Key Points
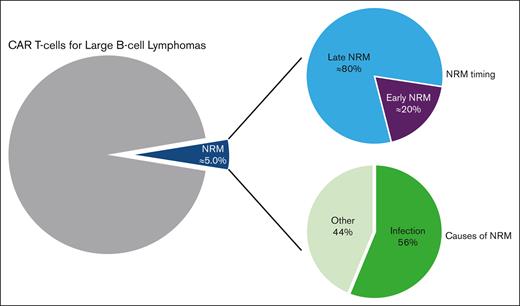
The NRM rate estimate after CAR T-cell therapy for large B-cell lymphomas was 5%, with ∼80% of deaths occurring beyond day 28 after infusion.
Infections are responsible for the majority of NRM after CAR T-cell infusion for large B-cell lymphomas (56%).
Abstract
CD19 chimeric antigen receptor (CAR) T cells can induce prolonged remissions and potentially cure a significant proportion of patients with relapsed/refractory large B-cell lymphomas. However, some patients may die of causes unrelated to lymphoma after CAR T-cell therapy. To date, little is known about the nonrelapse mortality (NRM) after CAR T-cell therapy. Using the French DESCAR-T registry, we analyzed the incidence and causes of NRM and identified risk factors of NRM. We report on 957 patients who received standard-of-care axicabtagene ciloleucel (n = 598) or tisagenlecleucel (n = 359) between July 2018 and April 2022, in 27 French centers. With a median follow-up of 12.4 months, overall NRM occurred in 48 patients (5.0% of all patients): early (before day 28 after infusion) in 9 patients (0.9% of all patients and 19% of overall NRM), and late (on/after day 28 after infusion) in 39 patients (4.1% of all patients and 81% of overall NRM). Causes of overall NRM were distributed as follows: 56% infections (29% with non–COVID-19 and 27% with COVID-19), 10% cytokine release syndromes, 6% stroke, 6% cerebral hemorrhage, 6% second malignancies, 4% immune effector cell associated neurotoxicities, and 10% deaths from other causes. We report risk factors of early NRM and overall NRM. In multivariate analysis, both diabetes and elevated ferritin level at lymphodepletion were associated with an increased risk of overall NRM. Our results may help physicians in patient selection and management in order to reduce the NRM after CAR T-cell therapy.
Introduction
CD19 chimeric antigen receptor (CAR) T-cell therapy has been approved for the treatment of relapsed/refractory large B-cell lymphoma (LBCL) after 1 or 2 prior lines of treatment.1-5 The predominant cause of death after CAR T-cell therapy is lymphoma relapse/progression. However, pivotal trials and preliminary data from the real-world experience have also reported a nonrelapse mortality (NRM) rate ranging from 0% to 6%.1-3,6-10 Thus, improving the overall survival (OS) of patients treated with CAR T cells can be achieved not only by improving the antilymphoma efficacy of CAR T cells but also by reducing the NRM. To date, there are limited data on NRM after CD19 CAR T-cell therapy in the real-world setting, which are mostly based on pharmacovigilance reports.11,12 However, such an approach does not inform about the incidence nor the risk factors associated with NRM. In this study, we aimed to describe the rates and causes of NRM associated with CAR T-cell therapy for LBCL and identify risk factors of NRM.
Methods
Data source
The DESCAR-T registry is the French national registry sponsored by the LYSARC for all patients treated with commercial CAR T cells across all hematologic malignancies. Here, we retrospectively analyzed data from all patients treated for a LBCL registered in the DESCAR-T registry who received standard-of-care CD19 CAR T cells between July 2018 and April 2022. A total of 27 French centers participated in this study. All patients received a consent letter before enrollment. The protocol was approved by the institutional review board, and the study was undertaken in accordance with the Declaration of Helsinki. DESCAR-T is registered under the ClinicalTrials.gov identifier NCT04328298.
Study design
Data on baseline patient and disease characteristics, cellular therapy, and outcome after CAR T-cell therapy were obtained from the DESCAR-T registry. Eligible patients were adults (aged ≥18 years), with LBCL treated with commercial axicabtagene ciloleucel (axi-cel) or tisagenlecleucel (tisacel), and registered in the DESCAR-T registry. Patients could be treated (1) under French Early Access Program, (2) under post–Early Access Program authorization, or (3) under Market Authorization covered by the French health insurance system in an approved center. Patients receiving CAR T cells in clinical trials or other CAR T-cell products were excluded from the analysis. Patients who died of a cause unrelated to lymphoma after having experienced a confirmed lymphoma progression were excluded from the analysis, whether they were offered a post–CAR T-cell salvage therapy or not. The objective of the study was to describe the rate and causes of NRM after CAR T cells and identify risk factors of NRM. NRM was defined as patients who died of causes unrelated to lymphoma relapse/progression (deaths of unknown origin were excluded) within 28 days of CAR T-cell infusion (early NRM) or beyond (late NRM). Overall NRM was defined as the sum of early and late NRMs.
Statistical analyses
Descriptive statistics of early and overall NRM were performed, and cumulative incidences calculated. Survival curves were generated using the Kaplan-Meier estimation method.
Risk factors for early NRM were identified by comparing the characteristics of the early NRM population with those of patients alive on day 28 after CAR T-cell infusion. Risk factors for overall NRM were identified by comparing the characteristics of the overall NRM population with patients alive at 1 year after CAR T-cell infusion. For discontinuous variables, we used a Fisher exact test. For continuous variables, we used a Wilcoxon test. For multiple categorical variables, we used a Cochran-Mantel-Haenszel row mean score. A 2-sided P value <0.05 was considered significant. Prognostic factors for overall NRM were defined using logistic regression. Statistical analyses were performed using SAS software version 9.3 and R version 4.2.0.
