Key Points
PAX5 expression and its targets are inhibited by AZD5153 combined with acalabrutinib in ABC-DLBCL.
Disruption of BCR signaling inhibits the functional activity of PAX5.
Abstract
B-cell receptor (BCR) signaling is essential for the diffuse large B-cell lymphoma (DLBCL) subtype that originates from activated B-cells (ABCs). ABC-DLBCL cells are sensitive to Bruton tyrosine kinase intervention. However, patients with relapsed or refractory ABC-DLBCL had overall response rates from 33% to 37% for Bruton tyrosine kinase inhibitors, suggesting the evaluation of combination-based treatment for improved efficacy. We investigated the efficacy and mechanism of the bromodomain and extraterminal motif (BET) inhibitor AZD5153 combined with the Bruton tyrosine kinase inhibitor acalabrutinib in ABC-DLBCL preclinical models. AZD5153 is a bivalent BET inhibitor that simultaneously engages the 2 bromodomains of BRD4. Adding AZD5153 to acalabrutinib demonstrated combination benefits in ABC-DLBCL cell line and patient-derived xenograft models. Differential expression analyses revealed PAX5 transcriptional activity as a novel downstream effector of this drug combination. PAX5 is a transcription factor for BCR signaling genes and may be critical for perpetually active BCR signaling in ABC-DLBCL. Our analyses further indicated significant alterations in BCR, RELB/alternative NF-κB, and toll-like receptor/interferon signaling. Validation of these results mapped a positive-feedback signaling loop regulated by PAX5. We demonstrated that AZD5153 decreased PAX5 expression, whereas acalabrutinib disruption of BCR signaling inhibited PAX5 activation. Furthermore, several interferon levels were decreased by AZD5153 and acalabrutinib in tumors. Adding interferon-beta1 (IFNβ1) to cells treated with acalabrutinib partially rescued PAX5 activation. Our results demonstrate that AZD5153 enhances the efficacy of acalabrutinib through PAX5 and BCR mechanisms that are critical for ABC-DLBCL.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma. DLBCL is subclassified as activated B-cell (ABC), germinal center B-cell (GCB), or unclassified based on gene expression profiles.1 Patients with ABC-DLBCL treated with the frontline rituximab-cyclophosphamide-doxorubicin-vincristine-prednisone regimen have a lower 5-year survival rate of 56% compared with 78% for GCB cases.2 The ABC subtype has chronic active B-cell receptor (BCR) signaling through the Bruton tyrosine kinase (BTK)/CARD11 pathway, and BTK knockdown is toxic to ABC-DLBCL cells.3 BCR signaling stimulates cell survival as well as activation and differentiation in nonmalignant cells. Acalabrutinib is a BTK inhibitor approved for chronic lymphocytic leukemia and relapsed/refractory (R/R) mantle cell lymphoma. A recent phase 1b clinical trial for patients with acalabrutinib R/R DLBCL (NCT02112526) demonstrated a 33% overall response rate (ORR) for ABC-DLBCL cases vs 16.7% for GCB/unclassified cases,4 and similar results were found in R/R DLBCL trials for ibrutinib and tirabrutinib BTK inhibitors.5,6 The aim of this study was to evaluate acalabrutinib-based drug combinations in ABC-DLBCL to improve its efficacy over monotherapy.
Inhibitors of the BRD4 bromodomain protein have pleiotropic effects and affect multiple cancer pathways, including apoptosis regulation through the Bcl-2 family as well as the BCR signaling genes SYK and BTK.7 AZD5153 is a unique bromodomain and extraterminal motif (BET) inhibitor with bivalent binding characteristics that engages BRD4 at both BD1 and BD2 bromodomains simultaneously.8 This small molecule inhibitor harbors superior potency and disrupts BRD4 foci in U2OS cell with an IC50 value of 1.7 nM compared with 36 nM for the I-BET762 BET inhibitor.9 AZD5153 is in clinical trials and has preclinically demonstrated antitumor activity in multiple xenograft models of acute myeloid leukemia, multiple myeloma, and DLBCL.9 We hypothesize that targeting BRD4, in addition to BTK, may enhance the ABC-DLBCL tumor response through distinct mechanisms in these pathways.
In this study, we demonstrate that AZD5153 enhances the ability of acalabrutinib to target constitutive BCR signaling in ABC-DLBCL. We identified PAX5 transcriptional activity as a novel target of this drug combination. PAX5 is a key regulator of B-cell activation/differentiation that has functional activity associated with malignant transformation10 and higher transcription factor activity in the ABC subtype than in GCB-DLBCL cells.11 The PAX5 gene contains a BRD4 binding region near the transcription start site, which has been characterized as a superenhancer element.12,13 We discovered that targeting BRD4 and BTK disrupts BCR/PAX5 signaling, in which PAX5 transcriptional functions regulate the BCR pathway, and this positive-feedback signaling loop contributes to the constitutive BCR signaling in ABC-DLBCL.
Materials and methods
Cell lines and reagents
See the supplemental Information, including supplemental Table 1, for the cell line sources.
Immunoblots, immunofluorescence, quantitative polymerase chain reaction, and cell viability assays
See the supplemental Information, including supplemental Table 2, for the antibodies used.
Animals and patient-derived tumor models
Female NSG mice aged 5 or 6 weeks from Jackson Laboratories (Bar Harbor, ME) were maintained under specific-pathogen-free conditions in an AAALAC-accredited facility. Animal protocols were approved by the AstraZeneca Institutional Animal Care and Use Committee. All animal experiments were conducted in accordance with Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.14 Patient-derived xenograft (PDX) tumors and genomic characterization15 were obtained from Crown Bio (San Diego, CA). The tissue of ∼8 mm3 was implanted subcutaneously into the right flanks. Mice were randomly assigned to groups based on the tumor volume of the drug or vehicle (0.5% hydroxymethylcellulose and 0.1% Tween80). Tumor sizes were measured using caliper, and volume was calculated as volume = width2 × length, with relative tumor volume (RTV) = final tumor volume ÷ initial tumor volume for individual animals. Tumor growth inhibition (TGI) was calculated as (RTVcontrol – RTVtreatment) × 100) ÷ (RTVcontrol – 1), in which RTVcontrol is the geometric mean of the control group, and RTVtreatment is the treatment group.
