Key Points
Respiratory infections predominate after BCMA CAR T-cell therapy, particularly as a late complication after day 100.
Hypogammaglobulinemia and early respiratory infections are key risk factors for late respiratory infections that may guide prevention.
Abstract
Infections are an important complication after B-cell maturation antigen (BCMA)–directed chimeric antigen receptor (CAR) T-cell therapy and risks may differ between the early and late periods. We evaluated infections in 99 adults who received a first BCMA–directed CAR T-cell therapy (commercial and investigational autologous BCMA CAR T-cell products at the recommended phase 2 dose) for relapsed/refractory multiple myeloma between November 2016 and May 2022. Infections were recorded until day 365, if patients experienced symptoms with a microbiologic diagnosis, or for symptomatic site-specific infections treated with antimicrobials. One-year cumulative incidence functions were calculated based on time to first respiratory infection using dates of infection-free death and receipt of additional antineoplastic therapies as competing risks. Secondary analysis evaluated risk factors for late respiratory infections using univariate and multivariable Cox regression models. Thirty-seven patients (37%) experienced 64 infectious events over the first year after BCMA–directed CAR T-cell therapy, with 42 early infectious events (days, 0-100), and 22 late infectious events (days, 101-365). Respiratory infections were the most common site-specific infection and the relative proportion of respiratory infections increased in the late period (31% of early events vs 77% of late events). On multivariable analysis, hypogammaglobulinemia (hazard ratio [HR], 6.06; P = .044) and diagnosis of an early respiratory viral infection (HR, 2.95; P = .048) were independent risk factors for late respiratory infection. Respiratory infections predominate after BCMA CAR T-cell therapy, particularly after day 100. Hypogammaglobulinemia and diagnosis of an early respiratory infection are risk factors for late respiratory infections that may be used to guide targeted preventive strategies.
Introduction
Chimeric antigen receptor (CAR) T-cell therapy is a novel immunotherapy that has revolutionized the treatment of lymphoid malignancies. CD19 CAR T-cell therapy was approved for the treatment of relapsed/refractory B-cell lymphoma and B-cell acute lymphoblastic leukemia in 2017 after publication of studies demonstrating rapid durable responses.1-3 More recently, B-cell maturation antigen (BCMA)–directed CAR T-cell therapy has emerged for the treatment of relapsed/refractory multiple myeloma (R/R MM) with 2 products, idecabtagene vicleucel (ide-cel) and ciltacabtagene autoleucel (cilta-cel), approved by the Food and Drug Administration since 2021.4-6 New products with unique binding domains aimed at reducing toxicity are currently in clinical trials.7
Although CAR T-cell therapy is highly effective and has been demonstrated to provide benefit even as early line therapy, it is frequently complicated by significant toxicities.8 The most well-known acute toxicities include cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS), which typically occur in the first 30 days after cell infusion and are attributed to profound immune activation triggered by the infused cells.4,9,10 Both CRS and ICANS may range from mild self-limited symptoms to life-threatening disease and have been reported to occur in up to 92% and 21% of BCMA CAR T-cell recipients, respectively, in clinical trials.4,6,11,12 Anti-inflammatory therapies including corticosteroids and interleukin-6 inhibitors, such as tocilizumab and siltuximab, can mitigate adverse outcomes related to these toxicities, although they may incur additional risk for infectious complications.9,11,13
Prolonged “on-target, off-tumor” effects such as hypogammaglobulinemia and delayed cytopenias unrelated to the previous lymphodepleting chemotherapy regimen are also widely observed after CAR T-cell therapy.14-17 Studies of recipients of CD19 CAR T cells have reported severe CRS and prior hematopoietic cell transplantation as key risk factors for delayed cytopenias.16,17 Although there is still limited understanding of the prevalence and severity of delayed cytopenias after BCMA–directed CAR T-cell therapy, the incidence of any grade ≥3 cytopenia at day 60 after cell infusion is as high as 33% in early reports.15 Hypogammaglobulinemia and associated humoral immune dysfunction are additional “on-target, off-tumor” effects related to profound plasma cell aplasia that can last >200 days after cell infusion. The impact of BCMA–directed CAR T cells on humoral immune dysfunction may be even more profound than that caused by CD19-directed T cells because of BCMA expression on long-lived bone marrow plasma cells that produce pathogen–specific antibody responses.18,19 Further investigation into the effects of these immune deficits on infection risk is needed.20
Infections are an important complication that can occur both early and late after BCMA–directed CAR T-cell therapy and remain poorly characterized. The overall state of immunosuppression as well as the prevalence and risk factors for specific infections may vary between the early and late period after cellular therapy. Small retrospective studies of variable follow-up durations have demonstrated that infections are most prevalent in the first 30 days after cell infusion.20-23 Bacterial infections tend to predominate in the early period after BCMA–directed CAR T-cell therapy, whereas viral infections are more prevalent after day 30, which is quite similar to the periods of infectious risk that have been described after CD19 CAR T-cell therapy.13,19,24-26 Clear risk factors for infection after BCMA–directed CAR T-cell therapy have not been identified, which may be related to differing risks during the early and late time periods. Previous studies that have attempted to define risk factors have been limited by small sample size and assessments that have combined these distinct risk periods.
This study assesses early and late infections with a particular focus on late infections after day 100 in a cohort of 99 patients who received BCMA–directed CAR T-cell products for treatment of MM.
