Key Points
Iron-deficient Tmprss6–/– mice show elevated circulating FGF23 levels.
BM-SECs upregulate Fgf23 in chronic iron deficiency anemia, in phlebotomy-induced anemia, and in response to erythropoietin treatment.
Abstract
Iron deficiency is a potent stimulator of fibroblast growth factor 23 (FGF23), a hormonal regulator of phosphate and vitamin D metabolism, that is classically thought to be produced by bone-embedded osteocytes. Here, we show that iron-deficient transmembrane serine protease 6 knockout (Tmprss6–/–) mice exhibit elevated circulating FGF23 and Fgf23 messenger RNA (mRNA) upregulation in the bone marrow (BM) but not the cortical bone. To clarify sites of Fgf23 promoter activity in Tmprss6–/– mice, we introduced a heterozygous enhanced green fluorescent protein (eGFP) reporter allele at the endogenous Fgf23 locus. Heterozygous Fgf23 disruption did not alter the severity of systemic iron deficiency or anemia in the Tmprss6–/– mice. Tmprss6–/–Fgf23+/eGFP mice showed green fluorescence in the vascular regions of BM sections and showed a subset of BM endothelial cells that were GFPbright by flow cytometry. Mining of transcriptomic data sets from mice with normal iron balance revealed higher Fgf23 mRNA in BM sinusoidal endothelial cells (BM-SECs) than that in other BM endothelial cell populations. Anti-GFP immunohistochemistry of fixed BM sections from Tmprss6–/–Fgf23+/eGFP mice revealed GFP expression in BM-SECs, which was more intense than in nonanemic controls. In addition, in mice with intact Tmprss6 alleles, Fgf23-eGFP reporter expression increased in BM-SECs following large-volume phlebotomy and also following erythropoietin treatment both ex vivo and in vivo. Collectively, our results identified BM-SECs as a novel site for Fgf23 upregulation in both acute and chronic anemia. Given the elevated serum erythropoietin in both anemic models, our findings raise the possibility that erythropoietin may act directly on BM-SECs to promote FGF23 production during anemia.
Introduction
The endocrine hormone fibroblast growth factor 23 (FGF23) plays a key role in skeletal health by inhibiting renal phosphate reabsorption and suppressing circulating levels of 1,25-dihydroxyvitamin D3 and parathyroid hormone.1 Cells of the bone cortex (ie, osteoblasts and osteocytes) have been viewed as major sites of FGF23 expression and upregulation in physiological and pathological states.2-4 FGF23 is secreted into the circulation both as a full-length bioactive hormone and peptide fragments generated from intracellular cleavage.5 Circulating forms can be measured using enzyme-linked immunosorbent assays (ELISAs) that detect either intact FGF23 (iFGF23) alone or both intact hormone and C-terminal cleaved fragments, with the latter assay (cFGF23) providing insight into the total amount of FGF23 produced.5
The levels of FGF23 messenger RNA (mRNA) and the circulating hormone are influenced by multiple factors, including the serum levels of phosphate,6-8 1,25-dihydroxyvitamin D3,3,8,9 parathyroid hormone,10-12 and inflammatory cytokines.13-18 FGF23 levels increase in patients with chronic kidney disease, where they are associated with an increased risk of cardiovascular events and mortality.19 Initial links between iron deficiency and FGF23 regulation were observed during the study of the clinical variability of autosomal dominant hypophosphatemic rickets (ADHR), a disorder of plasma FGF23 elevation caused by defective FGF23 proteolytic cleavage20,21; intriguingly, serum iron is negatively correlated with circulating FGF23 levels in this disorder.22 In the general population, serum cFGF23 shows inverse correlations with serum iron in healthy premenopausal women23 and with serum ferritin in middle-aged men and women.24 In older individuals, low serum iron is associated with high iFGF2325,26 and cFGF2326 independently from markers of inflammation25,26 or renal impairment.25 Furthermore, in a rural Kenyan population, with a high prevalence of iron deficiency, antenatal oral iron supplementation lowers maternal plasma cFGF23 at delivery compared with placebo.27
Iron deficiency also increases FGF23 levels in experimental mouse models. Both, in an ADHR model and in wild-type mice, induction of iron deficiency anemia (IDA) through dietary manipulation after weaning led to elevations in Fgf23 mRNA from homogenized femur/tibia and in serum cFGF23.28 In both ADHR and wild-type mice, induction of maternal iron deficiency yielded pups with elevations in femur/tibia Fgf23 mRNA, serum cFGF23, and serum iFGF23 levels as well as hypophosphatemia at weaning age.29 Furthermore, in wild-type mice, both inflammation-induced iron restriction and functional iron deficiency without superimposed inflammation (achieved via exogenous hepcidin administration) elevated both whole femur Fgf23 mRNA and serum cFGF23 levels.16
To clarify the cellular sites of FGF23 upregulation during IDA, we used mice with genetic loss of transmembrane serine protease 6 (Tmprss6), a model of the autosomal recessive disorder Iron-Refractory Iron Deficiency Anemia.30 Because TMPRSS6, which is expressed predominantly in the liver,31,32 dampens the hepatic production of hepcidin,32,33Tmprss6 mutants exhibit chronic IDA on a standard rodent diet because of impaired absorption of dietary iron.32 Hepcidin elevation in Tmprss6-knockout (Tmprss6–/–) mice begins prenatally, causing anemia by the early postnatal period.34 Notably, Tmprss6–/– mice show hepcidin elevation without markers of inflammation,35 and their IDA phenotype does not require local Tmprss6 expression in hematopoietic cells.36 Here, we showed that Tmprss6–/– mice exhibit elevated circulating FGF23, and using an endogenous locus enhanced green fluorescent protein (eGFP) reporter, we established sites of Fgf23 production (1) in chronic, genetically induced IDA, (2) in acute phlebotomy-induced anemia, and (3) after direct administration of erythropoietin (EPO).
Methods
Animal strains
Animal studies were approved by the Yale University Institutional Animal Care and Use Committee. Tmprss6-deficient mice (B6.129P2-Tmprss6tm1Dgen/Crl),33 obtained from Deltagen, and Fgf23eGFP mice (B6.129S-Fgf23tm1Sliu/Mmjax),37 obtained from MMRRC-JAX, were bred and housed in the Yale Animal Resource Center with free access to food (Envigo Teklad 2018S rodent diet: 200 mg/kg iron, 1.0% calcium, and 0.7% phosphorus) and water. Breeding, genotyping, and EPO treatments are described in the supplemental Methods. Retro-orbital phlebotomy (500 μL) with saline volume replacement was performed under isoflurane anesthesia, as previously described.38 Sex- and age-matched mice were compared, as indicated.