Methylcellulose colony formation assay
Cells were added to 36% MethoCult H4100 (Stemcell Technologies, Vancouver, BC, Canada) and transferred to 24-well plates at a final concentration of 2500 cells per well. The plates were incubated overnight, followed by the addition of the drugs. The plates were incubated using IncuCyte (Sartorius, Göttingen, Germany) for visualization and rough quantification. After 14 days, the final viability was measured with the help of Alamar blue reagent (Thermo Fisher, Waltham, MA) using a Synergy Neo2 plate reader.
RNA-sequencing
Approximately 50 mg of the tumor tissue was snap-frozen in liquid nitrogen. RNA was extracted from lysed tissues using RNeasy kit (Qiagen, Hilden, Germany) per the manufacturer’s instructions and quantified using a Qubit fluorometer (Thermo Fisher). Sequencing of 25M reads on the Illumina HighSeq was performed on Novogene (Durham, NC). Data were analyzed using bcbio (Blue Collar Bioinformatics, https://github.com/bcbio/bcbio-nextgen) with an alignment to mouse mm10 and human hg38 reference genomes using HISAT2 2.1.0 for disambiguation.16,17 Ensembl (version 79) transcript quantification using human disambiguated reads was performed using Salmon 0.13.1.18 The R package tximport was used to create a gene/sample count table of Ensembl annotated human protein coding genes and rounded to an integer count. Processed counts were loaded into OnRamp software (Rosalind, San Diego, CA), which performs comparisons using DESeq2.19 Filtering thresholds for significant gene expression changes were log2FC ± 1.5 and false discovery rate < 0.05 for differential expression. Pathway analyses were performed using Enrichr (maayanlab.cloud/Enrichr20,21).
Single guide RNA–mediated knockout, siRNA-mediated knockdown, and plasmid overexpression
For PAX5 knockout and knockdown, 3 × 106 cells were used per electroporation. Cells were washed, pelleted, and resuspended in a buffer from the Lonza SF kit V4XC-2024 (Lonza, Basel, Switzerland). The resuspended cells were combined with the ribonucleoprotein complex or small interering (siRNA). The ribonucleoprotein complex was generated by incubating TrueCut caspase 9 (Cas9) Protein V2 (5 μM final, Invitrogen, Waltham, MA), with PAX5 single guide RNA (10 μM final; target sequence ATCCTCTGGCGGACTACATC; Thermo Fisher) in kit buffer. Cells and the ribonucleoprotein complex were electroporated in 100 μL cuvettes using the preset program DN-100. For knockdown, 1 nmol PAX5 Silencer siRNA #s10064 or siRNA negative control #AM4611 (Invitrogen) was electroporated using the preset program DS-104.
For PAX5 overexpression, the PAX5 plasmid #SC304450 or the pCMV6-AC vector (OriGene, Rockville, MD) was transformed into DH5alpha Escherichia coli cells (OriGene), inoculated overnight, and streaked on lysogeny broth/ampicillin agar plates. Isolated clones were inoculated, followed by plasmid isolation using the Plasmid Midi Kit (Qiagen). Electroporation of 2×106 cells resuspended in buffer R and 25 μg plasmid DNA was performed with 3 pulses at 1400V for 10 milliseconds using a Neon Tip system (Thermo Fisher).
STAT5 time resolved-FRET
STAT5 was detected using the STAT5 (Total) and STAT5 (Phospho-Tyr694/699) TR-FRET Assay Kit #500217 (Cayman, Ann Arbor, MI) following the manufacturer’s instructions. Time-resolved fluorescence energy transfer (TR-FRET) was measured using a Synergy Neo2 plate reader. The results were reported as phos-STAT5 normalized to the total STAT5 detected on a parallel plate.
Reversion of the CD79B Y196H mutation to WT state in TMD8 cells
The CD79B Y196H allele reverted to its wild-type (WT) state in TMD8 cells using CRISPR-mediated gene editing. A 19 base pair synthetic CRISPR RNA was designed to target the CD79B locus with a protospacer adjacent motif site proximal to the Y196 codon (sequence GAGGAAGAUCACACCCACG). A 120 base pair single-stranded oligonucleotide donor repair template carrying the WT Y196 codon (TAT), a silent mutation (C>A) to enable restriction digestion detection, and mutated protospacer adjacent motif were designed (ACTCTGATCT CCATCCCTCT CCGCCCCCAG GATGACAGCA AGGCTGGCAT GGAGGAAGAT CACACATATG AAGTAAGGAG AGGGGCAGGC CCAGCAGCTC TGAGTCCTCG GGGTCAGTGG CCACTATCTG CTGGTGTGGT). TMD8 cells were coelectroporated with CRISPR RNA, tracerRNA, and recombinant Cas9 protein along with the repair template. The resultant pool of cells was subsequently single-cell cloned. Clones were selected, genomic DNA was extracted, and the mutation status was analyzed via tracking of indels by decomposition (TIDE) analysis.
Cas3/7 activity
Cells (10 000 cells per well) were lysed before the detection by adding equal amounts of the Caspase-Glo 3/7 Assay System (Promega) for 30 minutes, per the manufacturer’s protocol. The luminescence was measured using a Synergy Neo2 plate reader.
Superoxide detection by flow cytometry
Superoxide was detected using the ROS-ID Total ROS/Superoxide kit (Enzo, Farmingdale, NY), as previously described.22 Signal was detected using a MACSQuant MQ10 (Miltenyi Biotec, Bergisch Gladbach, Germany) flow cytometer. Analysis and gating were performed using the FlowJo software (BD Biosciences, San Jose, CA).
Cytokine detection
Cytokines were measured as previously described,23 using the MSD cytokine/chemokine kit (K1506L-2, Meso Scale Discovery, Rockville, MD). Xenograft tumor lysates were applied to MSD plates and read using MSD SECTOR Imager 2400. Macrophage colony-stimulating factor (M-CSF) was measured in lysed xenograft tumors using the SimpleStep ELISA kit ab245714 (Abcam, Waltham, MA) per the manufacturer’s instructions and read on a Synergy Neo2 plate reader.
Statistical data analyses
Statistical analyses were performed using 2-sided paired-sample t tests, unless otherwise indicated. Prism 8 (GraphPad Software, La Jolla, CA) was used to calculate significance at P < .05. For RNA-sequencing experiments, adjusted P < .05 and a fold-change threshold ≥1.5 or ≤−1.5 were calculated using the Onramp 3.35.11.0 software (Rosalind).
