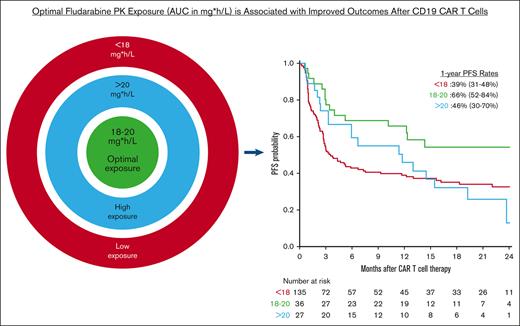
Key Points
Previous studies showed that fludarabine PK exposure is associated with key outcomes in pediatric patients after tisagenlecleucel.
In adults receiving axi-cel, an estimated fludarabine AUC of 18 to 20 mgh/L is associated with improved survival without increased toxicity.
Abstract
Fludarabine is one of the most common agents given for lymphodepletion before CD19 chimeric antigen receptor T cells, but its optimal therapeutic intensity is unknown. Using data from a multicenter consortium, we estimated fludarabine exposure (area under the curve [AUC]) using a population pharmacokinetic (PK) model in 199 adult patients with aggressive B-cell non-Hodgkin lymphomas who received commercial axicabtagene ciloleucel (Axi-cel). We evaluated the association of estimated fludarabine AUC with key outcomes, aiming to find an AUC that optimized efficacy and tolerability. We identified low (<18 mg × hour/L [mgh/L]), optimal (18-20 mgh/L), and high (>20 mgh/L) AUC groups for analyses; the 6-month cumulative incidences of relapse/progression of disease (relapse/POD) by AUC groups were 54% (45%-62%), 28% (15%-44%), and 30% (14%-47%), respectively; and the 1-year progression-free survival (PFS) rates were 39% (31%-48%), 66% (52%-84%), and 46% (30%-70%) and the overall survival (OS) rates were 58% (50%-67%), 77% (64%-92%), and 66% (50%-87%), respectively. In multivariable analyses compared with low AUC, an optimal AUC was associated with the highest PFS (hazard ratio [HR], 0.52; 0.3-0.91; P = .02) and lowest risk of relapse/POD (HR, 0.46; 0.25-0.84; P = .01) without an increased risk of any-grade cytokine release syndrome (HR, 1.1; 0.7-1.6; P = .8) or and immune effector cell–associated neurotoxicity syndrome (ICANS) (HR, 1.36; 0.83-2.3; P = .2). A high AUC was associated with the greatest risk of any-grade ICANS (HR, 1.9; 1.1-3.2; P = .02). Although the main cause of death in all groups was relapse/POD, nonrelapse-related deaths, including 3 deaths from ICANS, were more frequent in the high AUC group. These findings suggest that PK–directed fludarabine dosing to achieve an optimal AUC may result in improved outcomes for patients receiving axi-cel.
Introduction
CD19–directed chimeric antigen receptor (CAR) T-cell therapies have transformed the treatment landscape for relapsed/refractory aggressive B-cell non-Hodgkin lymphomas (r/r B-NHL). However, patients treated with CAR T cells may incur serious toxicities and up to 60% experience eventual disease relapse, often within 6 months.1-3 One component of CAR T-cell therapy is the use of immunosuppressive lymphodepleting chemotherapy (LD chemo) regimen that modifies the lymphoproliferative cytokine environment, depletes regulatory T-cell populations, and enhances T-cell expansion, potency, and efficacy.4,5 For example, in pediatric patients with B-cell acute lymphoblastic leukemia (B-ALL) treated with 19 to 28z+ CAR T cells, overall survival (OS) was superior when a higher-dose LD chemo was used, in part because of better T-cell expansion.6 In adults with r/r B-NHL receiving CD19 CAR T cells, higher intensity LD chemo was associated with favorable circulating cytokines, more robust T-cell expansion, and improved progression-free survival (PFS).7 Although these studies suggest that more intensive LD chemo is associated with better outcomes, the optimal therapeutic intensity remains undefined.
