Key Points
Nivolumab + BV showed durable safety and efficacy as salvage in patients with R/R PMBL after 3-year follow-up, with no new safety signals.
High 2-year complete response rates (80%-100%) after subsequent HCT indicate potential for nivo + BV as a bridging therapy to transplant.
Abstract
Patients with relapsed/refractory primary mediastinal large B-cell lymphoma (R/R PMBL) have poor responses to salvage therapy. Nivolumab and brentuximab vedotin (BV) showed promising early efficacy in patients with R/R PMBL in the phase 1/2 open-label, multicenter CheckMate 436 study; we report safety and efficacy findings from the 3-year follow-up. Patients who were eligible were aged ≥15 years with R/R PMBL previously treated with either high-dose chemotherapy plus autologous hematopoietic cell transplantation (HCT) or ≥2 prior multiagent chemotherapies, and had Eastern Cooperative Oncology Group performance status scores of 0 to 1 and CD30 expression of ≥1%. Patients were treated with nivolumab 240 mg and BV 1.8 mg/kg once every 3 weeks until disease progression or unacceptable toxicity. Primary end point was objective response rate (ORR); secondary end points included complete response rate, duration of response, progression-free survival (PFS), and overall survival (OS). Safety was monitored throughout. At final database lock (30 March 2022), 29 patients had received nivolumab plus BV; median follow-up was 39.6 months. Investigator-assessed ORR was 73.3%; median time to response was 1.3 months (range, 1.1-4.8). Median PFS was 26.0 months; median OS was not reached. PFS and OS rates at 24 months were 55.5% (95% confidence interval [CI], 32.0-73.8) and 75.5% (95% CI, 55.4-87.5), respectively. The most frequently occurring grade 3/4 treatment-related adverse event was neutropenia. Consolidative HCT was received by 12 patients, with a 100-day complete response rate of 100.0%. This 3-year follow-up showed long-term efficacy for nivolumab plus BV in R/R PMBL, with no new safety signals. This trial was registered at www.clinicaltrials.gov as #NCT02581631.
Introduction
Primary mediastinal large B-cell lymphoma (PMBL) is a mature, aggressive large B-cell lymphoma of thymic origin. PMBL occurs most frequently in young adults, with a median patient age of 35 years and a slight female predominance, and constitutes 2% to 4% of non-Hodgkin lymphoma (NHL).1,2 First-line treatment for PMBL typically consists of chemoimmunotherapy with rituximab and chemotherapeutic agents.3 After first-line chemoimmunotherapy, the 3-year progression-free survival (PFS) rate is 89.4%,4 and 5-year PFS and event-free survival are generally >80%.5,6 In contrast, patients with relapsed/refractory (R/R) PMBL have poor outcomes; the 2-year PFS rate after first disease progression is reported as 29%.4 The objective response rate (ORR) to salvage chemotherapy was 25%, and the 2-year overall survival (OS) rate after diagnosis of R/R disease was between 15% and 29%.7,8 These outcomes highlight a need for new therapeutic strategies for patients with R/R PMBL.
The checkpoint inhibitor nivolumab is a human immunoglobulin G4 monoclonal antibody that binds to the programmed death 1 (PD-1) protein receptor and blocks its interaction with programmed death ligand (PD-L) 1 and PD-L2.9,10 Brentuximab vedotin (BV), an anti-CD30 antibody conjugated to monomethyl auristatin E, induces apoptosis of CD30+ tumor cells by disrupting the microtubule network and inhibiting cell division.11,12 Nivolumab in combination with BV has shown promising efficacy in patients with R/R classical Hodgkin lymphoma (cHL), a disease that shares clinical and molecular features with PMBL, including abnormalities in chromosomes 9p and 2p, high mutational density, and expression of CD30.13-16 Phase 1/2 trials of nivolumab in combination with BV in R/R cHL have reported ORRs ranging from 82% to 89%,17,18 and a recent long-term follow-up of patients with R/R cHL treated with nivolumab plus BV showed durable efficacy, with an 85% ORR and an estimated 3-year PFS rate of 77% at a median follow-up of 34.3 months.19
In the treatment of R/R PMBL, BV monotherapy has shown an ORR of just 13.3% in a phase 2 study.20 An optimal treatment approach for R/R PMBL is not well established, but therapies with anti–PD-1 agents, such as nivolumab with or without BV and pembrolizumab, are included as treatment options based on their clinical efficacy in this setting.3 The phase 1b KEYNOTE-013 and phase 2 KEYNOTE-170 studies showed ORRs of 48% and 41.5%, respectively, in adult patients with R/R PMBL treated with pembrolizumab.21 The CheckMate 436 study is an open-label, multicenter, multicohort, phase 1/2 study of nivolumab in combination with BV in patients with R/R NHL. Initial results from 11.1 months median follow-up of the R/R PMBL cohort showed a 73% ORR, and a 37% complete response (CR) rate per investigator; 6-month PFS and OS rates were 63.5% and 86.3%, respectively.23 The safety profile was manageable and there were no treatment-related deaths.23 Here, we report the results from the 3-year follow-up of the PMBL expansion cohort of the CheckMate 436 trial.
