Key Points
The SALENTO model identified a low-risk group with a favorable prognosis with current therapy and a high-risk group with poor outcomes.
The SALENTO model is specific for limited-stage PTCL and has greater discriminatory power than established prognostic indices.
Abstract
The natural history of limited-stage peripheral T-cell lymphomas (PTCLs) remains poorly defined. We investigated outcomes and prognostic variables in patients registered in the T-Cell Project (TCP) (#NCT01142674) to develop a model to predict overall survival (OS) for the common nodal PTCL subtypes (PTCL-NOS, AITL, ALCL). The model was validated in an independent data set from Australian and Brazilian registries. 211 patients registered in the TCP between 2006-2018 were studied. The median age was 59 years (range 18-88) and median follow-up was 49 months. One hundred twenty-seven patients (78%) received anthracycline-based regimens, 5 patients (3%) radiotherapy alone (RT), 24 patients (15%) chemotherapy+RT. 5-year OS and PFS were 47% and 37%, respectively. Age >60 years, elevated LDH and low serum albumin were independent prognostic factors. The model identified 3 groups with low- (26%, score 0), intermediate- (41%, score 1), and high-risk (33%, score 2-3) with 5-year OS of 78% (95% confidence interval [95% CI], 29-127), 46% (95% CI, 24-68), and 25% (95% CI, 20-30), respectively (P < 0.001) and 5-year PFS of 66% (95% CI, 33-99), 37% (95% CI, 9-65), and 17% (95% CI, 9-25), respectively (P < 0.001). The model demonstrated greater discriminatory power than established prognostic indices and an analogous distribution and outcomes in the 3 groups in the validation cohort of 103 patients. The SALENTO Model (Limited Stage Peripheral T-Cell Lymphoma Prognostic Model) is an objective, simple and robust prognostic tool. The high-risk group has poor outcomes, comparable to advanced stage disease, and should be considered for innovative first-line approaches.
Introduction
Peripheral T-cell lymphomas (PTCLs) represent a heterogeneous disease group, accounting for 10% of non-Hodgkin lymphoma.1,2 At the time of diagnosis, most patients with PTCL present with advanced-stage disease; however, ∼25% to 30% present with stage I or II disease.1,3-6 Unsurprisingly, the natural history and outcomes of patients with limited-stage PTCL are poorly understood. Prior studies have demonstrated inferior outcomes for patients with limited-stage PTCL compared with those with limited-stage Hodgkin lymphoma or diffuse large B-cell lymphoma; however, a proportion of patients with limited-stage PTCL are cured with standard therapy.7,8
Traditionally, the treatment for PTCLs was extrapolated from clinical trials for diffuse large B-cell lymphoma; however, the results were poor, with inadequate responses to standard first-line treatment of 6 cycles of anthracycline-based regimens, such as cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone.5,9-13 In contrast to patients with Hodgkin lymphoma, diffuse large B-cell lymphoma, or follicular lymphoma, patients with limited-stage PTCLs are treated with combination chemotherapy, and remission is sometimes consolidated with high-dose chemotherapy/autologous stem cell transplantation (HDT/ASCT) or radiotherapy (RT).14,15
To better define the clinical features at presentation, management practices, and outcomes of patients with limited-stage PTCL, we identified patients with more frequent nodal subtypes (PTCL not otherwise specified [NOS], anaplastic lymphoma kinase–negative [ALK–], anaplastic large cell lymphoma [ALCL], and angioimmunoblastic T-cell lymphoma [AITL]) identified in the International T-Cell Lymphoma Project database (TCP 1). TCP 1 is a prospective registry that collected an exhaustive set of clinical data and biological information of more than 1500 patients with PTCL diagnosed and treated at 75 institutions worldwide and represents a unique opportunity to investigate and address several unmet medical needs in the management of patients with PTCL. Considering the paucity of literature, the purpose of this study was to assess the outcome of patients with limited-stage PTCL and investigate the potential prognostic factors. Here, we report the analysis performed in a cohort of 211 patients, which allowed us to build a new prognostic model specific to limited-stage PTCL.