Results
AZD5153 and acalabrutinib treatment demonstrates a combination benefit in ABC-DLBCL models
We evaluated acalabrutinib-based drug combinations to improve efficacy in DLBCL based on recent BTK inhibitor clinical trial results in DLBCL.4 AZD5153 is in clinical trials and has monotherapy activity against DLBCL cells.9 Boi et al had previously shown that BET inhibitor OTX015 and ibrutinib was synergistic in TMD8 and U2932 cells,24 and Ceribelli et al demonstrated JQ1 (BETi) and ibrutinib 96-hour treatment of TMD8 and OCI-Ly10 cells led to synergistic toxicity.25 A synergistic response was also explored between the BET inhibitor CPI203 and ibrutinib in a TMD8 xenograft model.25 Here, we evaluated the combination of acalabrutinib and AZD5153 across a wide range of DLBCL cell lines and PDX models.
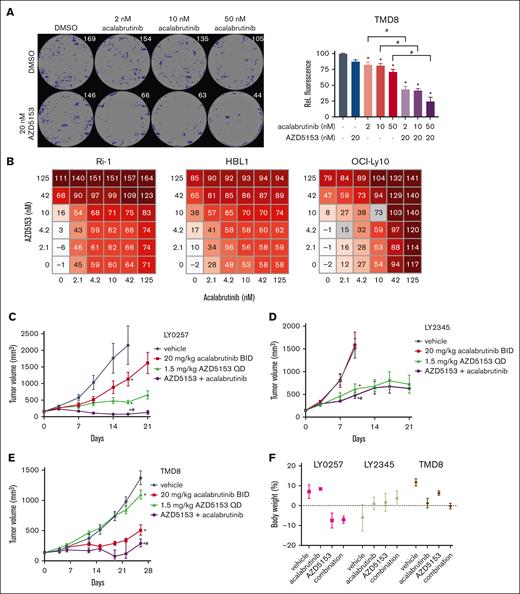
Cell lines originating from the ABC subtype were more sensitive to the AZD5153 and acalabrutinib combination than those from the GCB-DLBCL subtype. In a long-term colony formation assay, the ABC-DLBCL cell lines TMD8, HBL1, and Ri-1 showed less colony formation after treatment with the drug combination than the GCB-DLBCL OCI-Ly-19 and Farage cell lines (Figure 1A; supplemental Figure 1A-B). ABC-DLBCL cell lines HBL1, Ri-1, and OCI-Ly10 were also more sensitive to the highest single agent matrixes for both combination and monotherapy treatment compared with the 4 GCB-DLBCL cell lines (Figure 1B; supplemental Figure 1C), and the combination in ABC-DLBCL cells was synergistic (Loewe score ≥ 2.5; supplemental Figure 1D). To confirm the increased ABC-DLBCL sensitivity, we assessed AZD5153 and acalabrutinb combination in a larger panel of 15 DLBCL cell lines (supplemental Table 3). The GI50 values of ABC-DLBCL were lower than those of GCB-DLBCL cells based on 1:5 fixed-ratio doses of AZD5153 and acalabrutinib. Overall, we confirmed the synergistic toxicities across ABC-DLBCL cells and determined that nanomolar doses of AZD5153 and acalabrutinib were more cytotoxic in several ABC-DLBCL cells than in GCB-DLBCL cells.
Effect of AZD5153 and acalabrutinib combination treatment on ABC-DLBCL. (A) TMD8 colony formation assay with methylcellulose after 14-day dosing. (Left) Representative well images for each dose with artificially labeled colonies (blue). (Right) Quantification of colonies via Alamar blue staining. Error bars the standard error of the mean (SEM) of 4 wells per cell line. (B) Cell viability scoring (0-200) across multiple doses of both drugs for 5 days in ABC-DLBCL cell lines. (C) LY0257 ABC-DLBCL PDX and (D) LY2345 GCB-DLBCL PDX models dosed with 1.5 mg/kg AZD5153 and 20 mg/kg acalabrutinib for 21 days. (E) TMD8 cell line xenograft dosed with 0.15 mg/kg AZD5153 and 20 mg/kg acalabrutinib for 26 days. (F) Body weight changes on the last day of observation. Error bars represent the SEM; n = 5 mice per group. ∗ and # denote P < .05 for comparison between the control and acalabrutinib, respectively. DMSO, dimethyl sulfoxide.
Effect of AZD5153 and acalabrutinib combination treatment on ABC-DLBCL. (A) TMD8 colony formation assay with methylcellulose after 14-day dosing. (Left) Representative well images for each dose with artificially labeled colonies (blue). (Right) Quantification of colonies via Alamar blue staining. Error bars the standard error of the mean (SEM) of 4 wells per cell line. (B) Cell viability scoring (0-200) across multiple doses of both drugs for 5 days in ABC-DLBCL cell lines. (C) LY0257 ABC-DLBCL PDX and (D) LY2345 GCB-DLBCL PDX models dosed with 1.5 mg/kg AZD5153 and 20 mg/kg acalabrutinib for 21 days. (E) TMD8 cell line xenograft dosed with 0.15 mg/kg AZD5153 and 20 mg/kg acalabrutinib for 26 days. (F) Body weight changes on the last day of observation. Error bars represent the SEM; n = 5 mice per group. ∗ and # denote P < .05 for comparison between the control and acalabrutinib, respectively. DMSO, dimethyl sulfoxide.
The in vitro efficacy results were translated into multiple PDX DLBCL models. LY0257 (ABC-DLBCL) and LY2345 (GCB-DLBCL) models were dosed with a 1.5 mg/kg AZD5153 and 20 mg/kg acalabrutinib combination. The LY0257 model had a combination benefit with a TGI value of 97% (Figure 1C), whereas the drug combination in LY2345 achieved a lower TGI value of 74% (Figure 1D). In a TMD8 (ABC) xenograft model, we found that a low dose of 0.15 mg/kg AZD5153 combined with 20 mg/kg acalabrutinib enhanced TGI to 80% on day 26 of dosing (compared with 63% for acalabrutinib monotherapy; P = .022 for difference; Figure 1E). This was consistent with a previous TMD8 xenograft study that used CPI203 as a BET inhibitor and ibrutinib as a BTK inhibitor, which demonstrated a combination benefit in preventing tumor growth for 12 days.25 The combination regimen was well tolerated, and the body weight did not change >20% during these in vivo studies (Figure 1F). Additional PDX studies with LY6934 (ABC) and LY2214 (GBC) models resulted in 98% TGI (combination with 0.5 mg/kg AZD5153) and 90% TGI (combination with 1.5 mg/kg AZD5153), respectively (supplemental Figure 2A-B). The LY6934 model demonstrated a combination benefit, but the results for LY2214 were equivocal based on a robust AZD5153 monotherapy response. Unclassified DLBCL PDX models LY6933 and LY3604 also demonstrated a combination benefit from AZD5153/acalabrutinib but varied in the TGI responses of 96% and 65%, respectively (supplemental Figure 2B). Frequent non-Hodgkin lymphoma mutations reported in these PDX models (HuBase PDX database, Crown Bioscience) may contribute to AZD5153 and acalabrutinib sensitivity (supplemental Figure 2C). LY2345 has a CARD11 A647V mutation and ibrutinib resistance. LY2214 contains an MYC translocation that could regulate AZD5153 sensitivity. LY6933 and TMD8 harbor CD79B mutations of L180C and Y196H, respectively, which may alter the efficacy of AZD5153 and acalabrutinib. Taken together, these data suggest that the AZD5153 and acalabrutinib drug combination is more effective in PDX models of ABC-DLBCL than in PDX models of GCB-DLBCL. Consistent with the previous findings for these drug classes, we observed strong synergistic interactions between the 2 therapies. Next, we used TMD8 xenografts to explore the combination mechanism of AZD5153 and acalabrutinib.