Blood and tissue analysis
Terminal retro-orbital blood samples were collected under ketamine/xylazine anesthesia for blood counts and serum/plasma preparation (supplemental Methods). Serum was analyzed using an iron colorimetric assay (BioVision), mouse Erythopoietin/EPO Quantikine ELISA (R&D Systems), and hepcidin-murine compete ELISA (Intrinsic LifeSciences). Plasma was analyzed using mouse/rat FGF-23 (Intact) and FGF23 (C-Term) ELISAs (Quidel). Spot urine, collected immediately before dissection, was analyzed using a Creatinine Parameter assay (R&D Systems). Serum and urine phosphate were analyzed using Phosphorus Liqui-UV (Stanbio). After euthanization, organ nonheme iron concentrations were determined via bathophenanthroline quantification.39
Analysis of bone and BM RNA
Mouse femurs were flushed of bone marrow with RNAse-free phosphate-buffered saline. To eliminate residual BM cells, femurs were then flushed with RNAse-free water to promote cell lysis, and the marrow cavity was scraped with endodontic files before snap freezing. Total RNA from minced, flash-frozen bone was isolated using the miRNeasy Mini Kit with on-column DNase digestion (Qiagen). BM RNA was collected via RNeasy Mini Kit with on-column DNase digestion (Qiagen) in a prior study.40 RNA (0.5 μg) was reverse transcribed (Bio-Rad iScript cDNA Synthesis Kit) and amplified using an ABI7500 real-time polymerase chain reaction system per the supplemental Methods.
Confocal imaging
Green fluorescence in briefly fixed BM plugs was assessed via immediate imaging, using a Zeiss LSM 880 Airyscan confocal microscope. Colocalization of green fluorescence and endomucin immunofluorescence in fixed, decalcified femur cryosections was assessed using a Leica SP8 Gated STED 3× superresolution microscope (supplemental Methods).
Flow cytometry
BM endothelial cells were analyzed per the method described by Xu et al41 (see supplemental Methods for the detailed procedure). Cell fluorescence was captured on a BD LSR II flow cytometer (Yale Flow Cytometry Facility) and analyzed using FlowJo 10.5.2.
Immunohistochemistry
Whole femurs were fixed in 10% formalin at room temperature for 3 days. Formic acid decalcification, paraffin-embedding, 5 μm sectioning, and anti-GFP immunohistochemistry were performed by Yale Pathology Tissue Services (supplemental Methods). Brightfield images were acquired using an Olympus BX40 microscope with an attached Spot Insight 4.0 Mp color digital camera.
Statistical analyses
One-way or two-way analysis of variance with Tukey post hoc test and linear regression analyses were conducted using Prism 9. P < .05 was considered statistically significant. Analyses and visualization of RNA-sequencing (RNA-Seq) data were performed using R (version 4.1.1) in RStudio (version 1.4.1717).
Results
Iron-deficient Tmprss6–/– mice exhibit elevated FGF23
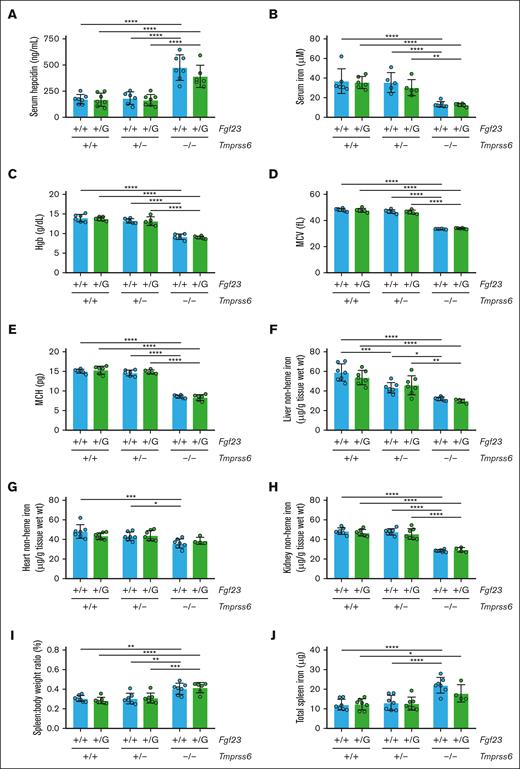
To characterize the effects of chronic IDA on phosphate-related parameters, we generated Tmprss6 wild-type (Tmprss6+/+), heterozygous (Tmprss6+/−), and knockout (Tmprss6–/–) offspring with C57BL/6N genetic background that underwent phenotypic characterization at 8 weeks. Consistent with their known hepcidin elevation,33Tmprss6–/– mice showed hypoferremia, microcytic anemia, elevated serum EPO, and markedly reduced nonheme iron concentrations in the liver, heart, and kidney (Figure 1A-H) compared with sex-matched littermate controls. Tmprss6–/– mice also showed a greater spleen-to-body weight ratio (Figure 1I), consistent with extramedullary hematopoiesis,33 and greater total spleen nonheme iron content (Figure 1J), consistent with macrophage iron retention.42
The perturbed iron homeostasis of Tmprss6–/– mice is accompanied by elevated FGF23 levels in circulation but unchanged Fgf23 mRNA levels in the cortical bone. Shown for 8-week-old Tmprss6+/+, Tmprss6+/−, and Tmprss6–/– male littermates are (A) serum iron, (B) Hgb, (C) erythrocyte mean corpuscular volume (MCV), (D) erythrocyte mean corpuscular hemoglobin (MCH), (E) serum EPO, (F) liver nonheme iron concentration, (G) heart nonheme iron concentration, (H) kidney nonheme iron concentration, (I) spleen-to-body-weight ratio, (J) total spleen nonheme iron content, (K) plasma C-terminal FGF23 (cFGF23), (L) plasma iFGF23, (M) urine phosphate-to-creatinine ratio, (N) phosphate excretion index, (O) serum phosphate, and (P) Fgf23 mRNA expression relative to Actb in the bone cortex. The mean mRNA ratio of the Tmprss6+/+ mice was normalized to 1. n = 4 to 11 per group. For all graphs, data represent mean ± standard deviation. The total spleen nonheme iron content was calculated by multiplying the measured nonheme iron concentration by the total spleen weight. The phosphate excretion index was calculated as (urine phosphate ÷ serum phosphate) ÷ urine creatinine.82 ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001 using one-way analysis of variance (ANOVA) with Tukey post hoc test.
The perturbed iron homeostasis of Tmprss6–/– mice is accompanied by elevated FGF23 levels in circulation but unchanged Fgf23 mRNA levels in the cortical bone. Shown for 8-week-old Tmprss6+/+, Tmprss6+/−, and Tmprss6–/– male littermates are (A) serum iron, (B) Hgb, (C) erythrocyte mean corpuscular volume (MCV), (D) erythrocyte mean corpuscular hemoglobin (MCH), (E) serum EPO, (F) liver nonheme iron concentration, (G) heart nonheme iron concentration, (H) kidney nonheme iron concentration, (I) spleen-to-body-weight ratio, (J) total spleen nonheme iron content, (K) plasma C-terminal FGF23 (cFGF23), (L) plasma iFGF23, (M) urine phosphate-to-creatinine ratio, (N) phosphate excretion index, (O) serum phosphate, and (P) Fgf23 mRNA expression relative to Actb in the bone cortex. The mean mRNA ratio of the Tmprss6+/+ mice was normalized to 1. n = 4 to 11 per group. For all graphs, data represent mean ± standard deviation. The total spleen nonheme iron content was calculated by multiplying the measured nonheme iron concentration by the total spleen weight. The phosphate excretion index was calculated as (urine phosphate ÷ serum phosphate) ÷ urine creatinine.82 ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001 using one-way analysis of variance (ANOVA) with Tukey post hoc test.