Results
Characteristics and outcome of the entire population
Between July 2018 and April 2022, 957 consecutive patients treated in 27 centers and registered in the DESCAR-T registry received either axi-cel (n = 598; 62%) or tisacel (n = 359; 38%). Patient characteristics are summarized in supplemental Figure 1. Briefly, the median age was 63 years (range, 18-82 years); 120 (12.5%) patients had an Eastern Cooperative Oncology Group performance status (ECOG PS) ≥ 2; 498 (52%) patients had an age-adjusted International Prognostic Index (aaIPI) ≥ 2; 471 (49.2%) patients had elevated lactate dehydrogenase (LDH) level; 459 (48%) patients had received ≥3 prior lines of treatment; 199 (20.8%) had failed a prior autologous stem cell transplant; and 768 (80.3%) received a bridging therapy.
Out of the 957 patients evaluable for response, 693 patients (72.4%) achieved an objective response (OR), including 520 (54.3%) complete responses. After a median follow-up of 12.4 months, the median progression-free survival and OS were 6.0 months (95% confidence interval [CI], 4.7-8.5) and 21.0 months (95% CI, 17.6-32.4), respectively (supplemental Figure 2). The estimated progression-free survival and OS rates at 12 months were 43.5% and 59.4%, respectively.
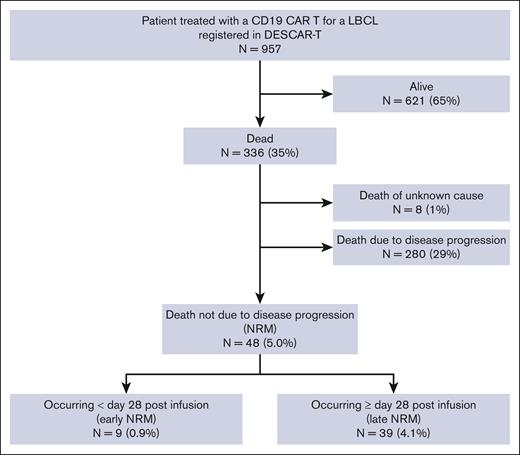
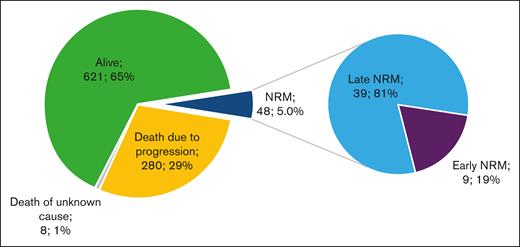
At the time of data cut-off (05/02/2022), 621 of 957 patients (65%) were alive and 336 (35%) had died: 280 (29%) because of lymphoma progression, 8 (1%) of an unknown cause, and 48 (5.0%) of causes unrelated to lymphoma relapse/progression, defining the overall NRM population (Figure 1). Among the overall NRM population, 9 deaths (0.9% of all patients and 19% of all NRMs) occurred before day 28 after infusion (early NRM population), and 39 deaths (4.1% of all patients and 81% of all NRMs) occurred beyond day 28 after infusion (late NRM population) (Figure 2).
Outcomes of the entire cohort of patients treated with commercial axi-cel or tisacel from the French DESCAR-T registry. (Left) Absolute number contribution to patient outcomes (N = 957). (Right) Proportion of early and late NRM among overall NRM. Early NRM is defined by NRM occurring before day 28 after CAR T-cell injection; late NRM is defined by NRM occurring beyond day 28 after CAR T-cell injection.
Outcomes of the entire cohort of patients treated with commercial axi-cel or tisacel from the French DESCAR-T registry. (Left) Absolute number contribution to patient outcomes (N = 957). (Right) Proportion of early and late NRM among overall NRM. Early NRM is defined by NRM occurring before day 28 after CAR T-cell injection; late NRM is defined by NRM occurring beyond day 28 after CAR T-cell injection.
Descriptive analysis of the NRM population
Patient characteristics of the early, late, and overall NRM populations are summarized in Table 1. Briefly, in the early NRM population, the median age was 73 years, 55.6% of the patients had a poor ECOG PS ≥ 2, and 83.4% had an aaIPI ≥ 2. In the late NRM population, the median age was 66 years, 28.9% of the patients had diabetes, 14.3% had a poor ECOG PS ≥ 2, and 61.1% had an aaIPI ≥ 2.