PAX5 signaling is interrupted by AZD5153 and acalabrutinib combination dosing in ABC-DLBCL
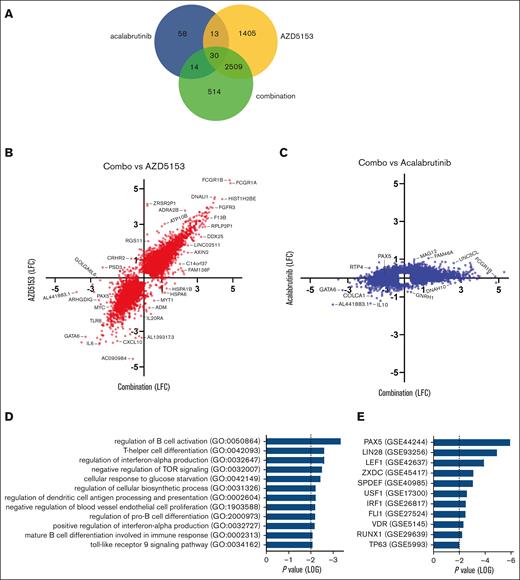
The mechanistic knowledge of BTK/BET inhibitors combined with ABC-DLBCL is limited and, to the best of our knowledge, no genomic studies on this combination for DLBCL have been reported. It was previously reported that the NF-κB inhibitory factor IκBα increases in TMD8 cells after treatment with JQ1/ibrutinib.25 Critical factors targeted by acalabrutinib/AZD5153 were identified using RNA sequencing as a comprehensive approach in TMD8 xenografts. Tumor tissue after treatment in mice with 0.72 mg/kg AZD5153 and 20 mg/kg acalabrutinib was sequenced at a 4-hour time point to enrich for primary transcriptome changes and transcription factors associated with BRD4.7,9,26 Most gene changes upon combination treatment were driven by AZD5153 dosing (Figure 2A-C). Differential expression pathway analysis of genes exclusive to the combination treatment indicated a significant alteration in B-cell activation/differentiation, interferon production, and toll-like receptor signaling (Figure 2D; supplemental Figure 3; supplemental Table 4). It was previously reported that the combination of JQ1 and ibrutinib altered NF-κB activation based on an overlap of monotherapy mechanisms,25 and we found that AZD5153 and acalabrutinib dosing decreased the expression of RELB in the alternative NF-κB pathway (supplemental Figure 3). The PAX5 transcription factor network was indicated as the top change for differential expression, as well as LEF1 (Figure 2E), a direct DNA binding target for PAX527 (supplemental Figure 3B). PAX5 is highly expressed in non-Hodgkin lymphoma cell lines and patient samples and shows codependency with BCR genes, such as SYK (supplemental Figure 4).
PAX transcriptional network is interrupted by AZD5153 and acalabrutinib in TMD8 tumor models. RNA-sequencing data generated from tumors extracted after 4 hours of drug dosing, n = 3 mice per group. (A) Venn diagram of genes with significant differential expression between treatment and vehicle. (B-C) Log fold-change (LFC) values for combination differential expression (x-axis) compared with (B) 0.72 mg/kg AZD5153 or C 20 mg/kg acalabrutinib (y-axis). All the genes were significant (P < .05) after at least 1 treatment and a 1.5-fold–change threshold was applied. (D-E) Pathway analysis of 514 differentially expressed genes exclusive of the combination treatment. (D) GO Biological and (E) Perturbed Transcription Factors databases. Statistical P values < 0.05 were considered significant.
PAX transcriptional network is interrupted by AZD5153 and acalabrutinib in TMD8 tumor models. RNA-sequencing data generated from tumors extracted after 4 hours of drug dosing, n = 3 mice per group. (A) Venn diagram of genes with significant differential expression between treatment and vehicle. (B-C) Log fold-change (LFC) values for combination differential expression (x-axis) compared with (B) 0.72 mg/kg AZD5153 or C 20 mg/kg acalabrutinib (y-axis). All the genes were significant (P < .05) after at least 1 treatment and a 1.5-fold–change threshold was applied. (D-E) Pathway analysis of 514 differentially expressed genes exclusive of the combination treatment. (D) GO Biological and (E) Perturbed Transcription Factors databases. Statistical P values < 0.05 were considered significant.
We validated that AZD5153 reduced PAX5 protein levels (Figure 3A; supplemental Figure 5A). Levels of RELB, which regulates PAX5 transcriptional activity in B cells28 and TLR3 proteins, was also decreased by AZD5153 and the combination treatment (Figure 3A). The combination of AZD5153 and acalabrutinib further limited the protein expression of PAX5-binding targets LEF1, EBF1, and SPI-B in ABC-DLBCL cells (Figure 3B; supplemental Figure 5B). In OCI-Ly19 cells (GCB), there was no decrease in PAX5, EBF1, and SPI-B protein levels after combination treatment (cellular Myc was used as control for AZD5153; supplemental Figure 5B). As expected for ABC-DLBCL cells, nuclear PAX5 and LEF1 levels were also decreased (Figure 3C; supplemental Figure 5C-D), which correlates with reduced transcriptional activity.29 In GCB-DLBCL OCI-Ly19 and Farage cells, there were limited changes to PAX5 and related proteins after AZD5153 and acalabrutinib (supplemental Figure 4B-D). Because AZD5153 induced a decrease in PAX5 messenger (mRNA), we used a PAX5 knockout model to confirm that the decrease in PAX5 expression could enhance the BTK inhibitor response. TMD8 cells electroporated with PAX5 single guide RNA were treated with acalabrutinib, and these cells were more sensitive to acalabrutinib than the nontargeting control (NTC) controls (Figure 3D); however, this was not observed in Raji lymphoma cells (supplemental Figure 5E). Likewise, Ri-1 cells with PAX5 siRNA knockdown also enhanced acalabrutinib activity (supplemental Figure 5F). These data suggest that AZD5153 and its combination with acalabrutinib decreased the expression of PAX5 and its downstream binding targets in ABC-DLBCL, but these drugs had only limited effects in GCB-DLBCL cells.