Fludarabine with cyclophosphamide (Flu/Cy) is the most widely used LD chemo regimen before CD19 CAR T cells. Fludarabine is administered by body-surface area (BSA)–based dosing. However, BSA-based dosing ultimately leads to highly variable cumulative pharmacokinetic (PK) exposure among patients because the main predictors of fludarabine exposure in a validated population PK model are weight and renal function (not BSA).8 Pharmacodynamic (PD) analyses reported that fludarabine underexposure or overexposure was associated with treatment failure and poorer survival after allogeneic hematopoietic cell transplantation.9 Similarly, fludarabine exposure may impact outcomes after CAR T-cell therapy. For example, in pediatric patients with B-ALL receiving tisagenlecleucel, an estimated fludarabine exposure, or area under the curve (AUC), of ≥13.8 mg × hour/L was associated with markedly lower rates of relapse and improved survival.10 Another study evaluating children and young adults with B-ALL reported a nearly identical fludarabine AUC threshold, based on measured fludarabine blood concentrations, which predictive of better disease-free survival.11
Thus, we hypothesized that variable fludarabine exposure also influences outcomes for adult patients with r/r aggressive B-NHL after CD19 CAR T-cell therapy. To test this hypothesis, we used a population PK model to estimate cumulative fludarabine AUC in these patients and then evaluated its association with key outcomes, aiming to identify an optimal therapeutic exposure target that maximized efficacy and tolerability.
Methods
This retrospective, multicenter study used data from the Cell Therapy Consortium (CTC) registry, a working group of 8 US academic institutions formed to aggregate data and resources to perform observational studies on comprehensive real-world data. Additional patient data from Hackensack University Medical Center were included. This study includes consecutive adult patients with r/r aggressive B-NHL who underwent apheresis for commercial CAR T-cell therapy from April 2018 to June 2021 with a data cutoff of March 2022. Clinical characteristics, laboratory data, pathology, CAR T-cell treatment details, toxicities, and responses from each institution were recorded in a centralized research electronic data capture (REDCap) database. The study was approved by the individual institutional review boards and was conducted according to the Declaration of Helsinki. Product and patient selection, bridging therapy, toxicity management, response assessment, and administration site (inpatient or outpatient) followed institutional practices. Cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS) were graded using the American Society for Transplantation and Cellular Therapy consensus criteria.12 Tumor responses after CAR T-cell infusion were assessed per Lugano criteria by the treating clinicians.13
Eligible patients included adults (aged ≥18 years) with r/r aggressive B-NHL who received Flu/Cy LD chemo followed by CD19-directed CAR T-cell therapy with axicabtagene ciloleucel (axi-cel). The patients received Flu/Cy dosing as per the Food and Drug Administration package insert: fludarabine at 30 mg/m2 and cyclophosphamide 500 mg/m2 daily for 3 days beginning on day –5 before axi-cel infusion. Any fludarabine dose adjustments made for individual patients was done per clinical discretion using local institutional guidelines. Patients were excluded from the analysis if they received an LD chemo regimen other than Flu/Cy, if their actual body weight was not supported by the population PK model (>130 kg), if the time between the end of LD chemo and axi-cel infusion was >3 days, and/or if the LD chemo was given on an interrupted schedule.10 Patients who received only 2 doses of fludarabine (n = 2) were included if their LD chemo was given in a sequential, uninterrupted schedule on the appropriate days. The cumulative fludarabine exposure, or AUC, of each patient was estimated via a validated population PK model using the InsightRX precision dosing software (InsightRX, Inc, San Francisco, CA).8 The patient variables required to estimate individual fludarabine exposures included estimated glomerular filtration rate (using the Cockcroft-Gault equation), actual body weight, height, and the daily dosage of fludarabine administered.