Compared with Tmprss6+/+ controls, Tmprss6–/– mice exhibited a marked (600%) increase in plasma cFGF23 (Figure 1K) and a moderate (50%) increase in iFGF23 (Figure 1L). Tmprss6+/− mice showed mildly reduced liver nonheme iron concentration and signs of slight iron-restricted erythropoiesis (Figure 1F,C) but retained plasma cFGF23 and iFGF23 levels comparable with those of Tmprss6+/+ controls (Figure 1K-L). Tmprss6–/– mice showed evidence of increased urinary phosphate excretion but appeared capable of compensating for the maintenance of normal serum phosphate (Figure 1M-O). Unexpectedly, Fgf23 mRNA levels in the bone cortex did not differ significantly between the Tmprss6–/– mice and littermate controls (Figure 1P). Younger Tmprss6–/– mice also showed microcytic anemia and elevated circulating FGF23 (supplemental Figure 1). Collectively, these findings validate Tmprss6–/– mice as a model of chronic IDA with systemic FGF23 elevation.
Heterozygous Fgf23 disruption does not alter the severity of iron deficiency in Tmprss6–/– mice
To facilitate the identification of FGF23 production sites in Tmprss6–/– mice, we introduced an established Fgf23 reporter allele37 in which the eGFP coding sequence is knocked in immediately after the endogenous Fgf23 start codon (Fgf23eGFP). This allele abolishes the transcription of endogenous Fgf23 mRNA and simultaneously enables the use of eGFP to mark cells in which the Fgf23 promoter is active. Because the Tmprss6 and Fgf23eGFP mouse strains differed in genetic background, we used a controlled, multigenerational breeding scheme to produce experimental Tmprss6+/+, Tmprss6+/−, and Tmprss6–/– littermates (F2 generation) that were either heterozygous for the Fgf23eGFP allele (Fgf23+/eGFP) or lacked the reporter (Fgf23+/+) (supplemental Figure 2A). Fgf23eGFP/eGFP homozygotes, which are, in effect, Fgf23 null, were not generated because this genotype exhibits marked growth retardation and significant mortality before weaning.37
Firstly, we examined whether the heterozygous loss of Fgf23 altered hematopoietic or iron status within any Tmprss6 genotype. Within each Tmprss6 genotype, heterozygous Fgf23 disruption did not significantly alter survival to weaning age (supplemental Figure 2A), adult body weight (supplemental Figure 2B-C), serum hepcidin, serum iron, hemoglobin (Hgb), red cell indices, tissue nonheme iron concentrations, spleen-to-body weight ratio, or total spleen iron content (male, Figure 2A-J; female, supplemental Figure 3A-J). Notably, the heterozygous loss of Fgf23 did not alter the degree of hypoferremia, anemia, tissue iron deficiency, or splenomegaly in the Tmprss6–/– mice.
Heterozygous Fgf23 disruption does not alter iron homeostasis in mice with 2, 1, or 0 wild-type Tmprss6 alleles. Shown for 8-week-old male mice of different Tmprss6-Fgf23 genotype combinations are (A) serum hepcidin, (B) serum iron, (C) Hgb, (D) MCV, (E) MCH, (F) liver nonheme iron concentration, (G) heart nonheme iron concentration, (H) kidney nonheme iron concentration, (I) spleen-to-body-weight ratio, and (J) total spleen nonheme iron content. n = 4 to 7 per group. Two-way ANOVA revealed significant effects of the Tmprss6 genotype on all parameters (P < .001), whereas no significant effect of the Fgf23 genotype on any parameter was detected. For all bar graphs, data represent the mean ± standard deviation. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001 using two-way ANOVA with Tukey post hoc test. G, eGFP allele.
Heterozygous Fgf23 disruption does not alter iron homeostasis in mice with 2, 1, or 0 wild-type Tmprss6 alleles. Shown for 8-week-old male mice of different Tmprss6-Fgf23 genotype combinations are (A) serum hepcidin, (B) serum iron, (C) Hgb, (D) MCV, (E) MCH, (F) liver nonheme iron concentration, (G) heart nonheme iron concentration, (H) kidney nonheme iron concentration, (I) spleen-to-body-weight ratio, and (J) total spleen nonheme iron content. n = 4 to 7 per group. Two-way ANOVA revealed significant effects of the Tmprss6 genotype on all parameters (P < .001), whereas no significant effect of the Fgf23 genotype on any parameter was detected. For all bar graphs, data represent the mean ± standard deviation. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001 using two-way ANOVA with Tukey post hoc test. G, eGFP allele.
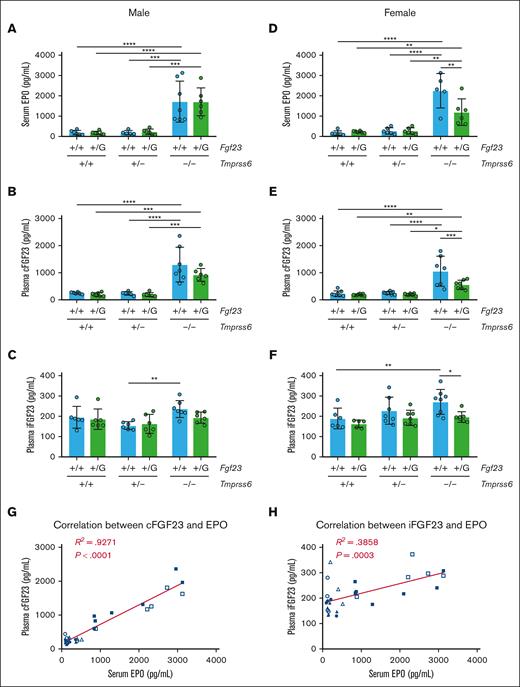
Compared with Tmprss6+/+Fgf23+/+ males, Tmprss6–/–Fgf23+/+ males showed significantly higher serum EPO and plasma cFGF23 and a trend toward higher plasma iFGF23 (P = .202; Figure 3A-C). Serum EPO, plasma cFGF23, and plasma iFGF23 in Tmprss6–/–Fgf23+/+ females were all significantly higher than those in the Tmprss6+/+Fgf23+/+ females (Figure 3D-F). In Tmprss6–/– females, the elevations in cFGF23 and iFGF23 were blunted by heterozygous Fgf23 disruption, suggesting an Fgf23 gene dosage effect. In other Tmprss6 genotypes, heterozygous Fgf23 disruption caused modest, nonsignificant reductions in mean cFGF23 levels (supplemental Figure 4A-B; note the log scales on y-axes). In Tmprss6–/–Fgf23+/eGFP mice, the cFGF23 level remained significantly higher than that in nonanemic genotypes (Figure 3B,D), suggesting that this genotype would be informative for interrogating sites of increased Fgf23 expression in the setting of IDA.