Methods
Patients and clinical protocols
This is a retrospective study of 99 sequential adults (age ≥18) who received first BCMA–directed CAR T-cell therapy (investigational autologous BCMA CAR T-cell products at the recommended phase 2 dose, commercial idecabtagene vicleucel, and commercial ciltacabtagene autoleucel) for R/R MM at 2 institutions between November 2016 and May 2022. This study was approved by the Dana-Farber Cancer Institute Office of Human Research Studies and Institutional Review Board, and the research was conducted in accordance with the Declaration of Helsinki. Patients received lymphodepletion with fludarabine (30 mg/m2 of body-surface area) and cyclophosphamide (300 mg/m2) daily for 3 consecutive days from days –5 to –3. Cell products were infused per institutional protocol from 5 to 7 days after lymphodepletion. CRS and ICANS were graded according to the Lee and American Society of Transplantation and Cellular Therapy consensus criteria.12 Granulocyte colony-stimulating factor and intravenous immunoglobulin (IVIG) were administered according to institutional protocols during CAR T-cell admission and according to provider discretion after discharge.
Anti-infective prophylaxis
Patients received routine antiviral prophylaxis with val/acyclovir for at least 1-year after infusion and Pneumocystis prophylaxis with trimethoprim-sulfamethoxazole or atovaquone from day 0 to at least day 180 after infusion. Prophylaxis was extended for patients with a low CD4 T-cell count of ≤200 cells/uL at individual provider discretion. Patients also received antibacterial prophylaxis with levofloxacin or ciprofloxacin per trial or institutional immune effector cell protocol from day 0 until count recovery (n = 26; 26%). Fungal prophylaxis was administered per institutional protocol (no routine prophylaxis at 1 institution or fluconazole administered for neutropenia ≥10 days or after development of CRS/ICANS requiring corticosteroid therapy at 1 institution; n = 9; 9%). Patients were additionally treated using a preemptive febrile neutropenia protocol in which empiric anti-Pseudomonas gram-negative antibacterial therapy including cefepime or ceftazidime were initiated at first onset of febrile neutropenia. Empiric antifungal therapy with micafungin was initiated for persistent neutropenic fevers ≥4 days after start of treatment or for recurrent fevers while neutropenic.
Definition of infection
Infections were documented from day 0 to last follow-up, receipt of additional antineoplastic therapy (not including maintenance therapy), or day 365. Bacterial and viral infections were recorded, if there was a microbiologic or histopathologic diagnosis in conjunction with symptoms, or for site-specific infections without a microbiologic diagnosis, if there was supporting radiographic or exam findings and treatment with antimicrobials. Invasive fungal disease was defined as proven or probable according to the Revised European Organization for Research and Treatment of Cancer and the Mycoses Study Group (EORTC/MSG) criteria.27 Infections were also categorized by organ sites including bloodstream, respiratory, genitourinary, abdominal or gastrointestinal, and skin and soft tissue infection. Respiratory infections were defined as microbiologically confirmed infections (bacterial, viral, or fungal) involving the lungs, upper respiratory tract, and sinuses, as well as pneumonia without microbiologic evidence but with supporting clinical symptoms, documented radiographic or exam findings, and treatment with directed antimicrobials. Date of infection diagnosis was reported as the first date of microbiologic diagnosis or if no microbiologic diagnosis was present on the date of initiation of antimicrobial therapy. Documented fever and neutropenia without a microbiologic diagnosis in the first 30 days after cell infusion were not included as infectious events because these were highly likely to correspond with CRS. Infection severity was determined28 as previously established with mild infections not requiring treatment, moderate infections requiring treatment with oral antimicrobials, severe infections requiring hospitalization or treatment with IV antimicrobials, life-threatening infections identified as those complicated by hypotension or other life-threatening events, and fatal infections defined as those that contributed significantly to death.29,30
Data collection and statistical analysis
Clinical information including baseline characteristics, disease history, cellular therapy-related toxicities, clinical course, and laboratory assessments was collected from the electronic medical record. Delayed hematologic toxicities and hypogammaglobulinemia were recorded for the cohort, with any absolute neutrophil count ≤1000 cells/uL, immunoglobulin G (IgG) ≤400 mg/dL, or CD4 T-cell count ≤200 cells/uL, recorded from day 30 to last follow-up, initiation of additional antineoplastic therapy, or day 365. Neutropenia was reported as severe if ≤500 cells/uL and moderate if ≤1000 but >500 cells/uL. Patients were also assessed for receipt of IVIG at any time point after cell infusion. Patients were followed for 1 year or till last follow-up, and they were censored at the time of receipt of any additional antineoplastic therapy (not including maintenance therapy) or death.
One-year cumulative incidence functions were calculated based on time to first respiratory infection using dates of infection-free death and receipt of additional antineoplastic therapies as competing risks. A secondary analysis was performed to evaluate risk factors for late respiratory infections after day 100 using univariate and multivariable Cox regression models. The end point was time to diagnosis of respiratory infection after day 100. Eighty-four patients were included with follow-up beyond day 100. Candidate risk factors included >5 lines of prior myeloma-directed therapy, autologous stem cell transplantation, diagnosis of CRS, diagnosis of ICANS, treatment of CRS with tocilizumab, treatment of CRS or ICANS with corticosteroids, CD4 T-cell count ≤200 cells/uL after day 30 and before day 100, hypogammaglobulinemia (IgG ≤ 400 mg/dL) after day 30 and before day 100, and diagnosis of an early respiratory infection from day 0 to day 100. Variables with a P values <.5 in univariate analyses were candidates for inclusion in the multivariable model. Analyses were performed using SAS Studio, version 9.4.