Methods
The methods for the CheckMate 436 study PMBL cohort were previously reported by Zinzani et al23 and are briefly described here.
Trial design and ethics
Checkmate 436 (ClinicalTrials.gov identifier: #NCT02581631) is an open-label, multicenter, multicohort, phase 1/2 study of nivolumab in combination with BV in adult patients with R/R NHL. The study consists of a phase 1 dose evaluation and a phase 2 efficacy and safety evaluation in expansion cohorts with R/R NHL subtypes: PMBL, diffuse large B-cell lymphoma, peripheral T-cell lymphoma, cutaneous T-cell lymphoma, and mediastinal gray zone lymphoma. Results from the R/R PMBL cohort are reported here.
All patients provided written informed consent. The study protocol was approved by the ethical review committee of all participating centers and was carried out in accordance with the principles of the Declaration of Helsinki.
Patients
Patients who were eligible in the PMBL cohort were aged ≥15 years, had Eastern Cooperative Oncology Group performance status scores of 0 to 1, had CD30 expression of ≥1% in the tumor or tumor-infiltrating lymphocytes (as measured by local immunohistochemistry), and had measurable disease per Lugano 2014 classification.24 Patients were required to have R/R disease after high-dose chemotherapy with autologous hematopoietic cell transplantation (auto-HCT) or >2 previous multiagent chemotherapies (for patients ineligible for auto-HCT).
Treatment
Doses for the phase 2 expansion cohorts were determined during the phase 1 dose-evaluation assessments.23 Patients received nivolumab 240 mg (flat dose) and BV 1.8 mg/kg once every 3 weeks and were treated until disease progression or unacceptable toxicity. Treatments were administered intravenously over 30 minutes. Patients received nivolumab on cycle 1, day 8, and BV on cycle 1, day 1. In subsequent cycles both treatments were given on the same day, with BV administered before nivolumab infusion, separated by a 30-minute rest period. Per protocol, dose modifications per body weight change were allowed for BV, and reduction to 1.2 mg/kg was permitted for prespecified toxicities. No dose modifications were permitted for nivolumab, but doses could be delayed for specified treatment-related adverse events (TRAEs). Follow-up visits were carried out 35 ± 7 days after the last dose and 80 ± 7 days after the first follow-up, with subsequent follow-up for survival every 3 months until death or loss to follow-up.
End points and assessments
The primary efficacy end point of the study was ORR assessed by investigator, with OR defined as the best overall response of confirmed CR, or partial response (PR) as assessed per Lugano 2014 classification24 between the date of first dose and either documented progression or subsequent therapy (including auto-HCT or allogeneic HCT). Secondary end points included duration of response (DOR), CR rate, duration of CR, PFS, and OS. DOR was calculated as time from initial documented CR or PR to the first evidence of progressive disease, relapse, or death. PFS was defined as the time from the first dose of study drug until the first evidence of progressive disease, relapse, or death. Patients who were progression-free and alive or had unknown status were censored at the last tumor assessment, and patients who received subsequent therapy (including HCT) before documented progression were censored on the last tumor assessment date before subsequent therapy. OS was defined as the time from first dose of study drug until the date of death for any reason; if the patient was alive or vital status was unknown, patients were censored at the last date on which they were known to be alive.