Protein validation of top differential expression results. (A) PAX5, TLR3, and RELB/p100 blots after 72-hour AZD5153 and acalabrutinib treatment. (B) Proteins of the PAX5 transcription factor direct binding targets after overnight drug treatment. (C) Representative images of PAX5 immunofluorescence (top) and quantification of nuclei detection of PAX5 and LEF1 (bottom) after a 72 hour treatment with 20 nM AZD5153, 10 nM acalabrutinib, or a combination. (D) PAX5 knockout in TMD8 cells. Cell viability was measured using CellTiterGlo after 7-day treatment with acalabrutinib. Error bars represent SEM; n = 6. (E) Immunoblot of PAX5 and related proteins from LY6934 tumor lysates after 5-day treatment. PAX5 quantification is normalized to GAPDH. ∗, #, and ## denote P < .05 for comparisons of the control, acalabrutinib, and AZD5153, respectively.
Protein validation of top differential expression results. (A) PAX5, TLR3, and RELB/p100 blots after 72-hour AZD5153 and acalabrutinib treatment. (B) Proteins of the PAX5 transcription factor direct binding targets after overnight drug treatment. (C) Representative images of PAX5 immunofluorescence (top) and quantification of nuclei detection of PAX5 and LEF1 (bottom) after a 72 hour treatment with 20 nM AZD5153, 10 nM acalabrutinib, or a combination. (D) PAX5 knockout in TMD8 cells. Cell viability was measured using CellTiterGlo after 7-day treatment with acalabrutinib. Error bars represent SEM; n = 6. (E) Immunoblot of PAX5 and related proteins from LY6934 tumor lysates after 5-day treatment. PAX5 quantification is normalized to GAPDH. ∗, #, and ## denote P < .05 for comparisons of the control, acalabrutinib, and AZD5153, respectively.
We further validated that AZD5153/acalabrutinib decreased PAX5 in PDX tumors. LY6934 tumors from the 25-day AZD5153/acalabrutinib efficacy study (supplemental Figure 2A) were analyzed via RNA sequencing (supplemental Figure 6A) and had 1162 significantly altered genes in common with AZD5153/acalabrutinib TMD8 xenografts (Figure 2). Pathway analysis from this 1162 gene data set indicated PAX5 and NF-κB2 as the top perturbed transcription factor networks (supplemental Figure 6B). The differential expression results between the data sets of these 2 models included many PAX5 direct binding targets, such as LEF1, IKZF3, NEDD9, and SIT1, (supplemental Figure 6C) and STAT5 signaling (supplemental Figure 6D). LEF1 is associated with lymphoma development,30 IKZF3 regulates B-cell proliferation/differentiation,31 NEDD9 controls B-cell trafficking,27 and SIT1 is an adapter protein for SHP2 phosphatase.32 PAX5 protein was decreased in LY6934 tumor lysates after 5-day AZD5153/acalabrutinib dosing and LEF1 protein was decreased to undetectable levels after 1.5 mg/kg AZD5153 with acalabrutinib (Figure 3E). Overall, these results confirm that the PAX5 transcription network is disrupted by the AZD5153 and acalabrutinib combination in ABC-DLBCL.
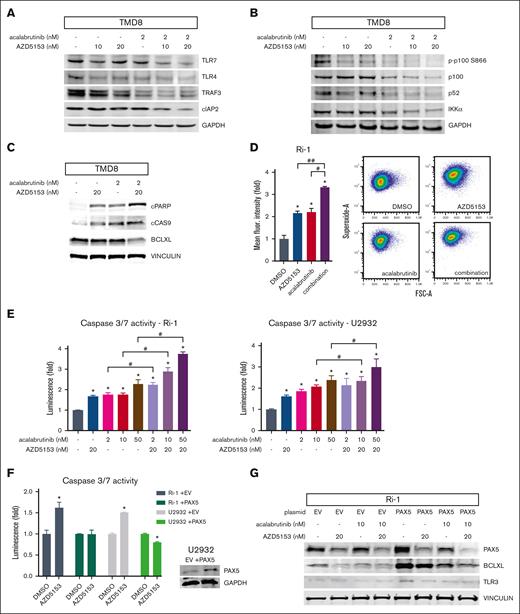
TLR pathways can support BTK-independent BCR signaling and engage the downstream type I interferon response through TRAF3 and RELB/alternative NF-κB signaling.33,34 The RELB/p52 complex is a transcriptional activator of several prosurvival and antiapoptotic genes. The AZD5153 and acalabrutinib combination decreased TLR3/4/7, TRAF3, cIAP2, RELB, and p100/52 in TMD8 but not in OCI-Ly19 (GCB) cells (Figures 2A and 4A-B; supplemental Figure 7A). BTK inhibitors and AZD5153 have individually been shown to induce apoptosis in ABC-DLBCL cell lines.9,12,35 This correlates with an increase in cleaved poly (ADP-ribose) polymerase (PARP) and cleaved Cas9 (Figure 4C), suggesting that AZD5153 acalabrutinib are involved in the intrinsic apoptosis pathway. The expression of antiapoptotic factor Bcl-XL, which is transcriptionally activated by PAX5, was decreased by the drug combination (Figure 4C). Increased cellular superoxide can indicate mitochondrial dysfunction and cellular stress, which is consistent with the induction of intrinsic apoptosis. AZD5153 and acalabrutinib-induced superoxide and Cas3/7 activity, which was enhanced by the drug combination (Figure 4D-E; supplemental Figure 7B). Ectopic overexpression of PAX5 decreased AZD5153-induced Cas3/7 activity (Figure 4F). Elevated PAX5 also increased TLR3 and Bcl-XL, whereas PAX5 knockdown decreased these protein expressions (Figure 4G; supplemental Figure 7C-D). These results suggest that AZD5153 and acalabrutinib combination promotes apoptosis through the intrinsic pathway, with PAX5, TLR3/4/7, and RELB signaling as major contributing factors.
