Key Points
CD19 CAR T-cell consolidation therapy combined with CD19+ FTCs and TKI had a manageable long-term safety profile.
CD19 CAR T-cell consolidation therapy combined with CD19+ FTCs and TKI yielded a high response rate and duration.
Abstract
We conducted a single-arm, open-label, single-center phase 1 study to assess the safety and efficacy of multicycle-sequential anti-CD19 chimeric antigen receptor (CAR) T-cell therapy in combination with autologous CD19+ feeding T cells (FTCs) and tyrosine kinase inhibitor (TKI) as consolidation therapy in patients under the age of 65 years with de novo Ph-positive CD19+ B-cell acute lymphoblastic leukemia. Participants were given induction chemotherapy as well as systemic chemotherapy with TKI. Afterward, they received a single cycle of CD19 CAR T-cell infusion and another 3 cycles of CD19 CAR T-cell and CD19+ FTC infusions, followed by TKI as consolidation therapy. CD19+ FTCs were given at 3 different doses. The phase 1 results of the first 15 patients, including 2 withdrawals, are presented. The most common adverse events were cytopenia (13/13) and hypogammaglobinemia (12/13). There was no incidence of cytokine release syndrome above grade 2 or immune effector cell-associated neurotoxicity syndrome or grade 4 nonhematological toxicities. All 13 patients achieved complete remission, including 12 patients with a complete molecular response (CMR) at the data cutoff. The relapse-free survival was 84%, and the overall survival was 83% with a median follow-up of 27 months. The total number of CD19-expressing cells decreased with an increasing CMR rate. CD19 CAR T cells survived for up to 40 months, whereas CD19+ FTCs vanished in 8 patients 3 months after the last infusion. These findings could form the basis for the development of an allo-HSCT–free consolidation paradigm. This trial was registered at www.clinicaltrials.gov as #NCT03984968.
Introduction
After the introduction of tyrosine kinase inhibitors (TKIs), a TKI-based regimen (in addition to chemotherapy or steroids) has become the backbone of therapy for Philadelphia-chromosome (Ph)+ acute lymphoblastic leukemia (ALL), allowing almost all patients with Ph+ ALL to achieve complete remission (CR) regardless of age. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is recommended as a postremission consolidation therapy for eligible patients, particularly for those with positive measurable residual disease (MRD) after induction therapy1,2 and has been shown to have an increased overall survival (OS) of ∼50%.3 However, treatment-related mortality and transplant-related morbidities jeopardize the efficacy and negatively impact the quality of life. Furthermore, some patients were unable to undergo allo-HSCT because they were unfit for the procedure or because the donor was unavailable. Therefore, alternative consolidation strategies are still urgently required for the maintenance of long-term remission. The emerging later-generation TKIs and immunotherapies are allowing us to investigate alternative approaches, while also challenging the role of allo-HSCT.4,5
Anti-CD19 chimeric antigen receptor (CD19 CAR) T cells have recently been shown to be highly effective in relapsed or refractory CD19+–B-cell ALL, with response rates ranging from 70% to 90%.6-10 However, the relapse rates of 20% to 70% and median leukemia-free survival of only 5.3 to 10.6 months9,11 suggest that CAR T-cell therapy’s high efficacy does not always translate into long-term survival benefits. The duration of the response is influenced by the in vivo kinetics of CAR T cells and is reflected by their expansion and persistence.12 Tumor burden decreases dramatically once the patients achieve CR after CAR T cells perform their function in vivo. Our unpublished preclinical research has demonstrated that antigen dose influences CD19 CAR T-cell activation and proliferation. The CD19 CAR T cells cannot be activated once the number of CD19+ blasts drop to a certain level, which might be one of the mechanisms of antigen-positive relapse in oncoimmunotherapy. The CD19 CAR T cells’ lost response to residual CD19+ blasts can be restored by supplementation with CD19+ feeding T cells (FTCs), which are T cells transduced with a CD19 gene expression vector. A study by Veatch et al13 has reported that autologous T cells modified with tumor antigens and additional adjuvant signals (Tvax) can elicit and boost effective antitumor immunity in transplantable models of local and systemic cancer in early treatment. Furthermore, a study by Finney et al14 has demonstrated that the cumulative burden of CD19-expressing leukemic cells and normal B cells is the primary driver of CAR T-cell expansion, enforcing prolonged B-cell aplasia. Therefore, we propose that stimulating reinfused CAR T cells with antigens recognized by CAR T cells could enhance CAR T-cell activation and maintain a potent CAR T-cell response, thereby extending their efficacy.
In this setting, autologous CD19+ FTCs were manufactured by transducing T cells with an overexpressed CD19 vector. These cells could imitate CD19+ blasts to provide sufficient antigens and were expected to stimulate the in vivo expansion of CAR T cells. Subsequent consolidation was achieved with multicycle-sequential administration of CD19 CAR T cells and CD19+ FTCs along with TKI in patients with Ph+ ALL who had achieved CR. This trial sought to determine the safety and efficacy of this consolidation strategy.
Methods
Study design and participants
This is a phase 1/2 single-arm, open-label, single-center study. The study has been authorized by the Institutional Review Board of the First Affiliated Hospital of Soochow University. Eligible patients were aged between 18 and 65 years and were diagnosed with de novo CD19+ Ph+ ALL and have been enrolled in this ongoing clinical trial since 2017. They were either unable to find a suitable donor or had refused to undergo allo-HSCT. Detailed inclusion criteria and exclusion criteria are shown in the supplemental Protocol. This trial is being conducted following the principles of the Declaration of Helsinki. All patients have signed an informed consent form before the commencement of the study.
Procedures
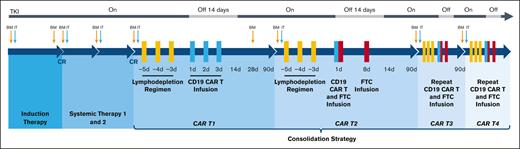
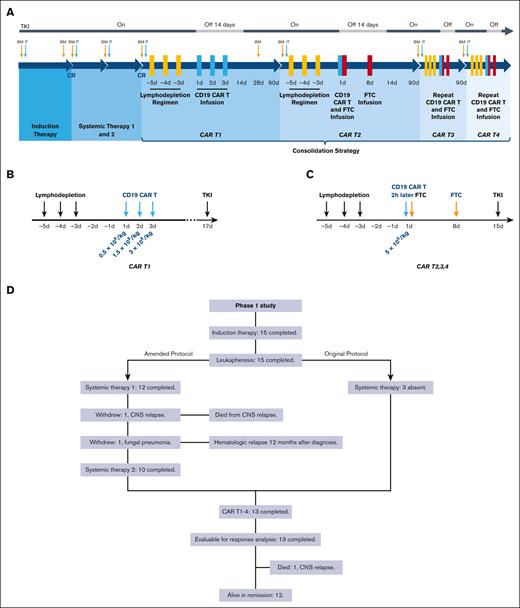
According to the protocol, before CD19 CAR T-cell consolidation, patients received induction therapy, TKI, and underwent leukapheresis. Induction therapy comprised vindesine (4 mg/d intravenously every week on days 1, 8, 15), idarubicin (6 mg/m2 per day intravenously every week on days 1, 8, 15), dexamethasone (10 mg/m2 per day intravenously daily on days 1-21, tapered on days 22-28), and imatinib (400 mg/d orally daily). TKI was continued indefinitely if tolerated according to the original protocol. To prevent central nervous system (CNS) leukemia, the protocol was amended on 3 November 2019. Two courses of systemic chemotherapies were added, which included 1 cycle of medium-dose cytarabine (2 g/m2 per day intravenously q12h on days 1-2) and 1 cycle of high-dose methotrexate (3 g/m2 per day intravenously on day 1) concurrent with TKI. Triple intrathecal therapy (methotrexate, cytarabine, and dexamethasone) was administered at the diagnosis, the start of systemic therapy, during each cycle of CAR T –cell infusion, and every 6 months during the 2-year follow-up for a total of 11 injections. The protocol was again amended with respect to TKIs on 13 April 2020. Imatinib was discontinued for 2 weeks after CD19 CAR T-cell infusion and was reinitiated during the interphase of CD19 CAR T cells (400 mg once daily). Dasatinib (100 mg once daily) was approved as a substitute when imatinib was not tolerated or if the disease progressed while on imatinib. The disease status and organ function were reevaluated before commencing CD19 CAR T-cell therapy. Patients with CR were initiated on multicycle-sequential CD19 CAR T-cell consolidation therapy. These patients received a single cycle of CD19 CAR T-cell infusion alone (CAR T1), followed by 3 cycles of CD19 CAR T-cell and CD19+ FTC infusion (CAR T2-T4) (Figure 1A). Figure 1B-C depicts the specifics of CAR T1 to T4. After completing the treatment, patients would be followed up for 15 years.
