor (VWF) levels and the concentration of thrombin-antithrombin (TAT) complexes were only increased in old mice subjected to stroke treated with IFN-α (Figure 2F-G). TAT complexes are an indirect marker of thrombin generation and thereby reflect a prothrombotic status. VWF is a prothrombotic and proinflammatory protein implicated in stroke pathophysiology.18 Circulating VWF levels increase with age,19 and brain endothelial cells from old mice have increased expression levels of VWF.20 IFN-α has been found to increase VWF-induced platelet activation in patients with myeloproliferative neoplasms.21 We speculate a similar phenomenon is occurring in the brain of old mice treated with IFN-α, in which the increased levels of VWF result in increased intravascular platelet deposition. Interestingly, we only observed this in old mice subjected to stroke (Figure 2D). This is likely because of increased type 1 IFN signaling in old mice,13,14,22 resulting in a more profound prothrombotic response. Additionally, IL-6, which was only increased in old mice subjected to stroke (Figure 2C), is known to induce the accumulation of VWF on the surface of endothelial cells to provoke platelet adhesion.23 Finally, IL-6 increases the expression of fibrinogen, tissue factor, and PAI-1.24 This is in line with increased fibrin(ogen) deposition (Figure 2E) and TAT levels (Figure 2G) observed in old mice treated with IFN-α, in which the IL-6 levels were the highest.
In conclusion, our results demonstrate a differential impact of type 1 IFNs on stroke outcomes in young and old mice. This is due to a distinct thromboinflammatory response of the young and old ischemic stroke brain, in which the IFNs have an antiinflammatory effect in young mice but a prothrombotic effect in old mice. Further mechanistic studies will be needed to dissect the cause of this. Nevertheless, these results warrant caution interpreting preclinical studies performed exclusively in young, healthy mice. Moreover, our findings may have implications for inflammatory diseases that have a vascular component and in which type 1 interferons are considered as therapeutic interventions, such as myeloproliferative neoplasms and several types of cancer.25
Acknowledgments: This work was supported by National Institutes of Health grants K01AG059892 and R01HL163019 (R.A.C.) and the American Heart Association (21POST830138; FD).
Contribution: F.D. designed experiments; F.D., I.A., M.J.C., and Y.K performed experiments; and F.D. and R.A.C analyzed results, prepared the figures, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Frederik Denorme, Division of Vascular Neurology, Department of Neurology, Eccles Institute of Human Genetics, The University of Utah, 15 North 2030 East, Bldg 533, Rm 4280, Salt Lake City, UT 84112; e-mail: Frederik.Denorme@utah.edu.
References
Author notes
Data are available on request from the corresponding author, Frederik Denorme (Frederik.Denorme@utah.edu).