CD30 and PD-L1 expression were measured in baseline tumor biopsy samples using validated immunohistochemistry assays (CD30, Mosaic laboratories; PD-L1, BMS and DAKO North America). PD-L1 and CD30 expression status was defined as the percentage of tumor cells exhibiting cell surface staining of each biomarker in a minimum of 100 evaluable tumor cells.
Patients discontinuing treatment for any reason entered the follow-up phase of the study and were monitored for long-term safety, survival status, disease progression, subsequent anticancer therapy, and occurrence of other primary malignancies. The first 2 follow-up visits were conducted in person and subsequent follow-up visits were conducted in person or by phone. All participants were followed-up for survival at least every 3 months until death or loss to follow-up.
Safety was monitored throughout the study. All AEs were tabulated using the worst grade per Common Terminology Criteria for Adverse Events version 4.03 criteria and Medical Dictionary for Regulatory Activities preferred terms. Safety analyses were performed per cohort and in all patients who were treated, combined.
Statistical analysis
A sample size of 30 patients with R/R PMBL was chosen based on a 2-sided 80% confidence interval (CI) for an ORR of 37% to 63% by assuming an observed ORR of 30%. ORR and CR were summarized by binomial response rate using the Clopper–Pearson method. PFS was summarized using the Kaplan–Meier product-limit method. Descriptive statistics of safety are presented. All statistical analyses were performed using SAS version 9.4.
Results
Baseline demographics and disease characteristics, patient disposition, and exposure
A total of 30 patients with R/R PMBL were enrolled and treated. The median patient age was 36 years (range, 19-83). Most patients had refractory disease at enrollment, and all had received prior first-line rituximab-based therapy (Table 1). At the final database lock (30 March 2022), all 30 patients had ended the treatment period. The most frequent reasons for treatment discontinuation were maximum clinical benefit (43.3%, n = 13) and disease progression (26.7%, n = 8) (supplemental Table 1). Twenty-four (80%) patients with R/R PMBL were continuing in the study (either for continued treatment or follow-up). Of the patients not continuing in the study, 4 (13.3%) died, 1 (3.3%) withdrew consent, and 1 (3.3%) was lost to follow-up. Nivolumab was received by 29 patients; 1 additional patient received BV in cycle 1, day 1 but discontinued before the first nivolumab dose. The median (range) number of doses received was 5 (1-35) and 5 (1-20) for nivolumab and BV, respectively, with 22 (75.9%) patients receiving a relative dose intensity between 90% and 110% for nivolumab.
Baseline demographics and disease characteristics
| Characteristic . | Patients (N = 30) . |
|---|---|
| Age, median (range), y | 36 (19-83) |
| Female, n (%) | 17 (56.7) |
| Disease stage at initial diagnosis,∗,†n (%) | |
| I–II | 16 (53.3) |
| III–IV | 13 (43.3) |
| Disease status at enrollment,†n (%) | |
| Relapsed | 7 (23.3) |
| Refractory | 18 (60.0) |
| Relapsed and refractory‡ | 5 (16.7) |
| Previous lines of systemic therapy, median (range)1§ | 2 (2-5) |
| First-line therapy,§n (%) | |
| R-CHOP | 8 (26.7) |
| R-EPOCH | 9 (30.0) |
| Other‖ | 13 (43.3) |
| Previous rituximab,† n (%) | 30 (100.0) |
| Previous auto-HCT,† n (%) | 4 (13.3) |
| Characteristic . | Patients (N = 30) . |
|---|---|
| Age, median (range), y | 36 (19-83) |
| Female, n (%) | 17 (56.7) |
| Disease stage at initial diagnosis,∗,†n (%) | |
| I–II | 16 (53.3) |
| III–IV | 13 (43.3) |
| Disease status at enrollment,†n (%) | |
| Relapsed | 7 (23.3) |
| Refractory | 18 (60.0) |
| Relapsed and refractory‡ | 5 (16.7) |
| Previous lines of systemic therapy, median (range)1§ | 2 (2-5) |
| First-line therapy,§n (%) | |
| R-CHOP | 8 (26.7) |
| R-EPOCH | 9 (30.0) |
| Other‖ | 13 (43.3) |
| Previous rituximab,† n (%) | 30 (100.0) |
| Previous auto-HCT,† n (%) | 4 (13.3) |
R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; R-EPOCH, rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin.