The main outcomes assessed were PFS, OS, and cumulative incidences of relapse or progression of disease (relapse/POD), CRS, and ICANS. OS and PFS were analyzed using Kaplan-Meier analyses, whereas the incidences of relapse/POD, CRS, and ICANS were analyzed using competing risks analysis with a competing risk of death. To define an optimal fludarabine AUC, univariable (UV) hazard ratio (HR) plots were drawn with P-splines and AUC groups were assigned by visually assessing where the HRs (and confidence intervals) crossed to <1 for relapse/POD, PFS, and OS, thereby signifying more favorable outcomes, and with no significant increase in CRS or ICANS (supplemental Figures 1-2).10 The AUC groups were used in the UV and multivariable (MV) Cox proportional hazards models (supplemental Tables 2-3) along with clinical and demographic variables that were significant on UV analysis (P < .05). These additional variables included in the analyses were age, sex (male or female), disease type, disease stage, International Prognostic Index score, number of lines of prior therapy, lactate dehydrogenase (LDH) levels before LD chemo, presence or absence of bulky disease, and receipt of bridging therapy. All analyses were conducted in R v.4.2.2.
Results
A total of 199 patients met the inclusion criteria for analyses and their characteristics are shown in Table 1. Patient characteristics stratified by fludarabine AUC group are shown in supplemental Table 1. The median age was 60 years (range, 18-86) and 149 patients (75%) were male. Disease types were diffuse large B-cell lymphoma in 131 patients (66%), transformed follicular lymphoma in 39 (20%), high-grade B-cell lymphoma in 19 (9%), and other aggressive B-NHL in 10 (5%). Most patients had stage IV disease (n = 126; 64%), had received 1 to 3 prior lines of therapy (121; 61%), and received bridging therapy (127; 64%) before axi-cel. The median LDH level at the time of LD chemo administration was 252 U/L (range, 115-4655). The median weight and creatinine level before LD chemo was 82 kg (range, 44-130) and 0.9 mg/dL (range, 0.3-1.9). Among all patients, the estimated cumulative fludarabine AUC was 16.5 mg × hour/L (mgh/L) (range, 9.3-23.3). Based on the results of P-splines curves, we observed and defined the following 3 fludarabine groups: low AUC (<18 mgh/L), optimal AUC (18-20 mgh/L), and high AUC (>20 mgh/L). Most patients (n = 135; 68%) were in the low AUC group, and 36 (18%) and 27 patients (14%) were in the optimal and high AUC groups, respectively. The median follow-up among survivors was 20.4 months (interquartile range, 15-25 months).
Patient characteristics
| Characteristic . | n (%) . |
|---|---|
| Total number of patients | 199 |
| Age, median (range; IQR) | 60 (18-86; 53-67) |
| Sex | |
| Male | 149 (75) |
| Female | 50 (25) |
| Pre-LDC weight, kg median (range; IQR) | 82 (44-130; 69-95) |
| Pre-LDC creatinine, mg/dL median (range; IQR) | 0.9 (0.3-1.9; 0.7-1.1) |
| Pre-LDC estimated GFR, ml/min Median (range; IQR) | 100 (80-128; 32-241) |
| Disease type | |
| DLBCL | 131 (66) |
| tFL | 39 (20) |
| HGBL | 19 (9) |
| Other | 10 (5) |
| Disease stage | |
| I | 14 (7) |
| II | 31 (16) |
| III | 27 (14) |
| IV | 126 (64) |
| Unknown | 1 |
| IPI | |
| 0-1 | 23 (19) |
| 2 | 37 (30) |
| 3 | 48 (39) |
| 4-5 | 16 (13) |
| Unknown | 75 |
| Prior lines of therapy | |
| 1-3 | 121 (61) |
| 4-5 | 55 (28) |
| >5 | 23 (12) |
| Bulky disease (≥10 cm) | |
| Yes | 30 (15) |
| No | 168 (85) |
| Unknown | 1 |
| Bridging therapy | |
| Yes | 127 (64) |
| No | 72 (36) |