Study oversight and consort diagram. (A) Treatment schema of the entire clinical trial. (B-C) Schema of CAR T1 to CAR T4. Before each cycle of CD19 CAR T cells, the FC regimen (fludarabine 30 mg/m2 daily and cyclophosphamide 300 mg/m2 daily) for lymphodepletion was given on days −5, −4, and −3. CD19 CAR T cells were infused at a total dose of 5 × 106 cells per kg on day 1 to day 3 of CAR T1 and day 1 of CAR T2 to T4, whereas CD19+ FTCs were infused 2 hours after the infusion of CD19 CAR T cells on day 1 and at the same dose alone on day 8 of CAR T2 to T4. Based on the preclinical data, the stimulation of CD19 CAR T cells via CD19+ FTCs at a ratio of 1:1 was seen to be the optimum ratio. Therefore, 5 × 106/kg of CD19+ FTCs was set as the starting dosage in our study. Moreover, considering the limited number of T cells harvested from a single apheresis from the patientsand the cost-effectiveness, we evaluated the minimum effective doses, ie, CD19+ FTCs (3.25 × 106/kg and 2 × 106/kg), to be used in our study. Therefore, CD19+ FTCs were administered at 3 different doses (5 × 106/kg, 3.25 × 106/kg, or 2 × 106/kg) to assess an optimal biologic CD19+ FTC dose. (D) Consort diagram describing the patient population. A total of 15 patients had enrolled in our phase I clinical trial. On 3 November 2019, we modified our original protocol owing to the observation of CNS leukemia in 1 patient (patient 4). Two cycles of systemic therapy after induction therapy and a total of 11 triple intrathecal injections (methotrexate, cytarabine, and dexamethasone) were included for the prevention of CNS leukemia. Three patients were already initiated on CAR T-cell therapy before the amendment, and therefore they were absent from the systemic chemotherapy. One patient (patient 7) withdrew because of fungal pneumonia that occurred after 1 cycle of systemic chemotherapy when he had a hematological CR. Patient 15 withdrew because of an isolated CNS relapse after 1 cycle of systemic chemotherapy. She achieved CR2 after multiple intrathecal therapies and underwent HSCT. Unfortunately, she died of a second relapse in the CNS.
Study oversight and consort diagram. (A) Treatment schema of the entire clinical trial. (B-C) Schema of CAR T1 to CAR T4. Before each cycle of CD19 CAR T cells, the FC regimen (fludarabine 30 mg/m2 daily and cyclophosphamide 300 mg/m2 daily) for lymphodepletion was given on days −5, −4, and −3. CD19 CAR T cells were infused at a total dose of 5 × 106 cells per kg on day 1 to day 3 of CAR T1 and day 1 of CAR T2 to T4, whereas CD19+ FTCs were infused 2 hours after the infusion of CD19 CAR T cells on day 1 and at the same dose alone on day 8 of CAR T2 to T4. Based on the preclinical data, the stimulation of CD19 CAR T cells via CD19+ FTCs at a ratio of 1:1 was seen to be the optimum ratio. Therefore, 5 × 106/kg of CD19+ FTCs was set as the starting dosage in our study. Moreover, considering the limited number of T cells harvested from a single apheresis from the patientsand the cost-effectiveness, we evaluated the minimum effective doses, ie, CD19+ FTCs (3.25 × 106/kg and 2 × 106/kg), to be used in our study. Therefore, CD19+ FTCs were administered at 3 different doses (5 × 106/kg, 3.25 × 106/kg, or 2 × 106/kg) to assess an optimal biologic CD19+ FTC dose. (D) Consort diagram describing the patient population. A total of 15 patients had enrolled in our phase I clinical trial. On 3 November 2019, we modified our original protocol owing to the observation of CNS leukemia in 1 patient (patient 4). Two cycles of systemic therapy after induction therapy and a total of 11 triple intrathecal injections (methotrexate, cytarabine, and dexamethasone) were included for the prevention of CNS leukemia. Three patients were already initiated on CAR T-cell therapy before the amendment, and therefore they were absent from the systemic chemotherapy. One patient (patient 7) withdrew because of fungal pneumonia that occurred after 1 cycle of systemic chemotherapy when he had a hematological CR. Patient 15 withdrew because of an isolated CNS relapse after 1 cycle of systemic chemotherapy. She achieved CR2 after multiple intrathecal therapies and underwent HSCT. Unfortunately, she died of a second relapse in the CNS.
Description of CD19 CAR T cells and CD19+ FTCs
T cells were isolated using anti-CD3 magnetic beads from the subjects using leukapheresis and then stimulated with anti-CD3/CD28 monoclonal antibodies. The cells were then transduced with recombinant lentiviral vectors encoding the CD19-specific CAR, which is composed of an anti-CD19 single-chain variable fragment, a 4-1BB costimulatory moiety, and a CD3 zeta activation domain with an interleukin-6 (IL-6) shRNA element that acts against the IL-6 gene. To generate CD19+ FTCs, T cells were transduced with recombinant lentiviral vectors encoding the CD19 antigen. CD19 CAR T cells and CD19+ FTCs were cultured in AIM-V media, containing 10% autologous serum, 100 IU/mL IL-2, 5 ng/mL IL-7, and 5 ng/mL IL-15 for 12 days. The transduction efficiency, percentage of CD3+, CD4/ CD8 ratios, and sterility (bacteria, endotoxin, and mycoplasma) were measured before the products were released. Our supplemental Protocol contains the methods for testing CD19 CAR T cells and CD19+ FTCs.
Outcomes
The primary end point was to assess the safety of CD19 CAR T cells in combination with CD19+ FTCs and TKI. The secondary end points were complete molecular response (CMR), relapse-free survival (RFS), and OS. The exploratory end points were the kinetics of CD19 CAR T cells and CD19+ FTCs.
The response was assessed on day 28 after the induction, systemic chemotherapies, and CAR T1 and on day 90 following CAR T1 to T4. All the analyzed samples had a sensitivity of 0.01% for real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR). On the basis of qRT-PCR, CMR was defined as a condition in which B-cell receptor (BCR)-Abelson tyrosine-protein kinase 1 (ABL1)/ABL ratio ≤0.01%. The definitions of MRD negativity, CR, OS, RFS, and molecular relapse are provided in the supplemental Protocol. All patients were to be followed up until relapse or death.