Methods
Patients and methods
The TCP 1 (NCT01142674) began in September 2006 as a prospective registry of patients with mature PTCLs. Data collection was performed via electronic case report forms using a dedicated website with the adoption of technology ensuring the protection of a subject’s data. This study was conducted in accordance with the principles of the Declaration of Helsinki. Approval was obtained from the institutional review board at the coordinating center (Modena Cancer Center, University of Modena, and Reggio Emilia, Modena, Italy) and at each participating center per institutional standards, with all patients signing an informed consent before registration. Eligible patients were adults (aged >18 years) with adequate tissue biopsy specimen for diagnosis and available clinical data, including baseline information at disease staging, laboratory parameters at diagnosis, treatment regimens received, and follow-ups. Survival data were updated until the database was locked on 30 March 2019. For this project, we searched through TCP 1 for all patients diagnosed with PTCL-NOS, AITL, or ALK– ALCL with stage I or II disease.
Statistical analysis
The primary end point of the study was overall survival (OS) at 5 years, measured from the date of diagnosis to death from any cause or the date of last known contact, for living patients. The secondary end point was progression-free survival (PFS) at 5 years, measured from the time of diagnosis to the date of progressive disease assessment or death from any cause. The full inclusion and exclusion criteria are provided in the TCP 1 study protocol (supplemental Materials). Standard descriptive analyses were performed for the clinical and demographic end points. Fisher exact tests were used to identify associations between categorical variables. Mann-Whitney tests were used to compare the median ages between the groups. Two-tailed P values < .05 were considered statistically significant. Survival estimates were calculated using the Kaplan-Meier method, and time-to-event distributions were compared using log rank tests (univariate regression).
To develop a prospective prognostic model, we included 9 variables selected from those reported in the literature to impact the survival of PTCL-NOS, including clinical (Eastern Cooperative Oncology Group performance status [ECOG PS], B symptoms, and number of extranodal sites), biological (lactate dehydrogenase [LDH], albumin serum level, platelet count, and hemoglobin level), and demographic (age and sex) factors. The final model obtained from Cox PH regression included covariates that showed a negligible difference between log-likelihood if it was compared with the full model including all 9 covariates.
Cox models were used to investigate the association between survival outcomes and covariates, with hazard ratios used as a summary measure. Covariates identified to have an independent prognostic effect on OS were used to create a novel prognostic model (limited-stage peripheral T-cell lymphoma prognostic model; SALENTO model). An external validation cohort of patients with newly diagnosed PTCLs registered in the Australian lymphoma and related diseases registry and the Brazilian T-cell lymphoma registry was used to validate the predictive model.
The performance of the SALENTO Model was compared with the previously published prognostic indices: prognostic index for T-cell lymphoma (PIT) and16 PIT and platelets (IPTCLP).17 We used a measure of global fit (Akaine information criterion [AIC]) and concordance index (c-Harrell), with low AIC values indicating a better fit and high c-Harrell values indicating better discrimination.18 High-risk disease was defined as SALENTO ≥2; PIT ≥2, or IPTCLP ≥2, as previously reported. Statistical analyses were performed using Stata (version 14.2) and SPSS (version 20.0).
Results
Patient characteristics and treatment details
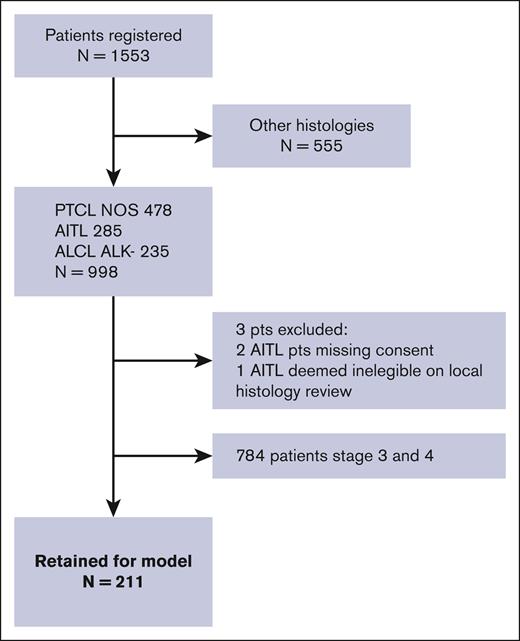
Between September 2006 and February 2018, 1553 patients were analyzed using the TCP 1 project. Of these, 998 patients were diagnosed with PTCL-NOS (n = 478; 48%), AITL (n = 282; 28%), and ALCL ALK– (n = 235; 24%). Of these, 211 (21%) were classified as having a limited-stage PTCL (stage I or II) and were considered for the analysis of prognostic factors (Figure 1; supplemental Table 1). Patient characteristics and treatment details are summarized in Table 1. Patient characteristics and treatment details for each histology are summarized in supplemental Table 1. The median age was 59 years (range, 18-88 years), 45% were male, and 51% of patients had stage I disease. Of the 211 limited-stage patients, treatment details were not available for 36 patients, and an additional 12 patients (5%) received the best supportive care. Thus, frontline treatment was available for 163 patients. Overall, 29 patients received RT in combination with chemotherapy (n = 24) or alone (n = 5). A minority (4%) of the cohort underwent HDT/ASCT as a first-line consolidation.