Patient characteristics of the NRM population
| . | Early NRM (n = 9) . | Late NRM (n = 39) . | All NRM (n = 48) . |
|---|---|---|---|
| Age, y | |||
| Median (range) | 73 (30-76) | 66 (41-79) | 67 (61-73) |
| ≥65, n (%) | 8 (88.9) | 22 (57.9) | 30 (63.8) |
| ≥70, n (%) | 5 (55.6) | 14 (35.9) | 19 (39.6) |
| Male sex, n (%) | 4 (44.4) | 25 (64.1) | 29 (60.4) |
| Diabetes, n (%) | 0 | 11 (28.9) | 11 (23.4) |
| ECOG PS, n (%) | |||
| 0-1 | 4 (44.4) | 30 (85.7) | 34 (77.3) |
| ≥2 | 5 (55.6) | 5 (14.3) | 10 (22.7) |
| Histology | |||
| DLBCL, n (%) | 6 (66.7) | 24 (61.5) | 30 (62.5) |
| PMBL, n (%) | 0 | 2 (5.1) | 2 (4.2) |
| HGBL, n (%) | 0 | 0 | 0 |
| tFL, n (%) | 1 (11.1) | 11 (28.2) | 12 (25.0) |
| Other, n (%) | 2 (22.2) | 2 (5.1) | 4 (8.3) |
| aaIPI, n (%) | |||
| 0 | 0 | 2 (5.6) | 2 (4.8) |
| 1 | 1 (16.7) | 12 (33.3) | 13 (31.0) |
| 2 | 4 (66.7) | 19 (52.8) | 23 (54.8) |
| 3 | 1 (16.7) | 3 (8.3) | 4 (9.5) |
| Elevated LDH level at lymphodepletion, n (%) | 8 (88.9) | 17 (45.9) | 25 (54.3) |
| Elevated ferritin level at lymphodepletion, n (%) | 4 (50.0) | 25 (67.6) | 29 (64.4) |
| Hypogammaglobulinemia < 5 g/L at lymphodepletion, n (%) | 4 (100) | 13 (48.1) | 17 (54.8) |
| Prior treatments | |||
| Number of prior lines, median (range) | 2 (2-5) | 3 (2-9) | 3 (2-9) |
| ASCT, n (%) | 0 | 14 (35.9) | 14 (29.2) |
| Allo-SCT, n (%) | 0 | 1 (2.6) | 1 (2.1) |
| Bridging therapy administered, n (%) | |||
| No bridging | 0 | 3 (8.8) | 3 (7.0) |
| Response to bridging (PR or CR) | 0 | 12 (35.3) | 12 (20.9) |
| No response to bridging (SD or PD) | 9 (100) | 19 (55.8) | 28 (65.1) |
| CAR T-cell product | |||
| Axi-cel, n (%) | 4 (44.4) | 27 (69.2) | 31 (64.6) |
| Tisacel, n (%) | 5 (55.6) | 12 (30.8) | 17 (35.4) |
| CRS, n(%) | |||
| Any grade | 9 (100) | 34 (87.2) | 43 (89.6) |
| Grade ≥3 | 6 (66.7) | 7 (17.9) | 13 (27.1) |
| ICANS, n (%) | |||
| Any grade | 6 (66.7) | 24 (61.5) | 30 (62.5) |
| Grade ≥3 | 4 (44.4) | 11 (28.2) | 15 (31.3) |
| . | Early NRM (n = 9) . | Late NRM (n = 39) . | All NRM (n = 48) . |
|---|---|---|---|
| Age, y | |||
| Median (range) | 73 (30-76) | 66 (41-79) | 67 (61-73) |
| ≥65, n (%) | 8 (88.9) | 22 (57.9) | 30 (63.8) |
| ≥70, n (%) | 5 (55.6) | 14 (35.9) | 19 (39.6) |
| Male sex, n (%) | 4 (44.4) | 25 (64.1) | 29 (60.4) |
| Diabetes, n (%) | 0 | 11 (28.9) | 11 (23.4) |
| ECOG PS, n (%) | |||
| 0-1 | 4 (44.4) | 30 (85.7) | 34 (77.3) |
| ≥2 | 5 (55.6) | 5 (14.3) | 10 (22.7) |
| Histology | |||
| DLBCL, n (%) | 6 (66.7) | 24 (61.5) | 30 (62.5) |
| PMBL, n (%) | 0 | 2 (5.1) | 2 (4.2) |
| HGBL, n (%) | 0 | 0 | 0 |
| tFL, n (%) | 1 (11.1) | 11 (28.2) | 12 (25.0) |
| Other, n (%) | 2 (22.2) | 2 (5.1) | 4 (8.3) |
| aaIPI, n (%) | |||
| 0 | 0 | 2 (5.6) | 2 (4.8) |
| 1 | 1 (16.7) | 12 (33.3) | 13 (31.0) |
| 2 | 4 (66.7) | 19 (52.8) | 23 (54.8) |
| 3 | 1 (16.7) | 3 (8.3) | 4 (9.5) |
| Elevated LDH level at lymphodepletion, n (%) | 8 (88.9) | 17 (45.9) | 25 (54.3) |
| Elevated ferritin level at lymphodepletion, n (%) | 4 (50.0) | 25 (67.6) | 29 (64.4) |
| Hypogammaglobulinemia < 5 g/L at lymphodepletion, n (%) | 4 (100) | 13 (48.1) | 17 (54.8) |
| Prior treatments | |||
| Number of prior lines, median (range) | 2 (2-5) | 3 (2-9) | 3 (2-9) |
| ASCT, n (%) | 0 | 14 (35.9) | 14 (29.2) |
| Allo-SCT, n (%) | 0 | 1 (2.6) | 1 (2.1) |
| Bridging therapy administered, n (%) | |||
| No bridging | 0 | 3 (8.8) | 3 (7.0) |
| Response to bridging (PR or CR) | 0 | 12 (35.3) | 12 (20.9) |
| No response to bridging (SD or PD) | 9 (100) | 19 (55.8) | 28 (65.1) |
| CAR T-cell product | |||
| Axi-cel, n (%) | 4 (44.4) | 27 (69.2) | 31 (64.6) |
| Tisacel, n (%) | 5 (55.6) | 12 (30.8) | 17 (35.4) |
| CRS, n(%) | |||
| Any grade | 9 (100) | 34 (87.2) | 43 (89.6) |
| Grade ≥3 | 6 (66.7) | 7 (17.9) | 13 (27.1) |
| ICANS, n (%) | |||
| Any grade | 6 (66.7) | 24 (61.5) | 30 (62.5) |
| Grade ≥3 | 4 (44.4) | 11 (28.2) | 15 (31.3) |
allo-SCT, allogeneic stem cell transplantation; ASCT, autologous stem cell transplantation; CR, complete response; DLBCL, diffuse large B-cell lymphoma; HGBL, high-grade B-cell lymphoma; PD, progressive disease; PMBL, primary mediastinal B-cell lymphoma; tFL, transformed follicular lymphoma; PR, partial response; SD, stable disease.