Tmprss6–/– mice, regardless of their Fgf23 genotype, show EPO elevation and increased circulating levels of cFGF23. (A) Serum EPO, (B) plasma cFGF23, and (C) plasma iFGF23 of 8-week-old male mice with different Tmprss6-Fgf23 genotype combinations (n = 5-7 per group). Using two-way ANOVA in male mice, Tmprss6 genotype had statistically significant effects on serum EPO (P < .0001), plasma cFGF23 (P < .0001), and plasma iFGF23 (P = .0081), whereas the Fgf23 genotype had no significant effect on these parameters. (D) Serum EPO, (E) plasma cFGF23, and (F) plasma iFGF23 of 8-week-old female mice with different Tmprss6-Fgf23 genotype combinations (n = 5-8 per group). Two-way ANOVA in female mice revealed statistically significant effects of both Tmprss6 and Fgf23 genotypes on serum EPO (Tmprss6 genotype: P < .0001; Fgf23 genotype: P = .0297), plasma cFGF23 (Tmprss6 genotype: P < .0001; Fgf23 genotype: P = .0075), and plasma iFGF23 (Tmprss6 genotype: P = .0191; Fgf23 genotype: P = .0063). For panels A-F, data represent mean ± standard deviation. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001 using two-way ANOVA with Tukey post hoc test. (G) Plasma cFGF23 vs serum EPO and (H) plasma iFGF23 vs serum EPO in individual Fgf23+/+ mice with different Tmprss6 genotypes (8-week-old mice, both sexes pooled): +/+ male (●), +/− male (▲), –/– male (■), +/+ female (○), +/− female (△), and –/– female (□). R2 represents the coefficient of determination using a linear regression model (ie, goodness of fit). The P value of the Pearson correlation is shown.
Tmprss6–/– mice, regardless of their Fgf23 genotype, show EPO elevation and increased circulating levels of cFGF23. (A) Serum EPO, (B) plasma cFGF23, and (C) plasma iFGF23 of 8-week-old male mice with different Tmprss6-Fgf23 genotype combinations (n = 5-7 per group). Using two-way ANOVA in male mice, Tmprss6 genotype had statistically significant effects on serum EPO (P < .0001), plasma cFGF23 (P < .0001), and plasma iFGF23 (P = .0081), whereas the Fgf23 genotype had no significant effect on these parameters. (D) Serum EPO, (E) plasma cFGF23, and (F) plasma iFGF23 of 8-week-old female mice with different Tmprss6-Fgf23 genotype combinations (n = 5-8 per group). Two-way ANOVA in female mice revealed statistically significant effects of both Tmprss6 and Fgf23 genotypes on serum EPO (Tmprss6 genotype: P < .0001; Fgf23 genotype: P = .0297), plasma cFGF23 (Tmprss6 genotype: P < .0001; Fgf23 genotype: P = .0075), and plasma iFGF23 (Tmprss6 genotype: P = .0191; Fgf23 genotype: P = .0063). For panels A-F, data represent mean ± standard deviation. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001 using two-way ANOVA with Tukey post hoc test. (G) Plasma cFGF23 vs serum EPO and (H) plasma iFGF23 vs serum EPO in individual Fgf23+/+ mice with different Tmprss6 genotypes (8-week-old mice, both sexes pooled): +/+ male (●), +/− male (▲), –/– male (■), +/+ female (○), +/− female (△), and –/– female (□). R2 represents the coefficient of determination using a linear regression model (ie, goodness of fit). The P value of the Pearson correlation is shown.
Because the mechanism leading to FGF23 upregulation in IDA is not yet known, we examined the relationship between plasma cFGF23/iFGF23 and circulating physiological parameters that are altered in IDA. When considering all mice from this cohort with 2 intact Fgf23 alleles, serum iron and Hgb both showed significant inverse correlations with cFGF23 and iFGF23 (supplemental Figure 4C-F), whereas serum EPO showed a significant positive correlation with cFGF23 and iFGF23 (Figure 3G-H). Under a linear regression model, the correlations of cFGF23 and iFGF23 with serum EPO showed a better goodness of fit (ie, higher R2 values) than their respective correlations with other circulating parameters (ie, serum iron and Hgb). Interestingly, EPO elevation in Tmprss6–/– females was blunted by the heterozygous Fgf23 disruption (Figure 3D). Collectively, these findings suggested that the regulation of FGF23 and EPO may be coupled.
Tmprss6–/– mice show increased Fgf23 expression in the BM
As our study was in progress, Rabadi et al43 reported that in C57BL/6 mice, acute blood loss elevates Fgf23 in total BM RNA. Therefore, we analyzed the Fgf23 mRNA expression in the total BM of Tmprss6+/+, Tmprss6+/−, and Tmprss6–/– littermates with 2 intact Fgf23 alleles. Tmprss6–/– mice showed significantly higher BM Fgf23 mRNA than Tmprss6+/+ controls (Figure 4A), suggesting that increased FGF23 production by 1 or more BM cell types may contribute to the elevated circulating FGF23 levels in Tmprss6–/– mice.
Tmprss6–/– mice show increased Fgf23 expression in the BM. (A) Fgf23 mRNA expression relative to Actb in the total BM mRNA of Tmprss6+/+, Tmprss6+/−, and Tmprss6–/– male mice (8-9 weeks old, C57BL/6 genetic background). The mean mRNA ratio of the Tmprss6+/+ mice was normalized to 1. n = 7 per group; 1 outlier per genotype was identified using the Robust regression followed by OUTlier identification (ROUT) method (Q = 1%) and removed from subsequent analysis. Data represent the mean ± standard deviation. ∗P < .05 using one-way ANOVA with Tukey post hoc test. (B) Confirmation that the Fgf23eGFP reporter allele yields green fluorescence in Tmprss6–/– BM. Shown are confocal images of briefly fixed BM core biopsies from Tmprss6–/–Fgf23+/eGFP and Tmprss6–/–Fgf23+/+ mice (19 weeks old). Original magnification ×20. (C) Localization of green fluorescence and endomucin expression in the BM of mice carrying the Fgf23eGFP reporter allele. Whole femurs from 6-month-male mice were fixed, decalcified, embedded, and cryosectioned before antiendomucin immunostaining. Pseudocolored merged images (GFP and endomucin) as well as individual channels in grayscale (GFP or endomucin) are presented in the same column; scale bar, 20 μm. Regions boxed in blue (Tmprss6–/–Fgf23+/eGFP) and yellow (Tmprss6+/−Fgf23+/eGFP) are presented at higher magnification in the adjacent column. The dashed line indicates the border between the bone (left) and BM (right).
Tmprss6–/– mice show increased Fgf23 expression in the BM. (A) Fgf23 mRNA expression relative to Actb in the total BM mRNA of Tmprss6+/+, Tmprss6+/−, and Tmprss6–/– male mice (8-9 weeks old, C57BL/6 genetic background). The mean mRNA ratio of the Tmprss6+/+ mice was normalized to 1. n = 7 per group; 1 outlier per genotype was identified using the Robust regression followed by OUTlier identification (ROUT) method (Q = 1%) and removed from subsequent analysis. Data represent the mean ± standard deviation. ∗P < .05 using one-way ANOVA with Tukey post hoc test. (B) Confirmation that the Fgf23eGFP reporter allele yields green fluorescence in Tmprss6–/– BM. Shown are confocal images of briefly fixed BM core biopsies from Tmprss6–/–Fgf23+/eGFP and Tmprss6–/–Fgf23+/+ mice (19 weeks old). Original magnification ×20. (C) Localization of green fluorescence and endomucin expression in the BM of mice carrying the Fgf23eGFP reporter allele. Whole femurs from 6-month-male mice were fixed, decalcified, embedded, and cryosectioned before antiendomucin immunostaining. Pseudocolored merged images (GFP and endomucin) as well as individual channels in grayscale (GFP or endomucin) are presented in the same column; scale bar, 20 μm. Regions boxed in blue (Tmprss6–/–Fgf23+/eGFP) and yellow (Tmprss6+/−Fgf23+/eGFP) are presented at higher magnification in the adjacent column. The dashed line indicates the border between the bone (left) and BM (right).