Results
Patient and disease characteristics
This study included 99 patients treated with 3 commercial and investigational autologous BCMA CAR T-cell products. Baseline and disease characteristics are shown in Table 1. The median age was 63 (range, 34-78) years, and 63% of patients were male. The majority of patients had IgG MM (56%). Patients had a median of 5 years from diagnosis to BCMA-directed therapy (range, 1-15 years) and had received median of 6 previous lines of therapy (range, 1-12 lines of therapy). After BCMA–directed CAR T-cell therapy, 53% of patients experienced disease relapse and 20% of patients died within the first year.
Baseline patient and disease characteristics in patients receiving BCMA–directed CAR T-cell therapy
| Baseline characteristics . | BCMA–directed CAR T-cell therapy (N = 99) . |
|---|---|
| Demographics and disease characteristics | |
| Age, median (range) | 63 (34-78) |
| Male sex, n (%) | 62 (63) |
| Multiple myeloma type | |
| IgG, n (%) | 55 (56) |
| IgA, n (%) | 17 (17) |
| Light chain, n (%) | 25 (25) |
| Nonsecretory, n (%) | 2 (2) |
| Year from diagnosis to BCMA therapy, median (range) | 5 (1-15) |
| Previous lines of therapy, median (range) | 6 (1-12) |
| Previous autologous stem cell transplant, n (%) | 67 (68) |
| Acute immune effector cell toxicity | |
| CRS diagnosis, n (%) | 85 (86) |
| CRS grade, n (%) | |
| Grade 1 | 47 (55) |
| Grade 2 | 33 (39) |
| Grade 3 | 5 (6) |
| Grade 4 | 0 (0) |
| ICANS diagnosis, n (%) | 15 (15) |
| ICANS grade, n (%) | |
| Grade 1 | 4 (27) |
| Grade 2 | 8 (53) |
| Grade 3 | 2 (13) |
| Grade 4 | 1 (7) |
| Tocilizumab administration, n (%) | 55 (56) |
| Median doses tocilizumab, range | 1 (1-4) |
| Median days to first tocilizumab dose, range | 3 (0-20) |
| Low-dose corticosteroid use, n (%) (≥10 mg/d dexamethasone) | 33 (33) |
| High-dose corticosteroid use, n (%) (≥500 mg/d methylprednisolone) | 5 (5) |
| BCMA CAR T-cell outcomes, n (%) | |
| Disease relapse | 52 (53) |
| Death | 20 (20) |
| Baseline characteristics . | BCMA–directed CAR T-cell therapy (N = 99) . |
|---|---|
| Demographics and disease characteristics | |
| Age, median (range) | 63 (34-78) |
| Male sex, n (%) | 62 (63) |
| Multiple myeloma type | |
| IgG, n (%) | 55 (56) |
| IgA, n (%) | 17 (17) |
| Light chain, n (%) | 25 (25) |
| Nonsecretory, n (%) | 2 (2) |
| Year from diagnosis to BCMA therapy, median (range) | 5 (1-15) |
| Previous lines of therapy, median (range) | 6 (1-12) |
| Previous autologous stem cell transplant, n (%) | 67 (68) |
| Acute immune effector cell toxicity | |
| CRS diagnosis, n (%) | 85 (86) |
| CRS grade, n (%) | |
| Grade 1 | 47 (55) |
| Grade 2 | 33 (39) |
| Grade 3 | 5 (6) |
| Grade 4 | 0 (0) |
| ICANS diagnosis, n (%) | 15 (15) |
| ICANS grade, n (%) | |
| Grade 1 | 4 (27) |
| Grade 2 | 8 (53) |
| Grade 3 | 2 (13) |
| Grade 4 | 1 (7) |
| Tocilizumab administration, n (%) | 55 (56) |
| Median doses tocilizumab, range | 1 (1-4) |
| Median days to first tocilizumab dose, range | 3 (0-20) |
| Low-dose corticosteroid use, n (%) (≥10 mg/d dexamethasone) | 33 (33) |
| High-dose corticosteroid use, n (%) (≥500 mg/d methylprednisolone) | 5 (5) |
| BCMA CAR T-cell outcomes, n (%) | |
| Disease relapse | 52 (53) |
| Death | 20 (20) |
Acute toxicities
In this cohort, 86% of patients developed CRS, with grade 1 to 2 occurring most commonly (n = 80; 94%), and only 5 patients developing grade ≥3 CRS as shown in Table 1. Fifty-five patients (56%) received tocilizumab for treatment of CRS at a median of 3 days after cell infusion (range, 0-20). Fifteen patients (15%) developed ICANS, with 12 patients with grade 1 to 2 (80%) and 3 patients with grade ≥3 (20%). Of those with CRS and/or ICANS (n = 85), 33% received low-dose corticosteroids (≥10 mg/day dexamethasone equivalent) and 5% received high-dose corticosteroids (≥500 mg/day methylprednisolone equivalent).