Disease stage at initial diagnosis not reported for 1 patient.
Data from December 2020 database lock, presented at ICML 2021.25
Defined as patients who relapsed after at least 1 prior line of therapy and had no response to the most recent prior therapy.
These data are from the January 2019 database lock.
Other anthracycline-containing therapies.
Efficacy
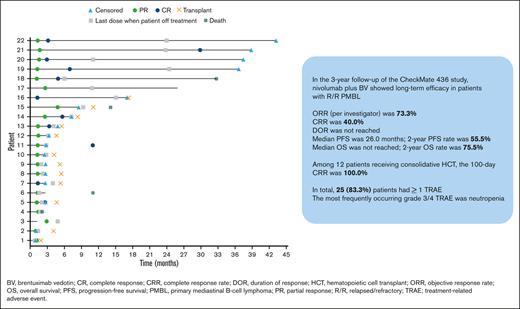
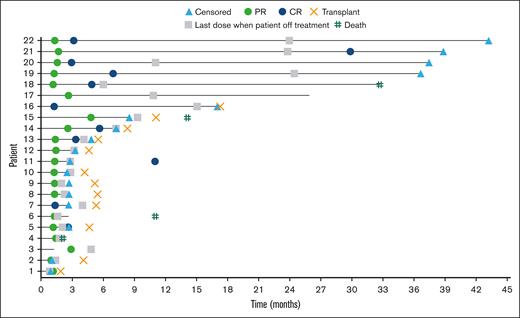
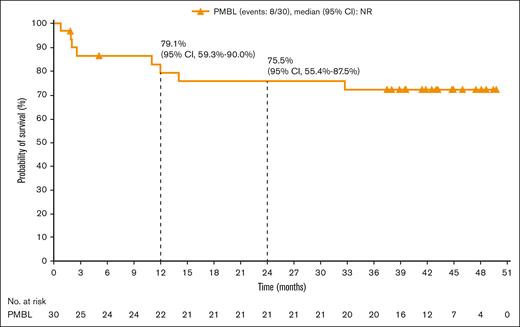
The ORR was 73.3% (95% CI, 54.1-87.7), with 40.0% and 33.3% of patients achieving CR and PR, respectively (Table 2). The median time to response was 1.3 months (range, 1.1-4.8), with 3.2 months (range, 1.2-11.0) to CR. At a median follow-up of 39.6 months, the median DOR was not reached. Four patients remained progression-free without subsequent HCT (Figure 1). Median PFS was 26.0 months (95% CI, 2.6-not reached); PFS rates at 6, 12, 18, and 24 months were 63.5% (95% CI, 42.5-78.6), 55.5% (95% CI, 32.0-73.8), 55.5% (95% CI, 32.0-73.8), and 55.5% (95% CI, 32.0-73.8), respectively (Figure 2). Median OS was not reached; OS rates at 6, 12, 18, and 24 months were 86.3% (95% CI, 67.5-94.6), 79.1% (95% CI, 59.3-90.0), 75.5% (95% CI, 55.4-87.5), and 75.5% (95% CI, 55.4-87.5), respectively (Figure 3).
Best overall response per investigator
| Best overall response∗ . | Patients (N = 30) . |
|---|---|
| ORR† | 22 (73.3) |
| 80% CI, % | 60.3-83.8 |
| 95% CI, % | 54.1-87.7 |
| CR | 12 (40.0) |
| PR | 10 (33.3) |
| Stable disease | 3 (10.0) |
| Relapsed or progressive disease | 3 (10.0) |
| Unable to determine (death before disease assessment) | 2 (6.7) |
| Best overall response∗ . | Patients (N = 30) . |
|---|---|
| ORR† | 22 (73.3) |
| 80% CI, % | 60.3-83.8 |
| 95% CI, % | 54.1-87.7 |
| CR | 12 (40.0) |
| PR | 10 (33.3) |
| Stable disease | 3 (10.0) |
| Relapsed or progressive disease | 3 (10.0) |
| Unable to determine (death before disease assessment) | 2 (6.7) |
Data are reported as number (percentage) unless otherwise stated.