| Pre-LDC LDH, U/L, median (range) | 252 (115-4655) |
| Estimated fludarabine exposure, mgh/L, median (range) | 16.5 (9.3-23.3) |
| Characteristic . | n (%) . |
|---|---|
| Total number of patients | 199 |
| Age, median (range; IQR) | 60 (18-86; 53-67) |
| Sex | |
| Male | 149 (75) |
| Female | 50 (25) |
| Pre-LDC weight, kg median (range; IQR) | 82 (44-130; 69-95) |
| Pre-LDC creatinine, mg/dL median (range; IQR) | 0.9 (0.3-1.9; 0.7-1.1) |
| Pre-LDC estimated GFR, ml/min Median (range; IQR) | 100 (80-128; 32-241) |
| Disease type | |
| DLBCL | 131 (66) |
| tFL | 39 (20) |
| HGBL | 19 (9) |
| Other | 10 (5) |
| Disease stage | |
| I | 14 (7) |
| II | 31 (16) |
| III | 27 (14) |
| IV | 126 (64) |
| Unknown | 1 |
| IPI | |
| 0-1 | 23 (19) |
| 2 | 37 (30) |
| 3 | 48 (39) |
| 4-5 | 16 (13) |
| Unknown | 75 |
| Prior lines of therapy | |
| 1-3 | 121 (61) |
| 4-5 | 55 (28) |
| >5 | 23 (12) |
| Bulky disease (≥10 cm) | |
| Yes | 30 (15) |
| No | 168 (85) |
| Unknown | 1 |
| Bridging therapy | |
| Yes | 127 (64) |
| No | 72 (36) |
| Pre-LDC LDH, U/L, median (range) | 252 (115-4655) |
| Estimated fludarabine exposure, mgh/L, median (range) | 16.5 (9.3-23.3) |
DLBCL, diffuse large B-cell lymphoma; GFR, glomerular filtration rate (by Cockcroft-Gault equation); HGBL, high-grade B-cell lymphoma; IPI, International Prognostic Index; IQR, interquartile range; LDC, lymphodepleting chemotherapy; tFL, transformed follicular lymphoma.
Treatment outcomes
A summary of the main clinical outcomes stratified by fludarabine AUC groups is shown in Table 2. Among all patients, the 6-month and 1-year cumulative incidences of relapse/POD were 46% (confidence interval [CI], 39-53) and 50% (CI, 43-57), respectively. The 6-month cumulative incidences of relapse/POD by low, optimal, and high AUC groups were 54% (CI, 45-62), 28% (CI, 15-44), and 30% (CI, 14-47), respectively. In UV analyses, an optimal fludarabine AUC was significantly associated with the lowest risk of relapse/POD, whereas a low fludarabine AUC was associated with the highest risk of relapse/POD. In multivariable analyses compared with low AUC, an optimal fludarabine AUC (HR, 0.5; CI, 0.25-0.84; P = .01) was associated with the lowest risk of relapse/POD, whereas higher LDH (HR, 2.2; CI, 1.7-2.9; P < .001) and presence of bulky disease (HR, 1.9; CI, 1.2-3.2; P = .01) were associated with a higher risk of relapse/POD.
Summary of main outcomes of interest by fludarabine exposure group
| Outcome . | AUC < 18 (n = 136), % (95% CI) . | AUC, 18-20 (n = 36), % (95% CI) . | AUC > 20 (n = 27), % (95% CI) . |
|---|---|---|---|
| 6-mo relapse/POD | 57 (48-65) | 32 (17-47) | 43 (23-62) |
| 1-y PFS | 39 (31-48) | 66 (52-84) | 46 (30-70) |
| 1-y OS | 58 (50-67) | 77 (64-92) | 66 (50-87) |
| 1-y NRM | 5 (2-9) | 3 (0.2-13) | 11 (3-26) |
| Day +30 all-grade CRS | 79 (71-85) | 78 (60-89) | 85 (63-95) |
| Day +30 all-grade ICANS | 44 (36-53) | 56 (38-70) | 70 (49-84) |
| Day +30 grade ≥3 ICANS | 30 (22-38) | 38 (23-55) | 37 (19-55) |
| Outcome . | AUC < 18 (n = 136), % (95% CI) . | AUC, 18-20 (n = 36), % (95% CI) . | AUC > 20 (n = 27), % (95% CI) . |
|---|---|---|---|
| 6-mo relapse/POD | 57 (48-65) | 32 (17-47) | 43 (23-62) |
| 1-y PFS | 39 (31-48) | 66 (52-84) | 46 (30-70) |
| 1-y OS | 58 (50-67) | 77 (64-92) | 66 (50-87) |
| 1-y NRM | 5 (2-9) | 3 (0.2-13) | 11 (3-26) |
| Day +30 all-grade CRS | 79 (71-85) | 78 (60-89) | 85 (63-95) |
| Day +30 all-grade ICANS | 44 (36-53) | 56 (38-70) | 70 (49-84) |
| Day +30 grade ≥3 ICANS | 30 (22-38) | 38 (23-55) | 37 (19-55) |
AUC in mgh/L.