Adverse events (AEs) were graded using the Common Terminology Criteria defined by the National Institute of Cancer (CTCAE version 5.0), except for cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS), which were graded using criteria specified by Lee et al15 and a revised grading system.16 Nonhematological dose-limiting toxicities (DLTs) were defined as any toxicities that were greater than or equal to grade 3 toxicities and were deemed potentially treatment-related and occurred within 28 days of CD19 CAR T-cell infusion. Hematological DLTs were grade 4 toxicities (except lymphopenia) that lasted ≥30 days and were not caused by other underlying diseases.
Statistical analysis
Overall RFS and OS were calculated using the reverse Kaplan-Meier method, although the distribution of the time-to-event end point was calculated using the Kaplan-Meier method. The differences between groups were examined using 2-sided log-rank tests. All the P values were two-sided and the results were deemed statistically significant when P < .05. The data were analyzed using the SPSS Statistics 22 software.
Results
Patient characteristics
Between 15 September 2017 and 30 April 2022, 33 adults with newly diagnosed Ph+ ALL were enrolled to participate in this trial. The data until 31 March 2022 has been presented in this study and is based on phase 1 results of the first 15 patients from the 3 CD19+ FTC groups, 13 of whom completed the study and 2 withdrew midway (Figure 1D). The patient demographics and clinical characteristics are depicted in Table 1 and supplemental Table 1. The median bone marrow burden was 70% (21%-95%), and no extramedullary diseases were found at diagnosis. Flow cytometry revealed that the mean percentage of CD19+ blasts in the bone marrow was 94%.
Characteristics of the patients at baseline
| Patient no. . | Age, y . | Sex . | WBC × 109/L . | Marrow blasts, % . | CD19+ blasts percentage . | Fusion proteins BCR-ABL . | Mutations . | CNS status∗ . | Time to achieve CR before CAR Ts, d . | FTC dose† cells per kg . | CART dose cells per kg . | TKIs . | TKIs duration . | Time to last follow-up, mo . | Status . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 52 | Female | 43 | 66 | 89 | P190 | Negative | 1 | 10 | 5 × 106 | 5 × 106 | Imatinib, Dasatinib | 55 | 57 | Alive in CR |
| 2 | 58 | Female | 3 | 72 | 100 | P190 | Negative | 1 | 9 | 5 × 106 | 5 × 106 | Imatinib | 54 | 55 | Alive in CR |
| 3 | 58 | Female | 19 | 65 | 90 | P190 | Negative | 1 | 50 | 5 × 106 | 5 × 106 | Imatinib | 54 | 54 | Alive in CR |
| 4 | 33 | Female | 39 | 70 | 89 | P210 | ASXL1 | 1 | 40 | 5 × 106 | 5 × 106 | Imatinib | 25 | 25 | Isolated CNS relapse, died |
| 5 | 34 | Female | 47 | 50 | 96 | P210 | RUNX1, JAK1 | 1 | 48 | 5 × 106 | 5 × 106 | Imatinib | 55 | 55 | Alive in CR |
| 6 | 48 | Female | 81 | 81 | 96 | P210 | PAX5 | 1 | 37 | 3.25 × 106 | 5 × 106 | Imatinib | 28 | 30 | Alive in CR |
| 7 | 55 | Male | 88 | 95 | 93 | P190 | Negative | 1 | 40 | 3.25 × 106 | 5 × 106 | Imatinib | 8 | 9 | Withdrew due to fungal pneumonia |
| 8 | 41 | Male | 88 | 84 | 99 | P190 | Negative | 1 | 14 | 3.25 × 106 | 5 × 106 | Imatinib | 26 | 28 | Alive in CR |
| 9 | 38 | Female | 120 | 50 | 86 | P210 | RUNX1, STAT1 | 1 | 90 | 3.25 × 106 | 5 × 106 | Dasatinib | 21 | 24 | Alive in CR |
| 10 | 29 | Female | 128 | 86 | 96 | P210 | GATA1, SETD2 | 1 | 50 | 3.25 × 106 | 5 × 106 | Imatinib | 20 | 22 | Alive in CR |
| 11 | 41 | Female | 15 | 93 | 100 | P190 | KMT2C | 1 | 21 | 2 × 106 | 5 × 106 | Imatinib | 24.5 | 27 | Alive in CR |
| 12 | 27 | Female | 7 | 80 | 96 | P190 | KMT2C | 1 | 31 | 2 × 106 | 5 × 106 | Imatinib | 23.5 | 26 | Alive in CR |
| 13 | 51 | Female | 1 | 21 | 95 | P190 | Negative | 1 | 80 | 2 × 106 | 5 × 106 | Dasatinib | 22 | 25 | Alive in CR |
| 14 | 34 | Male | 3 | 66 | 100 | P190 | ASXL1, TET2, BRCA2,EP300 | 1 | 50 | 2 × 106 | 5 × 106 | Imatinib | 22 | 24 | Alive in CR |
| 15 | 35 | Female | 34 | 63 | 92 | P210 | PDGFRB | 1 | 58 | 2 × 106 | 5 × 106 | Imatinib | 7 | 7 | Withdrew due to isolated CNS relapse |
| Patient no. . | Age, y . | Sex . | WBC × 109/L . | Marrow blasts, % . | CD19+ blasts percentage . | Fusion proteins BCR-ABL . | Mutations . | CNS status∗ . | Time to achieve CR before CAR Ts, d . | FTC dose† cells per kg . | CART dose cells per kg . | TKIs . | TKIs duration . | Time to last follow-up, mo . | Status . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 52 | Female | 43 | 66 | 89 | P190 | Negative | 1 | 10 | 5 × 106 | 5 × 106 | Imatinib, Dasatinib | 55 | 57 | Alive in CR |
| 2 | 58 | Female | 3 | 72 | 100 | P190 | Negative | 1 | 9 | 5 × 106 | 5 × 106 | Imatinib | 54 | 55 | Alive in CR |
| 3 | 58 | Female | 19 | 65 | 90 | P190 | Negative | 1 | 50 | 5 × 106 | 5 × 106 | Imatinib | 54 | 54 | Alive in CR |
| 4 | 33 | Female | 39 | 70 | 89 | P210 | ASXL1 | 1 | 40 | 5 × 106 | 5 × 106 | Imatinib | 25 | 25 | Isolated CNS relapse, died |
| 5 | 34 | Female | 47 | 50 | 96 | P210 | RUNX1, JAK1 | 1 | 48 | 5 × 106 | 5 × 106 | Imatinib | 55 | 55 | Alive in CR |
| 6 | 48 | Female | 81 | 81 | 96 | P210 | PAX5 | 1 | 37 | 3.25 × 106 | 5 × 106 | Imatinib | 28 | 30 | Alive in CR |
| 7 | 55 | Male | 88 | 95 | 93 | P190 | Negative | 1 | 40 | 3.25 × 106 | 5 × 106 | Imatinib | 8 | 9 | Withdrew due to fungal pneumonia |
| 8 | 41 | Male | 88 | 84 | 99 | P190 | Negative | 1 | 14 | 3.25 × 106 | 5 × 106 | Imatinib | 26 | 28 | Alive in CR |
| 9 | 38 | Female | 120 | 50 | 86 | P210 | RUNX1, STAT1 | 1 | 90 | 3.25 × 106 | 5 × 106 | Dasatinib | 21 | 24 | Alive in CR |
| 10 | 29 | Female | 128 | 86 | 96 | P210 | GATA1, SETD2 | 1 | 50 | 3.25 × 106 | 5 × 106 | Imatinib | 20 | 22 | Alive in CR |
| 11 | 41 | Female | 15 | 93 | 100 | P190 | KMT2C | 1 | 21 | 2 × 106 | 5 × 106 | Imatinib | 24.5 | 27 | Alive in CR |
| 12 | 27 | Female | 7 | 80 | 96 | P190 | KMT2C | 1 | 31 | 2 × 106 | 5 × 106 | Imatinib | 23.5 | 26 | Alive in CR |
| 13 | 51 | Female | 1 | 21 | 95 | P190 | Negative | 1 | 80 | 2 × 106 | 5 × 106 | Dasatinib | 22 | 25 | Alive in CR |
| 14 | 34 | Male | 3 | 66 | 100 | P190 | ASXL1, TET2, BRCA2,EP300 | 1 | 50 | 2 × 106 | 5 × 106 | Imatinib | 22 | 24 | Alive in CR |
| 15 | 35 | Female | 34 | 63 | 92 | P210 | PDGFRB | 1 | 58 | 2 × 106 | 5 × 106 | Imatinib | 7 | 7 | Withdrew due to isolated CNS relapse |
WBC, white blood cell.