Based on Lugano classification 2014.
CIs based on the Clopper–Pearson method.
Event chart for tumor response, tumor progression, duration of therapy, transplantation, and death for all responders with R/R PMBL, as assessed by investigator. Horizontal bar indicates PFS.
Event chart for tumor response, tumor progression, duration of therapy, transplantation, and death for all responders with R/R PMBL, as assessed by investigator. Horizontal bar indicates PFS.
PFS, per investigator, in all treated patients with R/R PMBL. NR, not reached.
OS, per investigator, in all patients with R/R PBML who received trearment.
Evaluation of response status per investigator vs CD30 expression in baseline tumor biopsies showed that patients across the entire range of CD30 expression were able to achieve CR or PR (supplemental Figure 1). All patients who received treatment had ≥5% PD-L1+ cells at baseline, however a PD-L1 expression subgroup deriving a higher or lower response benefit could not be identified (supplemental Figure 2).
Subsequent therapy and consolidative HCT
A total of 18 (60%) patients received subsequent anticancer therapy, including 7 patients (23%) who received nonpalliative radiotherapy and 6 (20%) who received systemic therapy. Among these patients, 13 received subsequent anticancer therapy without a previously reported progression event. Consolidative HCT was received by 12 (40%) patients: 6 (20%) receiving auto-HCT and 6 (20%) receiving allogeneic HCT (supplemental Table 2). Patients received between 3 and 20 cycles of nivolumab plus BV before auto-HCT, and 5 to 14 cycles of nivolumab plus BV before allogeneic HCT. Of the patients receiving auto-HCT or allogeneic HCT, 50% (3 of 6) of patients achieved CR at the time of transplantation in each group; patients not achieving CR had a best overall response of PR. After 100 days, 100% (6 of 6) and 100% (5 of 5) of patients assessed in the auto-HCT and allogeneic HCT groups, respectively, had CR. Two patients continued to investigational trials for chimeric antigen receptor (CAR) T-cell therapy.
Safety
A total of 25 (83.3%) patients experienced at least 1 TRAE, with 16 (53.3%) patients experiencing a grade 3/4 TRAE (Table 3). The most frequently occurring any-grade TRAEs were neutropenia (43.3%), pyrexia (30.0%), and arthralgia (20.0%). Neutropenia was also the most frequently occurring grade 3/4 TRAE (40.0%). There were no grade 5 TRAEs related to the study drug. The most common immune-mediated AEs were diarrhea/colitis and rash (supplemental Table 3).
Any-grade TRAEs reported in greater than or equal to 10% of patients who received treatment and grade 3/4 TRAEs in all patients with R/R PMBL who received treatment
| Any-grade TRAEs reported in ≥ 10% patients who received treatment, and grade 3/4 TRAEs in any patients, n (%) . | Any grade . | Grade 3/4 . |
|---|---|---|
| Total | 25 (83.3) | 16 (53.3) |
| Neutropenia | 14 (43.3) | 13 (40.0) |
| Pyrexia | 9 (30.0) | 1 (3.3) |
| Arthralgia | 6 (20.0) | 0 |
| Thrombocytopenia | 5 (16.7) | 3 (10.0) |
| Rash | 5 (16.7) | 1 (3.3) |
| Peripheral sensory neuropathy | 5 (16.7) | 0 |
| Peripheral neuropathy | 4 (13.3) | 3 (10.0) |
| Hyperthyroidism | 4 (13.3) | 0 |
| Decreased neutrophil count | 2 (6.7) | 2 (6.7) |
| Colitis | 1 (3.3) | 1 (3.3) |
| Immune-mediated hepatitis | 1 (3.3) | 1 (3.3) |
| Maculopapular rash | 1 (3.3) | 1 (3.3) |
| Any-grade TRAEs reported in ≥ 10% patients who received treatment, and grade 3/4 TRAEs in any patients, n (%) . | Any grade . | Grade 3/4 . |
|---|---|---|
| Total | 25 (83.3) | 16 (53.3) |
| Neutropenia | 14 (43.3) | 13 (40.0) |
| Pyrexia | 9 (30.0) | 1 (3.3) |
| Arthralgia | 6 (20.0) | 0 |
| Thrombocytopenia | 5 (16.7) | 3 (10.0) |
| Rash | 5 (16.7) | 1 (3.3) |
| Peripheral sensory neuropathy | 5 (16.7) | 0 |
| Peripheral neuropathy | 4 (13.3) | 3 (10.0) |
| Hyperthyroidism | 4 (13.3) | 0 |
| Decreased neutrophil count | 2 (6.7) | 2 (6.7) |