Next, we compared green fluorescence in briefly fixed BM core biopsies from Tmprss6–/– mice with or without the Fgf23eGFP allele. Using confocal microscopy, green fluorescence confirmed the reporter gene expression in a subset of cells distributed throughout the BM. The elongated shape and distribution of these cells suggested their localization to the vasculature (Figure 4B). Moreover, in fixed, decalcified femur cryosections from Tmprss6–/–Fgf23+/eGFP mice, fluorescence from eGFP (a cytosolic protein) in the BM showed partial overlap with the endothelial cell marker endomucin (a membrane-bound glycoprotein),44 whereas eGFP fluorescence was absent from most osteocytes in the bone cortex and trabeculae as well as the cell populations lining the bone surfaces (Figure 4C).
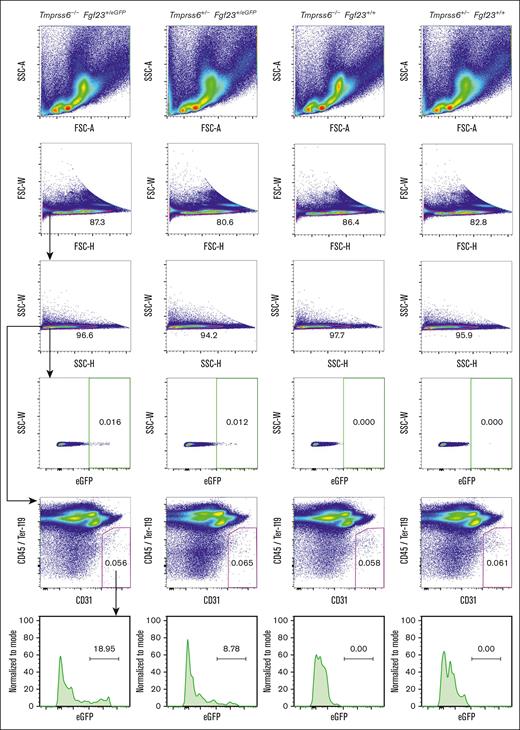
To determine whether BM endothelial cells could be a site of Fgf23 upregulation in Tmprss6–/– mice, we performed flow cytometry on BM cells harvested using an enzymatic digestion protocol that promotes the recovery of endothelial cells.41 Bright green fluorescence was detected in a very small fraction (<0.02%) of total BM cells from mice carrying the Fgf23eGFP allele (Figure 5). However, within the endothelial population (defined as CD45–TER-119–CD31+),41 cells showing bright green fluorescence were enriched (Figure 5), a finding that was confirmed using a different CD31 antibody clone (supplemental Figure 5). With both CD31 clones, the percentage of GFPbright endothelial cells was higher in Tmprss6–/–Fgf23+/eGFP mice than that in nonanemic mice carrying the Fgf23eGFP reporter allele.
A subset of BM endothelial cells exhibit bright green fluorescence in mice that carry the Fgf23eGFP allele. Fluorescence-activated cell sorter plots of BM cells from Tmprss6–/–Fgf23+/eGFP, Tmprss6+/−Fgf23+/eGFP, Tmprss6–/–Fgf23+/+, and Tmprss6+/−Fgf23+/+ male littermates (18 weeks old). The numbers represent the percentage of each gated population. Forward scatter (FSC) and side scatter (SSC) were used to exclude debris and cell doublets, and eGFPbright cells were assessed in each single-cell population (green gate). BM endothelial cells were identified as the CD45-TER-119-CD31+ population (magenta gate) as described,41 using CD31 antibody clone 390, and eGFP fluorescence was assessed in the endothelial cell gate. A total of 6 × 105 BM cells per mouse were analyzed.
A subset of BM endothelial cells exhibit bright green fluorescence in mice that carry the Fgf23eGFP allele. Fluorescence-activated cell sorter plots of BM cells from Tmprss6–/–Fgf23+/eGFP, Tmprss6+/−Fgf23+/eGFP, Tmprss6–/–Fgf23+/+, and Tmprss6+/−Fgf23+/+ male littermates (18 weeks old). The numbers represent the percentage of each gated population. Forward scatter (FSC) and side scatter (SSC) were used to exclude debris and cell doublets, and eGFPbright cells were assessed in each single-cell population (green gate). BM endothelial cells were identified as the CD45-TER-119-CD31+ population (magenta gate) as described,41 using CD31 antibody clone 390, and eGFP fluorescence was assessed in the endothelial cell gate. A total of 6 × 105 BM cells per mouse were analyzed.
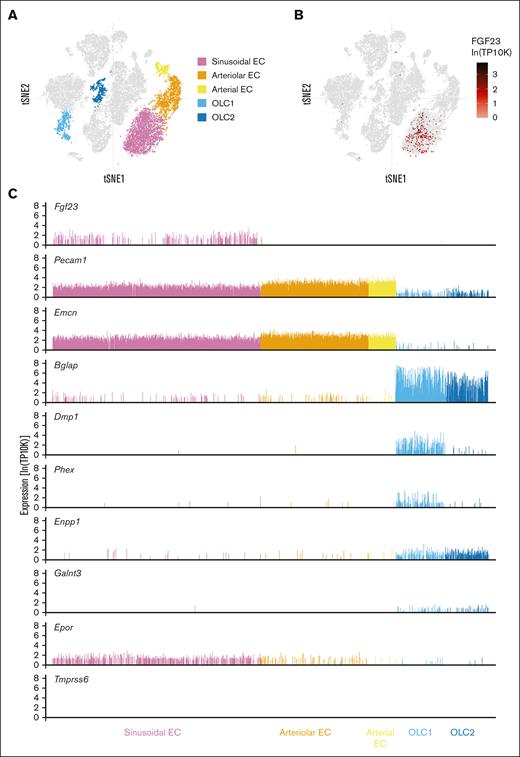
Sinusoidal endothelial cells show higher Fgf23 expression than other BM stromal cell populations in mice with a normal iron balance
To clarify the BM endothelial cell subtype that expresses Fgf23, we interrogated published single-cell RNA-Seq (scRNA-Seq) data from the BM stromal cells of C57BL/6 mice (ie, healthy mice with no defects in iron or phosphate balance); these data were generated in a study that used unbiased clustering to define 17 stromal cell subsets, including BM sinusoidal endothelial cells (BM-SECs), arterial endothelial cells, arteriolar endothelial cells, and 2 osteolineage populations.45 BM-SECs were the only prominent source of Fgf23 transcripts detected in single-cell analysis (Figure 6A-B). Cells expressing Fgf23 also expressed endothelial cell markers, such as Pecam1 (CD31) and Emcn (endomucin), whereas cells expressing osteolineage markers rarely expressed Fgf23 (Figure 6C). Several genes that lead to FGF23 dysregulation when mutated (eg, Dmp1,46Phex,37Enpp1,47 and Galnt348) were expressed in osteolineage cells but showed very low, if any, expression in BM-SECs, suggesting that FGF23 regulatory mechanisms might differ across cell types. Interestingly, mRNA encoding the erythropoietin receptor (Epor) was detected in BM-SECs. Tmprss6 mRNA was not detected in any of the BM stromal cell populations (Figure 6C; data not shown).