Causes of overall NRM were non–COVID-19 infections in 14 patients (Escherichia coli septic shock [n = 2], Pseudomonas aeruginosa pneumonia [n = 2], pulmonary mucormycosis [n = 2], undifferentiated pneumonias [n= 2], undifferentiated febrile neutropenia[n = 1], undifferentiated septic shock [n = 1], pulmonary aspergillosis [n = 1], cytomegalovirus encephalitis [n = 1], peritonitis [n = 1], and surgical site infection after total knee replacement [n = 1]), COVID-19 infection in 13 patients, cytokine release syndromes (CRS) in 5 patients, stroke in 3 patients, cerebral hemorrhage in 3 patients, second primary malignancy in 3 patients (acute myeloid leukemia [n = 1], myelodysplastic syndrome [n = 1], and breast cancer [n = 1]), immune effector cell associated neurotoxicity syndrome (ICANS) in 2 patients, and deaths from other causes in 5 patients (Figure 3). Other causes of death were sudden deaths in 3 patients, acute respiratory failure in 1 patient, and anasarca in 1 patient.
Causes of early NRM were CRS in 5 patients and non–COVID-19 infection in 4 patients (E coli septic shock [n = 2], P aeruginosa pneumopathy [n = 1], and undifferentiated febrile neutropenia [n = 1]). Other causes of NRM occurred beyond day 28.
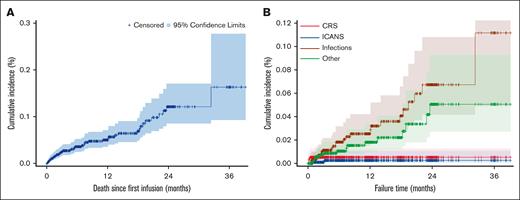
After a median follow-up of 12.4 months, 21 patients (43.8% of the overall NRM) died between 0 and 3 months, 7 patients (14.6%) between 3 nd 6 months, 5 patients (10.4%) between 6 and 9 months, 1 patient (2.1%) between 9 and 12 months, and 14 patients (29.2%) beyond 12 months. Regarding infection-related fatal events, 10 patients (37.0% of the infectious related NRM) died between 0 and 3 months, 4 patients (14.8%) between 3 and 6 months, 3 patients (11.1%) between 6 and 9 months, and 10 patients (37.0%) beyond 12 months. Cumulative incidence of total NRM and NRM according to the cause of death shows a sustained risk for fatal infection overtime (Figure 4). The 2 fatal ICANSs reported here occurred after day 28 after infusion and, as such, are defined as late NRM events, although their onset occurred on day 8 and day 11 after infusion.
Cumulative incidence of total NRM and NRM according to the cause of death in the entire population (N = 957). (A) Cumulative incidence of NRM in the overall population (N = 957). (B) Cumulative incidence of NRM based on the cause of death.
Cumulative incidence of total NRM and NRM according to the cause of death in the entire population (N = 957). (A) Cumulative incidence of NRM in the overall population (N = 957). (B) Cumulative incidence of NRM based on the cause of death.
Risk factors of NRM
To determine the risk factors for early NRM, we compared the characteristics of the early NRM population (n = 9) with patients alive at day 28 after CAR T-cell infusion (n = 891) in univariate analysis (Table 2; supplemental Table 3). Risk factors at lymphodepletion identified for early NRM were as follows: age (median, 73 vs 63 years; P = .020), body mass index (median, 22.5 vs 24.4; P = .040), ECOG PS ≥ 2 (55.6% vs 12.5%; P = .003), anemia (hemoglobin level median, 9.1 vs 10.5 g/dL; P = .020), LDH level (median, 408 vs 250 IU/L; P = .019), and ferritin level (median, 1837 vs 479 μg/L; P = .021). Risk factors at infusion identified for early NRM were as follows: thrombocytopenia (median platelet count, 90 vs 148 G/L; P = .035), LDH level (median, 354 vs 221 IU/L; P = .021), and ferritin level (median, 6686 vs 687 μg/L; P = .002). Risk factors after infusion identified for early NRM were as follows: CRS of grade ≥3 (66.7% vs 7.5%; P < .001) and ICANS grade ≥3 (44.4% vs 11.2%; P = .013). The composite CAR T-cell–related hematologic toxicity (CAR-HEMATOTOX) score was a risk factor for early NRM (P = .039).13 The type of CAR T-cell product (axi-cel vs tisacel) was not identified as a risk factor for early NRM (P = .313). A hypogammaglobulinemia (<5 g/L) was not identified as a risk factor for early NRM.
