TO THE EDITOR:
Cancer complicates ∼1 in 1000 pregnancies, which translates into ∼4000 cases annually in the United States.1 Lymphoma is the fourth most common malignancy and the most common hematological malignancy present during pregnancy.2 Although there are published reports of lymphoma occurring during pregnancy,3,4,5 most are among patients with newly diagnosed disease, and there is a near absence of data regarding the presentation, management, and maternal and neonatal outcomes of patients with relapsed/refractory lymphoma that occurs during pregnancy.
We performed a comprehensive retrospective analysis of patients diagnosed with relapsed/refractory lymphoma that occurred during pregnancy between 1989 and 2021 across 10 international academic centers, including the International Network on Cancer, Infertility and Pregnancy (https://www.cancerinpregnancy.org/). This study was approved by the institutional review board of each participating institution and was performed in accordance with the Declaration of Helsinki. Staging and therapy were performed at the discretion of the treating physician. Data on disease characteristics, treatment, obstetric, and neonatal outcomes were obtained from oncology and maternal-fetal medicine records and documented on a standardized data collection form. For patients who received therapy during pregnancy, data regarding the type and duration of treatment received during and after pregnancy were collected.
Progression-free survival (PFS) was calculated from the date of lymphoma relapse to the date of death or disease relapse or progression. Overall survival (OS) was computed from the date of lymphoma relapse to the date of death. PFS and OS rates were estimated using the Kaplan-Meier method. All statistical analyses were conducted using SAS v9.2 (SAS Institute, Cary, NC).
Twenty-three cases of relapsed/refractory lymphoma during pregnancy were identified, with the majority having classic Hodgkin lymphoma (cHL) (Table 1). The median time from initial lymphoma diagnosis to relapse was 38 months (range, 10-246) and the median age of patients at the time of relapse during pregnancy was 31 years (range, 21-35). The median gestation at the time of relapsed/refractory diagnosis was 22 weeks (range, 5-30).
Maternal characteristics of patients with relapsed lymphoma during pregnancy
| . | All patients N = 23 . |
|---|---|
| Diagnosis | |
| Hodgkin lymphoma | 18 (78) |
| PTCL∗ | 2 (9) |
| Diffuse large B-cell lymphoma | 1 (2) |
| Follicular lymphoma | 1 (2) |
| Marginal zone lymphoma | 1 (2) |
| Race/ethnicity | |
| White | 19 (82) |
| Hispanic | 1 (4.5) |
| Black | 1 (4.5) |
| American Indian | 1 (4.5) |
| Asian | 1 (4.5) |
| Previous therapy for initial lymphoma† | ABVD 13 (57%), other multiagent chemotherapy 3 (13%), BEACOPP 2 (9%), MACOP-B 2 (9%), and 1 each bendamustine/ obinutuzimab, R-CHOP, and radiotherapy |
| Median age at relapse, y | 31 (21-35) |
| Parity | |
| P0 | 9 (39) |
| P1 | 10 (43) |
| P2 | 4 (18) |
| Stage at relapse | |
| I | 3 (13) |
| II | 10 (44) |
| III | 4 (17) |
| IV | 4 (17) |
| B symptoms | 12 (53) |
| Anemia‡ | 9 (39) |
| Thrombocytopenia§ | 1 (4) |
| Lactase dehydrogenase|| | |
| Increased | 7 (30) |
| Normal | 8 (35) |
| Transplant after pregnancy¶ | |
| Autologous | 6 (31.6) |
| Allogeneic | 3 (15.8) |
| . | All patients N = 23 . |
|---|---|
| Diagnosis | |
| Hodgkin lymphoma | 18 (78) |
| PTCL∗ | 2 (9) |
| Diffuse large B-cell lymphoma | 1 (2) |
| Follicular lymphoma | 1 (2) |
| Marginal zone lymphoma | 1 (2) |
| Race/ethnicity | |
| White | 19 (82) |
| Hispanic | 1 (4.5) |
| Black | 1 (4.5) |
| American Indian | 1 (4.5) |
| Asian | 1 (4.5) |
| Previous therapy for initial lymphoma† | ABVD 13 (57%), other multiagent chemotherapy 3 (13%), BEACOPP 2 (9%), MACOP-B 2 (9%), and 1 each bendamustine/ obinutuzimab, R-CHOP, and radiotherapy |
| Median age at relapse, y | 31 (21-35) |
| Parity | |
| P0 | 9 (39) |
| P1 | 10 (43) |
| P2 | 4 (18) |
| Stage at relapse | |
| I | 3 (13) |
| II | 10 (44) |
| III | 4 (17) |
| IV | 4 (17) |
| B symptoms | 12 (53) |
| Anemia‡ | 9 (39) |
| Thrombocytopenia§ | 1 (4) |
| Lactase dehydrogenase|| | |
| Increased | 7 (30) |
| Normal | 8 (35) |
| Transplant after pregnancy¶ | |
| Autologous | 6 (31.6) |
| Allogeneic | 3 (15.8) |
ABVD, doxorubicin, bleomycin, vinblastine sulfate, and dacarbazine; BEACOPP, bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone; MACOP-B: methotrexate, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin; PTCL, peripheral T-cell lymphoma; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone.