NRM, nonrelapse mortality.
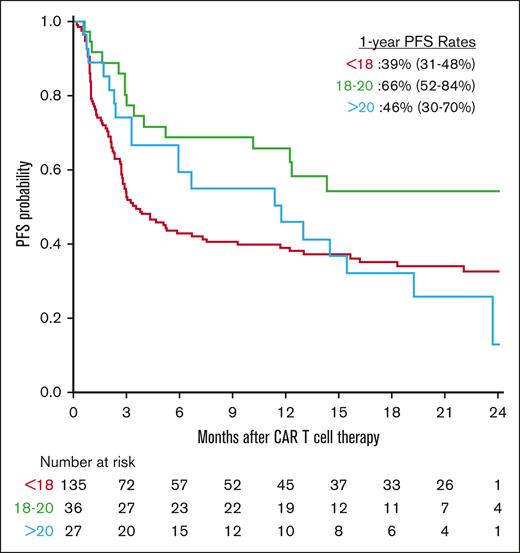
The 1-year PFS rates by low, optimal, and high AUC groups were 39% (CI, 31-48), 66% (CI, 52-84), and 46% (CI, 30-70), respectively, with corresponding median PFS in months of 3.6 (CI, 2.8-7.5), not reached (CI, 12-not reached), and 12 (CI, 6-not reached) (Figure 1). In UV analyses, an optimal fludarabine AUC was significantly associated with the most favorable PFS. In MV analyses compared with low AUC, an optimal fludarabine AUC (HR, 0.5; CI, 0.3-0.91; P = .02) was associated with improved PFS, whereas higher LDH (HR, 2.2; CI, 1.7-2.8; P < .001) and presence of bulky disease (HR, 1.8; CI, 1.1-2.9; P = .02) were associated with a poorer PFS.
The Kaplan-Meier plots show the PFS estimates stratified by the 3 fludarabine AUC groups. One-year PFS estimates are shown in the figure.
The Kaplan-Meier plots show the PFS estimates stratified by the 3 fludarabine AUC groups. One-year PFS estimates are shown in the figure.
The 1-year OS rates by low, optimal, and high AUC groups were 58% (CI, 50-67), 77% (CI, 64-92), and 66% (CI, 50-87), respectively, with corresponding median OS in months of 18 (CI, 12-not reached), not reached (CI, 16-not reached), and 16 (CI, 13-not reached) months (supplemental Figure 3). In UV analyses, an optimal fludarabine AUC was significantly associated with lowest risk of all-cause death. In MV analyses compared with low AUC, an optimal fludarabine AUC (HR, 0.75; CI, 0.4-1.4; P = .4) was not associated with improved OS compared with the other AUC groups. Higher LDH (HR, 2.6; CI, 1.9-3.5; P < .001) and presence of bulky disease (HR, 2.0; CI, 1.2-3.3; P = .01) were associated with a poorer OS.
Among all patients, the day +30 cumulative incidences of all-grade CRS and grade ≥3 CRS were 80% (CI, 74-85) and 8% (CI, 4-12), respectively. Among the low, optimal, and high AUC groups, the day +30 cumulative incidences of all-grade CRS were 79% (CI, 71-85), 78% (CI, 60-89), and 85% (CI, 63-95), respectively. In multivariable analyses, fludarabine AUC was not associated with an increased risk of CRS. Given the limited number of grade ≥3 CRS events, further statistical tests were not able to be performed. Among all patients, the day +30 cumulative incidences of all-grade ICANS and grade ≥3 ICANS were 50% (CI, 43-57) and 32% (CI, 26-39), respectively. Among the low, optimal, and high AUC groups, the day +30 cumulative incidences of all-grade ICANS were 44% (CI, 36-53), 56% (CI, 38-70), and 70% (CI, 49-84) and grade ≥3 ICANS were 30% (CI, 22-38), 39% (CI, 23-55), and 37% (CI, 19-55), respectively. In multivariable analyses compared with low AUC, a high fludarabine AUC (HR, 1.9; CI, 1.1-3.2; P = .02) was associated with an increased risk of all-grade ICANS. Receipt of 4 to 5 prior lines of therapy (HR, 1.8; CI, 1.2-2.8; P = .01) and >5 prior lines of therapy (HR, 2.6; CI, 1.5-4.6; P = .001) were associated with an increased risk of all-grade ICANS. A higher LDH (HR, 1.6; CI, 1.1-2.3; P = .02) was associated with an increased risk of grade ≥3 ICANS.