Hematologic toxicity
Delayed hematologic toxicity was frequent after BCMA–directed CAR T-cell therapy and is displayed in Table 2. Forty-five patients (45%) had moderate neutropenia documented after day 30, and 18 patients (18%) had severe neutropenia with absolute neutrophil count ≤500 cells/uL after day 30. Fifty-eight percent of recipients developed moderate neutropenia or received granulocyte colony-stimulating factor after day 30 after cell infusion. Prolonged T-cell depletion with CD4 T-cell count ≤200 cells/uL was also noted in 29 of 52 patients (56%) who had CD4 count documented after day 30 after cell infusion. Hypogammaglobulinemia with IgG level ≤400 mg/dL occurred in 70% of the recipients of BCMA CAR T-cell with median IgG at 3 months of 373 mg/dL (range, 51-7506) and median IgG level at 6 months of 504 mg/dL (range, 55-3346). At our center, IVIG administration and its timing was largely provider dependent. Overall, patients received a median of 2 doses of IVIG (range, 1-10). At least 1 dose of IVIG was administered to 62 patients (63%) with the first dose occurring at a median of 70 days after cell infusion (range, 9-301) and including 6 patients without any documented hypogammaglobulinemia. Thirty-nine patients (39%) received a second dose of IVIG at a median of 143 days after cell infusion, 28% received a third dose at a median of 185 days after cell infusion, and 15% received ≥4 doses within the first year after BCMA CAR T-cell therapy. Forty-two patients (42%) received ≥1 dose of IVIG before day 100.
Delayed hematologic toxicity after BCMA–directed CAR T-cell therapy
| Delayed hematologic toxicity day 30-365 . | BCMA–directed CAR T-cell therapy (N = 99) . |
|---|---|
| Neutropenia, n (%) | |
| Severe neutropenia (ANC ≤ 500 cells/uL) | 18 (18) |
| Moderate neutropenia (ANC ≤1000 and >500 cells/uL) | 45 (45) |
| Moderate neutropenia or receipt of granulocyte colony-stimulating factor | 57 (58) |
| T-cell depletion, n (%) | |
| CD4 T-cell count ≤200 cells/uL | 29 (29) |
| Hypogammaglobulinemia | |
| IgG ≤400 mg/dL, n (%) | 69 (70) |
| 3mo IgG, median (range) | 373 (51-7506) |
| 6mo IgG, median (range) | 504 (55-3346) |
| IVIG administration, n (%) | 62 (63) |
| Delayed hematologic toxicity day 30-365 . | BCMA–directed CAR T-cell therapy (N = 99) . |
|---|---|
| Neutropenia, n (%) | |
| Severe neutropenia (ANC ≤ 500 cells/uL) | 18 (18) |
| Moderate neutropenia (ANC ≤1000 and >500 cells/uL) | 45 (45) |
| Moderate neutropenia or receipt of granulocyte colony-stimulating factor | 57 (58) |
| T-cell depletion, n (%) | |
| CD4 T-cell count ≤200 cells/uL | 29 (29) |
| Hypogammaglobulinemia | |
| IgG ≤400 mg/dL, n (%) | 69 (70) |
| 3mo IgG, median (range) | 373 (51-7506) |
| 6mo IgG, median (range) | 504 (55-3346) |
| IVIG administration, n (%) | 62 (63) |
Early infections
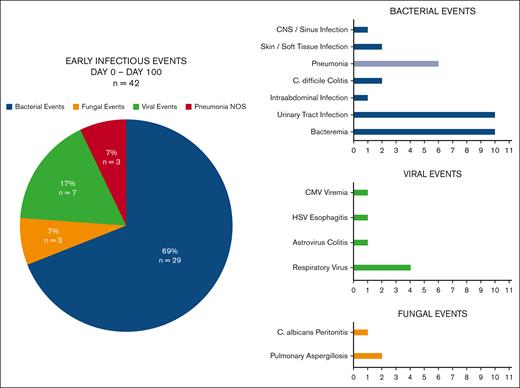
Early infections after BCMA–directed CAR T-cell therapy are described in Figure 1. Between days 0 and 100 after cell infusion, 26 patients (26%) experienced 42 infectious events. Seventeen of 26 patients with early infections (65%) experienced a single infectious event, whereas 9 patients (35%) experienced multiple infectious events (2 events, n = 5; 3 events, n = 1; and 4 events, n = 3). The median time to early infection was 18 days (range, 0-92). Infection severity and hospitalizations are displayed in Table 3. One-third (n = 14) of the early infectious events were mild or moderate, 43% (n = 18) were severe, 21% (n = 9) were life-threatening, and only 1 infection was fatal. Bacterial infections were the most frequent, composing 69% of infectious events in the first 100 days. Common sites of bacterial infection included bloodstream infections (n=10), urinary tract infections (n = 10), and pneumonia (n = 6). Of the early bloodstream infections identified, 6 were gram-negative organisms (Escherichia coli, n =2; Citrobacter freundii, n = 1; Klebsiella pneumoniae, n = 1; Enterobacter cloacae, n = 1; and unidentified anaerobic gram-negative organism, n = 1), 3 were gram-positive organisms (Enterococcus faecium, n = 2; and Parvimonas species, n = 1), and 1 infection was polymicrobial with gram-negative and gram-positive organisms identified (Streptococcus mitis and Stenotrophomonas maltophilia). Viral infections constituted the second most common early infectious event (17%; n = 7), primarily driven by respiratory viral infections (influenza A, n = 2; respiratory syncytial virus, n=1; and rhinovirus, n = 1). Fungal infections were rare, occurring in 3% of patients (probable invasive pulmonary aspergillosis, n = 2; and proven Candida peritonitis, n = 1), despite only 9 patients receiving fluconazole prophylaxis. Both patients with invasive mold infections had received high-dose corticosteroids preceding their diagnosis, including 1 patient who developed hemophagocytic lymphohistiocytosis as a toxicity of BCMA CAR T-cell therapy and died with active aspergillosis. Three patients were diagnosed with pneumonia via clinical and radiographic criteria that was not able to be delineated as bacterial versus viral. Overall, 13 of 42 (31%) of early infectious events were respiratory infections involving the lungs, upper respiratory tract, or sinuses.