Data are presented as number (percentage) or median (interquartile range).
One case each: PTCL not otherwise specified and ALK–negative anaplastic large-cell lymphoma.
Two patients with Hodgkin lymphoma had also received previous ifosfamide-based therapy (post-ABVD) followed by autologous transplantation.
Data available for n = 22.
Data available for n = 21.
Data available for n = 15.
Data available for n = 19.
Before relapse, the most common previous therapy received was doxorubicin, bleomycin, vinblastine sulfate, and dacarbazine (Table 1). The median number of previous treatments was 1 (range, 1-3). During pregnancy, 4 patients had termination or spontaneous abortion at a median gestation of 8 weeks (range, 7-14), 2 of whom conceived in the first trimester during aggressive multiagent chemotherapy. Fourteen (61%) patients had treatment deferred to the postpartum period, and 5 (22%) patients received therapy during pregnancy. The latter 5 patients were diagnosed at a median gestation of 25 weeks (range, 12-30), and treated at a median of 26 weeks (range, 20-32) owing to the life-threatening nature of the cancer (Table 2). Interestingly, the median gestation of pregnancy among the 18 patients who had treatment deferred was 19 weeks (range, 10-28.5).
Antenatal therapy
| Treatment . | Diagnosis . | Stage at relapse . | GA at relapse (wk) . | GA when therapy started (wk) . | Delivery (wk) . |
|---|---|---|---|---|---|
| Modified ESHAP; then nivolumab | cHL | IIIB | 12 | 20 and 25, respectively | 38∗ |
| DHAP, IGEV | cHL | NA | 20 | 25 | 36† |
| R-ICE | DLBCL | IIIA | 25 | 25 | 26† |
| ESHAP | PTCL NOS | II | 30 | 31 | 37∗ |
| Modified ESHAP (lowered cytarabine) | ALK negative ALCL | IIIA | 30 | 32 | 36† |
| Treatment . | Diagnosis . | Stage at relapse . | GA at relapse (wk) . | GA when therapy started (wk) . | Delivery (wk) . |
|---|---|---|---|---|---|
| Modified ESHAP; then nivolumab | cHL | IIIB | 12 | 20 and 25, respectively | 38∗ |
| DHAP, IGEV | cHL | NA | 20 | 25 | 36† |
| R-ICE | DLBCL | IIIA | 25 | 25 | 26† |
| ESHAP | PTCL NOS | II | 30 | 31 | 37∗ |
| Modified ESHAP (lowered cytarabine) | ALK negative ALCL | IIIA | 30 | 32 | 36† |
ALCL, anaplastic large cell lymphoma; ALK, anaplastic lymphoma kinase; DLBCL, diffuse large B-cell lymphoma; DHAP, dexamethasone, high dose cytarabine, cisplatin; ESHAP, etoposide, methylprednisolone, cytarabine, cisplatin; GA, gestational age; IGEV, ifosfamide + gemcitabine + vinorelbine; NA, not available; PTCL NOS, peripheral T-cell lymphoma not otherwise specified; R-ICE: rituximab, ifosfamide, carboplatin, etoposide.
Clinician-initiated delivery.
Spontaneous delivery.
Among the 5 patients who received antenatal therapy for relapsed/refractory lymphoma, 4 had a complete response (unconfirmed) by computerized tomography or magnetic resonance imaging, whereas 1 patient had a transient response followed by progression during pregnancy and had antenatal therapy changed to nivolumab (Table 2). The latter case was recently reported.6 She received 6 cycles of nivolumab throughout the second and third trimesters and delivered a healthy full-term infant. Overall, 9 (39%) patients underwent stem cell transplantation (SCT) postpartum, including 2 allogeneic SCT; 3 out of 5 patients treated during pregnancy underwent autologous SCT.