Classification of CNS status: CNS-1: no lymphoblasts in cerebrospinal fluid (CSF) regardless of the WBC count. CNS-2: WBC <5/mL in CSF with the presence of lymphoblasts. CNS-3: WBC ≥5/mL in CSF with the presence of lymphoblasts.
FTCs as a single dose at 5 × 106 cells per kg, 3.25 × 106 cells per kg, or 2 × 106 cells per kg were administered on days 1 and 8, respectively.
Safety
All 13 patients experienced AEs. CRS affected 9 patients in total. Out of the 13 patients, 69% (9/13) and 8% (1/13) experienced grade 1 CRS in CAR T1 and CAR T2, respectively (Table 2), although no CRS was observed in CAR T3 and CAR T4 (Table 3). There were no differences in the incidence or severity of CRS between the 3 doses of CD19+ FTCs (Table 4, P = .441; supplemental Figure 1A). No more than a grade 2 CRS or ICANS was observed during CAR T1-T4 (Table 2). Cytokine levels were measured in all patients and most had a slight elevation around the baseline, except for the patients who had infections (supplemental Figure 1B-C).
AEs among all the treated patients
| AEs . | Any grade . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|---|---|
| Number of patients . | |||||
| CRS | 9 | 9 | 0 | 0 | 0 |
| Fatigue | 9 | 6 | 3 | 0 | 0 |
| ICANS | 0 | 0 | 0 | 0 | 0 |
| Hypertension | 5 | 2 | 3 | 0 | 0 |
| Sinus tachycardia | 5 | 3 | 2 | 0 | 0 |
| Coagulation disorders | 4 | 4 | 0 | 0 | 0 |
| Increased aspartate aminotransferase | 2 | 1 | 1 | 0 | 0 |
| Increased alanine aminotransferase | 2 | 1 | 1 | 0 | 0 |
| Cytopenia | |||||
| Neutropenia | 13 | 0 | 1 | 6 | 6 |
| Anemia | 12 | 2 | 7 | 3 | 0 |
| Thrombocytopenia | 10 | 2 | 5 | 1 | 2 |
| Lymphopenia | 13 | 1 | 6 | 6 | 0 |
| Hypogammaglobulinemia | 12 | 12 | 0 | 0 | 0 |
| Infection | |||||
| Sepsis | 1 | 0 | 0 | 1 | 0 |
| Shingles | 1 | 0 | 0 | 1 | 0 |
| Upper respiratory infection | 2 | 0 | 2 | 0 | 0 |
| Bacteria | 1 | 0 | 0 | 1 | 0 |
| AEs . | Any grade . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|---|---|
| Number of patients . | |||||
| CRS | 9 | 9 | 0 | 0 | 0 |
| Fatigue | 9 | 6 | 3 | 0 | 0 |
| ICANS | 0 | 0 | 0 | 0 | 0 |
| Hypertension | 5 | 2 | 3 | 0 | 0 |
| Sinus tachycardia | 5 | 3 | 2 | 0 | 0 |
| Coagulation disorders | 4 | 4 | 0 | 0 | 0 |
| Increased aspartate aminotransferase | 2 | 1 | 1 | 0 | 0 |
| Increased alanine aminotransferase | 2 | 1 | 1 | 0 | 0 |
| Cytopenia | |||||
| Neutropenia | 13 | 0 | 1 | 6 | 6 |
| Anemia | 12 | 2 | 7 | 3 | 0 |
| Thrombocytopenia | 10 | 2 | 5 | 1 | 2 |
| Lymphopenia | 13 | 1 | 6 | 6 | 0 |
| Hypogammaglobulinemia | 12 | 12 | 0 | 0 | 0 |
| Infection | |||||
| Sepsis | 1 | 0 | 0 | 1 | 0 |
| Shingles | 1 | 0 | 0 | 1 | 0 |
| Upper respiratory infection | 2 | 0 | 2 | 0 | 0 |
| Bacteria | 1 | 0 | 0 | 1 | 0 |
Listed are the grade 1 to 2 AEs that occurred in at least 15% of the patients, and all events of grade 3 or higher occurred in patients from the time of infusion of CAR T cells until day 90. The severities of the AEs were graded according to the CTCAE version 5.0 except CRS and ICANS, which were graded according to the criteria of Lee et al10 and a revised grading system, respectively.
AEs by treatment cycle
| CAR T . | CRS . | Coagulation disorders . | Cytopenia . | Hypogamma-globulinemia . | Infections . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade 0 . | Grade 1 . | Above grade 1 . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | ||||
| Number of patients (percent) . | ||||||||||
| CAR T1 | 4 (31) | 9 (69) | 0 | 2 (15) | 0 | 3 (23) | 7 (54) | 3 (23) | 12 (92) | 2 (15) |
| CAR T2 | 12 (92) | 1 (8) | 0 | 0 | 0 | 4 (31) | 6 (46) | 3 (23) | 12 (92) | 0 |
| CAR T3 | 13 (100) | 0 | 0 | 1 (7) | 1 (8) | 6 (46) | 4 (31) | 2 (15) | 12 (92) | 1 (7) |
| CAR T4 | 13 (100) | 0 | 0 | 1 (7) | 1 (8) | 3 (23) | 8 (61) | 1 (8) | 12 (92) | 1 (7) |
| CAR T . | CRS . | Coagulation disorders . | Cytopenia . | Hypogamma-globulinemia . | Infections . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade 0 . | Grade 1 . | Above grade 1 . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | ||||
| Number of patients (percent) . | ||||||||||
| CAR T1 | 4 (31) | 9 (69) | 0 | 2 (15) | 0 | 3 (23) | 7 (54) | 3 (23) | 12 (92) | 2 (15) |
| CAR T2 | 12 (92) | 1 (8) | 0 | 0 | 0 | 4 (31) | 6 (46) | 3 (23) | 12 (92) | 0 |
| CAR T3 | 13 (100) | 0 | 0 | 1 (7) | 1 (8) | 6 (46) | 4 (31) | 2 (15) | 12 (92) | 1 (7) |
| CAR T4 | 13 (100) | 0 | 0 | 1 (7) | 1 (8) | 3 (23) | 8 (61) | 1 (8) | 12 (92) | 1 (7) |
Listed are grade 1 to 2 AEs that occurred in at least 15% of the patients and all events of grade 3 or higher occurred in patients from the time of infusion of CAR T cells until day 90. All were graded per CTCAE version 5.0 except CRS, which was graded according to the criteria of Lee et al10.