| Colitis | 1 (3.3) | 1 (3.3) |
| Immune-mediated hepatitis | 1 (3.3) | 1 (3.3) |
| Maculopapular rash | 1 (3.3) | 1 (3.3) |
Nine patients discontinued study treatment because of AEs; among these patients, 4 initially discontinued BV and remained on nivolumab, 4 discontinued both study drugs simultaneously, and 1 died. Six patients discontinued study treatment because of TRAEs, most commonly peripheral neuropathy (supplemental Table 4). There were 8 deaths reported, 2 of which occurred within 30 days of the last dose. The primary cause of death was disease progression (n = 5); other causes included sepsis (n = 1), cardiovascular disease (n = 1), and neurologic complications after allogeneic HCT (n = 1). No deaths were considered related to the study drug by the investigator.
Discussion
This 3-year follow-up of the CheckMate 436 study of nivolumab plus BV for the treatment of R/R PMBL represents one of the studies using checkpoint inhibitors that have the longest follow-ups in this setting, and shows one of the highest ORRs for this treatment setting.21,22,26 Efficacy results with nivolumab plus BV were consistent with previous reports from CheckMate 436,23 with a 73.3% ORR and a 40.0% CR rate. The 2-year OS rate of 75.5% indicates durable efficacy of nivolumab plus BV as salvage therapy in R/R PMBL. Safety data were also consistent with previous reports of CheckMate 436,23 with neutropenia, thrombocytopenia, and peripheral neuropathy among the most frequently occurring grade 3/4 TRAEs. This 3-year follow-up showed an increase in overall incidence of neutropenia compared with the previous report (any grade: 43% vs 30%; grade 3/4: 40% vs 30%).23 The reason for this increased incidence is unclear, and the association of neutropenic events with extended exposure to nivolumab has not been examined in NHL. Neutropenia has been observed after anti–PD-1 monotherapy in patients with R/R PMBL.22
Patients with R/R PMBL treated with nivolumab plus BV in this study achieved a 2-year PFS rate of 55.5%, which represents approximately twice the PFS rates previously reported for this population,4 and ORR and OS rates are more than doubled compared with prior reports of salvage chemotherapy in this population.7,8 Patients with R/R PMBL in the CheckMate 436 study were also able to achieve CR or PR across a range of CD30 expression levels.
These results also align with a previous study showing long-term efficacy of nivolumab plus BV in patients with R/R cHL, which reported a 3-year ORR of 85% and an OS rate of 93%.19 The ORR for nivolumab plus BV in this study was also higher than the 13.3% ORR for BV monotherapy in R/R PMBL20 and the 48% and 41.5% ORRs for anti-PD-1 monotherapy reported in the KEYNOTE-013 and KEYNOTE-170 trials, respectively,21 although direct comparisons between these studies should be approached with caution because of differences in design.22
The distinct antitumor mechanisms of action of BV and anti–PD-1 agents provide strong rationale for combining these therapies.27 BV complements the checkpoint inhibition of anti–PD-1 agents not only by disrupting the microtubule network of tumor cells and triggering apoptosis12 but also through activation of innate and adaptive immune cells. In mouse models, proinflammatory antitumor immune responses resulting from BV treatment are potentiated by combination anti–PD-1 therapy.28,29 Recent studies have also indicated clinical synergy between nivolumab and BV in R/R cHL. In the CheckMate 205 study, nivolumab showed efficacy in patients with R/R cHL who had previously been treated with BV,10 and a retrospective analysis of patients with R/R cHL treated with nivolumab plus BV indicated promising efficacy.30 R/R PMBL shares clinical and molecular features with R/R cHL, including high mutational density and expression of CD30,13-15,31 and data reported here for CheckMate 436 possibly indicate a similar synergy in patients with R/R PMBL.