There were 19 (83%) live births, 3 elective abortions (13%), and 1 (4%) spontaneous abortion (Table 3). Of the 19 live births, 13 (68%) were vaginal deliveries and 6 (32%) were cesarean deliveries. The indications for cesarean deliveries included 3 elective and 1 each of fetal compromise, breech presentation, and rapidly increasing liver function tests. The median gestational age at delivery was 36 weeks (range, 26-39). Eleven (58%) births occurred at <37 weeks and 8 (42%) occurred at ≥37 weeks of gestation (Table 3). Of the preterm births, 9 out of 11 (82%) were late preterm (between 35-36 weeks gestation) and 8 out of 11 (73%) were clinician-initiated, with the most common reason being the desire to initiate systemic chemotherapy. Overall, the median birth weight was 2777 grams (740-3900) with 2 (11%) infants being small for gestational age, 1 of whom had received antenatal therapy. There were no reports of fetal malformations or late neonatal death (up to 28 days of age) in all live births.
Obstetrical outcomes for patients with relapsed lymphoma during pregnancy
| . | All patients N = 23 . | Patients who had antenatal therapy N = 5 . | Patients without treatment in pregnancy N = 18∗ . |
|---|---|---|---|
| Median GA at recurrence, wk | 20 (13-28) | 25 (20-30) | 19 (10-28) |
| Median GA at treatment, wk | NA | 26 (25-30) | NA |
| Median GA at delivery, wk | 36 wk (35-38) | 36 (33.5-36.5) | 36.5 (35.7-38) |
| Median birthweight, grams | 2777 (2493-3373) | 2784 (2315-3373) | 2769 (2585-3292) |
| Elective abortion | 3 (14) | 0 | 3 |
| Miscarriage | 1 (5) | 0 | 1 |
| Live birth | 18 (82) | 5 | 14 |
| Cesarian delivery | 6 (33) | 0 | 6 |
| ≥37 wk | 8 (42) | 2 | 6 |
| <37 wk | 10 (53) | 2 | 8 |
| <32 wk | 1 (5) | 1 | 0 |
| Clinically initiated PTD | 7 (64) | 2 | 6 |
| Postpartum hemorrhage | 0 | 0 | 0 |
| Chorioamnionitis | 0 | 0 | 0 |
| Endometritis | 1 | 0 | 1 |
| Transfusion of blood products | 0 | 0 | 0 |
| GDM | 2 | 0 | 2 |
| Preeclampsia | 1 | 0 | 1 |
| . | All patients N = 23 . | Patients who had antenatal therapy N = 5 . | Patients without treatment in pregnancy N = 18∗ . |
|---|---|---|---|
| Median GA at recurrence, wk | 20 (13-28) | 25 (20-30) | 19 (10-28) |
| Median GA at treatment, wk | NA | 26 (25-30) | NA |
| Median GA at delivery, wk | 36 wk (35-38) | 36 (33.5-36.5) | 36.5 (35.7-38) |
| Median birthweight, grams | 2777 (2493-3373) | 2784 (2315-3373) | 2769 (2585-3292) |
| Elective abortion | 3 (14) | 0 | 3 |
| Miscarriage | 1 (5) | 0 | 1 |
| Live birth | 18 (82) | 5 | 14 |
| Cesarian delivery | 6 (33) | 0 | 6 |
| ≥37 wk | 8 (42) | 2 | 6 |
| <37 wk | 10 (53) | 2 | 8 |
| <32 wk | 1 (5) | 1 | 0 |
| Clinically initiated PTD | 7 (64) | 2 | 6 |
| Postpartum hemorrhage | 0 | 0 | 0 |
| Chorioamnionitis | 0 | 0 | 0 |
| Endometritis | 1 | 0 | 1 |
| Transfusion of blood products | 0 | 0 | 0 |
| GDM | 2 | 0 | 2 |
| Preeclampsia | 1 | 0 | 1 |
GA, gestational age; GDM, gestational diabetes mellitus; NA, not applicable; PTD, preterm delivery.
Data are presented as number (percentage) or median (interquartile range).
Two patients with Hodgkin lymphoma conceived during multiagent ifosfamide-based chemotherapy during the first trimester (at 5 and 8 weeks), with 1 patient having an elective abortion and the other a miscarriage; 1 patient with high-tumor burden follicular lymphoma had an elective abortion at week 7, and 1 patient was found to be pregnant in the first trimester during the final radiation treatment.
