Key Points
Elevated BMI is associated with increased toxicity and NRM, and decreased OS among AYAs treated on DFCI consortium pediatric ALL regimens.
Normal BMI is associated with excellent outcomes, regardless of age; the deleterious effect of increased BMI is more pronounced in older AYAs.
Abstract
Adolescent and young adults (AYAs) with acute lymphoblastic leukemia (ALL) treated with asparaginase-containing pediatric regimens are commonly overweight or obese. We studied the association of body mass index (BMI) on outcomes of 388 AYAs aged 15 to 50 years treated on Dana-Farber Cancer Institute (DFCI) consortium regimens (2008-2021). BMI was normal in 207 (53.3%) and overweight/obese in 181 (46.7%). Patients who were overweight or obese experienced higher nonrelapse mortality (NRM; 4-year, 11.7% vs 2.8%, P = .006), worse event-free survival (4-year, 63% vs 77%, P = .003), and worse overall survival (OS; 4-year, 64% vs 83%, P = .0001). Because younger (aged 15-29 years) AYAs more frequently had a normal BMI (79% vs 20%, P < .0001), we conducted separate analyses in each BMI group. We found excellent OS among younger and older (30-50 years) AYAs with normal BMI (4-year OS, 83% vs 85%, P = .89). Conversely, in AYAs who were overweight/obese, worse outcomes were seen in older AYAs (4-year OS, 55% vs 73%, P = .023). Regarding toxicity, AYAs who were overweight/obese experienced higher rates of grade 3/4 hepatotoxicity and hyperglycemia (60.7% vs 42.2%, P = .0005, and 36.4% vs 24.4%, P = .014, respectively) but had comparable rates of hypertriglyceridemia (29.5% vs 24.4%, P = .29). In a multivariable analysis, higher BMI was associated with worse OS, hypertriglyceridemia was associated with improved OS, and age was not associated with OS. In conclusion, among AYAs treated on DFCI Consortium ALL regimens, elevated BMI was associated with increased toxicity, increased NRM, and decreased OS. The deleterious effect of elevated BMI was more pronounced in older AYAs.
Introduction
Adolescent and young adult (AYA) patients with acute lymphoblastic leukemia (ALL) have favorable outcomes when treated with pediatric ALL regimens.1,2 Pediatric regimens are believed to be effective because of intensive use of the nonmyelosuppressive agents asparaginase, corticosteroids, and vincristine, in combination with other chemotherapies. Asparaginase is a uniquely effective drug for the treatment of ALL but is also associated with a distinct set of adverse events including metabolic toxicities (hepatotoxicity, hyperglycemia, and hypertriglyceridemia), hypersensitivity, venous thromboembolism, pancreatitis, and orthopedic complications.3-5 Although pediatric ALL regimens have been shown to be tolerable in older children, adolescents, and young adults, the regimens are known to incur more toxicity in AYAs compared with in younger children.6
Obesity is a serious and worsening noninfectious health pandemic. The rate of obesity, defined as a body mass index (BMI) of >30 mg/m2 in adults and in children, a BMI ≥ 95% percentile, is rising among children, adolescents, and adults. The estimated prevalence of obesity among adults rose from 30.5% in 1999 to 2000, to 41.9% in 2017 to 2020 and also affects 19.7% of children.7 Importantly, in addition to being associated with liver disease, diabetes, cardiovascular disease, and cancer,8-11 obesity has been specifically associated with adverse outcomes in children with ALL receiving asparaginase-containing chemotherapy regimens.12,13 Especially concerning for patients with ALL is the reported association of obesity with increased risk of death, an association shown in both pediatric and adult cohorts.14,15
Although both age and obesity are reportedly associated with increased risk of treatment-related adverse events and death in patients being treated for ALL, a detailed understanding of the influence of obesity on metabolic toxicities, disease response, and survival in AYAs with ALL treated with pediatric regimens is limited. Thus, we aimed to assess the association between obesity and outcomes among AYA patients aged 15 to 50 years with ALL treated in accordance with Dana-Farber Cancer Institute (DFCI) consortium pediatric ALL regimens, which incorporate 30 weeks of continuous asparagine depletion during postremission therapy.
Methods
Patients
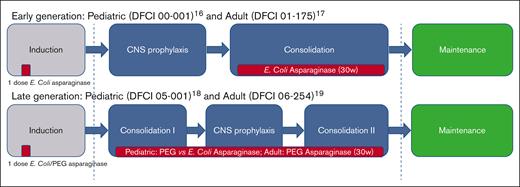
We included all patients aged 15 to 50 years (n = 308) treated on 4 sequential multicenter DFCI ALL Consortium protocols: Pediatric 00-00116 and Adult 01-17517 (older protocols); Pediatric 05-00118 and Adult 06-25419 (newer protocols). All protocols include an induction phase, a consolidation phase that includes 30 weeks of continuous asparaginase depletion, and a continuation phase that continues until 2 years from achievement of complete remission (CR, Figure 1). The older protocols used native Escherichia Coli asparaginase preparation, whereas the newer protocols incorporated pegylated asparaginase. In addition, we included all consecutive patients (n = 80) who were treated per these protocols at DFCI, Boston Children’s Hospital, and Massachusetts General Hospital between 2001 and 2021 with data extracted from the electronic medical record. BMI at diagnosis was used as a standardized measure of obesity and was calculated based on Center for Disease Control guidelines, per age-adjusted percentiles for patients aged 2 to 20 years (underweight: BMI < 5% percentile; normal: BMI within 5%-84.99% percentiles; overweight: BMI within 85%-94.99% percentiles; obese: BMI ≥95% percentile) and per absolute BMI (kg/m2) in patients aged ≥20 years (underweight: BMI <18.5 kg/m2; normal: BMI of 18.5-24.99 kg/m2; overweight: BMI of 25-29.99 kg/m2; and obese: BMI of ≥30 kg/m2). For this analysis, underweight and normal categories were unified.
ALL DFCI consortium treatment protocols. Including pediatric protocols (00-001 and 05-001) and pediatric-inspired protocols (01-175 and 06-254). Asparaginase administration is marked in red. CNS, central nervous system.