Early infections from days 0 to 100 after BCMA–directed CAR T-cell therapy.Figure 1 presents early infectious events from day 0 to day 100 (n = 42) by pathogen type and individual infection. Between day 0 and day 100 after cell infusion, 26 patients (26%) experienced 42 infectious events. Bacterial infections were the most frequent infectious event (69%), whereas viral infections were less common (17%) and fungal infections were rare (3%). Overall, 13 of 42 (31%) of early infectious events were respiratory infections involving the lungs, upper respiratory tract, or sinuses. “Pneumonia NOS” represents pneumonia diagnosed via clinical and radiographic criteria and not able to be delineated as bacterial vs viral. Amongst early infections, 3/6 cases of pneumonia were "Pneumonia NOS" and 3 cases had bacterial pathogens identified on microbiologic testing. NOS, Not otherwise specified.
Early infections from days 0 to 100 after BCMA–directed CAR T-cell therapy.Figure 1 presents early infectious events from day 0 to day 100 (n = 42) by pathogen type and individual infection. Between day 0 and day 100 after cell infusion, 26 patients (26%) experienced 42 infectious events. Bacterial infections were the most frequent infectious event (69%), whereas viral infections were less common (17%) and fungal infections were rare (3%). Overall, 13 of 42 (31%) of early infectious events were respiratory infections involving the lungs, upper respiratory tract, or sinuses. “Pneumonia NOS” represents pneumonia diagnosed via clinical and radiographic criteria and not able to be delineated as bacterial vs viral. Amongst early infections, 3/6 cases of pneumonia were "Pneumonia NOS" and 3 cases had bacterial pathogens identified on microbiologic testing. NOS, Not otherwise specified.
Infection severity and hospitalizations
| Infection severity and hospitalizations . | Early (day 0-100) n = 42 . | Late (day 101-365) n = 22 . |
|---|---|---|
| Infection severity | ||
| Mild | 3 (7) | 2 (9) |
| Moderate | 11 (26) | 12 (54.5) |
| Severe | 18 (43) | 7 (32) |
| L ife-threatening | 9 (21) | 1 (4.5) |
| Fatal | 1 (2) | 0 (0) |
| Hospitalization | ||
| Infection while hospitalized for other indication | 27 (64) | 0 (0) |
| Hospitalized for infection | 9 (22) | 7 (32) |
| Not hospitalized | 6 (14) | 15 (68) |
| Infection severity and hospitalizations . | Early (day 0-100) n = 42 . | Late (day 101-365) n = 22 . |
|---|---|---|
| Infection severity | ||
| Mild | 3 (7) | 2 (9) |
| Moderate | 11 (26) | 12 (54.5) |
| Severe | 18 (43) | 7 (32) |
| L ife-threatening | 9 (21) | 1 (4.5) |
| Fatal | 1 (2) | 0 (0) |
| Hospitalization | ||
| Infection while hospitalized for other indication | 27 (64) | 0 (0) |
| Hospitalized for infection | 9 (22) | 7 (32) |
| Not hospitalized | 6 (14) | 15 (68) |
Late infections
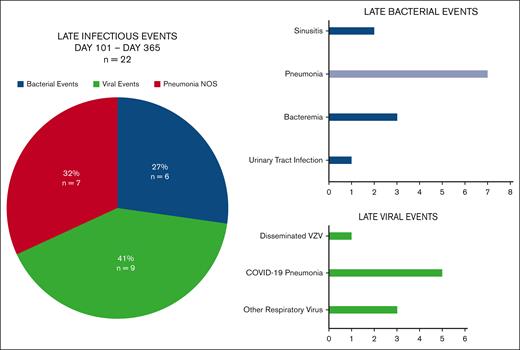
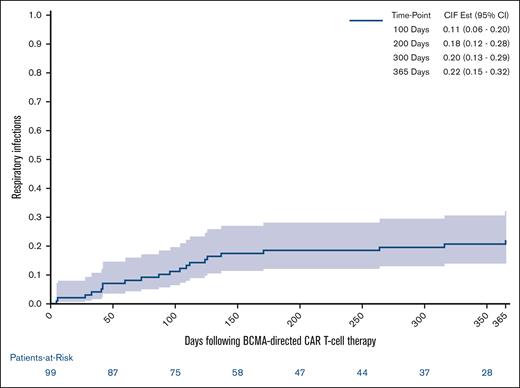
Late infections after BCMA–directed CAR T-cell therapy are described in Figure 2. Between days 101 and 365 after BCMA–directed CAR T-cell infusion, 15 patients (15%) experienced 22 infectious events. Eleven of 15 patients with late infections (73%) experienced a single infectious event, whereas 4 patients (27%) experienced multiple infectious events (2 events, n = 2; 3 events, n = 1; and 4 events, n = 1). The median time to late infection was 171 days (range, 104-365). The majority of late infectious events were mild or moderate (64%; n = 14), although there were 7 severe events (32%) and 1 life-threatening event. Viral infections were the most frequent infectious event after day 100 (41%; n = 9) with all but 1 event representing respiratory viral infections (COVID-19, n =5; rhinovirus, n = 2; influenza A, n = 1; and disseminated varicella-zoster virus, n = 1). Seven of the 22 late infectious events (32%) consisted of pneumonia that was not able to be delineated as bacterial vs viral. Bacterial infections were less common after day 100. Late bacterial events included bloodstream infections (n=3; Micrococcus lutae, n = 1; Escherichia coli, n = 1; and Citrobacter koseri, n = 1), sinusitis (n = 2), and 1 urinary tract infection. There were no fungal infections diagnosed after day 100 following BCMA–directed CAR T-cell therapy. Overall, 17 of 22 late infectious events (77%) consisted of respiratory infections involving the lungs, upper respiratory tract, or sinuses. The 1-year cumulative incidence of respiratory infections accounting for competing risks of infection-free death and disease relapse was 22% (95% confidence interval [CI], 15-32) and is displayed in Figure 3.