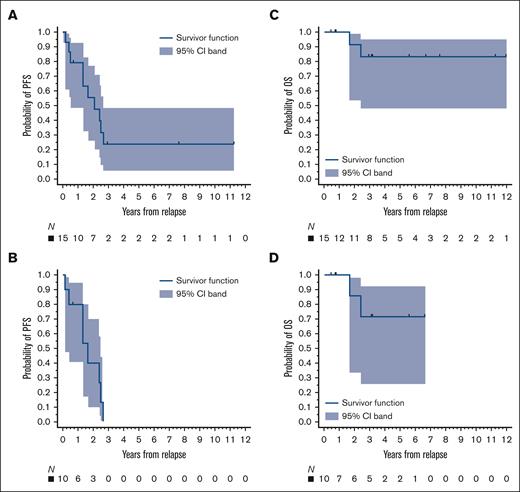
Maternal pregnancy–related complications were infrequent. One patient experienced preeclampsia, 2 had gestational diabetes, and 2 had infectious complications (1 case each of chorioamnionitis and postpartum endometritis). All patients received deferred therapy after delivery. Of the 15 patients with complete follow-up data, 10 relapsed and 2 died (1 of 4 patients treated antenatally progressed vs 9 of 11 patients who had therapy deferred). Three of the progressions occurred in the intrapartum (1 with and 2 without therapy). With a median follow-up of 37 months (range, 5-151), the 3-year PFS and OS estimates for all patients were 24% (95% confidence interval [CI], 5.8-48.5) and 83% (95% CI, 48.2-95.6), respectively, and 0% and 71% (95% CI, 25.8-92.0), respectively, for patients with cHL only (Figure 1). Finally, there were no obvious differences in maternal or neonatal complications between antepartum and postpartum therapies.
Survival. The 3-year PFS rates for (A) all patients and (B) patients with cHL were 24% and 0%, respectively, and the 3-year OS rates for (C) all patients and (D) patients with cHL were 83% and 71%, respectively. Survival times started on the date of diagnosis of relapsed lymphoma during pregnancy.
Survival. The 3-year PFS rates for (A) all patients and (B) patients with cHL were 24% and 0%, respectively, and the 3-year OS rates for (C) all patients and (D) patients with cHL were 83% and 71%, respectively. Survival times started on the date of diagnosis of relapsed lymphoma during pregnancy.
In a real-world international collaboration, we delineated patient and disease characteristics with associated gestational, fetal, and maternal outcomes among patients diagnosed with relapsed/refractory lymphoma during pregnancy. The median time from initial lymphoma diagnosis to relapse was ∼3 years, with a median gestation of 22 weeks at the time of diagnosis of relapsed/refractory disease. Most patients had therapy deferred to after delivery, whereas several patients were safely and successfully treated antenatally with platinum-based regimens, including 1 patient who received checkpoint inhibitor therapy. Overall, there were few obstetrical or neonatal complications and maternal OS was strong. However, the maternal PFS was poor, especially in patients with cHL, and most of the births were preterm, with most being clinician-initiated. In interpreting these observations, several factors should be considered.
Anticancer therapy during pregnancy presents a unique clinical and ethical dilemma of balancing maternal health and survival without endangering fetal wellbeing. Care should involve a multidisciplinary team, including hematology/oncology, radiation oncology, high-risk maternal-fetal medicine, and neonatology. The decision to treat antenatally is highly individualized, considering the tumor burden, disease location, disease-related symptoms, aggressiveness of lymphoma, gestation of pregnancy, and patient preference.7
A multitude of therapeutic options are available for the treatment of relapsed/refractory lymphoma, including multiagent chemotherapy and targeted therapeutic agents. However, there are sparse reports on the use of nonrituximab immunotherapy or other targeted therapeutic agents for the treatment of lymphoma during pregnancy.8,9,10 Patients treated antenatally in our report mostly received platinum-containing chemotherapy regimens. There have been reports of transient use of immune checkpoint inhibitors in patients with solid tumors with an unexpected pregnancy.11 The case herein was one of the first patients with lymphoma treated intentionally with a checkpoint inhibitor and the first known patient with cancer treated beyond 34 weeks of gestation.6 The article by Evens et al presents additional details, including maternal blood, cord, and placental pharmacokinetic analyses.6
Most deliveries among patients with relapsed/refractory lymphoma herein were preterm births, with most being clinician-initiated, primarily to initiate systemic chemotherapy. Whenever possible, in pregnancies complicated by cancer, a goal is to achieve a term birth. This may involve treatment of the malignancy during pregnancy as opposed to elective preterm birth to avoid treatment during pregnancy. Preterm birth can have a more significant effect on neonatal neurodevelopment than in utero exposure to chemotherapy (beyond the first trimester).2,3,7,12-14 In prospective studies, when children underwent developmental testing after chemotherapy exposure compared with a control group, for each additional gestational week gained, cognitive scores increased 2.3 points. Infants born at 34 to 36 weeks, which are considered late preterm, can have an increased risk of complications.14 When additional maternal therapy is required after delivery, pregnancy does not need to continue beyond 37 weeks.15,16 In addition, national cohort data have shown that preterm birth is an independent risk factor for maternal cardiovascular disease and premature mortality in mothers.17,18
It is likely that the compromised PFS observed for patients in this series was attributable to the delayed initiation of systemic therapy, which was over 4 months from diagnosis to the start of treatment for patients who had deferred therapy (ie, median gestational age at diagnosis of relapsed/refractory disease at 19 weeks with a median delivery of 36.5 weeks, etc). Although term delivery at ≥37 weeks is a general goal, when the maternal condition deteriorates and/or the use of novel targeted therapeutic agents (with unknown antenatal safety) is warranted, balancing expediting a clinician-assisted preterm delivery vs continuing the pregnancy must be discussed with the patient and a multidisciplinary team, including neonatal consultants.