AEs by dose level
| AEs . | 2 × 106 cells per kg n = 4 . | 3.25 × 106 cells per kg n = 4 . | 5 × 106 cells per kg n = 5 . | |||
|---|---|---|---|---|---|---|
| All grades n (%) . | Grade ≥3 n (%) . | All grades n (%) . | Grade ≥3 n (%) . | All grades n (%) . | Grade ≥3 n (%) . | |
| Lymphocyte count decreased | 4 (100) | 2 (50) | 4 (100) | 1 (25) | 5 (100) | 3 (60) |
| Neutrophil count decreased | 4 (100) | 3 (75) | 4 (100) | 4 (100) | 5 (100) | 5 (100) |
| White blood cells decreased | 4 (100) | 4 (100) | 4 (100) | 4 (100) | 5 (100) | 4 (80) |
| Anemia | 4 (100) | 1 (25) | 4 (100) | 0 | 4 (80) | 2 (40) |
| Platelet count decreased | 3 (75) | 1 (25) | 4 (100) | 1 (25) | 4 (80) | 1 (25) |
| Bacteremia | 1 (25) | 1 (25) | 0 | 0 | 0 | 0 |
| Sepsis | 2 (50) | 2 (50) | 0 | 0 | 0 | 0 |
| Shingles | 0 | 0 | 0 | 0 | 1 (20) | 1 (20) |
| Grade | 1–2 n (%) | 1–2 n (%) | 1–2 n (%) | |||
| CRS | 4 (100) | 2 (50) | 3 (60) | |||
| Fatigue | 3 (75) | 2 (50) | 4 (80) | |||
| Hypertension | 2 (50) | 1 (25) | 2 (40) | |||
| Sinus tachycardia | 2 (50) | 1 (25) | 2 (40) | |||
| Upper respiratory infection | 0 | 1 (25) | 1 (20) | |||
| Aspartate aminotransferase increased | 1 (25) | 1 (25) | 0 | |||
| Alanine aminotransferase increased | 1 (25) | 1 (25) | 0 | |||
| Activated partial thromboplastin time prolonged | 1 (25) | 1 (25) | 2 (40) | |||
| AEs . | 2 × 106 cells per kg n = 4 . | 3.25 × 106 cells per kg n = 4 . | 5 × 106 cells per kg n = 5 . | |||
|---|---|---|---|---|---|---|
| All grades n (%) . | Grade ≥3 n (%) . | All grades n (%) . | Grade ≥3 n (%) . | All grades n (%) . | Grade ≥3 n (%) . | |
| Lymphocyte count decreased | 4 (100) | 2 (50) | 4 (100) | 1 (25) | 5 (100) | 3 (60) |
| Neutrophil count decreased | 4 (100) | 3 (75) | 4 (100) | 4 (100) | 5 (100) | 5 (100) |
| White blood cells decreased | 4 (100) | 4 (100) | 4 (100) | 4 (100) | 5 (100) | 4 (80) |
| Anemia | 4 (100) | 1 (25) | 4 (100) | 0 | 4 (80) | 2 (40) |
| Platelet count decreased | 3 (75) | 1 (25) | 4 (100) | 1 (25) | 4 (80) | 1 (25) |
| Bacteremia | 1 (25) | 1 (25) | 0 | 0 | 0 | 0 |
| Sepsis | 2 (50) | 2 (50) | 0 | 0 | 0 | 0 |
| Shingles | 0 | 0 | 0 | 0 | 1 (20) | 1 (20) |
| Grade | 1–2 n (%) | 1–2 n (%) | 1–2 n (%) | |||
| CRS | 4 (100) | 2 (50) | 3 (60) | |||
| Fatigue | 3 (75) | 2 (50) | 4 (80) | |||
| Hypertension | 2 (50) | 1 (25) | 2 (40) | |||
| Sinus tachycardia | 2 (50) | 1 (25) | 2 (40) | |||
| Upper respiratory infection | 0 | 1 (25) | 1 (20) | |||
| Aspartate aminotransferase increased | 1 (25) | 1 (25) | 0 | |||
| Alanine aminotransferase increased | 1 (25) | 1 (25) | 0 | |||
| Activated partial thromboplastin time prolonged | 1 (25) | 1 (25) | 2 (40) | |||
Listed are the grade 1 to 2 AEs that occurred in at least 15% of the patients and all events of grade 3 or higher occurred in patients from the time of infusion of CAR T cells until day 90. All were graded per the CTCAE version 5.0 except CRS, which was graded according to the criteria of Lee et al10.
AEs occurred in all CAR T-cell phases and dosage subgroups, with no significant differences observed between cycles or subgroups (Tables 3 and 4). The early AEs were related to the process of CD19 CAR T-cell infusion. Within 2 weeks after the commencement of treatment, 4 patients reported less than grade 3 coagulopathy and 2 patients reported liver function abnormalities, which resolved spontaneously.
Cytopenia was an expected side effect of lymphodepleting chemotherapy, which was observed in all patients. During each cycle, the median onset of grade 4 neutropenia was observed on day 5 (range, 3-7), with a median duration of 3 days (range, 0-5), requiring granulocyte colony-stimulating factor. The median onset of anemia and thrombocytopenia was observed on day 4 (range, 1-7) and on day 5 (range, 1-7), with median durations of 7 days (range, 5-24) and 9 days (range, 5-20), respectively, when the patients were given blood transfusion support. One patient with thrombocytopenia developed hemoptysis and recovered after hemostatic therapy. When the patients were discharged from the hospital, 7 of them experienced a recurrence of neutropenia (grade >3), including 4 with grade 4 neutropenia. The median onset of the second neutropenia event occurred on day 35 (range, 28-45), with a 7-day median duration (range, 3-21). However, prolonged (>14 days) neutropenia (greater than grade 3) was observed in only 3 of the 13 patients (Table 2).
Efficacy
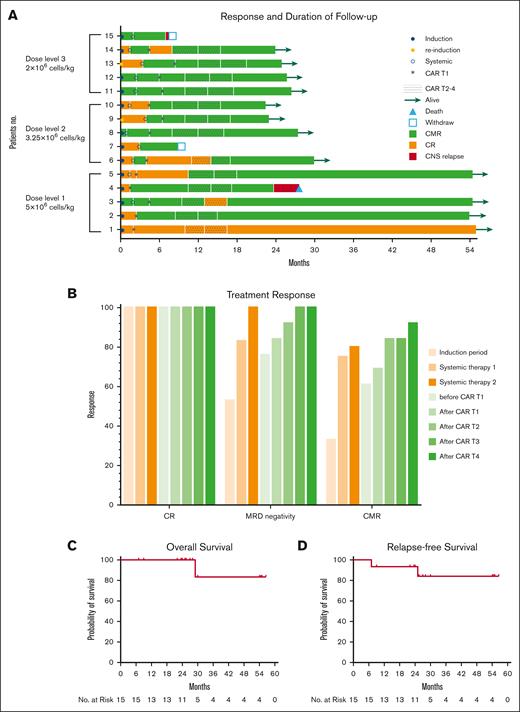
For the efficacy analysis, 15 subjects were included, of which 2 withdrew early. At the end of the induction phase, all patients, including the 2 who dropped out of the trial, had hematological CR and 33% (5/15) had a CMR. Before CAR T1, all 13 patients were evaluated again and 62% (8/13) had achieved CMR. The median time to CR was 40 days (range, 14-90 days). At the end of CAR T1, 69% (9/13) of the patients had achieved CMR. The percentage of patients who achieved CMR increased to 92% (12/13) after subsequent CAR T2 to T4 and TKI. It is worth noting that the T315I mutation was discovered in patient 2 ∼3 months after CAR T3, but it was eliminated after CAR T4, and it continues to remain negative. Patients 3 and 14 experienced molecular relapses, and patient 6 was seen to have a BCR/ABL elevation during the consolidation phases. However, all patients achieved molecular remission after the subsequent CAR T-cell infusion. On the data cutoff date of 31 March 2022, 92% (12/13) of the patients remained in CMR. Eight percent (1/13) of the patients maintained hematological CR despite detectable BCR/ABL fusion. One patient (patient 4) developed CNS leukemia (CD19+ relapse) 6 months after CAR T4 before the protocol was changed to include active CNS prophylaxis, and she died of CNS involvement despite receiving several salvage chemotherapies after CAR T-cell therapy (Figure 2A-B).