Late infections from days 101 to 325 after BCMA–directed CAR T-cell therapy. The figure presents late infectious events from days 101 to 365 (n = 22) by pathogen type and individual infection. Between days 101 and 365 after cell infusion, 15 patients (15%) experienced 22 infectious events. Viral infections were the most frequent infectious event after day 100 (41%) and bacterial infections were less common after day 100 (27%). A total of 32% of infections were clinical pneumonia that was not delineated as bacterial or viral. There were no fungal infections identified after day 100. The case of disseminated varicella-zoster virus developed in a patient admitted to an outside facility with a vertebral fracture who was briefly off prophylaxis in that setting, and there was no evidence of acyclovir resistance and he improved on IV acyclovir. Overall, 17 of 22 (77%) of late infectious events were composed of respiratory infections involving the lungs, upper respiratory tract, or sinuses. Pneumonia NOS represents pneumonia diagnosed via clinical and radiographic criteria that could not be delineated as bacterial or viral. Amongst late infections, 7/7 cases of pneumonia were "Pneumonia NOS".
Late infections from days 101 to 325 after BCMA–directed CAR T-cell therapy. The figure presents late infectious events from days 101 to 365 (n = 22) by pathogen type and individual infection. Between days 101 and 365 after cell infusion, 15 patients (15%) experienced 22 infectious events. Viral infections were the most frequent infectious event after day 100 (41%) and bacterial infections were less common after day 100 (27%). A total of 32% of infections were clinical pneumonia that was not delineated as bacterial or viral. There were no fungal infections identified after day 100. The case of disseminated varicella-zoster virus developed in a patient admitted to an outside facility with a vertebral fracture who was briefly off prophylaxis in that setting, and there was no evidence of acyclovir resistance and he improved on IV acyclovir. Overall, 17 of 22 (77%) of late infectious events were composed of respiratory infections involving the lungs, upper respiratory tract, or sinuses. Pneumonia NOS represents pneumonia diagnosed via clinical and radiographic criteria that could not be delineated as bacterial or viral. Amongst late infections, 7/7 cases of pneumonia were "Pneumonia NOS".
One-year cumulative incidence of respiratory infections after BCMA–directed CAR T-cell therapy. The 1-year cumulative incidence of respiratory infections accounting for competing risks of infection-free death and disease relapse requiring antineoplastic treatment was 22% (95% CI, 15-32).
One-year cumulative incidence of respiratory infections after BCMA–directed CAR T-cell therapy. The 1-year cumulative incidence of respiratory infections accounting for competing risks of infection-free death and disease relapse requiring antineoplastic treatment was 22% (95% CI, 15-32).
Univariate and multivariable Cox regression analyses for late respiratory infection
Respiratory infections were prevalent after day 100 after BCMA–directed CAR T-cell therapy. Univariate and multivariable Cox regression analyses for late respiratory infections is presented in Table 4. Eighty-four patients with follow-up beyond day 100 were included. Hypogammaglobulinemia with IgG ≤400 mg/dL between days 30 and 100 (hazard ratio [HR], 5.027; 95% CI, 0.66-38.24; P = .12) and diagnosis of an early respiratory infection (HR, 2.73; 95% CI, 0.81-9.23; P = .11) showed a trend toward increased risk for late respiratory infections on univariate analysis. Notably, all but 1 patient who was diagnosed with a late respiratory infection had documented hypogammaglobulinemia with IgG ≤400 mg/dL after day 30. The 1 patient who did not have documented hypogammaglobulinemia before day 100, developed IgG of 366 mg/dL on day 119, one month before diagnosis of her respiratory infection. On multivariable analysis, hypogammaglobulinemia (HR, 6.06; 95% CI, 1.05-35.08; P = .044) and diagnosis of an early respiratory viral infection (HR, 2.95; 95% CI, 1.01-8.63; P = .048) were independent risk factors for respiratory infection after day 100 after BCMA–directed CAR T-cell therapy. CRS showed a trend toward a decreased risk of late respiratory infections, although this was not significant (HR, 0.51; 95% CI, 0.17-1.51; P = .22).