ALL DFCI consortium treatment protocols. Including pediatric protocols (00-001 and 05-001) and pediatric-inspired protocols (01-175 and 06-254). Asparaginase administration is marked in red. CNS, central nervous system.
Outcomes
Overall survival (OS) was defined as time from diagnosis to death from any cause, censored at date of last follow-up. Event-free survival (EFS) was defined as time from diagnosis to relapse, second cancer, or death, censored at date of last follow-up. Toxicities were determined by Common Terminology Criteria for Adverse Events 2.0 and 3.0, per the specific trial guidelines or by Common Terminology Criteria for Adverse Events 3.0 for patients treated per protocols. Hepatotoxicity was defined as either elevated alanine aminotransferase (ALT), aspartate aminotransferase (AST), and/or total bilirubin. Toxicity grade was determined both as the highest (per patient) per each treatment phase and highest (per patient) per entire treatment course.
Statistics
Categorical variables are summarized as numbers and percentages, and comparisons were made by Pearson χ2 or Fisher exact tests, as appropriate. Continuous variables are summarized as median and range, and comparisons were made by Mann-Whitney tests. OS and EFS were estimated by the Kaplan-Meier method, with the log-rank test used to compare survival curves. Cox proportional hazard regression models were fitted to assess the effect of covariates on survival outcomes in univariate and multivariable models. Covariates, which were found to be significant in a univariate setting at a significance level of P < .05, were included in the initial multivariable model. Backward selection was performed, retaining variables with a P < .05 in the final model. Allogeneic stem cell transplantation (alloSCT) was included as a time-varying covariate. The cumulative incidence of relapse (CIR) and nonrelapse mortality (NRM) were estimated by the cumulative incidence method, identifying death by any cause or relapse as a competing risk, respectively, and tested using the Gray test. For all analyses, confidence intervals (CI) were calculated at the (2-sided) 95% level of confidence. A 2-sided P value of < .05 was considered statistically significant. All statistics were performed with STATA version 17.0, SAS version 9.4, and the “cmprsk” package in R.
This study was conducted with the approval of the institutional review board at the DFCI.
Results
Patients and treatment
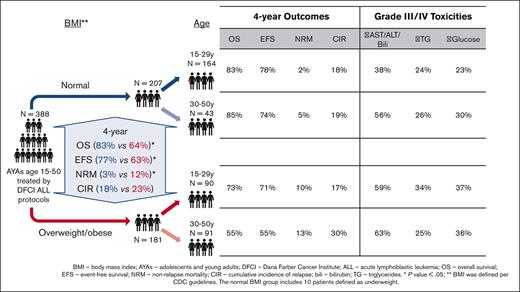
Overall, 388 patients were included in our analysis. Most patients were male (n = 240, 61.9%) and the median age was 24 years (range, 15-50 years), with 254 (65%) aged 15 to 29 years (younger AYAs) and 134 (35%) aged 30 to 50 years (older AYAs). BMI at diagnosis was underweight (n = 10, 2.6%) or normal (n = 197, 50.7%) in slightly more than half of patients (n = 207, 53.3%, normal BMI group) with the remainder of patients having a BMI of overweight (n = 97, 25%) or obese (n = 84, 21.7%; combined overweight/obese group, n = 181, 46.7%, Table 1). Patients in the normal BMI vs overweight/obese groups were more likely to be female (44% vs 32%, P = .02) and were younger (median age, 20 years [range, 15-50 years] vs 30 years [range, 15-50 years]; P < .001). Given that patients in the normal BMI group were younger compared with those in the overweight/obese group, they had lower rates of BCR::ABL1 translocation (7% vs 19%, P < .001) and higher rates of hyperdiploid karyotype (14% vs 6%, P = .006). Central nervous system involvement was present in 56 patients (14%) at diagnosis and was not different between normal and overweight/obese BMI groups (14% vs 15%, P = .7).
Patient, disease, and treatment characteristics of patients with ALL treated on DFCI ALL protocols
| . | All patients N = 388 . | Normal BMI group, n = 207 . | Overweight/obese BMI groups, n = 181 . | P value . |
|---|---|---|---|---|
| Age group (2 groups) | <.001 | |||
| 15-29 y | 254 (65) | 164 (79) | 90 (50) | |
| 30-50 y | 134 (35) | 43 (21) | 91 (50) | |
| Age group (4 groups) | <.001 | |||
| 15-19 y | 138 (36) | 100 (48) | 38 (21) | |
| 20-29 y | 116 (30) | 64 (31) | 52 (29) | |
| 30-39 y | 72 (19) | 20 (10) | 52 (29) | |
| 40-49 y | 62 (16) | 23 (11) | 39 (22) | |
| Sex (male) | 240 (62) | 117 (56) | 123 (68) | .021 |
| Immunophenotype∗ | .75 | |||
| B-ALL | 288 (74) | 155 (75) | 133 (73) | |
| T-ALL | 100 (26) | 52 (25) | 48 (27) | |
| Anterior mediastinal mass | 77 (20) | 42 (20) | 35 (19) | .96 |
| CNS involvement | .70 | |||
| CNS1 | 303 (78) | 165 (80) | 138 (76) | |
| CNS2 | 43 (11) | 23 (11) | 20 (11) | |
| CNS3 | 13 (3) | 6 (3) | 7 (4) | |
| Traumatic/unknown | 29 (8) | 13 (6) | 16 (9) | |
| WBC (×109/L, median, IQR) | 11.8 (4.1-45.2) | 11.95 (4-46.8) | 11.55 (4.9-44.4) | .94 |
| Cytogenetics | ||||
| Normal | 102 (26) | 55 (27) | 47 (26) | .89 |