Causes of death
In the low, optimal, and high AUC groups, there were 65, 12, and 14 patient deaths, respectively. In the low AUC group, causes of death (COD) were relapse/POD (n = 57; 88%), infection (n = 3; 5%), and toxicity (n = 4; 6%); in the optimal AUC group, COD were relapse/POD (n = 8; 67%), infection (n = 3; 25%), and toxicity (n = 1; 8%); and in the high AUC group, COD were relapse/POD (n = 7; 50%), infection (n = 2; 14%), and toxicity (n = 5; 36%), with 3 of these deaths resulting from ICANS. One-year nonrelapse mortality rates by low, optimal, and high AUC group were 5% (CI, 2-9), 3% (CI, 0.2-13), and 11% (CI, 3-26), respectively.
Discussion
By using a population PK model for estimation, we observed highly variable fludarabine exposures among a large cohort of patients who received standard Flu/Cy before axi-cel for r/r aggressive B-NHL, likely because of a wide range of weights and renal function (as estimated by glomerular filtration rate). Moreover, a cumulative fludarabine AUC window of 18 to 20 mgh/L was associated with improved PFS and expected rates of CRS and ICANS.2 Patients with a low fludarabine AUC (<18 mgh/L) had a higher risk of lymphoma–related treatment failure, whereas patients with high fludarabine AUC (>20 mgh/L) had a higher risk of all-grade ICANS and similar survival to those in the low exposure group owing to a higher proportion of deaths because of nonrelapse mortality. The results of our PK/PD study, which to the best of our knowledge is the largest analysis of its kind, add to a growing body of evidence suggesting that, at least for certain CAR T-cell products, there are optimal fludarabine therapeutic exposures that are associated with more favorable outcomes.
It has been long known that LD chemo enhances the activity of adoptive T-cell therapies.14 Numerous LD chemo regimens have been studied both preclinically and in practice, and although the ideal LD regimen is unknown, Flu/Cy has emerged as a mainstay. In fact, the Food and Drug Administration package inserts for all 3 commercially approved CD19 CAR T-cell products for aggressive B-NHL include recommendations to use Flu/Cy LD chemo before cell infusion, albeit at different doses.1-3 Previous studies suggested that higher-dosed (more intensive) LD chemo before CD19 CAR T cells led to a favorable cytokine milieu and, importantly, more effective antitumor activity. A recent analysis in patients with B-ALL receiving tisagenlecleucel estimated fludarabine exposure using a population PK model to help define a favorable therapeutic exposure threshold.10 Dekker et al took this a step further by more precisely measuring fludarabine PK concentrations in similar patients, confirming these findings.11 Interestingly, this group noted that although the fludarabine exposure ranges estimated by the population PK model were comparable with measured exposures, there was some individual patient variability. Although the reasons for this are not entirely clear, it is likely that measured PK–directed fludarabine dosing can overcome this potential barrier.