A limitation of this retrospective study was the small cohort size. Given the highly selective nature of the cases, these data should be interpreted with caution. This in part reflects the rare occurrence of relapsed lymphoma during pregnancy. Furthermore, there should be caution in drawing significant conclusions regarding the consequences of delaying therapy on lymphoma outcomes as well as on the findings of maternal outcomes. In addition, the period examined included an era of newer targeted therapeutics that may have affected the outcomes. As the therapeutic indications for immunotherapy broaden across several different cancers and the integration of targeted therapeutic agents has become a part of the standard therapy, there is an increased need to understand the efficacy and safety of these agents in pregnant people with cancer.
Collectively, we identified cHL as the dominant relapsed/refractory histologic subtype observed in patients with lymphoma occurring during pregnancy. Therapy was commonly deferred, whereas several patients with life-threatening diseases received intentional antenatal therapy, including checkpoint inhibition in 1 case, which was effective and well tolerated. Most births were iatrogenic preterm births (not spontaneous), and there were few associated obstetrical or neonatal complications. Despite modest maternal PFS, the OS was robust. Continued emphasis on the importance of full-term birth in pregnancies complicated by cancer is necessary and research on these complex cancer cases is warranted.5
Acknowledgment: No financial support was necessary for the preparation of this manuscript or acquiring data.
Contribution: F.F. contributed to writing the original draft and data curation; J.S.B. contributed to the writing of the original draft; E.C., E.P., J.V., S.A., P.R.G., A.J.O., H.Y., U.F., N.H., C.M., R.F., M.M.G., K.D.S., and F.A. contributed to the writing, review, and editing of this article; Y.L. contributed to the formal analysis, writing, review, and editing of this article; and A.M.E. contributed to the conceptualization, formal analysis, and writing of the manuscript.
Conflict-of-interest disclosure: A.J.O. reports research funding from Precision Bio, Adaptive Biotechnologies, Celdex, Acrotech Biopharma, Genmab, and Genentech, and consultancy for Schrodinger, TG Therapeutics, and Genmab. S.A. reports research support to institution for clinical trials from Seattle Genetics, Merck, Xencor, Chimagen and Tessa Therapeutics; has membership on Tessa Therapeutic’s and Chimagen scientific advisory committee; serves on data safety monitoring board for Myeloid Therapeutics; and is a consultant for ADC Therapeutics and Kite/Gilead. P.R.G. reports consultancy services to Kite Pharma, Bristol Myers Squibb, and Rafael Pharma, and served on the advisory boards of Pharmacyclics LLC, ADC Therapeutics, Cellectar Biosciences, and Ono Pharma. A.M.E. reports consulting services to SeaGen, HUTCHMED, Incyte, Epizyme, Pharmacyclics, Novartis, AbbVie, and Daiichi Sankyo. The remaining authors declare no competing financial interests.
Correspondence: Andrew M. Evens, Division of Blood Disorders, Rutgers Cancer Institute of New Jersey, 195 Little Albany St, New Brunswick, NJ 08903; e-mail: andrew.evens@rutgers.edu.
References
Author notes
Presented in abstract form at the 63rd annual meeting of the American Society of Hematology, Atlanta, GA, 12 December 2021.
Data are available on request from the corresponding author, Andrew M. Evens (andrew.evens@rutgers.edu).