Baseline characteristics of patients in the training cohort (n = 211) including variables with a possible impact on survival analysis
| Factor . | N . | % . |
|---|---|---|
| Median age, y (range) | 59 (18-88) | |
| Age ≥60 y | 104 | 49 |
| Sex, male | 95 | 45 |
| B symptoms present | 73 of 202 | 36 |
| Extranodal involvement | 115 | 55 |
| ECOG PS >1 | 27 of 199 | 14 |
| LDH > ULN | 72 of 175 | 41 |
| Albumin <35 g/L | 58 of 175 | 33 |
| Hb >12g/dL | 66 of 196 | 34 |
| Platelet count <150 × 109 cells/L | 111 of 195 | 57 |
| ANC >6.5 × 103/ml | 55 of 188 | 29 |
| Histotypes | ||
| PTCL-NOS | 129 of 211 | 61 |
| AITL | 28 of 211 | 13 |
| ALCL ALK– | 54 of 211 | 26 |
| Therapy | 175 of 211 | 83 |
| Best supportive care | 12 | 7 |
| CHT alone | 127 of 163 | 78 |
| RT alone | 5 of 163 | 3 |
| CHT/RT | 24 of 163 | 15 |
| CHT and HDT/ASCT | 7 of 1163 | 4 |
| CHT regimens | 161 of 211 | 76 |
| Anthracycline | 119 of 161 | 75 |
| Anthracycline/etoposide | 24 of 161 | 15 |
| Etoposide | 7 of 161 | 4 |
| Other | 11 of 161 | 6 |
| Response to treatment | 156 of 211 | 74 |
| CR | 97 of 156 | 63 |
| PR | 24 of 156 | 15 |
| PD | 35 of 156 | 22 |
| Factor . | N . | % . |
|---|---|---|
| Median age, y (range) | 59 (18-88) | |
| Age ≥60 y | 104 | 49 |
| Sex, male | 95 | 45 |
| B symptoms present | 73 of 202 | 36 |
| Extranodal involvement | 115 | 55 |
| ECOG PS >1 | 27 of 199 | 14 |
| LDH > ULN | 72 of 175 | 41 |
| Albumin <35 g/L | 58 of 175 | 33 |
| Hb >12g/dL | 66 of 196 | 34 |
| Platelet count <150 × 109 cells/L | 111 of 195 | 57 |
| ANC >6.5 × 103/ml | 55 of 188 | 29 |
| Histotypes | ||
| PTCL-NOS | 129 of 211 | 61 |
| AITL | 28 of 211 | 13 |
| ALCL ALK– | 54 of 211 | 26 |
| Therapy | 175 of 211 | 83 |
| Best supportive care | 12 | 7 |
| CHT alone | 127 of 163 | 78 |
| RT alone | 5 of 163 | 3 |
| CHT/RT | 24 of 163 | 15 |
| CHT and HDT/ASCT | 7 of 1163 | 4 |
| CHT regimens | 161 of 211 | 76 |
| Anthracycline | 119 of 161 | 75 |
| Anthracycline/etoposide | 24 of 161 | 15 |
| Etoposide | 7 of 161 | 4 |
| Other | 11 of 161 | 6 |
| Response to treatment | 156 of 211 | 74 |
| CR | 97 of 156 | 63 |
| PR | 24 of 156 | 15 |
| PD | 35 of 156 | 22 |
ANC, absolute neutrophil count; CHT, chemotherapy; CR, complete response; CRP, C-reactive protein; Hb, hemoglobin; PD, progressive disease; PR, partial response; ULN, upper limit of normal.