Risk factors for early NRM by univariate analysis
| . | . | Early NRM . | Comparative group . | P value . |
|---|---|---|---|---|
| n = 9 . | n = 891 . | |||
| Patient characteristics | Age, median, y | 73 | 63 | .020 |
| BMI, median | 22.5 | 24.4 | .040 | |
| At lymphodepletion | ECOG PS, n (%) | .003 | ||
| 0-1 | 4 (44.4) | 732 (87.5) | ||
| ≥2 | 5 (55.6) | 105 (12.5) | ||
| Hemoglobin level (g/dL), median | 9.1 | 10.5 | .020 | |
| LDH level (IU/L), median | 408 | 250 | .019 | |
| Ferritin level (μg/L), median | 1837 | 479 | .021 | |
| Hypogammaglobulinemia, n (%) | 4 (100) | 324 (48.1) | .054 | |
| CAR-HEMATOTOX, n (%) | .039 | |||
| Low | 2 (22.2) | 522 (58.7) | ||
| High | 7 (77.8) | 368 (41.3) | ||
| At infusion | CAR T-cell product, n (%) | .313 | ||
| Axi-cel | 4 (44.4) | 553 (62.1) | ||
| Tisacel | 5 (55.6) | 337 (37.9) | ||
| Platelet count (G/L), median | 90 | 148 | .035 | |
| LDH level (IU/L), median | 354 | 221 | .021 | |
| Ferritin level (μg/L), median | 6686 | 687 | .002 | |
| After infusion | CRS grade ≥3, n (%) | 6 (66.7) | 67 (7.5) | <.001 |
| ICANSgrade ≥3, n (%) | 4 (44.4) | 100 (11.2) | .013 |
| . | . | Early NRM . | Comparative group . | P value . |
|---|---|---|---|---|
| n = 9 . | n = 891 . | |||
| Patient characteristics | Age, median, y | 73 | 63 | .020 |
| BMI, median | 22.5 | 24.4 | .040 | |
| At lymphodepletion | ECOG PS, n (%) | .003 | ||
| 0-1 | 4 (44.4) | 732 (87.5) | ||
| ≥2 | 5 (55.6) | 105 (12.5) | ||
| Hemoglobin level (g/dL), median | 9.1 | 10.5 | .020 | |
| LDH level (IU/L), median | 408 | 250 | .019 | |
| Ferritin level (μg/L), median | 1837 | 479 | .021 | |
| Hypogammaglobulinemia, n (%) | 4 (100) | 324 (48.1) | .054 | |
| CAR-HEMATOTOX, n (%) | .039 | |||
| Low | 2 (22.2) | 522 (58.7) | ||
| High | 7 (77.8) | 368 (41.3) | ||
| At infusion | CAR T-cell product, n (%) | .313 | ||
| Axi-cel | 4 (44.4) | 553 (62.1) | ||
| Tisacel | 5 (55.6) | 337 (37.9) | ||
| Platelet count (G/L), median | 90 | 148 | .035 | |
| LDH level (IU/L), median | 354 | 221 | .021 | |
| Ferritin level (μg/L), median | 6686 | 687 | .002 | |
| After infusion | CRS grade ≥3, n (%) | 6 (66.7) | 67 (7.5) | <.001 |
| ICANSgrade ≥3, n (%) | 4 (44.4) | 100 (11.2) | .013 |
Risk factors for early NRM were identified by comparing the characteristics of the early NRM population with those of patients alive on day 28 after CAR T-cell infusion.
BMI, body mass index.
To determine the risk factors for overall NRM, we compared the characteristics of the overall NRM population (n = 48) with patients alive at 1 year (n = 191) after CAR T-cell infusion on univariate analysis (Table 3; supplemental Table 4). Risk factors at lymphodepletion identified for overall NRM were as follows: age (median, 67 vs 63 years; P = .003), diabetes (23.4% vs 10.1%; P = .025), ECOG PS ≥ 2 (22.7% vs 4.9%; P < .001), anemia (median hemoglobin level, 9.6 vs 10.7 g/dL; P=.002), thrombocytopenia (median platelet count, 156 vs 185 G/L; P=.034), C-reactive protein level (median, 17 g/dL vs 5 mg/L; P < .001), and ferritin level (median, 732 vs 393 μg/L; P = .002). Risk factors at infusion identified for overall NRM were as follows: anemia (median hemoglobin 9.3 vs 10.0 g/dL, P = .002), thrombocytopenia (median platelet count, 117 vs 153 G/L; P = .007), C-reactive protein level (median, 21 vs 8 mg/L; P = .001), LDH level (median, 354 vs 221 IU/L; P = .021), and ferritin level (median, 897 vs 539 μg/L; P < .001). Postinfusion risk factors identified for overall NRM were as follows: CRS of grade ≥3 (27.1% vs 5.2%; P < .001), ICANS grade ≥3 (31.3% vs 10.5%; P < .001), grade 4 neutropenia at M1 (23.5% vs 10.5%; P = .047), grade 4 thrombocytopenia at M1 (28.2% vs 12.3%; P = .024), anemia at M1 (median 9.7 vs 10.8 g/dL; P = .001), and grade 4 thrombocytopenia at M3 (12% vs 2.2%; P = .039). The composite CAR-HEMATOTOX and endothelial activation and stress index (EASIX) scores were risk factors for overall NRM (P < .001).13-15 There was no correlation between response to bridging therapy and overall NRM risk (data not shown). The CAR T-cell products (axi-cel vs tisacel) were not identified as a risk factor for overall NRM (P = .287). A hypogammaglobulinemia (<5 g/L) was not identified as a risk factor for overall NRM. Furthermore, we conducted a univariate analysis on these factors restricted to death of specific infectious cause (supplemental Table 5).