| t(9;22) | 48 (12) | 14 (7) | 34 (19) | <.001 |
| Complex | 12 (3) | 6 (3) | 6 (3) | .82 |
| Hyperdiploid | 39 (10) | 29 (14) | 10 (6) | .006 |
| MLL | 24 (6) | 12 (6) | 12 (7) | .73 |
| Other abnormalities | 122 (31) | 69 (33) | 53 (29) | .39 |
| Unknown | 51 (13) | 27 (13) | 24 (13) | .95 |
| Treatment type | .24 | |||
| On trial | 308 (79) | 169 (82) | 139 (77) | |
| Per protocol | 80 (21) | 38 (18) | 42 (23) | |
| Protocols | .12 | |||
| Older (00-001 & 01-175) | 127 (33) | 75 (36) | 52 (29) | |
| Newer (05-001 & 06-254)/as per newer protocols | 261 (67) | 132 (64) | 129 (71) |
| . | All patients N = 388 . | Normal BMI group, n = 207 . | Overweight/obese BMI groups, n = 181 . | P value . |
|---|---|---|---|---|
| Age group (2 groups) | <.001 | |||
| 15-29 y | 254 (65) | 164 (79) | 90 (50) | |
| 30-50 y | 134 (35) | 43 (21) | 91 (50) | |
| Age group (4 groups) | <.001 | |||
| 15-19 y | 138 (36) | 100 (48) | 38 (21) | |
| 20-29 y | 116 (30) | 64 (31) | 52 (29) | |
| 30-39 y | 72 (19) | 20 (10) | 52 (29) | |
| 40-49 y | 62 (16) | 23 (11) | 39 (22) | |
| Sex (male) | 240 (62) | 117 (56) | 123 (68) | .021 |
| Immunophenotype∗ | .75 | |||
| B-ALL | 288 (74) | 155 (75) | 133 (73) | |
| T-ALL | 100 (26) | 52 (25) | 48 (27) | |
| Anterior mediastinal mass | 77 (20) | 42 (20) | 35 (19) | .96 |
| CNS involvement | .70 | |||
| CNS1 | 303 (78) | 165 (80) | 138 (76) | |
| CNS2 | 43 (11) | 23 (11) | 20 (11) | |
| CNS3 | 13 (3) | 6 (3) | 7 (4) | |
| Traumatic/unknown | 29 (8) | 13 (6) | 16 (9) | |
| WBC (×109/L, median, IQR) | 11.8 (4.1-45.2) | 11.95 (4-46.8) | 11.55 (4.9-44.4) | .94 |
| Cytogenetics | ||||
| Normal | 102 (26) | 55 (27) | 47 (26) | .89 |
| t(9;22) | 48 (12) | 14 (7) | 34 (19) | <.001 |
| Complex | 12 (3) | 6 (3) | 6 (3) | .82 |
| Hyperdiploid | 39 (10) | 29 (14) | 10 (6) | .006 |
| MLL | 24 (6) | 12 (6) | 12 (7) | .73 |
| Other abnormalities | 122 (31) | 69 (33) | 53 (29) | .39 |
| Unknown | 51 (13) | 27 (13) | 24 (13) | .95 |
| Treatment type | .24 | |||
| On trial | 308 (79) | 169 (82) | 139 (77) | |
| Per protocol | 80 (21) | 38 (18) | 42 (23) | |
| Protocols | .12 | |||
| Older (00-001 & 01-175) | 127 (33) | 75 (36) | 52 (29) | |
| Newer (05-001 & 06-254)/as per newer protocols | 261 (67) | 132 (64) | 129 (71) |
BMI groups were calculated per CDC guidelines.
CDC, Center for Disease Control; CNS, central nervous system; IQR, interquartile range 25% to 75%; MLL, mixed lineage leukemia.
One and 3 patients were diagnosed with T-/myeloid and B-/myeloid mixed phenotype acute leukemia, respectively.
Approximately one-third (n = 127, 33%) of patients were treated on earlier E coli asparaginase-based DFCI protocols (00-001 and 01-175, Figure 1; supplemental Table 1). The remaining patients (n = 261, 67%) were treated on (n = 181), or per (n = 80) later protocols (05-001 and 06-254), primarily receiving pegylated asparaginase, without any difference between the normal vs overweight/obese BMI groups (P = .12).
Toxicities
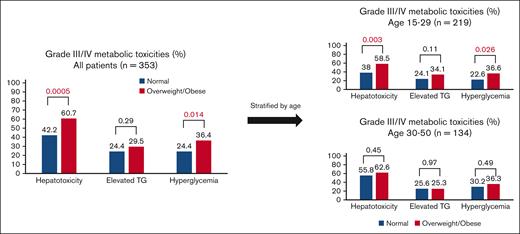
Comprehensive toxicity data were available for all patients except 35 patients enrolled in the older pediatric 00-001 trial. Thus, the toxicity analysis was based on 353 (91%) patients. During the entire treatment course, the rate of any grade 3/4 hepatotoxicity (elevation of AST, ALT, and/or bilirubin) was higher in patients who were overweight/obese vs those with a normal BMI (60.7% vs 42.2%, P = .0005, Figure 2). The rate of each individual grade 3/4 liver toxicity was also higher in patients who were overweight/obese vs those with a normal BMI (AST: 26.6% vs 14.4%, P = .005; ALT: 50.3% vs 38.9%, P = .031; and bilirubin: 23.1% vs 6.7%, P < .0001, respectively). Likewise, the rate of grade 3/4 hyperglycemia was higher among patients who were overweight/obese vs those with normal BMI (36.4% vs 24.4%, P = .014). Conversely, no significant difference in grade 3/4 hypertriglyceridemia was seen in patients who were overweight/obese vs those with a normal BMI (29.5% vs 24.4%, P = .29, Table 2). A direct association between BMI and toxicity rates was also seen when BMI was stratified by 3 groups (normal, overweight, and obese, supplemental Table 2). Of note, the type of asparaginase (pegylated vs nonpegylated) did not affect hepatotoxicity and hyperglycemia toxicity rates, but the rate of grade 3/4 hypertriglyceridemia was higher among patients treated with pegylated vs nonpegylated asparaginase (supplemental Table 3). In addition, being treated on protocol vs as per protocol was associated with lower rates of grade 3/4 toxicities (supplemental Table 4).
Comparison of grade 3/4 toxicity rates between BMI groups (normal vs overweight/obese). TG, hypertriglyceridemia.
Comparison of grade 3/4 toxicity rates between BMI groups (normal vs overweight/obese). TG, hypertriglyceridemia.