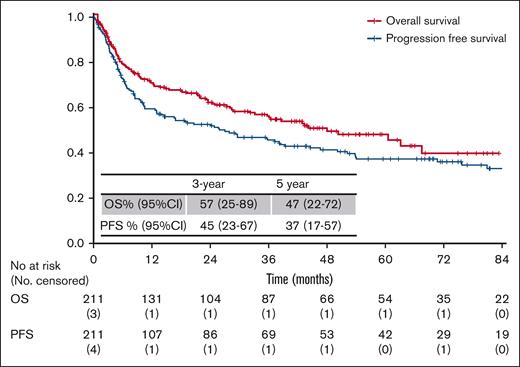
After a median follow-up of 49 months (range, 1-94 months), 101 (48%) deaths were recorded, mostly due to lymphoma (74%), followed by infections (12%), treatment-related toxicity (7%), or other causes (7%). The overall response rate to first-line therapy (as assessed by local investigators) was 78% (n = 156): 63% of the patients (n = 97) achieved a complete response, and 15% (n = 24) achieved a partial response (Table 1). The 5-year OS and PFS rates were 47% (95% confidence interval [95% CI], 22-72) and 37% (95% CI, 17-57), respectively (Figure 2).
Kaplan-Meier curves of OS and PFS for all patients in the training sample (n = 211).
Kaplan-Meier curves of OS and PFS for all patients in the training sample (n = 211).
Prognostic model development
In the univariate analysis, age >60 years, serum albumin level <35 g/L, elevated LDH levels, B symptoms, ECOG PS ≥2, hemoglobin <12 g/L, and platelet count >150 × 109 had a statistically significant impact on the OS (Table 2). The 82 reported events corresponded to an event/variable ratio of 12, which was acceptable for the multivariate analysis. From the multiple Cox PH regression analysis, 3 factors were predictive of OS: age ≥60 years, serum albumin level <35 g/L, and LDH level greater than the upper limit of normal (Table 2).
Univariate and multivariate Cox PH regression for OS in the training cohort (n = 211)
| . | . | 5 years OS % (95% CI) . | Univariate . | Multivariate . | ||
|---|---|---|---|---|---|---|
| Overall . | ||||||
| Factor . | Status . | . | HR (95% CI) . | P value . | HR (95% CI) . | P value . |
| Age, y | <60 | 61 (39-83) | 1.00 | .001 | 1.00 | .01 |
| >60 | 23 (14-32) | 1.6 (1.3-2.1) | 1.55 (1.3-2.1) | |||
| Albumin, g/L | >35 | 56 (32-80) | 1.00 | .003 | 1.00 | .004 |
| <35 | 25 (20-30) | 2.7 (1.8-4.2) | 2.13 (1.2-3.5) | |||
| LDH > ULN | <ULN | 58 (41-75) | 1.00 | .001 | 1.00 | .001 |
| >ULN | 28 (19-37) | 2.3 (1.4-3.4) | 2.02 (1.1-3.2) | |||
| Sex | Female | 45 (39-51) | 1.00 | .91 | ||
| Male | 45 (39-51) | 1.01 (0.7-1.5) | ||||
| B symptoms | No | 68 (42-94) | 1.00 | .001 | ||
| Yes | 27 (24-30) | 2.1 (1.3-3.1) | ||||
| ECOG PS | 0-1 | 51 (35-67) | 1.00 | .001 | ||
| 2-4 | 20 (14-26) | 2.7 (1.7-4.5) | ||||
| Extranodal involvement | No | 49 (36-62) | 1.00 | .82 | ||
| Yes | 46 (30-62) | 0.71 (0.6-1.4) | ||||
| Hb, g/L | >120 | 57 (36-78) | 1.00 | .001 | ||
| <120 | 29 (19-39) | 2.2 (1.5-3.3) | ||||
| Platelet count, ×109/L | >150 | 50 (31-69) | 1.00 | .02 | ||
| <150 | 29 (20-38) | 1.8 (1.1-3.7) | ||||
| . | . | 5 years OS % (95% CI) . | Univariate . | Multivariate . | ||
|---|---|---|---|---|---|---|
| Overall . | ||||||
| Factor . | Status . | . | HR (95% CI) . | P value . | HR (95% CI) . | P value . |
| Age, y | <60 | 61 (39-83) | 1.00 | .001 | 1.00 | .01 |
| >60 | 23 (14-32) | 1.6 (1.3-2.1) | 1.55 (1.3-2.1) | |||
| Albumin, g/L | >35 | 56 (32-80) | 1.00 | .003 | 1.00 | .004 |
| <35 | 25 (20-30) | 2.7 (1.8-4.2) | 2.13 (1.2-3.5) | |||
| LDH > ULN | <ULN | 58 (41-75) | 1.00 | .001 | 1.00 | .001 |
| >ULN | 28 (19-37) | 2.3 (1.4-3.4) | 2.02 (1.1-3.2) | |||
| Sex | Female | 45 (39-51) | 1.00 | .91 | ||
| Male | 45 (39-51) | 1.01 (0.7-1.5) | ||||
| B symptoms | No | 68 (42-94) | 1.00 | .001 | ||
| Yes | 27 (24-30) | 2.1 (1.3-3.1) | ||||
| ECOG PS | 0-1 | 51 (35-67) | 1.00 | .001 | ||
| 2-4 | 20 (14-26) | 2.7 (1.7-4.5) | ||||
| Extranodal involvement | No | 49 (36-62) | 1.00 | .82 | ||
| Yes | 46 (30-62) | 0.71 (0.6-1.4) | ||||
| Hb, g/L | >120 | 57 (36-78) | 1.00 | .001 | ||
| <120 | 29 (19-39) | 2.2 (1.5-3.3) | ||||
| Platelet count, ×109/L | >150 | 50 (31-69) | 1.00 | .02 | ||
| <150 | 29 (20-38) | 1.8 (1.1-3.7) | ||||
HR, hazard ratio; ULN, upper limit of normal.