Risk factors for overall NRM by univariate analysis
| . | . | All NRM N = 48 . | Comparative group n = 191 . | P value . |
|---|---|---|---|---|
| Patient characteristics | Age, median, y | 67 | 63 | .003 |
| Diabetes, n (%) | 11 (23.4) | 19 (10.1) | .025 | |
| At lymphodepletion | ECOG PS, (%) | <.001 | ||
| 0-1 | 34 (77.3) | 176 (95.1) | ||
| ≥2 | 10 (22.7) | 9 (4.9) | ||
| Hemoglobin level (g/dL), median | 9.6 | 10.7 | .002 | |
| Platelet count (G/L), median | 156 | 185 | .034 | |
| C-reactive protein level (mg/L), median | 17 | 5 | <.001 | |
| Ferritin level (μg/L), median | 732 | 393 | .002 | |
| Hypogammaglobulinemia, n (%) | 17 (54.8) | 72 (48) | .556 | |
| CAR-HEMATOTOX, n (%) | <.001 | |||
| Low | 20 (41.7) | 136 (71.2) | ||
| High | 28 (58.3) | 55 (28.8) | ||
| EASIX score, median | 17.1 | 10.0 | <.001 | |
| At infusion | CAR T-cell product, n (%) | .287 | ||
| Axi-cel | 31 (64.6) | 139 (72.8) | ||
| Tisacel | 17 (35.4) | 52 (27.2) | ||
| Hemoglobin level (g/dL), median | 9.3 | 10.0 | .002 | |
| Platelet count (G/L), median | 117 | 153 | .007 | |
| C-reactive protein level (mg/L), median | 21 | 8 | .001 | |
| LDH level (IU/L), median | 354 | 221 | .021 | |
| Ferritin level (μg/L), median | 897 | 539 | <.001 | |
| Postinfusion | CRSgrade ≥3, n (%) | 13 (27.1) | 10 (5.2) | <.001 |
| ICANSgrade ≥3, n (%) | 15 (31.3) | 20 (10.5) | <.001 | |
| Grade 4 neutropenia at month 1, n (%) | 8 (23.5) | 19 (10.5) | .047 | |
| Grade 4 thrombocytopenia at month 1, n (%) | 11 (28.2) | 23 (12.3) | .024 | |
| Hemoglobin level (g/dL) at month 1, median | 9.7 | 10.8 | .001 | |
| Grade 4 neutropenia at month 3, n (%) | 2 (7.7) | 5 (2.7) | .213 | |
| Grade 4 thrombocytopenia at month 3, n (%) | 3 (12) | 4 (2.2) | .039 | |
| Hemoglobin level (g/dL) at month 3, median | 11.1 | 11.6 | .139 | |
| Grade 4 neutropenia at month 6, n (%) | 2 (11.1) | 5 (2.9) | .135 | |
| Grade 4 thrombocytopenia at month 6, n (%) | 2 (11.1) | 3 (1.8) | .072 | |
| Hemoglobin level (g/dL) at month 6, median | 11.3 | 12.3 | .105 | |
| Grade 4 neutropenia at month 12, n (%) | 1 (9.1) | 3 (1.8) | .225 | |
| Hemoglobin level (g/dL) at month 12, median | 12.7 | 12.9 | .571 |
| . | . | All NRM N = 48 . | Comparative group n = 191 . | P value . |
|---|---|---|---|---|
| Patient characteristics | Age, median, y | 67 | 63 | .003 |
| Diabetes, n (%) | 11 (23.4) | 19 (10.1) | .025 | |
| At lymphodepletion | ECOG PS, (%) | <.001 | ||
| 0-1 | 34 (77.3) | 176 (95.1) | ||
| ≥2 | 10 (22.7) | 9 (4.9) | ||
| Hemoglobin level (g/dL), median | 9.6 | 10.7 | .002 | |
| Platelet count (G/L), median | 156 | 185 | .034 | |
| C-reactive protein level (mg/L), median | 17 | 5 | <.001 | |
| Ferritin level (μg/L), median | 732 | 393 | .002 | |
| Hypogammaglobulinemia, n (%) | 17 (54.8) | 72 (48) | .556 | |
| CAR-HEMATOTOX, n (%) | <.001 | |||
| Low | 20 (41.7) | 136 (71.2) | ||
| High | 28 (58.3) | 55 (28.8) | ||
| EASIX score, median | 17.1 | 10.0 | <.001 | |
| At infusion | CAR T-cell product, n (%) | .287 | ||
| Axi-cel | 31 (64.6) | 139 (72.8) | ||
| Tisacel | 17 (35.4) | 52 (27.2) | ||
| Hemoglobin level (g/dL), median | 9.3 | 10.0 | .002 | |
| Platelet count (G/L), median | 117 | 153 | .007 | |
| C-reactive protein level (mg/L), median | 21 | 8 | .001 | |
| LDH level (IU/L), median | 354 | 221 | .021 | |
| Ferritin level (μg/L), median | 897 | 539 | <.001 | |
| Postinfusion | CRSgrade ≥3, n (%) | 13 (27.1) | 10 (5.2) | <.001 |
| ICANSgrade ≥3, n (%) | 15 (31.3) | 20 (10.5) | <.001 | |
| Grade 4 neutropenia at month 1, n (%) | 8 (23.5) | 19 (10.5) | .047 | |
| Grade 4 thrombocytopenia at month 1, n (%) | 11 (28.2) | 23 (12.3) | .024 | |
| Hemoglobin level (g/dL) at month 1, median | 9.7 | 10.8 | .001 | |
| Grade 4 neutropenia at month 3, n (%) | 2 (7.7) | 5 (2.7) | .213 | |
| Grade 4 thrombocytopenia at month 3, n (%) | 3 (12) | 4 (2.2) | .039 | |
| Hemoglobin level (g/dL) at month 3, median | 11.1 | 11.6 | .139 | |
| Grade 4 neutropenia at month 6, n (%) | 2 (11.1) | 5 (2.9) | .135 | |
| Grade 4 thrombocytopenia at month 6, n (%) | 2 (11.1) | 3 (1.8) | .072 | |
| Hemoglobin level (g/dL) at month 6, median | 11.3 | 12.3 | .105 | |
| Grade 4 neutropenia at month 12, n (%) | 1 (9.1) | 3 (1.8) | .225 | |
| Hemoglobin level (g/dL) at month 12, median | 12.7 | 12.9 | .571 |
Risk factors for overall NRM were identified by comparing the characteristics of the overall NRM population with those of patients alive at 1 year after CAR T-cell infusion.