AZD5153 enhances acalabrutinib-induced apoptosis. (A) Type 1 interferon response pathway protein detection of TLR4, TLR7, TRAF3, and cIAP2. (B) Alternative NF-κB pathway protein detection of p100 (including phosphorylation), p52, and IKKα. (C) Intrinsic apoptosis-related protein detection of cleaved PARP (cPARP), cleaved Cas9 (cCAS9), and Bcl-XL. (D) Superoxide detection after treatment with 20 nM AZD5153, 50 nM acalabrutinib, or a combination of both. Error bars represent SEM; n = 3. Representative histogram (middle) and pseudocolor density plots (right). (E) Cas3/7 activity normalized to CellTiter-Glo cell viability. Error bars represent SEM; n = 3. (F) Cas3/7 activity after AZD5153 treatment in cells overexpressing PAX5. Error bars represent SEM, n = 3. ∗, #, and ## denote P < .05 for comparisons of the control, acalabrutinib, and AZD5153, respectively.
AZD5153 enhances acalabrutinib-induced apoptosis. (A) Type 1 interferon response pathway protein detection of TLR4, TLR7, TRAF3, and cIAP2. (B) Alternative NF-κB pathway protein detection of p100 (including phosphorylation), p52, and IKKα. (C) Intrinsic apoptosis-related protein detection of cleaved PARP (cPARP), cleaved Cas9 (cCAS9), and Bcl-XL. (D) Superoxide detection after treatment with 20 nM AZD5153, 50 nM acalabrutinib, or a combination of both. Error bars represent SEM; n = 3. Representative histogram (middle) and pseudocolor density plots (right). (E) Cas3/7 activity normalized to CellTiter-Glo cell viability. Error bars represent SEM; n = 3. (F) Cas3/7 activity after AZD5153 treatment in cells overexpressing PAX5. Error bars represent SEM, n = 3. ∗, #, and ## denote P < .05 for comparisons of the control, acalabrutinib, and AZD5153, respectively.
Disruption of BCR signaling inhibits PAX5 activation
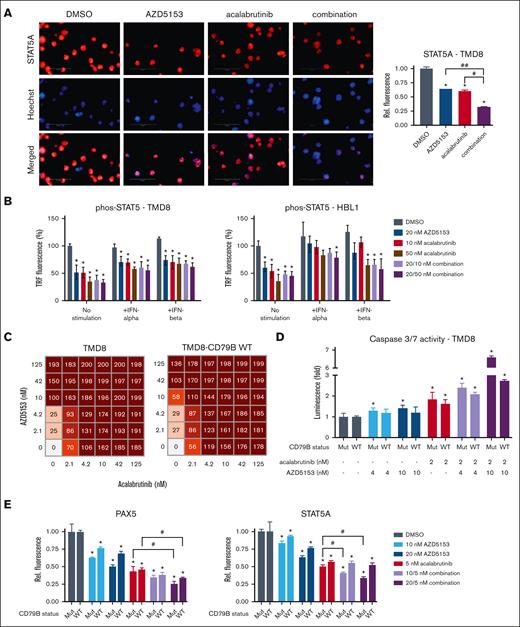
We hypothesized that the downstream targets of PAX5 may be regulated by BCR signaling. PAX5 nuclear translocation and its corresponding activity can be regulated by STAT5,29 where STAT5 Tyr694/699 phosphorylation induces translocation and PAX5 stimulation.36 We found that acalabrutinib can reduce STAT5A in the nuclei (Figure 5A; supplemental Figure 8A), which is consistent with BCR induction of the JAK/STAT pathway. Acalabrutinib also decreased phospho-STAT5 levels, and this reduction could be partially rescued by interferon-alfa (IFNα) or IFNβ (Figure 5B; supplemental Figure 8B). Moreover, AZD5153 caused a reduction in phospho-STAT5 levels, particularly in TMD8 and HBL1 cell lines with a CD79B mutation (Figure 5B). The AZD5153-induced downregulation of PAX5 expression, which transcriptionally regulates CD79B, may be contributing to the reduction in phospho-STAT5 levels.
Inhibition of BCR prevents STAT5 activation in ABC-DLBCL. (A) Representative images of STAT5A immunofluorescence (left) and quantification for nuclei detection (right) after treatment with 20 nM AZD5153, 10 nM acalabrutinib, or a combination. n = 3. (B) TR-FRET detection of phos-STAT5 with stimulation by 0.1 ng/mL IFNα or 1 ng/mL IFNβ. n = 6 (C) Cell viability scoring (0-200) and (D) Cas3/7 activity across multiple doses of both drugs in TMD8 and TMD8-CD79B WT cell lines. n = 6. € PAX5 and STAT5A immunofluorescence quantification for nuclei detection comparing TMD8 (Mut) and TMD8-CD79B WT cell lines. Error bars represent SEM. ∗, #, and ## denote P < .05 for comparison of the control, acalabrutinib, and AZD5153, respectively.
Inhibition of BCR prevents STAT5 activation in ABC-DLBCL. (A) Representative images of STAT5A immunofluorescence (left) and quantification for nuclei detection (right) after treatment with 20 nM AZD5153, 10 nM acalabrutinib, or a combination. n = 3. (B) TR-FRET detection of phos-STAT5 with stimulation by 0.1 ng/mL IFNα or 1 ng/mL IFNβ. n = 6 (C) Cell viability scoring (0-200) and (D) Cas3/7 activity across multiple doses of both drugs in TMD8 and TMD8-CD79B WT cell lines. n = 6. € PAX5 and STAT5A immunofluorescence quantification for nuclei detection comparing TMD8 (Mut) and TMD8-CD79B WT cell lines. Error bars represent SEM. ∗, #, and ## denote P < .05 for comparison of the control, acalabrutinib, and AZD5153, respectively.