The prognostic SALENTO model was developed considering each adverse factor having a weightage of 1.
Risk groups were defined by comparing the relative risk of disease progression in patients with each possible number of presenting risk factors and by combining categories of similar relative risks.
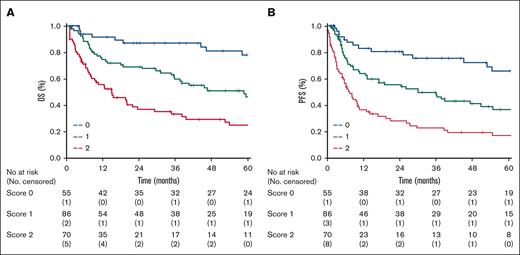
The patients were then stratified into the following 3 risk groups: those at low-risk (LR, 55 patients; 26%; score, 0), intermediate-risk (IR, 86 patients; 41%; score, 1), and high-risk (HiR, 70 patients; 33%; score, 2 -3). The 3 risk groups had a 2-year and 5-year OS of 87% (95% CI, 81-93) and 78% (95% CI, 29-127), 67% (95% CI, 60-74) and 46% (95% CI, 24-68), 36% (95% CI, 20-52) and 25% (95% CI, 20-30) for patients at LR, IR, and HiR, respectively (P < .001; log rank 34.4; Figure 3A-B). The model also proved to be a robust tool for PFS: the 5-year PFS was 66% (95% CI, 33-99), 37% (95% CI, 9-65), and 17% (95% CI, 9-25) in LR, IR, and HiR groups, respectively (P < .001; log rank 36.7). The performance of the SALENTO model was compared with the published prognostic scores. In comparison with the PIT and IPTCLP, the SALENTO score demonstrated the greatest discriminant power with a lower AIC (509) and higher Harrell c-statistic (0.689) than the other prognostic indices (PIT, AIC 609; Harrell c-statistic 0.620 vs IPTCLP, AIC 574; Harrell c-statistic 0.576) (Table 3).
Kaplan-Meier curves. (A) Overall survival and (B) progression free survival by risk groups for all patients in the training sample (score 0 = 55 patients, score 1 = 86 patients, score 2 = 70 patients) identified by the Salento Model.
Kaplan-Meier curves. (A) Overall survival and (B) progression free survival by risk groups for all patients in the training sample (score 0 = 55 patients, score 1 = 86 patients, score 2 = 70 patients) identified by the Salento Model.