Preliminary data showed that 4 patients in this study had a durable PFS without consolidative HCT; however, 60% of all patients in the study received subsequent anticancer therapy, and 40% received either allogeneic or auto-HCT. Further analysis is warranted to explore survival outcomes in patients treated with nivolumab plus BV with vs without consolidation therapy.
In patients with R/R PMBL, response to salvage therapy is a prognostic factor for the success of subsequent HCT but patients treated with ifosfamide, carboplatin, etoposide, or rituximab plus etoposide as salvage therapy before auto-HCT previously showed an estimated 3-year OS rate of just 65%.32 In the CheckMate 436 study, the ORR was 73.3%, with a 24-month OS rate of 55.5%. Of the patients treated with nivolumab plus BV in the PMBL cohort who underwent HCT, CR rates were high, with a 100% CR rate after 100 days and 2-year CR rates of 100% and 80% for auto-HCT and allogeneic HCT, respectively. Results from the CheckMate 436 study indicate that nivolumab plus BV can potentially be a bridging therapy before auto-HCT and allogeneic HCT for patients with R/R PMBL. In addition, CAR T-cell therapy is emerging as an effective treatment option for R/R lymphomas,33-36 and a preliminary study has shown the safety and efficacy of CD30-directed CAR T-cell therapy in cHL.37 Recent studies have shown a potential positive impact for radiotherapy compared with systemic therapy as bridging treatment between leukapheresis and CAR T-cell infusion for large B-cell lymphoma,38 whereas patients receiving high intensity chemotherapy as bridging therapy showed lower OS and increased rates of infection.39 To our knowledge, no studies have assessed chemoimmunotherapy with checkpoint inhibitor plus BV as bridging therapy to CAR T-cell therapy. Based on the preliminary success of consolidative HCT in patients treated with nivolumab plus BV, this combination regimen could be considered as a bridging therapy for CAR T-cell therapy in future studies in R/R lymphoma.
This study has a few limitations. The sample size was small (N = 30) and there is no comparator arm; this limits the interpretability of results and possibility of subgroup analyses and comparison with a control population. The study design evaluated only the efficacy and safety of nivolumab plus BV before consolidation; survival outcomes after consolidation were not examined. There was also no central pathology review for efficacy assessment. Both median DOR and median OS were not reached in this extended follow-up, indicating that further long-term studies would provide more insight into survival outcomes.
Overall, this 3-year follow-up of the CheckMate 436 study shows durable safety and efficacy for nivolumab plus BV in patients with R/R PMBL and identifies no new safety signals. These results support nivolumab plus BV as a salvage therapy option in PMBL, and this regimen could be considered as a bridging therapy for stem cell transplantation and CAR T-cell therapy.
Acknowledgments
The authors thank the patients and their families for making this study possible, and all coinvestigators and clinical study teams who participated in the trial.
Direct funding was provided by Bristol Myers Squibb through the joint financial support of Bristol Myers Squibb and Seagen. Professional medical writing and editorial support were provided by Katie Walwyn-Brown, of Caudex, funded by Bristol Myers Squibb.
Authorship
Contributions: A.S. conceptualized and designed the study; J. Kuruvilla provided study matierials and/or recruited/enrolled patients; A.A. collected and assembled data; A.S., J. Kuruvilla, N.M.-S., and A.A. analyzed and interpreted the data; P.L.Z., A.S., G.G., P.B., P.M.B., J. Kuruvilla, D.C., J. Kline, N.A.J., N.M-S., J.L., R.W., A.A., and A.J.M. wrote the manuscript; P.L.Z., A.S., G.G., P.B., P.M.B., J. Kuruvilla, D.C., J. Kline, N.A.J., N.M.-S., J.L., R.W., A.A., and A.J.M. provided final approval of the manuscript; and P.L.Z., A.S., G.G., P.B., P.M.B., J. Kuruvilla, D.C., J. Kline, N.A.J., N.M.-S., J.L., R.W., A.A., and A.J.M. are accountable for all aspects of the work.