Our study has several unique strengths. First, these are multicenter, real-world data from the CTC registry that evaluated outcomes in adult patients with r/r aggressive B-NHL, the most common indication for CD19-directed CAR T-cell therapy globally. Furthermore, our results suggest a potential optimal therapeutic window, rather than a threshold, associated with improved outcomes, wherein excessive LD chemo intensity may also be detrimental. Whether high fludarabine exposure may be associated with excessive toxicities outside of CRS and ICANS, such as specific infections and/or prolonged cytopenias, although not addressed by our analysis, is a hypothesis that we intend to evaluate in future studies.15-17 Our study also has several limitations. Given its retrospective nature, we could not collect fludarabine PK levels in real-time to internally confirm the precision of the fludarabine AUC estimates for each patient, although these efforts are ongoing. Moreover, we did not evaluate the estimated PK exposure of cyclophosphamide, a prodrug with highly complex pharmacologic metabolism for which contemporary population PK models are unavailable.18 The study patients were mostly male, and patients with heavier weights (>130 kg) were excluded as estimation of their fludarabine exposure is poorly defined by the population PK model. As was done by Fabrizio et al, we restricted the analysis to patients who received uninterrupted Flu/Cy and those who did not have a prolonged time between the end of LD chemo and CAR T-cell infusion.10 Whether a longer duration of time influences the association of fludarabine exposure with outcomes requires further inquiry. Influential pretreatment factors such as overall metabolic tumor volume and detailed cytokine profiles were unavailable for inclusion in the analysis.19,20 We did not have more granular details on the COD including, for example, the specific infections that led to death in some. The sample size of our optimal AUC group was relatively small, which possibly limited the appropriate necessary statistical power to observe significant differences in certain outcomes such as OS. Most importantly, our findings require independent external validation, which is ongoing, followed by prospective evaluation in a clinical trial.
Despite these limitations, our data suggest that a more personalized fludarabine exposure is achievable using population PK–directed dosing, thus representing a novel and easily modifiable strategy to improve outcomes after CD19 CAR T-cell therapy. We intend to evaluate the association of fludarabine exposure and outcomes in other CD19 CAR T-cell products, such as tisagenlecleucel and lisocabtagene maraleucel, and other indications, such as indolent B-NHL. Future research efforts will include real-time fludarabine PK concentration measurement to correlate these with estimated exposure using the model. We will also address unanswered questions, including whether there is an association between fludarabine exposure and organ toxicities, cytokine profiles, and CAR T-cell expansion kinetics. In addition, comparisons of population PK-based fludarabine (in the context of Flu/Cy) with other LD-chemo regimens, such as single-agent bendamustine, will be of considerable interest.
Acknowledgments
The authors thank the data managers at each Cell Therapy Consortium member institution who collected patient data and maintained the database.
This research was supported in part by a grant from Novartis Pharmaceuticals, Inc (D.L.P.), along with National Institutes of Health, National Cancer Institute P30 CA008748 (all Memorial Sloan Kettering Cancer Center authors), and the National Center for Advancing Translational Sciences of the National Institutes of Health award number UL1-TR002494 (V.B.). The content is solely the responsibility of the authors.
Authorship
Contribution: M.S. was responsible for the study conception and design; M.S., J.R.F., S.M.D., M.G., A.P., A.A.T., R.S., J.B., and P.A.R. were responsible for data acquisition and/or data analyses; M.S. was responsible for writing the manuscript; and all authors reviewed the results, provided critical feedback on data interpretation, manuscript editing, approved the final version of the manuscript, and confirmed the decision to submit the manuscript for publication.