Clinical outcome of treatment. (A) Swimmer plot showing the clinical response and follow-up of the individual patients, as denoted with different colors in the swimmer lanes. Each bar represents 1 patient. The indicated responses include a CR and a CMR. Before the systemic chemotherapies, imatinib was replaced with dasatinib for 2 patients. Patient 9, with no ABL mutations, had a poor response after 1 cycle of induction with imatinib but demonstrated a molecular response after reinduction with dasatinib. Patient 13 was intolerant of imatinib owing to severe rash and edema. (B) Treatment response at the end of induction therapy, each systemic therapy, and each cycle of CAR T-cell infusion. (C-D) Kaplan-Meier estimates of OS and RFS for the 15 patients who were included in the primary efficacy analysis.
Clinical outcome of treatment. (A) Swimmer plot showing the clinical response and follow-up of the individual patients, as denoted with different colors in the swimmer lanes. Each bar represents 1 patient. The indicated responses include a CR and a CMR. Before the systemic chemotherapies, imatinib was replaced with dasatinib for 2 patients. Patient 9, with no ABL mutations, had a poor response after 1 cycle of induction with imatinib but demonstrated a molecular response after reinduction with dasatinib. Patient 13 was intolerant of imatinib owing to severe rash and edema. (B) Treatment response at the end of induction therapy, each systemic therapy, and each cycle of CAR T-cell infusion. (C-D) Kaplan-Meier estimates of OS and RFS for the 15 patients who were included in the primary efficacy analysis.
The median follow-up was 27 months (range, 7-57). The median duration of remission was 27 months, and the median CMR duration was 21.5 months. The OS and RFS were 83% (95% confidence interval, 58-100) and 84% (95% confidence interval, 66-100), respectively (Figure 2C-D). Given the small sample size, reaching a conclusion was difficult, but patients with p190 tended to have a better overall OS and RFS than patients with p210 (supplemental Figure 2A-B).
Expansion and persistence
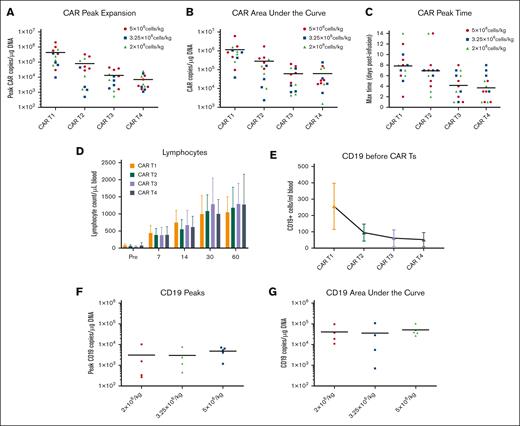
The mean transduction efficiency of CD19 CAR T cells was 46.0%, whereas that of CD19+ FTCs was 34.56% (supplemental Tables 2 and 3). The CD19 CAR T cells expanded rapidly in the peripheral blood of patients in the CAR T1 cycle, reaching peak copies within 1 week and then declining by day 28, but they persisted in all patients. In CAR T2 to T4 cycle, CD19 CAR T cells had lower peaks than in the CAR T1 cycle, with the second and third peaks occurring 1 week later (supplemental Figure 3). The peak expansion and area under the curve (AUC) of CD19 CAR T cells for 3 months after administration, gradually decreased after subsequent cycles (Figure 3A-C). The number of CD19 CAR T cells increased most significantly in the first month of CAR T1 to T2 but decreased rapidly in the second and third months (supplemental Figure 4).
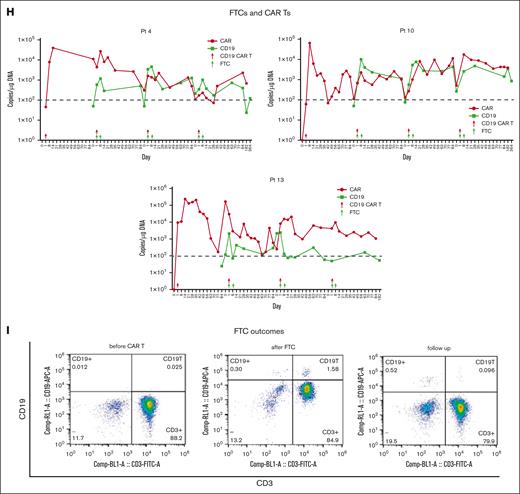
CD19 CAR T-cell and CD19+ FTC kinetics. (A-C) CD19 CAR T-cell expansion and persistence during CAR T1 to T4. Kinetics of the CAR vector transgene copies per microgram of the genomic DNA of peripheral blood mononuclear cells in the individual patients, as assessed using quantitative PCR, according to cycles of CARTs. (D) Lymphocyte counts during CAR T1 to T4 were checked from a complete blood count. “Pre” refers to day 1. (E) The cumulative CD19+ cells (B cells and CD19+ FTCs) from peripheral blood were evaluated via flow cytometry on the day before each cycle of the lymphodepletion regimen. (F-G) Aggregate peaks and AUC of CD19+ cell (CD19+ FTCs) of CAR T2 to T4 infusion in peripheral blood were examined using quantitative PCR (n = 4, 4, and 5 for low-, medium-, and high-dose groups, respectively). The CD19+ cells are referred to as CD19+FTCs. The CD19 transgene was specific. CD19+ FTCs could be determined by primer spanning the exon-exon junction of the CD19 gene. (H) The trends of the CD19 CAR T cells and CD19+ FCTs in patients 4, 10, and 13 in the high-, medium-, and low-dose groups are shown in different colors, respectively. (I) CD19+ FTCs (CD3+CD19+CD45+) in the peripheral blood of patient 10 was evaluated 7 months after CAR T4 infusion using flow cytometry.
CD19 CAR T-cell and CD19+ FTC kinetics. (A-C) CD19 CAR T-cell expansion and persistence during CAR T1 to T4. Kinetics of the CAR vector transgene copies per microgram of the genomic DNA of peripheral blood mononuclear cells in the individual patients, as assessed using quantitative PCR, according to cycles of CARTs. (D) Lymphocyte counts during CAR T1 to T4 were checked from a complete blood count. “Pre” refers to day 1. (E) The cumulative CD19+ cells (B cells and CD19+ FTCs) from peripheral blood were evaluated via flow cytometry on the day before each cycle of the lymphodepletion regimen. (F-G) Aggregate peaks and AUC of CD19+ cell (CD19+ FTCs) of CAR T2 to T4 infusion in peripheral blood were examined using quantitative PCR (n = 4, 4, and 5 for low-, medium-, and high-dose groups, respectively). The CD19+ cells are referred to as CD19+FTCs. The CD19 transgene was specific. CD19+ FTCs could be determined by primer spanning the exon-exon junction of the CD19 gene. (H) The trends of the CD19 CAR T cells and CD19+ FCTs in patients 4, 10, and 13 in the high-, medium-, and low-dose groups are shown in different colors, respectively. (I) CD19+ FTCs (CD3+CD19+CD45+) in the peripheral blood of patient 10 was evaluated 7 months after CAR T4 infusion using flow cytometry.