Distribution, OS and PFS for patients in the training cohort based on the SALENTO model and previously proposed risk scores (PIT and IPTCLP)
| . | Score . | N (%) . | 5-year OS (%) (95% CI) . | Log rank . | P value . | HR (95% CI) . | 5-year PFS (%) (95% CI) . | HR (95% CI) . | Log rank . | P value . |
|---|---|---|---|---|---|---|---|---|---|---|
| SALENTO | ||||||||||
| 0 | 55 (26) | 78 (29-127) | 34.4 | < .001 | 1.00 | 66 (33-99) | 1.00 | 36.7 | < .001 | |
| 1 | 86 (41) | 46 (24-68) | 2.2 (1.6-2.9) | 37 (9-65) | 2.3 (1.6-2.9) | |||||
| 2 | 70 (33) | 25 (20-30) | 1.9 (1.5-2.8) | 17 (9-25) | 2.1 (1.5-2.9) | |||||
| PIT | ||||||||||
| 0 | 49 (32) | 79 (27-131) | 29.5 | .001 | 1.00 | 62 (25-99) | 1.00 | 26, 2 | .001 | |
| 1 | 64 (42) | 44 (24-64) | 2.2 (1.4-3.2) | 37 (15-74) | 2.0 (1.5-2.7) | |||||
| 2 | 39 (26) | 22 (19-25) | 2.7 (2.1-3.1) | 16 (14-21) | 2.1 (1.4-2.6) | |||||
| IPTCLP | ||||||||||
| 0 | 20 (10) | 91 (23-159) | 18.2 | .001 | 1.00 | 84 (31-137) | 1.00 | 13.7 | .001 | |
| 1 | 89 (42) | 58 (19-97) | 1.8 (1.1-2.7) | 40 (13-67) | 1.5 (1.1-2.2) | |||||
| 2 | 102 (48) | 32 (14-50) | 2.1 (1.6-3.1) | 28 (15-41) | 1.9 (1.6-2.9) |
| . | Score . | N (%) . | 5-year OS (%) (95% CI) . | Log rank . | P value . | HR (95% CI) . | 5-year PFS (%) (95% CI) . | HR (95% CI) . | Log rank . | P value . |
|---|---|---|---|---|---|---|---|---|---|---|
| SALENTO | ||||||||||
| 0 | 55 (26) | 78 (29-127) | 34.4 | < .001 | 1.00 | 66 (33-99) | 1.00 | 36.7 | < .001 | |
| 1 | 86 (41) | 46 (24-68) | 2.2 (1.6-2.9) | 37 (9-65) | 2.3 (1.6-2.9) | |||||
| 2 | 70 (33) | 25 (20-30) | 1.9 (1.5-2.8) | 17 (9-25) | 2.1 (1.5-2.9) | |||||
| PIT | ||||||||||
| 0 | 49 (32) | 79 (27-131) | 29.5 | .001 | 1.00 | 62 (25-99) | 1.00 | 26, 2 | .001 | |
| 1 | 64 (42) | 44 (24-64) | 2.2 (1.4-3.2) | 37 (15-74) | 2.0 (1.5-2.7) | |||||
| 2 | 39 (26) | 22 (19-25) | 2.7 (2.1-3.1) | 16 (14-21) | 2.1 (1.4-2.6) | |||||
| IPTCLP | ||||||||||
| 0 | 20 (10) | 91 (23-159) | 18.2 | .001 | 1.00 | 84 (31-137) | 1.00 | 13.7 | .001 | |
| 1 | 89 (42) | 58 (19-97) | 1.8 (1.1-2.7) | 40 (13-67) | 1.5 (1.1-2.2) | |||||
| 2 | 102 (48) | 32 (14-50) | 2.1 (1.6-3.1) | 28 (15-41) | 1.9 (1.6-2.9) |
External validation
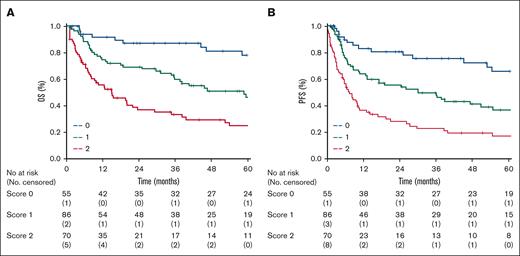
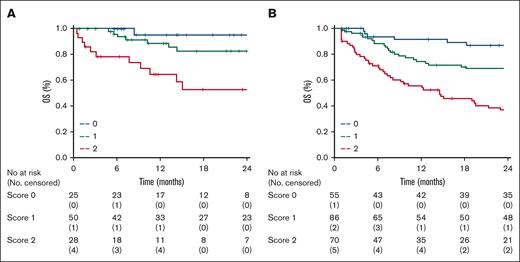
A total of 103 patients registered in the Australian lymphoma and related diseases registry or Brazilian T-cell lymphoma registry fulfilled the inclusion criteria and represented an external validation cohort. The median follow-up duration of the validation cohort was 22 months (range, 20-27 months), and 49 events (47% of the patients) were recorded. The baseline patient characteristics and treatment received are shown in supplemental Table 2. Compared with the training cohort, there were no significant differences in baseline characteristics or treatment. There was a trend toward the use of more RT in the validation cohort. Therefore, the model was tested in terms of 2-year OS: 25 patients (24%) were classified as LR; 50 patients (49%) as IR; and 28 patients (27%) as HiR. The distribution of the different risk groups was almost superimposable between the training and validation cohorts. The 2-year OS of the 3 groups was 95% (95% CI, 82 to not reached), 82% (95% CI, 72-86), and 53% (95% CI, 24-82) for LR, IR, and HiR, respectively (P < .01; log rank, 14.4, hazard ratio = 3.5; 95% CI, 1.7-7.6) in the validation cohort. The SALENTO model was prognostic for OS and identified 3 risk groups in the training and validation cohorts (Figures 4A-B).