Conflict-of-interest disclosure: M.S. served as a paid consultant for McKinsey & Company, Angiocrine Bioscience, Inc, and Omeros Corporation; received research funding from Angiocrine Bioscience, Inc, and Omeros Corporation; served on ad hoc advisory boards for Kite – A Gilead Company; and received honoraria from i3Health and Medscape for CME-related activity. D.L.P. has received honoraria for consulting or advisory board participation from Novartis, Kite/Gilead, Incyte, Janssen (Johnson and Johnson), Jazz, DeCART, Instill Bio, Bristol Myers Squibb, bluebird bio, Angiocrine, Mirror Biologics, and Capstan Therapeutics; research support from Novartis and patent/royalties for IP from Novartis and Tmunity. S.J.S. has served as a consultant for MorphoSys, MustangBio, Janssen, Legend Biotech, Loxo, Acerta, BiGene, Nanovecter, Pharmacyclics, Regeneron, Nordic; has served as a consultant and received research support from Incyte, Genentech/Roche, Celgene, Novartis; and has received research funding from Merck, DTRM, Juno Therapeutics, Abbvie, Adaptive Biotechnologies, and TG Therapeutics. V.B. has received funding from Gamida Cell, Citius, and Incyte; and has received honoraria for serving on advisory boards for AstraZeneca, ADC Therapeutics, Takeda, and Kite; and is a DSMB member of Miltneyi Biotec. J.M. received research funding from Gilead, Atara, CRISPR, Precision Biosciences, Scripps, Fate Therapeutics, and ADC Therapeutics. R.T.M. has served as consultant for Novartis, Artiva, Incyte, Kite, and Bristol Myers Squibb; received research support from Novartis, AlloVir, and Orca; and has served on DSMB for Novartis, Century Therapeutics, VOR pharma, and Athersys. A.I.C. has served as a consultant for Kite, Intellia, and Elsevier; and has received research support from Fate Therapeutics, Novartis, Kite, and AstraZeneca. L.J.N. has received honorarium from AbbVie, Atarra, Bristol Myers Squibb, Caribou Biosciences, Epizyme, Genentech, Genmab, Gilead/Kite, Janssen, Novartis, and Takeda; and research support from Bristol Myers Squibb, Caribou Biosciences, Epizyme, Genentech, Genmab, Gilead/Kite, IGM Biosciences, Janssen, Novartis, and Takeda. J.P.M. reports receiving consultancy fee from and/or advisory board participation for Envision, Kite/Scimentum, AlloVir, Bristol Myers Squibb, Novartis, CRISPR, Nektar, Caribou Biosciences, Sana Technologies, and Legend Biotech. O.O.O. reports receiving consultancy fee from and advisory board participation for Pfizer, Kite, Gilead, AbbVie, Janssen, TGR therapeutics, ADC, Novartis, Epizyme, Curio science, Nektar, Cargo, and Caribou; has received institution funding from Kite, Pfizer, Daichi Sankyo, and Allogen; and has honoraria from Pfizer and Gilead. L.A.L. has received honorarium and/or consulting fees from AbbVie, ADC Therapeutics, AstraZeneca, BeiGene, Celgene/BMS, Eli Lilly, Epizyme, Karyopharm, Kite/Gilead, Janssen, Merck, Pharmacyclics, SeaGen, and TG Therapeutics. M.R.B. has served as a consultant for or on the advisory board for Kite – A Gilead Company, Novaritis, Bristol Myers Squibb, CRISPR Therapeutics, Autolus, In8bio, Sana Biotechnology, Chimeric Therapeutics, Arcellx, and Achieve Clinics; and has received honoraria from Bristol Myers Squibb, Kite – A Gilead Company, Novartis, Incyte, Servier, Sanofi, and ADC Therapeutics. P.A.R. serves as a consultant and/or is on the advisory board for AbbVie, Genmab, ADC Therapeutics, Pharmacyclics, Novartis, Bristol Myers Squibb, Kite/Gilead, Nurix Therapeutics, Nektar Therapeutics, Takeda, Intellia Therapeutics, Sana Biotechnology, BeiGene, Janssen, and CVS Caremark; serves as a speaker for Kite Pharma; receives honoraria from Novartis; and research support from Bristol Myers Squibb, Kite/Gilead, Novartis, MorphoSys, CRISPR Therapeutics, Calibr, Xencor, Fate Therapeutics, and Tessa Therapeutics. M.A.P. reports honoraria from AbbVie, Astellas, Bristol Myers Squibb, Celgene, Equilium, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Novartis, Nektar Therapeutics, Omeros, OrcaBio, Takeda, and VectivBio AG, Vor Biopharma; serves on the DSMB for Cidara Therapeutics, Medigene, Sellas Life Sciences, and Servier; is on the scientific advisory board of NexImmune; has ownership interests in NexImmune and Omeros; and has received research support for clinical trials from Incyte, Kite – A Gilead Company, Miltenyi Biotec, and Novartis. The remaining authors declare no competing financial interest.
Correspondence: Michael Scordo, Adult Bone Marrow Transplant Service, Cellular Therapy Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, 530 E. 74th Street, New York, NY 10021; e-mail: scordom@mskcc.org.
References
Author notes
Data are available on request from the corresponding author, Michael Scordo (scordom@mskcc.org).
The full-text version of this article contains a data supplement.