Sinusoidal endothelial cells show higher Fgf23 expression than other BM stromal cell populations in mice with normal iron balance. Visualization of scRNA-Seq data from 20 896 nonhematopoietic cells (mixed bone and BM fractions) generated by Baryawno et al.45 Data were downloaded from the Broad Institute Single Cell Portal (https://singlecell.broadinstitute.org/single_cell/study/SCP361/mouse-bone-marrow-stroma-in-homeostasis). T-distributed stochastic neighbor embedding (tSNE) plots (A) highlighting the 5 cell clusters of interest and (B) showing the Fgf23 expression. (C) The expression of selected genes is shown for each individual cell within the 5 cell clusters highlighted in A. TP10K transcripts per 10 thousand transcripts. EC, endothelial cell; OLC, osteolineage cell.
Sinusoidal endothelial cells show higher Fgf23 expression than other BM stromal cell populations in mice with normal iron balance. Visualization of scRNA-Seq data from 20 896 nonhematopoietic cells (mixed bone and BM fractions) generated by Baryawno et al.45 Data were downloaded from the Broad Institute Single Cell Portal (https://singlecell.broadinstitute.org/single_cell/study/SCP361/mouse-bone-marrow-stroma-in-homeostasis). T-distributed stochastic neighbor embedding (tSNE) plots (A) highlighting the 5 cell clusters of interest and (B) showing the Fgf23 expression. (C) The expression of selected genes is shown for each individual cell within the 5 cell clusters highlighted in A. TP10K transcripts per 10 thousand transcripts. EC, endothelial cell; OLC, osteolineage cell.
By mining a different study involving bulk RNA-Seq of 4 principal stromal cell populations from wild-type murine BM,49 we identified higher Fgf23 expression in BM-SECs than that in BM arterial endothelial cells in mice of 3 different ages (supplemental Figure 6). Furthermore, Fgf23 transcripts were undetectable in 2 mixed mesenchymal cell populations associated with marrow vasculature (CXCL12-abundant reticular cells and PDGFRα+Sca1+ cells). Collectively, our data mining suggests that BM-SECs may produce FGF23 in mice with normal iron and phosphate balance.
BM-SECs are a site of Fgf23 upregulation in Tmprss6–/–Fgf23+/eGFP mice
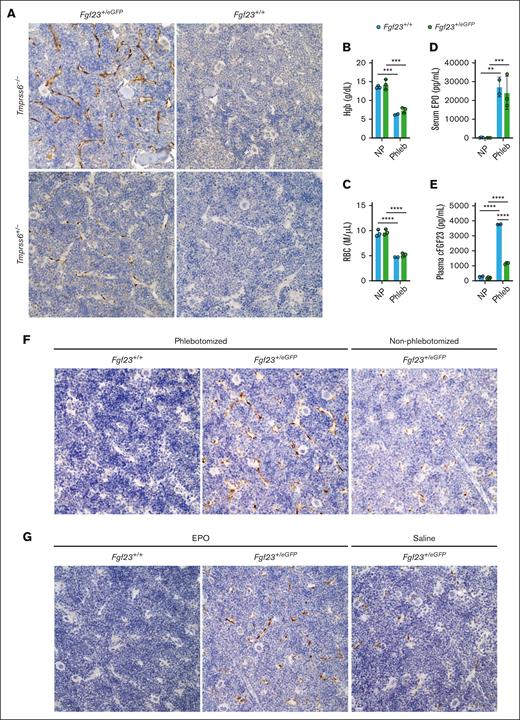
To assess Fgf23eGFP reporter allele expression in the context of BM architecture, we used immunohistochemistry with an anti-GFP antibody in formalin-fixed, paraffin-embedded tissue sections, an approach that provides greater sensitivity for GFP detection than the direct visualization of fluorescence in unfixed tissue sections.50Tmprss6–/–Fgf23+/eGFP mice showed GFP expression in BM-SECs throughout the BM, which was more intense than that in nonanemic Tmprss6+/−Fgf23+/eGFP mice, and which was absent in mice lacking the Fgf23eGFP allele (Figure 7A; supplemental Figure 7). These data identified BM-SECs as a site of Fgf23 upregulation in mice with chronic IDA.
BM-SECs are a site of Fgf23 upregulation in genetically induced IDA, in phlebotomy-induced anemia, and after the direct administration of EPO. (A) Immunohistochemical staining with an anti-GFP antibody in the BM of formalin-fixed, decalcified femur sections of selected Tmprss6-Fgf23 genotype combinations (6-month-old male mice). Original magnification ×40. Brown staining highlights anti-GFP immunoreactivity; blue staining reflects hematoxylin counterstain. (B-E) Circulating parameters in Fgf23+/eGFP and Fgf23+/+ mice evaluated at the 18-hour time point after a large-volume phlebotomy, compared with nonphlebotomized, genotype-matched controls, validating the expected effects of phlebotomy on (B) Hgb, (C) red blood cell (RBC) count, (D) serum EPO, and (E) plasma cFGF23. Fgf23+/eGFP and Fgf23+/+ female mice (8-weeks-old; 2-4 per group) were either not previously phlebotomized (NP) or underwent a single 500 μL phlebotomy with saline volume replacement 18 hours earlier ("Phleb"). For all graphs, data represent mean ± standard deviation. ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001 using a two-way ANOVA with Tukey post hoc test. (F) Immunohistochemical staining with anti-GFP antibody in the BM of formalin-fixed, decalcified femur sections harvested 18 hours after a 500 μL phlebotomy (Fgf23+/+ or Fgf23+/eGFP mice) or harvested from nonphlebotomized Fgf23+/eGFP control mice. Original magnification ×40. (G) Immunohistochemical staining with anti-GFP antibody in the BM of formalin-fixed decalcified femur sections harvested 6 hours after injection of EPO (Fgf23+/+ or Fgf23+/eGFP mice) or saline vehicle (Fgf23+/eGFP control mice). Original magnification ×40.
BM-SECs are a site of Fgf23 upregulation in genetically induced IDA, in phlebotomy-induced anemia, and after the direct administration of EPO. (A) Immunohistochemical staining with an anti-GFP antibody in the BM of formalin-fixed, decalcified femur sections of selected Tmprss6-Fgf23 genotype combinations (6-month-old male mice). Original magnification ×40. Brown staining highlights anti-GFP immunoreactivity; blue staining reflects hematoxylin counterstain. (B-E) Circulating parameters in Fgf23+/eGFP and Fgf23+/+ mice evaluated at the 18-hour time point after a large-volume phlebotomy, compared with nonphlebotomized, genotype-matched controls, validating the expected effects of phlebotomy on (B) Hgb, (C) red blood cell (RBC) count, (D) serum EPO, and (E) plasma cFGF23. Fgf23+/eGFP and Fgf23+/+ female mice (8-weeks-old; 2-4 per group) were either not previously phlebotomized (NP) or underwent a single 500 μL phlebotomy with saline volume replacement 18 hours earlier ("Phleb"). For all graphs, data represent mean ± standard deviation. ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001 using a two-way ANOVA with Tukey post hoc test. (F) Immunohistochemical staining with anti-GFP antibody in the BM of formalin-fixed, decalcified femur sections harvested 18 hours after a 500 μL phlebotomy (Fgf23+/+ or Fgf23+/eGFP mice) or harvested from nonphlebotomized Fgf23+/eGFP control mice. Original magnification ×40. (G) Immunohistochemical staining with anti-GFP antibody in the BM of formalin-fixed decalcified femur sections harvested 6 hours after injection of EPO (Fgf23+/+ or Fgf23+/eGFP mice) or saline vehicle (Fgf23+/eGFP control mice). Original magnification ×40.