Univariate and multivariable Cox regression analyses for late respiratory infections after day 100 after BCMA–directed CAR T-cell therapy
| Variable . | Univariate HR . | 95% CI . | P value . | Multivariable HR . | 95% CI . | P value . |
|---|---|---|---|---|---|---|
| Lines of treatment >5 | 0.77 | 0.26–2.31 | .646 | — | — | — |
| Previous autologous hematopoietic cell transplantation | 0.86 | 0.29–2.57 | .790 | — | — | — |
| CRS diagnosis | 0.60 | 0.14–2.58 | .496 | 0.506 | 0.17--1.51 | .223 |
| Tocilizumab administration | 0.80 | 0.26–2.40 | .686 | — | — | — |
| ICANS diagnosis | 1.13 | 0.24–5.36 | .881 | — | — | — |
| Corticosteroid administration | 1.17 | 0.38–3.57 | .787 | — | — | — |
| CD4 T-cell ≤ 200 cells/uL (day 30-100) | 1.21 | 0.36–4.07 | .757 | — | — | — |
| IgG ≤400 mg/dL (day 30-100) | 5.03 | 0.66–38.24 | .119 | 6.06 | 1.05–35.08 | .044 |
| Early respiratory infection | 2.74 | 0.81–9.23 | .105 | 2.95 | 1.01–8.63 | .048 |
| Variable . | Univariate HR . | 95% CI . | P value . | Multivariable HR . | 95% CI . | P value . |
|---|---|---|---|---|---|---|
| Lines of treatment >5 | 0.77 | 0.26–2.31 | .646 | — | — | — |
| Previous autologous hematopoietic cell transplantation | 0.86 | 0.29–2.57 | .790 | — | — | — |
| CRS diagnosis | 0.60 | 0.14–2.58 | .496 | 0.506 | 0.17--1.51 | .223 |
| Tocilizumab administration | 0.80 | 0.26–2.40 | .686 | — | — | — |
| ICANS diagnosis | 1.13 | 0.24–5.36 | .881 | — | — | — |
| Corticosteroid administration | 1.17 | 0.38–3.57 | .787 | — | — | — |
| CD4 T-cell ≤ 200 cells/uL (day 30-100) | 1.21 | 0.36–4.07 | .757 | — | — | — |
| IgG ≤400 mg/dL (day 30-100) | 5.03 | 0.66–38.24 | .119 | 6.06 | 1.05–35.08 | .044 |
| Early respiratory infection | 2.74 | 0.81–9.23 | .105 | 2.95 | 1.01–8.63 | .048 |
Discussion
To our knowledge, this is the largest study to date of infections in patients receiving BCMA–directed CAR T-cell therapy. In this cohort, more than half of the patients who developed infection experienced a respiratory infection, and respiratory infections represented the most common site-specific infection during the first year. Most notably, although the overall number of infectious events decreased after day 100, the relative proportion of respiratory infections increased (13/42 [31%] early infectious events vs 17/22 [77%] late infectious events), representing an important shift in infection risk and epidemiology.18,19,31-38
Previous studies evaluating infections after BCMA–directed CAR T-cell therapy in smaller cohorts have shown an incidence of infections ranging from 53% to 58% over varying time periods of follow-up (100 days to >16 months).20,21,39 The incidence of infections in this cohort was lower than what has previously been reported with 37 of 99 patients (37%) experiencing 64 infectious events over the first year after BCMA–directed CAR T-cell therapy. Nonetheless, the breakdown of infections in this study is similar to what has previously been described with bacterial infections frequently occurring in the early period and viral infections predominating later.13,19-22,24,39 Fungal infections continue to be rarely reported in these populations.21,39,40 Interestingly, previous studies have primarily focused on dividing infectious events by pathogen and have not clearly evaluated site-specific infections across pathogen groups. When viewed in this way, it is clear that respiratory infections represent the leading cause of infectious events in patients who have undergone BCMA–directed CAR T-cell therapy with ∼58% to 83% of infectious events in prior large studies involving the sinuses and upper or lower respiratory tracts.20,21,39 In this study, respiratory infections composed 47% of infectious events, by far the most frequent site-specific event across both time periods. This effect became even more prominent in the late period, with respiratory infections representing 77% of infectious events that occurred after day 100. Furthermore, it is possible that additional mild respiratory infections were not diagnosed in patients who did not seek care or did not live locally, suggesting that this may be lower estimate of the true incidence of respiratory infections in this population. Importantly, respiratory infections may be more severe or prolonged in these patients as has been demonstrated with reports of severe and protracted COVID-19 in patients with B-cell depletion including those who received CAR T-cell therapy.41-44 In this study, although the majority of severe infections occurred before day 100, almost one-third of the late infections were severe, demonstrating the critical need to identify patients at high risk and mitigate any factors that may predispose them to infection.
Identification of risk factors for infections after BCMA–directed CAR T-cell therapy has previously been limited without any clear risk factors for infection identified, likely because of small cohort sizes, and the combined analysis of early and late infectious events, which may be driven by unique risk factors. In this study, we focused on evaluating risk factors for late respiratory infections. Early infectious risk is likely defined by the period of neutropenia as well as acute toxicities such as ICANS or CRS, the latter of which has been previously associated with an increased risk for early infections after CD19 CAR T-cell therapy in multiple studies.13,24,26,45 These acute toxicities can lead to prolonged hospitalization, administration of additional immunosuppressive therapies and long-term antimicrobials, and critical illness requiring intensive care with placement of IV catheters, all of which may play an important role in risk for early infections. Risk factors for late infections, including those we identified, represent an important area for prevention in patients who are otherwise doing well after CAR T-cell therapy.