Kaplan-Meier curves of 2-year overall survival by risk groups. (A) Validation cohort (score 0 = 25 patients, score 1 = 50 patients, score 2 = 28 patients) compared with the (B) training sample.
Kaplan-Meier curves of 2-year overall survival by risk groups. (A) Validation cohort (score 0 = 25 patients, score 1 = 50 patients, score 2 = 28 patients) compared with the (B) training sample.
Discussion
The SALENTO model represents a novel and validated prognostic index specific to limited-stage PTCL. The model was developed from patients prospectively registered in the TCP 1 and divided patients into 3 risk groups based on 3 independent objective and universally available factors (age, LDH level, and albumin level). It is an objective, simple, and robust prognostic tool that identifies patients at low or high risk of treatment failure. The prognostic value of the score was confirmed in a validation cohort. Compared with the prior scores, the SALENTO model demonstrated the greatest discriminatory power. The model was developed using real-world data pooled from multiple countries. This enhances its applicability in clinical practice and potential for use in clinical trial design.
Our model was developed using TCP 1, a large international prospective registry of patients with mature PTCLs. We focused on the common subtypes (PTCL-NOS, AITL, and ALK– ALCL) for which treatments have been uniform and examined the prognostic significance of common clinical and laboratory variables in limited-stage PTCL. Considering the favorable prognosis of ALK+ ALCL, we omitted this subtype from the analysis.1,13 We conducted an external validation of the score in an independent international data set from the Australian and Brazilian registries within the TCP 2. TCP 1 and 2 represent unprecedented databases to examine and address this unmet medical need. Frontline therapy was homogeneous in the training cohort, with ∼80% of the patients receiving anthracycline-based therapy. Despite being with limited-stage disease, few patients received RT. Similarly, a few patients underwent HDT/ASCT. Management practices were similar in the validation cohort, with the majority receiving chemotherapy only.
The prognostic factors in the model assess age, which is generally a marker of fitness for treatment, proliferation (LDH), and inflammation (albumin). The model identified 3 groups with different rates of 5-year OS: LR (n = 26%; score, 0) 78%, IR (n = 41%; score, 1) 46%, HiR (n = 33%; score, 2-3) 25%. Furthermore, the score stratified the patients into 3 groups of similar sizes. The goal of a prognostic score is to identify patients at high risk with expected poor outcomes for the consideration of alternative treatment approaches and to identify patients at low risk with potentially curable disease. Here, the LR category identified 26% of patients, who were young, with normal LDH and albumin levels with a high likelihood of cure. We grouped the patients with scores of 2 or 3 to reduce the number of groups to enable the development of a model.
Age and LDH have been recognized as prognostic factors in PTCL and have already been included in previously reported indices as significant predictors for both OS and PFS.16,19 Low-serum albumin has been reported as an adverse prognostic factor in PTCLs.20,21 Low-serum albumin is a potential marker of cachexia, a multifactorial state of involuntary weight loss driven by inflammation in the context of cancer. Cancer-associated inflammation is a key determinant of survival in aggressive lymphoma.22 A cachexia index that includes albumin was demonstrated to predict inferior OS in aggressive B-cell lymphomas.23 Albumin is an independent prognostic factor in the international prognostic score in Hodgkin lymphoma.24 The adverse prognostic impact of hypoalbuminemia in PTCLs and Hodgkin lymphoma likely represents a shared systemic inflammatory state in untreated lymphoma.