Grade 3/4 metabolic toxicities during different phases of treatment
| Grade 3/4 toxicity . | Induction phase . | Consolidation phase . | Maintenance/ posttreatment phase . | Overall . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal, % . | Overweight/obese, % . | P∗ value . | Normal, % . | Overweight/obese, % . | P∗ value . | Normal, % . | Overweight/obese, % . | P value . | Normal, % . | Overweight/obese, % . | P value . | |
| Elevated AST | 6.0 | 10.4 | .14 | 9.9 | 19.3 | .023 | 5.5 | 9.5 | .23 | 14.4 | 26.6 | .005 |
| Elevated ALT | 13.7 | 22.1 | .046 | 29.1 | 37.9 | .12 | 19.5 | 24.1 | .38 | 38.9 | 50.3 | .031 |
| Elevated bilirubin | 4.2 | 14.7 | .001 | 3.3 | 11.4 | .011 | 0 | 2.6 | .11 | 6.7 | 23.1 | <.0001 |
| Elevated TG | 1.2 | 6.1 | .019 | 27.8 | 32.1 | .42 | 0 | 4.3 | .023 | 24.4 | 29.5 | .29 |
| Elevated glucose | 18.5 | 28.8 | .026 | 12.6 | 20.7 | .06 | 2.3 | 8.6 | .028 | 24.4 | 36.4 | .014 |
| Grade 3/4 toxicity . | Induction phase . | Consolidation phase . | Maintenance/ posttreatment phase . | Overall . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal, % . | Overweight/obese, % . | P∗ value . | Normal, % . | Overweight/obese, % . | P∗ value . | Normal, % . | Overweight/obese, % . | P value . | Normal, % . | Overweight/obese, % . | P value . | |
| Elevated AST | 6.0 | 10.4 | .14 | 9.9 | 19.3 | .023 | 5.5 | 9.5 | .23 | 14.4 | 26.6 | .005 |
| Elevated ALT | 13.7 | 22.1 | .046 | 29.1 | 37.9 | .12 | 19.5 | 24.1 | .38 | 38.9 | 50.3 | .031 |
| Elevated bilirubin | 4.2 | 14.7 | .001 | 3.3 | 11.4 | .011 | 0 | 2.6 | .11 | 6.7 | 23.1 | <.0001 |
| Elevated TG | 1.2 | 6.1 | .019 | 27.8 | 32.1 | .42 | 0 | 4.3 | .023 | 24.4 | 29.5 | .29 |
| Elevated glucose | 18.5 | 28.8 | .026 | 12.6 | 20.7 | .06 | 2.3 | 8.6 | .028 | 24.4 | 36.4 | .014 |
TG, triglycerides.
Fisher exact test used.
When analyzed separately by age group, younger AYAs with overweight/obese vs normal BMI were more likely to experience grade 3/4 hepatotoxicity (58.5% vs 38.0%, P = .003). Conversely, in older AYAs, no significant difference in grade 3/4 hepatotoxicity was seen between the different BMI groups (62.6% vs 55.8%, P = .45). Similarly, regarding hyperglycemia, in younger AYAs, the rate of grade 3/4 hyperglycemia was higher among patients who were overweight/obese vs those with a normal BMI (36.6% vs 22.6%, P = .026) but no significant difference was seen in the older AYAs (36.3% vs 30.2%, P = .49). Grade 3/4 hypertriglyceridemia rates did not differ by BMI groups in the entire cohort or when separated by age group (Figure 2).
Bacterial or fungal infection were reported in 52 patients (13%) and did not differ between patients with a normal BMI vs those with overweight/obese BMI (15% [n = 32] vs 11% [n = 20], P = .20).
The timing of toxicities varied. Grade 3/4 hepatotoxicity was common in all treatment phases: present in 23.6%, 38.5%, and 23.8% of AYAs during induction, consolidation, and continuation, respectively. Grade 3/4 hyperglycemia was most common during induction (23.6%), less common during consolidation (16.5%), and rare during continuation (5.3%). Grade 3/4 hypertriglyceridemia was rare during induction (3.6%) and maintenance (2%), but common during consolidation (29.9%).
Response, survival, and relapse
CR was achieved in 87% of patients, without a difference between normal and overweight/obese BMI groups (P = .84). Similarly, induction failure and early death rates (within 30 days) were comparable between patients in the normal vs overweight/obese BMI groups (6% vs 8%, P = .42 and 2% in both groups [P = .4], respectively). AlloSCT was performed in 70 patients, with 79% (55/70) performed at CR1. Transplant rates at CR1 were comparable between patients with normal (75%, 24/32) vs overweight/obese BMI (82% [31/38], P = .5).
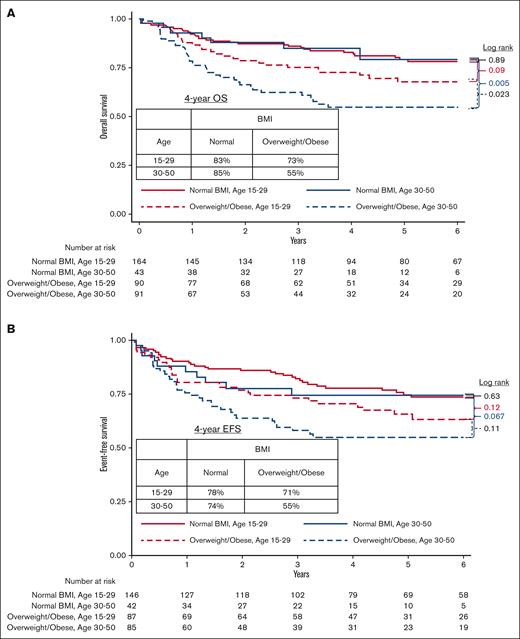
With a median follow-up of 5.5 years, the 4-year OS was 74% (95% CI, 69-78), higher in younger vs older AYA patients (79% [95% CI, 73-84] vs 64% [95% CI, 55-72], P = .003), and higher in patients with normal vs overweight/obese BMI (83% [95% CI, 77-88] vs 64% [95% CI, 56-71], P = .0001). Because of the association between age group and BMI group, we conducted separate analyses in each BMI group. In patients with normal BMI, the 4-year OS was similarly excellent between younger vs older AYAs (83% [95% CI, 76-88] vs 85% [95% CI, 69-93], P = .89). In contrast, in patients who were overweight/obese, the 4-year OS was higher in younger vs older AYAs (73% [95% CI, 62-81] vs 55% [95% CI, 43-65], P = .023; Figure 3A). Older AYAs in the obese BMI category (n = 39) had a particularly poor 4-year OS of 47% (95% CI, 28-64) vs 72% (95% CI, 56-83) in younger AYAs in the obese group (P = .025).