Here, hypogammaglobulinemia (IgG ≤400 mg/dL from days 30 to 100) and the diagnosis of a respiratory infection before day 100 were identified as independent risk factors. Remarkably, every patient with a late respiratory infection had documented hypogammaglobulinemia before infection onset. In this study, IVIG strategies including administration and timing was provider dependent and at times given to patients without documented hypogammaglobulinemia. For this reason, given the low overall number of events, we chose to focus on the presence of hypogammaglobulinemia before day 100 regardless of IVIG administration in our multivariable analysis. Nonetheless, the association of hypogammaglobulinemia and late respiratory infections raises an important question of whether administration of IVIG may mitigate or alleviate the humoral immune defects caused by BCMA–directed cell therapy and reduce risk or severity of respiratory infections after cell infusion. Furthermore, although there remains a lack of randomized controlled data evaluating the use of IVIG to prevent infections after CAR T-cell therapy, studies do support the use of IVIG to prevent infections in other secondary immunodeficiencies with associated hypogammaglobulinemia, including chronic lymphocytic leukemia and MM.46-48 These studies have specifically demonstrated reductions in bacterial infections after use of IVIG, but severity and incidence of viral infections may also be impacted via transfer of pathogen-specific antibodies or “passive immunization” and could benefit from further study.18,19 Additionally, although the traditional recommended dose of IVIG for secondary immunodeficiency is 0.4 g/kg every 3 to 6 weeks, it remains to be seen whether higher dosing or more frequent dosing intervals could benefit patients at high risk, particularly if hypogammaglobulinemia persists despite routine adminsitration.31,49,50 In primary immunodeficiency syndromes, a “wear off” phenomenon with increased rates of infections from weeks 3 to 4 compared with week 1 after dosing has been demonstrated, suggesting a possible benefit of dosing changes for those patients with recurrent infections despite routine IVIG therapy.51 Subcutaneous immunoglobulin replacement that results in steady state pharmacokinetics and little fluctuation in IgG levels, could also be a consideration in patients who are high risk.52-54 These findings may also be applicable to secondary immunodeficiencies such as those caused by BCMA–directed CAR T-cell therapy, and prospective studies are needed to better characterize the potential benefit of IVIG to prevent late respiratory infections in this population and to delineate the optimal IVIG dose and frequency. Other preventive strategies include vaccination both before and after cell infusion, although optimal timing of post–CAR T-cell vaccination has not been studied in randomized clinical trials.18,19,31,32 The development of additional messenger RNA vaccines against respiratory viruses such as RSV and others will likely play an important role in the prevention of respiratory infections in this population moving forward.33-36 Finally, novel therapies in the pipeline including cytotoxic T-cell therapies and monoclonal antibody therapies targeting respiratory viruses may play a future role to address deficiencies in humoral and cell-mediated immunity and prevent or treat serious respiratory infections in this population.37,38
Diagnosis of an early respiratory infection before day 100 was a second risk factor for late respiratory infections in this study. Viral infections such as influenza and COVID-19 have previously been shown to be associated with an increased risk of secondary bacterial pneumonia through impacts on the host immune system.55-57 In the post–cell therapy setting, shifts in host immune pathways and dysregulation of respiratory microbiota triggered by an early respiratory infection may similarly lead to increased risk for subsequent infections.58 It is also possible that an early respiratory infection does not directly increase risk but rather serves as a marker of a high-risk host with increased propensity for respiratory infections after cell therapy. Regardless, identification of key risk factors, such as this one, can aid in recognizing and stratifying patients at higher risk for late infections, enabling targeted preventive strategies such as preemptive IVIG administration, increased immunologic monitoring, or alternative approaches to vaccination or anti-infective prophylaxis.59
Respiratory infections are a leading cause of infectious complications after BCMA CAR T-cell therapy, and this risk remains elevated even after day 100 after infusion. Hypogammaglobulinemia and diagnosis of an early respiratory infection are important actionable risk factors for late respiratory infections, and identification of these factors can be used to guide targeted preventive strategies such as IVIG replacement, vaccination, and antimicrobial prophylaxis in this population. Prospective studies evaluating the benefit of IVIG in this setting are needed.
Acknowledgments
This study was supported (in part) by research funding from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (grant K23AI16335) to S.B. for designing the research, providing support for data collection, and editing the manuscript.
Authorship
Contribution: J.S.L. designed the research, performed data collection, analyzed data, and wrote the manuscript; M.T. performed data collection and edited the manuscript; J.S.H performed data collection and edited the manuscript. O.N contributed to manuscript content and edited the manuscript. A.S.S contributed to manuscript content and edited the manuscript. N.R. helped to design the research and edited the manuscript; N.M. helped to design the research and edited the manuscript; M.F. helped to design the research and edited the manuscript; S.B. helped to design the research and edited the manuscript; and S.P.H. helped to conceptualize the project and design the research, as well as assisted in writing and editing the manuscript.
Conflict-of-interest disclosure: O.N. serves on advisory board of Janssen, BMS, Karyopharm, GPCR therapeutics, GSK, and Sanofi; and consults for GPCR therapeutics and Janssen. A.S. receives consulting fee from Novartis and Roche. N.R. receives consulting/advisory board membership fee from Pfizer, BMS, Janssen, Sanofi, AbbVie, Regeneron, Amgen, Caribou, and Immuneel; research funding from Pfizer and 2Seventybio. N.M. receives consulting fee from Takeda, Janssen, OncoPeptides, AbbVie, Adaptive, Amgen, BeiGene, Karyopharm Therapeutics, BMS, Celgene, Legend Biotech, Novartis, Sebia, Raqia, and Pfizer; has stock/patents/royalties in OncoPeptides, C4 therapuetics, and Raquia; is on the board of directors of OncoPeptides; and is the President of the International Myeloma Society. M.F. receives consulting fee from Celgene, Novartis, Kite, and Iovance. S.P.H. receives consulting from Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Jessica S. Little, Division of Infectious Diseases, Brigham and Women’s Hospital, 75 Francis St, PBB-A4, Boston, MA 02115; e-mail: jlittle@bwh.harvard.edu.
References
Author notes
Publication-related data will be shared upon request to the corresponding author, Jessica S. Little (jlittle@bwh.harvard.edu).