We then performed a multivariate analysis using logistic regression on variables collected at lymphodepletion. After testing these factors for the overall NRM population (n = 48) and for patients alive without progression at 1 year after CAR T-cell infusion (n = 191), 2 parameters were associated with an increased risk of overall NRM: diabetes (OR, 4.417; 95% CI, 1.651-11.819) and elevated ferritin level at lymphodepletion (OR, 2.716; 95% CI, 1.003-7.350; Table 4). In a subgroup multivariate analysis, diabetes was associated with an increased risk of overall NRM of infectious cause (OR, 8.089; 95% CI, 2.865-22.840).
Risk factors of NRM at lymphodepletion by multivariate analysis
| Parameter . | Odds ratio estimate . | Lower 95% CI limit for odds ratio . | Upper 95% CI limit for odds ratio . |
|---|---|---|---|
| Age, y, ≥65, yes vs no | 1.033 | 0.992 | 1.075 |
| Diabetes, yes vs no | 4.417 | 1.651 | 11.819 |
| ECOG PS of ≥2 at lymphodepletion, yes vs no | 2.354 | 0.607 | 9.126 |
| Platelets < 50 G/L at lymphodepletion, yes vs no | 1.133 | 0.446 | 2.878 |
| Elevated ferritin level at lymphodepletion, yes vs no | 2.716 | 1.003 | 7.350 |
| C-reactive protein level > 30 mg/L at lymphodepletion, yes vs no | 1.246 | 0.399 | 3.892 |
| Parameter . | Odds ratio estimate . | Lower 95% CI limit for odds ratio . | Upper 95% CI limit for odds ratio . |
|---|---|---|---|
| Age, y, ≥65, yes vs no | 1.033 | 0.992 | 1.075 |
| Diabetes, yes vs no | 4.417 | 1.651 | 11.819 |
| ECOG PS of ≥2 at lymphodepletion, yes vs no | 2.354 | 0.607 | 9.126 |
| Platelets < 50 G/L at lymphodepletion, yes vs no | 1.133 | 0.446 | 2.878 |
| Elevated ferritin level at lymphodepletion, yes vs no | 2.716 | 1.003 | 7.350 |
| C-reactive protein level > 30 mg/L at lymphodepletion, yes vs no | 1.246 | 0.399 | 3.892 |
Logistic regression for the prediction of NRM comparing the overall NRM population with patients alive without progression at 1 year after CAR T-cell infusion.
Discussion
CAR T cells have dramatically improved the outcome of relapsed/refractory LBCL. Accumulating evidence from the real-world setting enable a better understanding of their efficacy and toxicity. However, little is known about the NRM after CAR T-cell therapy.
The aim of our study was to describe the rates and causes of NRM associated with CD19 CAR T-cells in patients with LBCL and identify risk factors of NRM. The NRM reported in our study (5.0%) is in line with the NRM reported in pivotal trials (0%-3.7%)1-3 and other real-world cohorts (1.2%-6%).6-10 To the best of our knowledge, this is the largest cohort focusing on NRM after CAR T-cell therapy, with 48 NRMs of 957 patients who received infusion.
Overall, 19% of NRMs occurred before day 28, and 81% beyond day 28 after infusion. Interestingly, early NRM represents <1% of all treated patients, highlighting the fact that fatal acute toxicities of CAR T cells are uncommon. Overall, the vast majority of NRMs occur at later time points, usually months after CAR T-cell infusion. We found that NRM was predominantly due to infections (56% of all NRMs). Infections were responsible for 44% (4 of 9) and 59% (23 of 39) of the deaths observed before day 28 and beyond day 28 after infusion, respectively. This could prompt clinicians to use granulocyte colony-stimulating factor early, from day 2 after infusion, which was associated with a significantly reduced febrile neutropenia incidence after CAR T-cell infusion without increasing other toxicity rates or affecting response rates.16 Interestingly, no fatal COVID-19 occurance was reported in the early NRM population. This could be due to preventive procedures, such as preadmission severe acute respiratory syndrome coronavirus 2 nasal testing and intrahospital barriers, to prevent nosocomial cases. In the late NRM population, among 23 fatal infections, 13 (57%) were due to COVID-19, and 10 (43%) were non–COVID-19. Based on these observations, physicians should consider patients undergoing CAR T-cell therapy to be at risk of severe and potentially lethal infections at any time after infusion. Specifically, COVID-19 vaccination before and after CAR T-cell infusion as well as curative treatment in the case of COVID-19 occurring after CAR T-cell infusion should be encouraged. It was previously reported that the CAR-HEMATOTOX score, composed of 5 prelymphodepletion variables, can be used to identify patients at high risk for severe infections between days 0 nd 90 after infusion.17 In our study, a high CAR-HEMATOTOX score was a risk factor for both early NRM and overall NRM in univariate analysis.