B-cell reconstitution may serve as a surrogate end point for effective T-cell therapy.14 Total lymphocytes decreased after the lymphodepletion regimen and accumulated to normal levels after 1 month (Figure 3D). This was consistently observed across 4 cycles. B-cell aplasia continued for more than 3 months although B cells could be detected via flow cytometry (supplemental Figure 5). Furthermore, as the number of CAR T-cell administration cycles increased, the cumulative number of CD19+ cells in patients decreased, implying the effect and durability of functional CD19 CAR T cells (Figure 3E). No differences in the CD19+ peaks (CD19+ FTCs) or AUC were observed among the 3 CD19+ FTC groups (Figure 3F-G). In each cycle, the CD19+ cells (CD19+ FTCs) increased after CD19+ FTC infusion and decreased later, paralleling the fluctuation of CD19 CAR T cells (Figure 3H) and indicating a mutual effect between the two.
The CD19 CAR T cells were durable, with all patients including patient 4 who had an isolated CNS relapse, having detectable CD19 CAR T cells by the cutoff date (supplemental Figure 5). As the treatment progressed, CD19+ FTCs were undetectable after ∼3 months after the last infusion in 8 patients and detectable in 5 patients (including detectability at very low percentages in 4 patients) via flow cytometry (Figure 3I; supplemental Figure 5). However, 1 patient (patient 14) had higher detectable CD19+ FTCs than the others. According to flow cytometry, majority of the detectable CD19+ FTCs in patient 14 were T memory stem cells and T central memory cells (supplemental Figure 6).
Dose evaluation
The kinetics of CD19+ FTCs were generally similar in the 3 groups. No clear correlation was found between the dose and the persistence of CD19+ FTCs within 3 months. The peak expansion and AUC of CD19 CAR T cells for 3 months in the 5 × 106/kg CD19+ FTC group appeared to be higher than in the other 2 groups, but no significant differences were observed because of the small sample size (supplemental Figures 7 and 8). Similarly, there were no significant differences in CMR or toxicities between the 3 groups owing to the small sample size.
Discussion
In our ongoing phase 1/2 trial that sought to enhance the prognosis of adults with newly diagnosed Ph+ ALL who did not undergo allo-HSCT, patients received chemotherapy induction and 2 courses of systemic chemotherapies plus TKI. It was followed by a novel consolidation strategy with 1 cycle of CD19 CAR T-cell therapy and 3 cycles of sequential therapies with CD19 CAR T cells and CD19+FTCs, which are autologous T cells with overexpressed CD19 via recombinant engineering technology, along with TKI (imatinib or dasatinib). To our knowledge, this was the first study to enforce consolidation without allo-HSCT using a multicycle-sequential CD19 CAR T-cell therapy.
The trial's primary end point analysis showed that multicycle-sequential CD19 CAR T-cell consolidation therapy was safe and feasible. The adverse effects seen in this trial were similar to those seen in other CD19 CAR T-cell studies.8-10,17 CRS was observed within a week and was mild throughout the 4 cycles. Infusion of CD19+ FTCs in CAR T2-T4 did not worsen CRS but only slightly increased cytokine levels. No ICANS was observed during CAR T1 to T4. Cytopenia was the most common and obvious but reversible AE among the patients. The first occurrence of cytopenia within 2 weeks was linked to fludarabine and cyclophosphamide, and it was alleviated by granulocyte colony-stimulating factor administration and blood transfusion. The second neutropenia, which occurred ∼1 month after CD19 CAR T-cell infusion, was most likely caused by the side effects of CD19 CAR T cells as well as TKIs, as neutropenia was the most common grade 3/4 hematological AE associated with TKI.18 One patient suffered from infections, when discharged from the hospital, which manifested as viral infections, necessitating periodic hospitalization and IVIG injections because of hypogammaglobulinemia. Regardless of the above observations, the potential risks of multiple infusions of CD19 CAR T cells and CD19+FTCs, such as tumorigenicity caused by lentiviral vectors, must always be borne in mind, and patients should be monitored for these risks for as long as possible.
CMR is an important indicator for predicting RFS and OS.19-21 For patients who underwent allo-HSCT as consolidation therapy after a TKI-based regimen, the CMR rate varied between generations of TKIs,22-24 with the highest occurring with ponatinib, which had an associated CMR of 73% at ∼3 months of therapy.5 Similarly, after excluding patients who underwent allo-HSCT, the CMR rates at 3 months for patients receiving imatinib, dasatinib, or ponatinib were 39%, 54%, and 87%, respectively; improving with each successive generation of TKIs.20 In our study, patients in CR received multicycle-sequential CD19 CAR T-cell consolidation therapy. Our data indicated improvement in our patients, with a CMR of 69% at 3 months after CAR T1, and it increased to 92% at 3 months after CAR T4, even though the TKI that we primarily used was imatinib, a first-generation TKI.
It should be noted that attaining CMR is associated with improved OS and RFS.19-21 The 1-, 2-, and 4-year OS rates in this study were 100%, 100%, and 83%, respectively, whereas the 1-, 2-, and 4-year RFS rates were 100%, 93%, and 84%, respectively. In contrast, previous studies have reported a consolidation strategy with allogenic or autologous HSCT or TKI. After allogeneic HSCT, The 1-, 2-, and 5-year OS rates were 69.6%, 61.1%, and 50.3%, respectively.25 In the autologous cohort with MRD negativity, the posttransplant RFS and OS rates were 46.1% and 55.1%,26 respectively. The 5-year OS was 30% to 40% with imatinib and 40% to 50% with dasatinib in the patients without HSCT,19 and the overall 4-year OS and RFS rates for patients who had achieved CMR at 3 months were 66% and 63% without HSCT, respectively.20 CD19 CAR T cells were particularly active against the ABL1 mutation clone because ABL1 was only mutated after CAR T3 in patient 2 but became negative again after CAR T4, indicating a complementary effect between CD19 CAR T cells and TKIs. Taken together, analysis of the secondary end point in our trial demonstrated that multicycle-sequential CD19 CAR T-cell consolidation therapy achieved favorable CMR, RFS, and OS among the reported strategies of HSCT and TKI, or their combination.
The CAR copies were monitored using qRT-PCR during the entire multicycle-sequential therapy and follow-up. The kinetics of CAR T cells in the first cycle with CD19 CAR T cells alone were similar to previous studies.27,28 Although the peak expansion and AUC of CD19 CAR T cells were lower after CAR T2 to T4 than after CAR T1, CD19 CAR T cells were seen to persist for up to 40 months after the last infusion as per our results. Furthermore, multiple peaks of CD19 CAR T cells were observed in all patients, indicating that the stimulation of supplemented CD19+ FTCs to CD 19 CAR T cells likely played a key role in the long-term expansion of CD19 CAR T cells. The cumulative number of CD19-expressing cells decreased with each cycle of CD19 CAR T infusion, indicating that leukemia clearance was effective. Interestingly, even after the addition of CD19+ FTCs, CD19 CAR T-cell expansion gradually declined over 4 cycles, indicating that the CAR T-cell expansion was not entirely dependent on CD19+FTCs but rather on the gradual consumption and reconstruction of total CD19+ cells in the body. Furthermore, the endogenous immune response against murine CAR may also contribute to the declining CAR T-cell expansion as evidenced by prior studies.29,30 Although this could be mitigated using an FC (fludarabine + cyclophosphamide) lymphodepletion regimen in our study, the development of humanized single-chain variable fragment is essential for reducing CAR immunogenicity. CD19+ FTCs were almost undetectable after 3 months, except in 1 patient who had 2% to 3% CD19+ FTCs and an elevated proportion of memory T cells in the peripheral blood sample. In conclusion, to our knowledge, this was the first study to investigate the kinetic parameters of CD19 CAR T cells plus CD19+ FTCs as continuous consolidation therapy in patients with Ph+ ALL with CR, and it provides a foundation for efficacy and safety.