The 4-year EFS was 70% (95% CI 65-75) and was higher in patients with a normal BMI vs those with an overweight/obese BMI (77% [95% CI, 70-83] vs 63% [95% CI, 55-70], P = .003). In patients with a normal BMI, the 4-year EFS was similar between younger vs older AYAs (78% [95% CI, 70-84] vs 74% [95% CI, 57-85], P = .63), whereas in patients in the overweight/obese group, the 4-year EFS was numerically higher in younger vs older AYAs, without statistical significance (71% [95% CI, 60-79] vs 55% [95% CI, 43-65], P = .11, Figure 3B). Four-year EFS was very poor among older AYA patients with obese BMI (49%, 95% CI, 29-66).
In a sensitivity analysis excluding 48 AYAs with BCR::ABL1 translocation (Philadelphia-negative ALL cohort, n = 340), higher OS were seen among AYAs with normal vs overweight/obese BMI (4-year OS, 85% [95% CI, 78-89] vs 67% [95% CI, 58-74], P = .0004). As was seen in the entire cohort, OS among AYAs with normal BMI was comparable in younger vs older AYA patients (4-year OS, 84% [95% CI, 77-89] vs 88% [95% CI, 71-95], P = .43), whereas younger AYA patients with obese BMI had better OS compared with older AYA patients in the obese category (74% [95% CI, 56-85] vs 48% [95% CI, 37-67], P = .05).
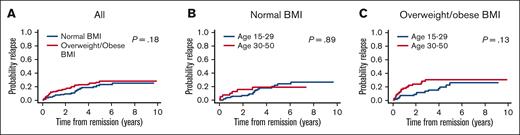
The 4-year CIR in the entire cohort was 20.7% (95% CI, 15.9-25.9) and was not statistically different between normal vs overweight/obese BMI groups (18.2% [95% CI, 11.8-25.4] vs 23.4% [95% CI, 16.6-31.0], P = .18, Figure 4A). In the normal BMI group, the 4-year CIR was not different between younger and older AYAs (17.9%, [95% CI, 10.6-26.9] vs 18.8%, [95% CI, 8.0-33.2], P = .89, Figure 4B). Conversely, among patients who were overweight/obese, the 4-year CIR was numerically lower in younger patients (17.1% [95% CI, 9.0-27.5]) vs older patients (30.1% [95% CI, 19.6-41.2, P = .13], Figure 4C).
CIR by BMI and age group. (A) All patients by BMI group. (B) Patients with normal BMI by age group. (C) Overweight/obese patients by age group.
CIR by BMI and age group. (A) All patients by BMI group. (B) Patients with normal BMI by age group. (C) Overweight/obese patients by age group.
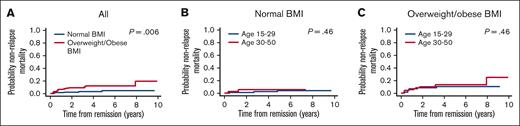
The 4-year cumulative incidence of NRM was 7.3% [95% CI, 4.6-10.8] and was lower in patients with normal BMI vs those with overweight/obese BMI (2.8% [95% CI, 0.9-6.7] vs 11.7% [95% CI, 6.9-17.8], P = .006, Figure 5A). Within each BMI group, the cumulative incidence of NRM was similar between younger and older AYAs: normal BMI group: 2.1% (95% CI, 0.4-6.7) vs 5.2% (95% CI, 0.9-15.5, P = .46, Figure 5B); overweight/obese BMI group: 10.1% (95% CI, 4.3-18.6) vs 13.4% (95% CI, 6.5-22.9, P = .46), respectively (Figure 5C).
Nonrelapse mortality by BMI and age group. (A) All patients by BMI group. (B) Patients with normal BMI by age group. (C) Overweight/obese patients by age group.
Nonrelapse mortality by BMI and age group. (A) All patients by BMI group. (B) Patients with normal BMI by age group. (C) Overweight/obese patients by age group.
In univariate analyses, patient (age and BMI), disease (white blood cell count [WBC], immunophenotype, and BCR::ABL1 translocation), treatment (older vs newer protocols; as per protocol vs on protocol, and alloSCT), and toxicity (hyperglycemia and hypertriglyceridemia) covariates were found to be significantly associated with OS. In the multivariable Cox regression analysis, BMI (overweight/obese vs normal), WBC (≥30 000 × 109/L vs <30 000 × 109/L) and immunophenotype (B vs T) were significantly associated with worse OS, whereas hypertriglyceridemia was significantly associated with improved OS (Table 3). Of note, alloSCT and age were significantly associated with worse OS in the univariate analysis, but not in the multivariable analysis. In addition, there was a trend toward worse OS with grade 3/4 hyperglycemia in the multivariable analysis (hazard ratio [HR], 1.51; 95% CI, 0.99-2.28, P = .054).