To date, the identification of risk factor for NRM has been limited by the study of small cohorts of patients or pharmacovigilance databases.11,12 Wudhikarn et al described a high burden of organ toxicities in a cohort of 60 patients with diffuse LBCL treated with CD19 CAR T cells.18 They reported a total of 289 grade ≥3 toxicities including hematologic, metabolic, infectious, and neurologic complications, with a 1-year cumulative incidence of 57.7%, 54.8%, 35.4%, and 18.3%, respectively. However, the majority of toxicities were manageable, and NRM occurred only in 2 patients after a median follow-up of 9 months, resulting in an estimated 1-year NRM of 1.7% (95% CI, 0.1-8.0). In a univariate analysis, a severe pulmonary complication (grade ≥3) increased the NRM risk of a given patient by 3 times. Conversely, neither severe CRS nor neurologic toxicities were associated with an increased risk of mortality. In our study, we identified both diabetes and an elevated ferritin level at lymphodepletion as risk factors of overall NRM in a multivariate analysis. Interestingly, diabetes was associated with an increased risk of lethal infection in a subgroup multivariate analysis. Although it is well known that, in the general population, diabetes and poor glycemic control are associated with an increased risk of infection, specific studies on patients treated with CAR T cells are lacking.19,20 Future studies should investigate whether infectious NRM after CAR T-cell therapy may be reduced by better glycemic control in patients who are diabetic. Elevated ferritin level at lymphodepletion was shown to correlate with severe CRS and ICANS in patients treated with CAR T cells for LBCL and was incorporated into the EASIX–combined with ferritin and EASIX–combined with CRP and ferritin scores to predict these complications, respectively.21 Furthermore, elevated ferritin level at lymphodepletion is one of the parameters of the CAR-HEMATOTOX score, which is used to stratify the risk of prolonged cytopenia after CAR T-cell therapy. Importantly, a high CAR-HEMATOTOX score is associated with a higher risk of severe infections between days 0 and 90 after infusion.13,17
When comparing axi-cel vs tisacel in the DESCAR-T registry using propensity score matching, Bachy et al observed no significant difference with regard to grade 5 adverse events. Similarly, in our study, we did not observe differences in NRM when comparing these CAR T-cell products (axi-cel and tisacel).22
Our study has several strengths. To the best of our knowledge, this is the largest series focusing on NRM after CAR T-cell therapy. We used the French national registry (DESCAR-T), which collects data for all patients treated with CAR T cells in France, in an exhaustive manner. Furthermore, real-world cohorts are more relevant than clinical trials to study NRM because they include less selected patients who are more representative of current practice, including patients who are older, frail, and/or with comorbidities. Nevertheless, our study also has some limitations. The analysis focused on NRM experienced by patients with LBCL treated with CAR T cells between July 2018 and April 2022 and is affected by deaths attributable to COVID-19. Thus, the COVID-19 pandemic may overestimate the NRM due to infectious fatal events. It is well known that patients treated with CAR T cells are at risk of severe/fatal COVID-19, even if vaccinated, because of an impaired immune response to COVID-19 vaccination.23,24 The development of preexposure prophylaxis of COVID-19 for patients with frail immune systems could be of interest even if it has not been studied in much detail among patients treated with CAR T cells.25 Furthermore, management of patients with COVID-19 may include anti–severe acute respiratory syndrome coronavirus 2 monoclonal antibodies,26,27 antiviral therapy,28-30 dexamethasone,31 and convalescent plasma therapy,32 depending on whether they require hospitalization or not. This therapeutic arsenal was not available at the beginning of the pandemic, and recommendations have evolved throughout the development of these prophylactic and curative strategies. Thus, from the beginning of the pandemic, COVID-19 management in our cohort might have been suboptimal and heterogeneous across centers and time periods. Furthermore, overestimation of COVID-19–attributable deaths in our study may underestimate NRM due to alternative causes, especially for late NRM. Indeed, we did not report any fatal COVID-19 occurance before day 28 after infusion.
To the best of our knowledge, this is the largest study focusing on NRM after CAR T-cell therapy in the real-world setting. This analysis confirms a NRM rate of ∼5% in patients with LBCL treated with CD19 CAR T cells. Improving our knowledge on NRM may help guide the selection and management of patients and eventually improve the outcome of patients after CAR T-cell therapy.
Acknowledgments
The authors thank the patients whose data were collected in the DESCAR-T registry and their families. The authors thank everyone from the LYSARC DESCAR-T study group who actively participated in the study and T. Fradon and E. Gat from the biostatistics department.
The DESCAR-T registry was partly funded by Gilead and Novartis; however, they did not participate in the study design, data collection, statistical analysis, or interpretation; and they did not provide assistance for manuscript writing or editorial support.
Authorship
Contribution: J.L. and R.H. contributed to study design; E.B., G.C., D.B., T.G., R.D.B., M.T.R, S.G., M.M., R.-O.C., M.J., C.C.-L, C. Herbaux, O.H., M.L., S.C., P.B., C. Haioun, P.S., S.L.G., F.M., C.T., and R.H. enrolled and treated patients; T.F. performed statistical analyses; all authors analyzed and interpreted the data; and all authors contributed to the writing of the manuscript and approved its final version.
Conflict-of-interest disclosure: E.B. received honoraria from Kite, a Gilead Company, and Novartis. G.C. received honoraria from Kite, a Gilead Company, and Novartis. T.G. received honoraria from Kite, a Gilead Company. R.D.B. received honoraria from Kite, a Gilead Company, and Novartis. S.G. received honoraria from Kite/Gilead, Incyte, Takeda, and Janssen. M.M. received honoraria from Kite, a Gilead Company, and Novartis. R.-O.C. received honoraria from Kite, a Gilead Company. C.C.-L. received honoraria from Kite, a Gilead Company. M.L. received honoraria from Kite, a Gilead Company, and Novartis. S.L.G. reports consultancy for Kite, a Gilead Company, and Novartis. C.T. received honoraria from Kite, a Gilead Company, and Novartis. R.H. received honoraria from Kite, a Gilead Company, and Novartis. The remaining authors declare no competing financial interests.
Correspondence: Jean Lemoine, Service d’hématologie, CHU Rennes, 2 rue Henri le Guilloux, 35000 Rennes, France; e-mail: jean.lemoine@aphp.fr.
References
Author notes
Presented previously as “Causes and risk factors of early and late nonrelapse mortality after CD19 CAR T-cell therapy for diffuse large B-cell lymphoma (DLBCL): a LYSA study from the DESCAR-T registry.” Blood. 2022;140 (suppl 1):1859–1861.
Data are available on request from the corresponding author, Jean Lemoine (jean.lemoine@aphp.fr).
The full-text version of this article contains a data supplement.