BM-SECs are a site of Fgf23 upregulation in acute phlebotomy-induced anemia and after EPO injection
Because IDA in Tmprss6–/– mice results from pathologic hepcidin elevation, we also sought to determine whether BM-SECs are a site of Fgf23 upregulation in anemic mice with intact hepcidin regulation (ie, with 2 intact Tmprss6 alleles). We subjected Fgf23+/eGFP mice and Fgf23+/+ controls to an established 500 μL phlebotomy protocol51 (with saline volume replacement) known to induce marked anemia, EPO elevation, and hepcidin suppression. Pilot studies confirmed marked anemia and elevations of serum EPO and plasma cFGF23 in Fgf23+/+ mice at 18 hours after phlebotomy (Figure 7B-E). The trajectories of hematologic recovery and EPO elevation after phlebotomy were not markedly altered by the heterozygous Fgf23 disruption; however, phlebotomized Fgf23+/eGFP mice showed blunted plasma FGF23 responses, suggesting an Fgf23 gene dosage effect (Figure 7E; supplemental Figure 8). Therefore, we used anti-GFP immunohistochemistry to localize the BM expression of the Fgf23eGFP allele at baseline and 18 hours after phlebotomy. Phlebotomized Fgf23+/eGFP mice showed GFP expression in BM-SECs throughout the BM, which was more intense than that in nonphlebotomized Fgf23+/eGFP mice and absent in phlebotomized Fgf23+/+ controls (Figure 7F).
Because serum erythropoietin elevation is a feature shared by Tmprss6–/– mice and mice with phlebotomy-induced anemia, we asked whether direct administration of EPO could induce Fgf23 upregulation in BM-SECs of nonanemic mice. In BM plugs isolated from Fgf23eGFP mice, the GFP intensity of BM-SECs was significantly higher after 18 hours of ex vivo treatment with EPO than after treatment with vehicle (supplemental Figure 9A). In addition, we assessed Fgf23+/eGFP and Fgf23+/+ mice for Fgf23 upregulation 6 hours after injection with recombinant human EPO or saline vehicle. EPO injection significantly increased plasma cFGF23 levels (supplemental Figure 9B), consistent with results of a previous study.52Fgf23+/eGFP mice treated with EPO showed GFP expression in BM-SECs throughout the BM, which was more intense than that in Fgf23+/eGFP mice treated with saline and absent in EPO-treated Fgf23+/+ controls (Figure 7G).
Discussion
Although iron deficiency has been associated with increased levels of circulating FGF23 in humans and mouse models, the cell type(s) contributing to the increase in FGF23 production in IDA remain unclear. Traditionally, cells of the bone cortex have been viewed as major sites of FGF23 production.2-4 Indeed, conditional deletion of Fgf23 in murine osteoblasts/osteocytes revealed that Fgf23 expression in these cells maintains basal circulating FGF23 levels53 and contributes to FGF23 elevation in response to challenges such as high-phosphate feeding53 and induction of kidney disease.54 However, selective deletion of Fgf23 in osteoblasts and osteocytes reduced circulating levels of the active FGF23 hormone by only 40% or 50%,53 suggesting that other cell types contribute to FGF23 in circulation under baseline conditions. In this study, using the Fgf23eGFP reporter allele, we identified BM-SECs as a site of Fgf23 upregulation in chronic genetically induced IDA and acute phlebotomy-induced anemia.
The Fgf23eGFP reporter allele in this study was previously used by Liu et al37 to verify osteocytes as a site of Fgf23 upregulation in mice with pathological FGF23 elevation55 and hypophosphatemia56 due to the mutation of Phex57 (PhexHyp allele). In PhexHyp mutants carrying the Fgf23eGFP allele, green fluorescence was observed in the femur cortex and BM (colocalizing with CD31) as well as the thymus and brain.37 In Fgf23+/eGFP mice with intact Phex, however, green fluorescence was apparent in the BM but not in the mineralized bone.37 Compared with Fgf23+/eGFP mice with intact Phex, Fgf23+/eGFPPhexHyp mice showed marked upregulation of green fluorescence in bone-embedded osteocytes but not in the BM.37 A subsequent study found that pathological FGF23 upregulation in PhexHyp mice was eliminated by the selective deletion of Fgf23 in osteoblasts/osteocytes.53
In our study, iron-deficient Tmprss6–/– mice showed increased circulating FGF23 compared with nonanemic controls (Figure 1K-L), which could not be attributed to higher Fgf23 expression in osteocytes (Figure 1P). Rather, compared with nonanemic controls, Tmprss6–/– mice showed increased Fgf23 RNA in the BM (Figure 4A) and greater Fgf23eGFP reporter expression in BM endothelial cells (Figures 5 and 7A; supplemental Figure 5). We also observed Fgf23eGFP reporter allele expression in rare cells of the thymus (supplemental Figure 10) but not in the liver, spleen, heart, muscle, or kidney (data not shown). Similarly, Fgf23+/eGFP mice (with intact Tmprss6 alleles) with phlebotomy-induced anemia and elevated circulating FGF23 showed upregulation of Fgf23eGFP reporter expression in BM-SECs compared with nonanemic Fgf23+/eGFP controls (Figure 7E).
Several groups have demonstrated that circulating FGF23 levels rise under conditions of increased erythropoietic drive. Wild-type C57BL/6 mice showed an acute increase in plasma cFGF23 (but not iFGF23) 6 hours after phlebotomy.43 In healthy rodents, injection of recombinant human erythropoietin (rhEPO) caused significant increases in circulating cFGF23 levels43,52,58-61 with less pronounced52,58-61 or nonsignificant43 changes in iFGF23 levels. Administration of rhEPO increased circulating cFGF23 levels in healthy human subjects60 and elevated both cFGF23 and iFGF23 levels in patients with anemia of unclear etiology and normal renal function.59 In RNA harvested from the total murine BM, upregulation of Fgf23 mRNA was detected in response to both rhEPO injection52,59,60 and phlebotomy,43 and pretreatment with the BM-ablative agent carboplatin blunted the induction of circulating cFGF23 by rhEPO.59 Different experimental approaches have suggested that erythroid,43,60 myeloid,60 and Lin–Sca1+c-Kit+ cells59 are sites of FGF23 upregulation by EPO.
In contrast, in our study, iron-deficient Tmprss6–/– mice, which also displayed EPO elevation, showed upregulated Fgf23 reporter expression in endothelial cells in the context of intact BM architecture as well as via flow cytometry. In addition, we detected Fgf23 mRNA in the BM-SECs of wild-type mice using scRNA-Seq45 and bulk RNA-Seq49 transcriptomic data sets. Our BM flow cytometric analyses used methods to enhance endothelial cell recovery (ie, enzymatic digestion and 100 μM filtering), with gating to exclude cell doublets. Notably, in our preliminary analyses conducted without enzymatic digestion, GFPbright cells were not detected in the BM of the mice carrying the Fgf23eGFP reporter allele (data not shown).