Univariate and multivariable Cox regression analysis for OS
| . | Univariate HR (95% CI) . | P value . | Multivariable HR (95% CI) . | P value . |
|---|---|---|---|---|
| Age as continuous variable (y) | 1.03 (1.01-1.05) | .003 | - | - |
| Sex (male vs female) | 1.06 (.70-1.60) | .79 | - | - |
| BMI (overweight/obese vs normal) | 2.26 (1.48-3.45) | <.0001 | 2.38 (1.56-3.63) | <.0001 |
| WBC (>30 vs ≤30 × 109/L) | 1.92 (1.29-2.87) | .001 | 1.79 (1.20-2.69) | .005 |
| CNS-2 or CNS-3 vs CNS-1 | 1.55 (0.94-2.55) | .08 | - | - |
| Immunophenotype B- vs T-ALL | 2.52 (1.41-4.53) | .002 | 2.27 (1.26-4.09) | .006 |
| BCR::ABL1 translocation (yes vs no) | 2.40 (1.46-3.94) | .001 | - | - |
| Complex karyotype (yes vs no) | 0.61 (0.15-2.48) | .49 | - | - |
| Hyperdiploid karyotype (yes vs no) | 0.81 (.39-1.67) | .57 | - | - |
| MLL rearrangement (yes vs no) | 1.58 (0.77-3.27) | .22 | - | - |
| Other karyotype abnormality (yes vs no) | 0.81 (0.52-1.26) | .35 | - | - |
| Treatment type (per protocol vs on protocol) | 0.49 (0.27-0.91) | .023 | - | - |
| Treatment protocols (newer vs older) | 0.60 (0.39-0.92) | .019 | - | - |
| Hepatotoxicity, grade 3/4 (yes vs no) | 0.68 (0.45-1.02) | .06 | - | - |
| Hyperglycemia, grade 3/4 (yes vs no) | 1.61 (1.07-2.44) | .023 | - | - |
| Hypertriglyceridemia, grade 3/4 (yes vs no) | 0.33 (0.18-0.60) | <.0001 | 0.33 (0.18-0.61) | <.0001 |
| AlloSCT (as time dependent variable) | 2.04 (1.26-3.30) | .004 | 1.31 (.80-2.14) | .28 |
| . | Univariate HR (95% CI) . | P value . | Multivariable HR (95% CI) . | P value . |
|---|---|---|---|---|
| Age as continuous variable (y) | 1.03 (1.01-1.05) | .003 | - | - |
| Sex (male vs female) | 1.06 (.70-1.60) | .79 | - | - |
| BMI (overweight/obese vs normal) | 2.26 (1.48-3.45) | <.0001 | 2.38 (1.56-3.63) | <.0001 |
| WBC (>30 vs ≤30 × 109/L) | 1.92 (1.29-2.87) | .001 | 1.79 (1.20-2.69) | .005 |
| CNS-2 or CNS-3 vs CNS-1 | 1.55 (0.94-2.55) | .08 | - | - |
| Immunophenotype B- vs T-ALL | 2.52 (1.41-4.53) | .002 | 2.27 (1.26-4.09) | .006 |
| BCR::ABL1 translocation (yes vs no) | 2.40 (1.46-3.94) | .001 | - | - |
| Complex karyotype (yes vs no) | 0.61 (0.15-2.48) | .49 | - | - |
| Hyperdiploid karyotype (yes vs no) | 0.81 (.39-1.67) | .57 | - | - |
| MLL rearrangement (yes vs no) | 1.58 (0.77-3.27) | .22 | - | - |
| Other karyotype abnormality (yes vs no) | 0.81 (0.52-1.26) | .35 | - | - |
| Treatment type (per protocol vs on protocol) | 0.49 (0.27-0.91) | .023 | - | - |
| Treatment protocols (newer vs older) | 0.60 (0.39-0.92) | .019 | - | - |
| Hepatotoxicity, grade 3/4 (yes vs no) | 0.68 (0.45-1.02) | .06 | - | - |
| Hyperglycemia, grade 3/4 (yes vs no) | 1.61 (1.07-2.44) | .023 | - | - |
| Hypertriglyceridemia, grade 3/4 (yes vs no) | 0.33 (0.18-0.60) | <.0001 | 0.33 (0.18-0.61) | <.0001 |
| AlloSCT (as time dependent variable) | 2.04 (1.26-3.30) | .004 | 1.31 (.80-2.14) | .28 |
CNS, central nervous system; MLL, mixed lineage leukemia.
In a univariable analysis for EFS, BMI (overweight/obese vs normal), WBC (>30 × 109/L vs ≤30 × 109/L) and immunophenotype (B- vs T-ALL) were associated with worse survival (BMI: HR, 1.94; 95% CI, 1.18-3.17; P = .008; WBC: HR, 2.50; 95% CI, 1.53-4.08; P < .0001; and immunophenotype: HR, 2.87; 95% CI, 1.46-5.62; P = .002; supplemental Table 5). Conversely, treatment on newer protocols (vs older protocols), which primarily used a pegylated asparaginase as opposed to native asparaginase preparation, and grade 3/4 hypertriglyceridemia were associated with better OS (HR, 0.45; 95% CI, 0.27-0.74; P = .002 and HR, 0.46; 95% CI, 0.25-0.84; P = .011), respectively.
Effect of hypertriglyceridemia and hyperglycemia on survival and relapse
Given the association between grade 3/4 hypertriglyceridemia and hyperglycemia with survival, we performed an exploratory analysis comparing OS, EFS, CIR, and NRM between patients with vs without grade 3/4 hypertriglyceridemia or hyperglycemia.
The 4-year OS and EFS were higher among patients who experienced grade 3/4 hypertriglyceridemia: OS, 88.4% (95% CI, 79.4-93.6) vs 69.6% (95% CI, 63.2-75.0, P = .0001, supplemental Figure 1A); EFS, 83.9% (95% CI, 74.2-90.2) vs 64.4% (95% CI, 57.6-70.5), P = .002, supplemental Figure 1B). The CIR rates were lower among patients who experienced grade 3/4 hypertriglyceridemia vs those that did not (11.4%, [95% CI, 5.5-19.7] vs 25%, [95% CI, 18.8-31.7], P = .048, supplemental Figure 1C). Conversely, the rate of NRM was comparable between patients with vs without grade 3/4 hypertriglyceridemia (5.1%, [95% CI, 1.6-11.7] vs 8.2%, [95% CI, 4.9-12.7], respectively, P = .39).