To date, only 1 patient (patient 4) who did not receive systemic therapies relapsed with isolated CNS disease while still having CMR in the bone marrow and CD19 CAR T cells in her peripheral blood. Most importantly, no CNS relapse was observed after active prophylaxis against the CNS sanctuary was implemented because of the preceding observation, even though most patients (10/13) received imatinib. In contrast, in a previous study, isolated relapses in the CNS were reported in 11% of the patients treated with dasatinib and chemotherapy,24 even though, dasatinib penetrates the blood-brain barrier better than imatinib. Furthermore, 2 isolated CNS relapses were observed in patients treated with dasatinib and blinatumomab, although 12 lumbar punctures were performed for a total of 12 procedure.4 Moreover, during the 40-month update of D-ALBA (dasatinib-blinatumomab), 9 relapses have been documented, including 4 hematological, 4 CNS, and 1 nodal in nature.31 Several factors may have contributed to our finding of fewer CNS relapses, which comprise (1) CNS prophylaxis, including systemic chemotherapies and intrathecal chemotherapies; (2) the ability of CD19 CAR T cells to migrate to the cerebrospinal fluid and eradicate CNS MRD;27,28,32 and (3) the potential synergy between CD19 CAR T cells and TKIs via blood-brain barrier penetration. More research into their synergy is required.
Repeated infusions of CAR T cells were previously reported for the treatment of relapsed/refractory ALL,30,33-36 and we investigated their potential as first-line consolidation therapy for patients with CR. Only 1 study examined relapse prevention with secondary CD19 CAR T-cell infusion, which showed limited efficacy, with only 52% patients achieving CR on day 28% and 33% remaining in remission after a median follow-up of 39 months.37 Therefore, repeated CAR T-cell infusions alone were not sufficient to extend the duration of efficacy. The robust proliferation of reinfused CD19 CAR T cells is essential for long-term efficacy, which necessitates continuous stimulation by sufficient CD19+ cells.30 Supplementation with CD19+ FTCs may compensate for target cell deficiency under CR conditions. In our study, CD19 CAR T cells were combined with CD19+FTCs. In our trial, CMR increased to 84% after CAR T2. As a result of the multicycle-sequential CD19 CAR T cells treatment followed by CD19+ FTCs and TKI administration, not only did all patients maintain a longer duration of CR until the last follow-up, but the CMR rate continuously increased up to 92%, indicating that the residual leukemia cells could be deeply cleared by the multicycle-sequential consolidation strategy. In particular, patients 3 and 14 had molecular relapses, and patient 6 had a BCR/ABL elevation during the consolidation phases. However, all patients achieved molecular remission after the subsequent CD19 CAR T-cell infusion, demonstrating the efficacy of multicycle-sequential CD19 CAR T-cell infusions aided by the CD19+FTCs strategy. The “pulsed” supplementary CD19 antigen and expandable CD19 CAR T cells, similar to the boosting mechanism of vaccination, may form long-term immune monitoring to eliminate MRD.
TKIs are widely thought to inhibit T-cell activation.38,39 However, TKI plays an immune modulatory role by selectively depleting Treg cells and increasing the number of effector/memory CD8+ T cells to elicit effective immune responses to various cancers.40 Furthermore, TKI could reduce the differentiation and exhaustion of 4-1BB/CAR T cells during ex vivo expansion, enhancing their therapeutic efficacy in mouse models.41 Therefore, we believe that rational TKI administration is critical for eliciting their beneficial effects on CAR T cells. Because the peak expansion of CD19 CAR T cells inthe patients in our study was noted ∼1 week after infusion, TKI (imatinib or dasatinib) was designed to be administered 2 weeks after infusion to enable it to exert its immune moderation benefits at the appropriate time and avoid damaging the CD19 CAR T-cell expansion.
There were no DLTs found in the three-dose subgroups of CD19+ FTCs. Hematological toxicity was the most common AE across all three-dose subgroups, with no statistically significant differences in incidence observed. The AUC and peak expansion of CD19 CAR T cells were greater in the high-dose group than in the other 2 groups. However, there were no significant differences owing to the small sample size. Based on the safety and efficacy profile and kinetics results, we chose the high-dose CD19+ FTC group for the ongoing extended clinical trial.
The results presented in this study have demonstrated potent durable CMR, and an encouraging safety profile, achieved by multicycle-sequential anti-CD19 CAR T-cell/CD19+ FTCs consolidation strategy in patients with Ph+ ALL. However, the benefits of this allo-HSCT-free consolidation strategy require confirmation through an ongoing phase 2 trial, and a randomized trial to be conducted at our center in the near future. The infusion of CD19+ FTCs alone as vaccination is also intriguing, but according to our knowledge, investigators are conducting a clinical trial to observe if administering T-cell–antigen-presenting cells expressing truncated CD19 (T-APCs) after CAR T-cell treatment can improve CD 19 CAR T-cell persistence and reduce the incidence of leukemia relapse (NCT03186118). We eagerly await the release of their data. Other future directions we intend to pursue could include the optimization of the antigen vaccination platform.
In conclusion, our findings indicated that multicycle-sequential CD19 CAR T-cell consolidation therapy combined with autologous CD19+ FTCs and TKI was safe. CRS was mild, there were no ICANS, and the hematological and nonhematological toxicities were manageable and reversible. A high CMR rate and a long duration of remission was achieved, and few relapses were reported.
Acknowledgments
This study was supported by the grants from National Natural Science Foundation of China (grant numbers 81970138, 82270165, 82200249), Jiangsu Province Natural Science Foundation (No. BK20210091, BK20221235), Translational Research Grant of NCRCH (grant numbers 2020WSC01, 2020ZKMB05), Social Development Project of the Science and Technology Department of Jiangsu (BE2021649), Jiangsu Province “333” project, and Gusu Key Medical Talent Program (GSWS2019007).
The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the article, or decision to submit the article for publication.
Authorship
Contribution: L.-Y.C. and W.-J.G. wrote the first draft of the manuscript; W.-J.G. and L.-Y.C. revised the manuscript; M.-H.L. was responsible for the laboratory analysis; S.-L.X. was the principal investigator and designed the trial and study protocol; D.-P.W. and L.Y. were both principal investigators; L.-Y.C., D.-P.W., A.-N.S., L.-Q.K., L.Y., and Z.-L.T. contributed to the study protocol amendment; L.-Y.C., W.-J.G., H.-X.Z., M.-Z.X., C.-S.Q., M.Q., T.-T.Z., L.Z., and S.-L.X. enrolled and treated patients; N.X. and Z.Y. contributed to the revision of the manuscript; and S.-L.X. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict-of-interest disclosure: M.-H.L., L.-Q.K., N.X., Z.Y., and L.Y. are employees of Shanghai Unicar-Therapy Bio-medicine Technology Co, Ltd. Z.-L.T. is an employee of Gobroad Medical Group. The remaining authors declare no competing financial interests.
Correspondence: Sheng-Li Xue, National Clinical Research Center for Hematologic Diseases, Jiangsu Institute of Hematology, The First Affiliated Hospital of Soochow University, Shizi St 188, Suzhou 215006, China; e-mail: slxue@suda.edu.cn.
References
Author notes
∗L.-Y.C., W.-J.G., and M.-H.L. are joint first authors.
†L.Y., D.-P.W., and S.-L.X. contributed equally to this work.
Most of the data are presented in the main article and the extensive supplemental tables, figures, and protocol of the article (open access); any additional data will be made available on request from the corresponding author, Sheng-Li Xue (slxue@suda.edu.cn).
The full-text version of this article contains a data supplement.