CD79 is the transmembrane base of the BCR, and CD79B mutations can enhance BCR signaling.3 CD79B mutations are frequent in patients with DLBCL (13.51% mutation rate in DLBCL TGCA samples; accessed September 202237,38). TMD8 cells have a CD79B Y196H allele gain-of-function mutation. We had the Y196H mutation in TMD8 cells revert to a Y196 WT allele (supplemental Figure 8C) to assess the extent to which this CD79B mutation altered AZD5153 and acalabrutinib/AZD5153 responses. TMD8 CD79B-WT cells showed reduced cell death sensitivity and Cas3/7 activation upon AZD5153 treatment (Figure 5C-D). The 10 nM AZD5153 and 2 nM acalabrutinib combination also led to a marked reduction in Cas3/7 induction in TMD8 CD79B-WT cells compared with unchanged TMD8 cells (CD79B mutant). PAX5, STAT5, and LEF1 protein decreases induced by AZD5153 were greater for unchanged TMD8 cells than for TMD8 CD79B-WT cells (for 20 nM AZD5153, mutant vs WT: PAX5 -49.7 vs −31.2%, STAT5A −36.9 vs 23.5%, and LEF1 −0.51 vs −31.5%; Figure 5E; supplemental Figure 8F). Immunofluorescence results for AZD5153 in TMD8 CD79B-WT cells were similar to those in Ri-1 cells without a CD79B mutation (PAX5: −28.6%, STAT5A: −26.1%, and LEF1: −39.3%; supplemental Figure 7D-F). Ri-1 cells have a CD79A copy number variation (CNV), and HBL1 cells have a heterogenous Y196F CD79B mutation.3,39 It is interesting that Ri-1 and HBL1 were less sensitive to AZD5153 for the reduction of nuclear PAX5/LEF1/STAT5A, but the AZD5153/acalabrutinib combination significantly reduced these proteins in the nucleus. To assess whether the CD79B-WT reversion alters the dependency on IRAK1/4 signaling, we used the tool compound AZ1495, which has been demonstrated to enhance acalabrutinib cytotoxicity in DLBCL.40 We found a modest increase in AZ1495 sensitivity for the CD79B-WT cells (supplemental Figure 8G), which suggests a minor shift toward a dependence on the IRAK1/4 pathway in cells with WT CD79B. Overall, the results from our TMD8 isogenic pairs suggest that CD79B mutations can be more sensitive to AZD5153 and the combination with acalabrutinib than cells with WT CD79B, but ABC-DLBCL cells without this mutation still show a combination-induced reduction in nuclear PAX5/LEF1/STAT5A levels.
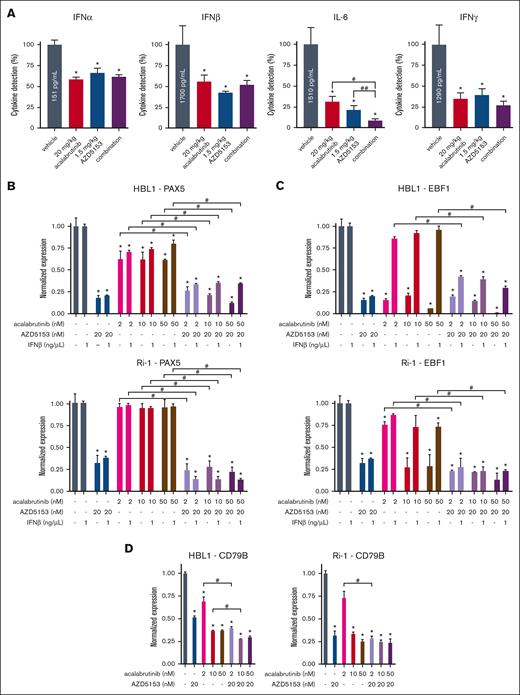
To understand the link between BCR signaling and STAT5 activation, we examined the cytokines that were decreased by AZD5153 and acalabrutinib. We focused on type 1 interferons indicated by RNA-sequencing data as well as cytokines regulated by alternative NF-κB/RELB/TLR pathways. Interleukin-6 (IL-6; IFNβ2) was included, whose expression is reduced by AZD5153.41 IFNs IFNα2, IFNβ1, IFNβ2 (IL-6), and IFNγ (Figure 6A) as well as the chemokine IP-10 (activator of IFNγ) and cytokine M-CSF (supplemental Figure 9A) had decreased expressionsin TMD8 tumors after 5-day treatment with AZD5153 and acalabrutinib. IL-6 promoted survival in DLBCL and 2 ng/mL recombinant IL-6 significantly inhibited the AZD5153 response but had a limited effect on acalabrutinib cytotoxicity (supplemental Figure 9B-C). As a type 1 interferon, IFNβ1 level was decreased by acalabrutinib as well as AZD5153, and we sought to determine whether this cytokine could regulate PAX5 signaling in vitro by using EBF1 mRNA as a marker for PAX5 activation. AZD5153 induced a decrease in PAX5 expression as expected (Figure 6B; supplemental Figure 9D), and EBF1 could be decreased by acalabrutinib or the combination with AZD5153 (Figure 6C; supplemental Figure 9E). The addition of IFNβ1 prevented the acalabrutinib-induced reduction in EBF1 mRNA. Levels of other PAX5 targets, LEF1 and CD79B, were also decreased by the drug combination (Figure 6D; supplemental Figure 9F-H). Taken together, these data confirm that disrupting BCR signaling in ABC-DLBCL cells attenuates interferons, which can then regulate the STAT5/PAX5 control of BCR/BTK signaling.
Tumor interferon and PAX5 transcriptional network validation. (A) Cytokines IFNα2, IFNβ1, IL-6 (IFNβ2), and IFNγ measured via the meso scale discovery assay using TMD8 tumor lysates after 5-day treatment. Error bars represent SEM, n = 4. (B-D) ABC-DLBCL cell mRNA expression after 24-hour drug treatment with or without IFNβ1 stimulation for (B) PAX5, (C) EBF1, and (D) CD79B. Error bars represent SEM, n = 5. ∗, #, and ## denote P < .05 for comparison of the control, acalabrutinib, and AZD5153, respectively.
Tumor interferon and PAX5 transcriptional network validation. (A) Cytokines IFNα2, IFNβ1, IL-6 (IFNβ2), and IFNγ measured via the meso scale discovery assay using TMD8 tumor lysates after 5-day treatment. Error bars represent SEM, n = 4. (B-D) ABC-DLBCL cell mRNA expression after 24-hour drug treatment with or without IFNβ1 stimulation for (B) PAX5, (C) EBF1, and (D) CD79B. Error bars represent SEM, n = 5. ∗, #, and ## denote P < .05 for comparison of the control, acalabrutinib, and AZD5153, respectively.