Conversely, the 4-year OS and EFS were lower among patients who experienced grade 3/4 hyperglycemia vs those who did not: 71.0% (95% CI, 60.8-79.0) vs 76.3% (95% CI, 70.2-81.3, P = .022, supplemental Figure 2A); and 62.9% (95% CI, 52.3-71.8) vs 73.6% (95% CI, 67.0-79.1, P = .006, supplemental Figure 2B), respectively. The 4-year CIR was 25.5% (95% CI, 16.3-35.8) among patients who experienced grade 3/4 hyperglycemia and 18.5% (95% CI, 13.2-24.5) among patients who did not (P = .32). The rate of NRM in patients with vs without grade 3/4 hyperglycemia was 9.5% (95% CI, 4.7-16.5) vs 6.1% (95% CI, 3.2-10.4) respectively, P = .14.
Of note, no association was seen between grade 3/4 hypertriglyceridemia and pancreatitis (30% with vs 70% without hypertriglyceridemia, respectively, P = .6).
Discussion
In this study we examined the association between BMI, treatment-related toxicity, and survival among AYA patients with ALL treated on, or per, DFCI consortium pediatric protocols. Among 388 AYA patients, being overweight or obese was associated with higher NRM (11.7% vs 2.8%, P = .006), worse EFS (4-year EFS, 63% vs 77%, P = .003), and worse OS (4-year OS, 64% vs 83%, P = .0001).
Given the association between elevated BMI and older age, we conducted separate analyses within each BMI group to understand the contribution of each of these factors to patient outcomes. We found excellent outcomes among AYAs with normal BMI, regardless of age. Younger and older AYAs with normal BMI had 4-year NRM of 2.1% vs 5.2%, P = .46; CIR of 17.9% vs 18.8%, P = .89; and 4-year OS of 83% vs 85%, P = .89. AYAs, particularly older AYAs, with an overweight or obese BMI had inferior outcomes. Among AYAs with an overweight or obese BMI, those in older vs younger AYA groups were more likely to not survive (4-year OS, 55% vs 73%, P = .023), which appeared to be principally driven by an increased 4-year CIR (30.1 vs 17.1%, P = .13). NRM mortality was similar between younger and older AYAs with an overweight or obese BMI (10% vs 13%, P = .46). The lack of statistical difference in CIR between older vs younger AYAs in the overweight/obese group is because of late relapse rates (after 4 years) as seen in Figure 5.
Our finding that elevated BMI adversely affects OS is consistent with analyses of patients enrolled on the Cancer and Leukemia Group B (CALGB) 10403 trial in which higher BMI was also associated with inferior outcomes.14 We also show that the impact of obesity is most severe in older AYAs. In the CALGB analysis, obesity was associated with disease-free survival on multivariable analysis but it was not significant for OS. In our cohort, obesity was associated with both EFS and OS.
We investigated the association between BMI and age and risk for specific metabolic toxicities, the pattern of those toxicities, and association with survival outcomes. We found that AYA patients with elevated BMI compared with those with normal BMI were more likely to experience metabolic toxicities, including grade 3/4 hepatotoxicity (60.7 vs 42.2%, P = .0005) and hyperglycemia (36.4 vs 24.4%, P = .014). Conversely, no association was seen between BMI group and rates of grade 3/4 hypertriglyceridemia (29.5% vs 24.4%, P = .29). When stratified by age group, higher rates of grade 3/4 metabolic toxicities were seen only in the younger but not in older AYAs with elevated BMI. This might be related to higher incidence of hyperglycemia and hepatotoxicity among older patients, irrespective of BMI, because the rate of these abnormalities increase with age, regardless of treatment.20,21
The association between BMI and metabolic adverse events has been previously reported in children and AYAs treated for ALL.13,22 A recent analysis of 1443 children aged 2 to 18 years treated on the NOPHO ALL2008 protocol demonstrated a higher rate of toxicity overall among children who were obese (defined in the study as a BMI of ≥30 kg/m2).23 A post hoc analysis of the CALGB 10403 and AALL0232 trials showed that elevated BMI was associated with increased grade ≥3 adverse events in both cohorts.6
Of note, a recent report of younger patients (aged 1-22 years) treated on DFCI consortium protocols did not find association between BMI and several toxicities.24 This difference was likely because of the younger age of the patient cohort and a different approach to classifying toxicities, specifically considering all asparaginase toxicities together instead of analyzing each asparaginase-related toxicity separately.
Regarding the timing of toxicities, the pattern varied: grade 3/4 hepatotoxicity and hypertriglyceridemia were highest during consolidation whereas hyperglycemia was most common during induction. This pattern differs from the post hoc analysis of the CALGB 10403 and AALL0232 trials, which demonstrated that toxicities were most common during induction.6 The difference may relate, in part, to the differences between protocols: in CALGB 10403 and AALL0232, asparaginase is given intermittently, whereas DFCI protocols include 30 continuous weeks of asparaginase during the postremission consolidation phase.
Finally, we found an association between several grade 3/4 toxicities and survival. Grade 3/4 hypertriglyceridemia was associated with lower cumulative relapse rates and improved EFS and OS, including in the multivariable model. In addition, similar to previous studies, no association was seen between grade 3/4 hypertriglyceridemia and pancreatitis.25 Hypertriglyceridemia in our patients is believed to indirectly reflect therapeutic antileukemic asparaginase activity. Further correlative studies with direct measures of asparaginase activity could confirm the utility of triglyceride measurement as an inexpensive and accessible laboratory measurement for clinical asparaginase activity. Interestingly, we have previously shown in our DFCI AYA cohort that osteonecrosis, which is, at least in part, due to asparaginase-associated toxicity, is associated with improved survival.5,26 As per worse OS seen in patients with grade 3/4 hyperglycemia, previous studies demonstrated association between hyperglycemia, higher rates of infections, and worse survival in both the pediatric population27,28 and in adults treated with hyper-CVAD regimen.29
The mechanism connecting higher BMI with worse survival is largely unknown. Our findings on the association between BMI and higher NRM suggest that a portion of the impact of higher BMI is related to inferior ability to tolerate chemotherapy. This is likely a multifactorial effect, including a possible increased risk for hyperglycemia, infection, and less resilience in the setting of severe complications of treatment, such as sepsis.