Multiple studies have attempted to identify the prognostic scores or factors in PTCLs. The internal prognostic index is commonly used; however, it is not specific for PTCLs as it was developed in a cohort of patients with aggressive lymphoma, largely of B-cell origin25. It is familiar, easy to use, and broadly applicable in PTCLs.26,27 Prognostic scores have been developed specifically for PTCL. The PIT score was developed for PTCL-NOS based on age, ECOG PS, LDH, and bone marrow involvement.16 The IPTCLP developed a score for PTCL-NOS and AITL based on age, ECOG PS, and platelet count.17 Elevated β2-microglubulin and age >40 years were prognostic for OS in ALCL.28 In AITL, age, ECOG, elevated CRP, and β2-microglubulin were prognostic for OS.11 The SALENTO model was tested against other PTCL-specific scores. It was superior to PIT and IPTCLP and overall demonstrated good discriminatory ability (c-value 0.689). Although therapeutic developments in PTCLs have not mirrored those in aggressive B-cell lymphomas (anti-CD20 monoclonal antibodies, bispecific T-cell engager therapies, or chimeric antigen receptor T-cell therapy), the value of a robust prognostic score remains.
The development of this model has several limitations. Similar to all models, comorbidities, which are particularly important in the elderly, were not incorporated. The patients were managed in the era of computed tomography imaging for staging and response assessment. The use of positron emission tomography might have resulted in stage migration with an improved outcomes in patients with truly limited-stage disease. The prognostic impact of the molecular events was not examined in this cohort. Genetic heterogeneity with prognostic significance is known to exist in PTCL-NOS based on gene expression profiling (GATA-3 vs TBX21) and in ALCL based on fluorescence in situ hybridization findings (DUSP22 vs TP63 vs triple negative).29,30 Similar to previous models, the SALENTO score is based on clinical variables and does not account for known prognostic molecular findings, which could have significant clinical relevance. The integration of molecular events with clinical factors can further strengthen this model. Owing to the rarity and patient numbers, histological subanalysis was not possible. We acknowledge the biological differences between PTCLs.
This study has several strengths. A central pathology review was performed for both training and validation cohorts. This is critical in PTCLs considering that significant variation has been reported even among expert hematopathologists.31 The validation cohort was assembled from national registries from the prospective TCP 2. The model was developed from real-world data pooled across multiple countries, enhancing its applicability in clinical practice and potential for clinical trial design. Finally, the score only requires 3 objective criteria: age and universally available laboratory values.
Our goal was to develop and validate a prognostic score specific to limited-stage PTCLs. Although PIT and IPTCLP remain useful in defining risk, the SALENTO model developed in a prospectively collected data set better stratified patients. This model is an objective, simple, and robust prognostic tool. The model identifies patients with a favorable prognosis with the current therapy. Despite the superior 5-year OS for the LR group, the deescalation of therapy should not be considered without further study. Conversely, the model identifies a high-risk group with poor outcomes that warrants studies with innovative first-line approaches. Future studies incorporating biological variables and clinical factors are needed to determine whether clinical risk can be further refined to allow better risk stratification.
Acknowledgments
The authors thank Eliza Chung, Eliza Hawkes, Erica Wood, Zoe McQuilten, and all participating sites and principal investigators at the Australian Lymphoma and Related Diseases Registry and Eliana Miranda at the Brazilian T-Cell Lymphoma Registry for their valuable assistance assembling the validation cohort.
Authorship
Contribution: G.H., M.C., T.S., and M.F. designed the project, analyzed the data, and wrote the manuscript; Y.S., J.V., M.E.C., R.H.A., S.A.P., S.M.H., F.M.F., F.H., J.R., I.D., C.C., W.S.K., A.N., J.T., S.L., and M.F. provided data; and all authors approved the final manuscript.
A complete list of the members of the International T-Cell Project appears in the supplemental Appendix.
Conflict-of-interest disclosure: S.M.H. received grant or research support from Kyowa, Takeda Pharmaceuticals, Secura, Ono, Daiichi, Acrotech, CRISPR, C4, and Yingli. J.T. reported research funding to the institution from Roche, BeiGene, Takeda, Cellectar, Bristol Myers Squibb (BMS), PCYC, and Janssen for the clinical trials. S.L. is an adviser for Roche, Janssen, Gilead/Kite, BMS/Celgene, Regeneron, and Genmab. The remaining authors declare no competing financial interests.
Correspondence: Greg Hapgood, Princess Alexandra Hospital, 199 Ipswich Rd, Woolloongabba, Brisbane 4102, Australia; e-mail: greg.hapgood@health.qld.gov.au.
References
Author notes
Data are available on request from the corresponding author, Greg Hapgood (greg.hapgood@health.qld.gov.au).
The full-text version of this article contains a data supplement.