Conflict-of-interest disclosure: P.L.Z reports honoraria from AstraZeneca, Bristol Myers Squibb, Gilead, Incyte, Kyowa Kirin, Merck, Novartis, Rocke, Takeda, and Sanofi. A.S. reports honoraria from AbbVie, Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Celgene, Eisai, Gilead, Lilly, Merck Sharp and Dohme, Novartis, Pfizer, Roche, Sandoz, Servier, and Takeda; serves on the advisory boards for Bayer, Bristol Myers Squibb, Eisai, Gilead, Merck Sharp and Dohme, Pfizer, and Servier; and reports consultancy for Incyte and Sanofi. G.G. reports honoraria from BeiGene, Clinigen, Ideogen, Incyte, Roche, and Takeda; serves on the advisory boards for Genmab, Ideogen, IQVIA, Italfarmaco, Kite-Gilead, Roche, and Takeda; reports consultancy for Takeda; and reports meeting/travel support from Roche and Sandoz. P.B. reports honoraria from Takeda. P.M.B. serves on the advisory board for TG Therapeutics; and reports consultancy for AbbVie, AstraZeneca, BeiGene, Bristol Myers Squibb, Genentech, Gilead, Janssen, Merck, and TG Therapeutics. J. Kuruvilla reports honoraria from AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Gilead, Incyte, Janssen, Karyopharm, Merck, Novartis, Pfizer, Roche, and Seattle Genetics; serves on advisory boards for Karyopharm; reports consultancy for AbbVie, Antengene, Bristol Myers Squibb, Gilead, Karyopharm, Medison Ventures, Merck, Roche, and Seattle Genetics; reports research support (institution) from AstraZeneca and Merck; and is an advisory board chair for Lymphoma Canada. D.C. serves on advisory boards for OVIBIO; and reports research support (institution) from 4SC, Bayer, Celgene, Clovis, Eli Lilly & Co, Leap, MedImmune, and Roche. J. Kline reports honoraria from Kite-Gilead; consultancy for Merck, Morphoys, Seagen, and Secura Bio; and research support (institution) from Secura Bio. N.A.J. reports honoraria from AbbVie and BeiGene; reports consultancy for AbbVie, AstaZeneca, BeiGene, Merck, Roche, and Seattle Genetics; reports research support from Incyte; and reports board membership for Lymphoma Canada. N.M.-S. reports consultancy for AstraZeneca, C4 Therapeutics, Daiichi Sankyo, Genentech/Roche, Karyopharm, Kyowa Kirin, Ono Pharmaceuticals, and Secura Bio/Verastem; and reports research support (institution) from AstraZeneca, Bristol Myers Squibb, Celgene, C4 Therapeutics, Corvus Pharmaceuticals, Daiichi Sankyo, Dizal Pharmaceuticals, Genetech/Roche, Innate Pharmaceuticals, Secura Bio/Verastem, and Yingli Pharmaceuticals. J.L. is an employee and has stock in Seagen. R.W. is a Bristol Myers Squibb employee. A.A. is a Bristol Myers Squibb employee. A.J.M. reports honoraria from Affined, Imbrium Therapeutics, Janpix, Merck, miRagen, Seattle Genetics, and Takeda; reports consultancy for Affimed, Imbrium Therapeutics, Janpix, Merck, Seattle Genetics, and Takeda; reports research support (institution) from ADC Therapeutics, BeiGene, Bristol Myers Squibb, Incyte, Leukemia and Lymphoma Society, Merck, Miragen, NIH/NCI Cancer Center, Seattle Genetics, and Secura Bio; reports expert testimony for New England Cancer Specialists; serves as review editor for Hematologic Malignancies; is a SAB member for Lymphoma Hub; and is a scientific review committee member for Gilead Science.
Correspondence: Pier Luigi Zinzani, Lymphoma and Chronic Lymphoproliferative Syndromes Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Istituto di Ematologia “Seràgnoli,” Dipartimento di Medicina Specialistica, Diagnostica e Sperimentale, Università di Bologna, Via Massarenti 9, 40138, Bologna, Italy; e-mail: pierluigi.zinzani@unibo.it.
References
Author notes
The Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
The full-text version of this article contains a data supplement.