Another potential cause for poorer survival could be through disease resistance. Although the association of obesity and relapse in our cohort was of borderline significance, high CIR rates were seen in the older patients in the overweight/obese category, for whom the cumulative risk of relapse reached 30% in 4 years compared with 17% in younger patients in the same BMI category. This association is intriguing given the significant literature suggesting an association between elevated adipose tissue and mechanisms that might predict treatment failure. One proposed explanation is that adipose tissue protects lymphoblasts from anthracyclines30 or asparaginase,31,32 by providing metabolic “fuel” to leukemia cells or promoting inflammatory response.33 Another hypothesis is that patients with high BMI receive insufficient exposure to chemotherapy because chemotherapy is administered based on body surface area, which does not increase proportionally to body mass.34 Finally, 2 drugs that form the backbone of ALL regimens, asparaginase and vincristine, are “dose capped,” which may result in more underdosing in patients with elevated BMI.35,36
Because obesity is a serious disease with increasing rates in the last 20 years,37,38 the challenge of treating patients with ALL who are overweight or obese will be more common, and the optimal therapy, especially for older patients with obesity, has yet to be defined. Given that AYAs with obesity fare more poorly with asparaginase-based pediatric regimens as compared with AYAs with a normal BMI,14 alternative approaches including but not limited to alternative asparaginase dose and schedules should be explored. It is not known whether these patients would have improved outcomes with nonasparaginase-based regimens, such as hyper-CVAD (cyclophosphamide, vincristine sulfate, doxorubicin hydrochloride [Adriamycin], and dexamethasone) or approaches currently being evaluated in adults that rely on novel agents.39
In contrast, we found excellent outcomes in older AYA patients with normal BMI, suggesting that fit adults aged ≤50 years with normal BMI might be considered for pediatric regimens in which they may expect to be cured without need for transplantation.40,41 Indeed, other groups have demonstrated the ability to apply pediatric regimens to older adults with dose modifications of asparaginase.42
Overall, our findings emphasize that clinicians and clinical investigators should consider both age and BMI in making treatment decisions. This will be best implemented if ongoing and future studies report results based on both age and BMI.
Our study is limited by it is retrospective nature and heterogeneous population, which was partially addressed by stratification, and regression and sensitivity analyses. A second limitation is the fact that we conducted our analysis only considering BMI at diagnosis; there may be an additional role for studying weight gain during treatment but such data were not available to us in this cohort. Furthermore, our cohort did not include systematic evaluation of measurable residual disease, which plays a role in decision making throughout the course of treatment in the current era. The association between BMI and persistence of measurable residual disease should be pursued in future studies. Finally, our study has limited data on Hispanic ethnicity, which is associated with both obesity38 and higher rates of ALL.43,44 Additional studies evaluating our findings in cohorts with varied ethnic representation are needed. Because prospective clinical trials might not enroll representative populations,45 research using registry and retrospective chart review, although imperfect, will also be informative to understand outcomes in populations particularly affected by ALL and obesity, especially the Hispanic community. Efforts to enroll diverse, representative populations in ALL clinical trials should be prioritized.46
In conclusion, we show that among AYAs with ALL treated on a pediatric regimen, obesity at diagnosis was associated with increased NRM and inferior EFS and OS. An elevated BMI was associated with particularly high relapse rates and poor survival among AYAs aged >30 years. In contrast, AYAs with normal BMI, regardless of age, had favorable outcomes when treated on this pediatric-inspired regimen. Moreover, we demonstrate, to our knowledge, for the first time, that hypertriglyceridemia is associated with improved survival and decreased risk of relapse, most likely reflecting asparaginase activity and thus should not be viewed as an adverse event. Future trials of ALL treatments among patients of all ages should study the impact of obesity on treatment toxicity and outcomes.
Acknowledgment
This work was supported by the Foley Family Research Fund.
Authorship
Contribution: S.S., D.J.D., and M.R.L. designed the research; S.S. and Y.K.V. performed data extraction; S.S., Y.F., and D.S.N. analyzed the data; S.S. and M.R.L. wrote the initial draft of the manuscript; Y.F., Y.K.V., A.E.P., L.B.S., L.M.V., A.M.B., S.E.S., R.M.S., M.W., D.S.N., and D.J.D. reviewed the manuscript and contributed to its final version; and all authors reviewed the final version of the manuscript and agreed on submission.
Conflict-of-interest disclosure: L.B.S. reports serving on advisory boards for Jazz, Servier, and Syndax. A.M.B. reports research support from AstraZeneca, Novartis, Roivant, Takeda, Celgene/Bristol Myers Squibb (BMS), GlaxoSmithKline (GSK), and Janssen, and consulting fees from Agios, AbbVie, Acceleron, BMS/Celgene, Novartis, Gilead, Keros Therapeutics, and Taiho Oncology. S.E.S. reports honoraria from Jazz and Servier. R.M.S. reports consulting fees from AbbVie, AbbVie/Genetech, Actinium, Amgen, Aptevo, Aprea, Arog, AvenCell, BerGenBio, BMS, Boston Pharmaceuticals, Cellularity, CTI Pharma, Epizyme, Foghorn Therapeutics, Gemoab, GSK, Innate, Janssen, Jazz, Kura Oncology, Novartis, Onconova, Rigel, Syntrix, Syros, and Takeda. D.S.N. reports consultancy with The American Society of Hematology Research Collaborative as a senior scientific adviser and reports stock ownership in Madrigal Pharmaceuticals. D.J.D. has served as a consultant for Amgen, Autolos, Agios, Blueprint Pharmaceuticals, Forty-Seven, Gilead, Incyte, Jazz, Novartis, Pfizer, Servier, and Takeda, and received research funding from AbbVie, Glycomimetics, Novartis, and Blueprint Pharmaceuticals. M.R.L. receives research support from AbbVie and Novartis, and has served on advisory boards for Novartis, Jazz, and Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Marlise R. Luskin, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: marlise_luskin@dfci.harvard.edu.
References
Author notes
The data that support the findings of this study are available on request from the corresponding author, Marlise R. Luskin (marlise_luskin@dfci.harvard.edu). The data are not publicly available due to privacy or ethical restrictions.
The full-text version of this article contains a data supplement.