Discussion
The BCR pathway is a tumor driver for ABC-DLBCL and is targeted by BTK inhibitors. These inhibitors are more effective in patients with ABC than those with GCB; however, BTK inhibitors do not achieve robust response rates in DLBCL. For patients with R/R DLBCL, acalabrutinib had an ORR of 33.0% (3/9) for ABC vs 16.7% (1/6) for GCB,4 ibrutinib had an ORR of 37% (14/38) for ABC vs 5% (1/20) for GCB,5 and tirabrutinib had an ORR of 35% (11/31) for ABC vs no response (0/2) for patients with GCB.6 These trials with BTK inhibitors have demonstrated more responses from patients with ABC-DLBCL and highlighted the need for evaluating combination regimens to further enhance efficacy with BTK inhibitors.4
We found that the BET inhibitor AZD5153 enhanced efficacy when combined with acalabrutinib in ABC-DLBCL PDX models, which was consistent with previous BET and BTK inhibitor studies on cell line models.24,25 An increase of IκBα activity from JQ1 and ibrutinib had also been reported in TMD8 cells.25 To better understand the mechanism of AZD5153 combined with acalabrutinib in ABC-DLBCL, we analyzed differential expression data from TMD8 tumors and identified the that PAX5 transcriptional network was regulated by this drug combination. We confirmed that PAX5 expression was decreased by AZD5153 and that PAX5 activation (nuclear localization and expression of targets) could be inhibited by disrupting BCR signaling. AZD5153 also disrupted BCR signaling, partly through the transcriptional dependency of CD79 on PAX5. These findings uncovered the PAX5-BCR-IFN/STAT5 positive-feedback loop, which we suggest is the underlying mechanism of constitutive BCR signaling in ABC-DLBCL.
Our differential expression data also indicated significant changes in the type 1 interferon/TLR and RELB/alternative NF-κB pathways upon AZD5153 and acalabrutinib combination treatment. Type 1 interferon/TLR signaling stimulates the RELB/alternative NF-κB pathway through TRAF3,33 and TLR signaling can be a BTK-independent mechanism for BCR signaling.34 Acalabrutinib could cause a decrease in type 1 interferons that could be rescued with additional IFNβ1. We suggest that RELB/alternative NF-κB pathway modulation may be a parallel antiapoptotic mechanism that regulates classical NF-κB activity25 thwarted by the AZD5153 and acalabrutinib combination.
CD79 mutations are frequent in ABC-DLBCL but less common in GCB-DLBCL. CD79B mutations in the immunoreceptor tyrosine-based motif (ITAM), such as Y196H in TMD8 and Y196F in HBL1, can increase BCR expression on the surface of cells yet are not required for chronic active BCR signaling.3 PAX5 regulates CD79A and CD79B transcription, which can be inhibited by AZD5153 through a reduction in PAX5 mRNA. Our results support that the ITAM mutation in TMD8 cells is a sensitizing factor for AZD5153 and AZD5153/acalabrutinib. AZD5153 induced less cell death and less Cas3/7 induction, and the inhibition of PAX5 activation was partially alleviated in CD79B-WT TMD8 compared with that in native cells. However, PAX5/LEF1/STAT5A nuclear detection of HBL1 and Ri-1 (with a CD79A CNV) cells was not reduced by AZD5153 to the same extent as in TMD8 cells, but the AZD5153 and acalabrutinib combination still led to marked nuclear protein reduction. Furthermore, the drug combination was effective in reducing nuclear PAX5/LEF1/STAT5A in OCI-Ly10 cells (CD79A and CD79B WT). TMD8 cells have a PAX5 high copy number,39 which may also influence sensitivity to AZD5153/acalabrutinib. Overall, our isogenic TMD8 experiments demonstrated that CD79B is a sensitizing factor for AZD5153; however, CD79 mutations are not required for AZD5153/acalabrutinib combination-induced inhibition of PAX5 signaling.
In summary, PAX5 is a critical transcription factor for BET inhibition combined with acalabrutinib to drive responses in ABC-DLBCL. An in-depth mechanistic investigation uncovered a BCR signaling positive-feedback loop through PAX5. We found that AZD5153 reduced PAX5 expression and attenuated BCR signaling by regulating CD79 among other key factors. Disruption of BCR signaling with acalabrutinib and AZD5153 prevented PAX5 activation. Our data indicated that this mechanism may also be present in GCB-DLBCL; however, the drug combination has less efficacy and requires higher concentrations to induce a response. This suggests that the combination of BET and BTK inhibitors may require a dependency on high PAX5 expression and BCR signaling for their effectiveness, which is more frequent in ABC-DLBCL. Overall, targeting the STAT5/PAX5 component of constitutive BCR signaling is an approach to further enhance the therapeutic impact of BTK inhibitors in ABC-DLBCL.
Acknowledgments
The authors thank Caroline Fawcett for assistance with imaging, Alan Rosen for assistance with assay automation, Bethany Kaplan for assistance with methylcellulose colony formation assays, Brandon Willis for assistance with mouse study designs, and Daniel Karl for submitting data to the Gene Expression Omnibus database. This research was funded by AstraZeneca.
Authorship
Contribution: D.B.O. designed and performed the experiments, analyzed the data, and wrote the initial manuscript. S.S. performed the cytokine assays and analyzed the data; M.M.H. and M.D. set up, executed, and oversaw the mouse model experiments; S.W.C. oversaw the RNA-sequencing experiments and data processing; L.P. assisted in the design, setup, and data processing for flow cytometry assays; A.U.G. generated and validated the TMD8 CD79B reversion to WT cells; Y.Y., J.Z., L.D., and H.C. supervised the project and contributed to the experimental design and data analyses; and all authors edited and reviewed the manuscript.
Conflict-of-interest disclosure: All authors were employees of AstraZeneca at the time of this study and have held or currently hold stock in AstraZeneca.
The current affiliation for A.U.G. is AstraZeneca, Oncology R&D, Precision Medicine and Biosamples, Diagnostic Development Unit, Cambridge, United Kingdom.
The current affiliation for Y.Y. is HotSpot Therapeutics, Cambridge, MA.
Correspondence: Derek B. Oien, Early Oncology, AstraZeneca, 35 Gatehouse Dr, Waltham, MA 02451; e-mail: derek.oien@astrazeneca.com; and Ho Man Chan, Early Oncology, AstraZeneca, 35 Gatehouse Dr, Waltham, MA 02451; e-mail: homan.chan@astrazeneca.com.
References
Author notes
All RNA-sequencing data reported in this article have been deposited in the NCBI Gene Expression Omnibus database (accession number GSE211549).
Data are available on request from the corresponding authors, Derek B. Oien (derek.oien@astrazeneca.com) and Ho Man Chan (homan.chan@astrazeneca.com).
The full-text version of this article contains a data supplement.