An essential role for FGF23 in phosphate and vitamin D metabolism was demonstrated in mice with a global loss of Fgf23 (Fgf23–/–), which showed hyperphosphatemia, hypervitaminosis D, reduced bone mineral content, and profound growth retardation.62 These mice also developed other metabolic abnormalities (hypoglycemia, hypotriglyceridemia, and hypercholesterolemia), lymphoid organ atrophy, and a markedly reduced lifespan.62 Additionally, altered frequencies of BM cell populations and aberrant red blood cell indices have been described in Fgf23–/– mouse models.63,64 However, these hematopoietic alterations were not observed in lethally irradiated wild-type mice after transplantation with Fgf23–/– BM nucleated cells (ie, Fgf23–/– BM chimeras), suggesting that the loss of Fgf23–/– in hematopoietic cells is insufficient to alter steady-state hematopoiesis.64 These findings in Fgf23–/– BM chimeras do not exclude the possibility that BM-SEC-derived FGF23 contributes to steady-state hematopoiesis because BM-SECs are predominantly host-derived when vascular homeostasis is restored after BM transplantation.65,66
Recently, a local role of FGF23 in the BM was elucidated by Ishii et al,64 who demonstrated that FGF23 promotes the mobilization of hematopoietic progenitor cells (HPCs) into the circulation.64 Global Fgf23–/– mice and Fgf23–/– BM chimeras exhibit impaired HPC mobilization in response to granulocyte colony–stimulating factor (G-CSF) treatment.64 Additionally, in wild-type mice, G-CSF treatment increased Fgf23 mRNA levels in the total BM and FGF23 protein levels in the BM extracellular fluid and peripheral blood.64 After G-CSF treatment, Fgf23–/– BM chimeras also showed significant iFGF23 in the BM extracellular fluid, suggesting that nonhematopoietic cells contribute to local iFGF23 levels. In wild-type mice, Fgf23 mRNA induction was observed in both CD45–Ter-119+CD71+ and CD45–Ter-119–cell populations; we note that the latter population is expected to include BM-SECs, raising the possibility that BM-SEC–derived FGF23 may influence HPC mobilization. Indeed, of the different vessel types comprising the BM vascular network, sinusoids are the sites of immature and mature leukocyte trafficking between the BM and circulation.67 Additionally, the BM microvasculature is a critical component of the hematopoietic stem cell niche.68,69 In the BM, endothelial cells directly contact stem and progenitor cells, and endothelial cell-derived paracrine factors, known as angiocrine factors, orchestrate the self-renewal and differentiation of stem and progenitor cells.70 BM-SECs also actively controls erythropoiesis, as illustrated by the impaired terminal erythroid differentiation and fatal anemia observed in mice with selective overexpression of β-catenin in BM-SECs.71 Interestingly, these mutants also showed elevated serum FGF23 with Fgf23 mRNA upregulation in BM-SECs, as demonstrated in isolated BM-SECs by quantitative polymerase chain reaction and in BM sections by RNAScope fluorescence in situ hybridization.71
In bone, the regulation of FGF23 production involves transcription and translation, posttranslational modification, and cleavage of the mature peptide.1 Similar to other mouse models with increased erythropoietic drive,16,43,52,58-61,72 mice in our study with chronic IDA showed markedly increased circulating cFGF23 (intact hormone and C-terminal cleaved fragments) but only modestly increased iFGF23 (intact hormone). Such an ELISA profile has been interpreted to reflect increased FGF23 synthesis offset by increased FGF23 cleavage.5 This profile may also reflect local consumption of the intact hormone in the BM. Indeed, Ishii et al64 found that after G-CSF treatment, both iFGF23 and cFGF23 levels were elevated in BM extracellular fluid but only cFGF23 levels were elevated in the circulation.
FGF23 has been found to directly target different cell types in several organs via pathways that may or may not require the coreceptor Klotho.73 Although Tmprss6–/– mice showed elevated urine phosphate-to-creatinine ratios, compatible with the known phosphaturic effects of FGF23, they were not hypophosphatemic (Figure 1M-O). Tmprss6–/– bones were brittle, requiring less work to fracture while retaining their stiffness (supplemental Figure 11); this contrasts with the characteristics of bones of PhexHyp mice, which tolerate less force but are pliable.74 Although FGF23 has been shown to induce left ventricular hypertrophy in mice,75Tmprss6–/– mice showed cardiomegaly with right ventricular dilatation (supplemental Figure 12), a phenotype observed in mice exposed to chronic hypoxia.76 Thus, although elevated FGF23 might contribute to abnormal phenotypes in Tmprss6–/– mice, we cannot exclude the potential effects of anemia and/or iron deficiency.77,78
Several systemic and cellular changes associated with IDA have been proposed and investigated as stimulators of Fgf23 expression, including decreased iron availability, hypoxia, and elevated EPO levels.79 In our study, plasma cFGF23 and iFGF23 both showed stronger linear correlations with serum EPO than with serum iron or blood Hgb (Figure 3G-H; supplemental Figure 4C-F). Interestingly, our data mining of scRNA-Seq45 of BM stroma from wild-type mice detected both Fgf23 and Epor expression in BM-SECs (Figure 6C). Additionally, in nonanemic Fgf23+/eGFP mice, we found that direct injection of EPO increased Fgf23eGFP reporter expression in BM-SECs relative to vehicle-treated controls (Figure 7G). These findings suggest a model in which elevated circulating levels of EPO, which were observed in both chronic genetically induced IDA and acute phlebotomy-induced anemia, act directly on BM-SECs to promote FGF23 production. Intriguingly, while this manuscript was in revision, Aprile et al80 reported that EPO treatment upregulates Fgf23 mRNA via the ERK1/2 and STAT5 pathways in bone-derived cells and TER119-enriched BM cells. Future studies will be required to dissect the mechanisms mediating Fgf23 upregulation in BM-SECs and the local consequences of FGF23 upregulation in BM-SECs during anemic states. Additionally, it would be interesting to determine whether the induction of circulating FGF23 and hypophosphatemia by certain parenteral iron formulations81 is mediated by BM-SECs.
Acknowledgments
The authors thank Mark Lessard for confocal imaging, Eileen Chua for technical assistance, and Diane Krause, Vanessa Scanlon, Yi-Chien Lu, Chunliang Xu, and Clemens Bergwitz for helpful discussions. The visual abstract was created with BioRender.com.
This work was supported by a Gruber Foundation Gruber Science fellowship (X.L.), American Heart Association Predoctoral fellowship 18PRE33960343 (X.L.), a Burroughs Wellcome Career Award for Medical Scientists (K.E.F.), and National Institutes of Health grant U54 DK106857 (Yale Cooperative Center for Excellence in Hematology).
Authorship
Contribution: X.L., L.L., S.M.T., J.F., and K.E.F. performed the research and analyzed the data; X.L., J.F., and K.E.F. wrote the manuscript and prepared the figures; and K.E.F. supervised the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for X.L. is Department of Cell Biology, Harvard Medical School, Boston, MA.
Correspondence: Karin E. Finberg, Department of Pathology, Yale School of Medicine, 310 Cedar St, PO Box 208023, New Haven, CT 06520-8023; e-mail: karin.finberg@yale.edu.
References
Author notes
∗J.F. and K.E.F. contributed equally to this study.
Presented in abstract form at the 59th annual meeting of the American Society of Hematology, Atlanta, GA, 9 December 2017, and the 63rd annual meeting of the American Society of Hematology, Atlanta, GA, 13 December 2021.
Data are available on request from the corresponding author, Karin E. Finberg (karin.finberg@yale.edu).
The full-text version of this article contains a data supplement.