Abstract
Chronic graft-versus-host disease (cGvHD) remains a prominent barrier to allogeneic hematopoietic stem cell transplantion as the leading cause of nonrelapse mortality and significant morbidity. Tremendous progress has been achieved in both the understanding of pathophysiology and the development of new therapies for cGvHD. Although our field has historically approached treatment from an empiric position, research performed at the bedside and bench has elucidated some of the complex pathophysiology of cGvHD. From the clinical perspective, there is significant variability of disease manifestations between individual patients, pointing to diverse biological underpinnings. Capitalizing on progress made to date, the field is now focused on establishing personalized approaches to treatment. The intent of this article is to concisely review recent knowledge gained and formulate a path toward patient-specific cGvHD therapy.
Introduction
Chronic graft-versus-host disease (cGvHD) remains the leading cause of long-term morbidity and mortality after allo hematopoietic stem cell transplantation (alloHSCT).1,2 Significant progress has been made in understanding cGvHD pathophysiology,3,4 leading to preclinical testing and clinical translation of agents that are now Food and Drug Administration (FDA)-approved for steroid-resistant disease,5-7 as well as the potential development of new approaches for prophylaxis and first-line treatment. As a result of the National Institutes of Health (NIH)-supported cGvHD consensus development project, disease definition, clinical features and severity, and criteria for treatment response have been established.8,9 The 2020 NIH consensus development project refined various aspects of cGvHD biology, clinical spectra, current status, and mapped future advancements in therapy.10 The goal of the present article is to summarize how recent understandings of cGvHD mechanisms may facilitate personalized treatment and prevention strategies.
cGvHD can affect many organs and systems, typically involving barrier sites that are entry points for pathogens and home to cellular populations within the adaptive as well as innate immune systems.4,11,12 Human and animal studies have implicated inflammatory mediators, cytokines, danger-associated molecular patterns, and metabolites as contributors to cGvHD pathogenesis.4,13-17 The immune system dysregulation that leads to cGvHD can be mediated by T cells, B cells, natural killer (NK) cells, NKT cells, monocytes, and macrophages, and can be ameliorated by regulatory populations of T cells, B cells, myeloid cells (monocytes, macrophages, myeloid derived suppressor cells) and NK cells4,11,12 (Figure 1). Whether the affected organ systems share pathogenic mechanisms that initiate and sustain cGvHD is not yet fully elucidated, albeit certain immune populations have been shown in animal models to mediate cGvHD in multiple target organs.4,18,19 Whether clinical symptoms in patients represent protean manifestations of a limited set of pathophysiological processes or diverse mechanisms that invoke specific cGvHD manifestations is unclear. Clinically, variability in organ involvement is compounded by a spectrum of clinical subtypes and variable posttransplant timing, which adds to the complexity of discerning underlying mechanisms and devising strategies to overcome cGvHD. Taken together, these studies support the conclusion that the biology of cGvHD is complex and likely to be heterogenous. New treatment approaches for cGvHD focused around the concept of personalized therapy are greatly needed.
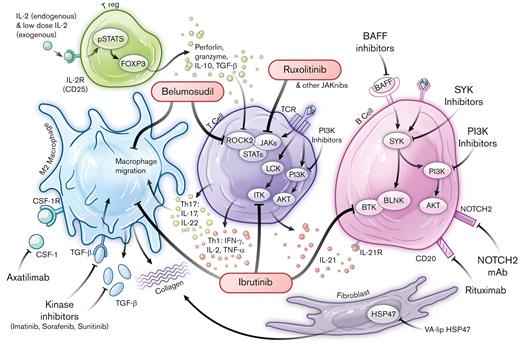
Pathophysiological networks and targeted approaches for prevention and treatment of cGvHD. FDA-approved drugs are highlighted within red bubbles. Ag, antigen; AKT, Ak strain transforming; BAFF, B-cell activating factor; BAFF R, BAFF receptor; BCR, B-cell receptor; BLNK, B-cell linker; BTK, Bruton’s Tyrosine Kinase; BTK/ITK, Bruton’s Tyrosine Kinase /IL-2 Inducible T-cell Kinase; CD, cluster of differentiation; FOXP3, forkhead box P3; HSP47, heat shock protein 47; INF-γ, interferon gamma; ITK, IL-2-inducible T-cell kinase; Jakinibs, Janus kinase inhibitors; JAKs, Janus kinases; LCK, lymphocyte-specific protein tyrosine kinase; NOTCH2, Neurogenic locus notch homolog protein 2; NOTCH2 mAb, neurogenic locus notch homolog protein 2 mAb; PI3K, phosphoinositide 3-kinase; pSTAT5, phosphorylated signal transducer and activator of transcription 5; ROCK2, Rho associated coiled-coil containing protein kinase 2; STATs, signal transducers and activators of transcription; SYK, spleen associated tyrosine kinase; TCR, T-cell receptor; TGF-β, transforming growth factor beta; Th, T helper; TNF-α, tumor necrosis factor alpha; Treg, T regulatory cell; VA-lip HSP47, vitamin A coupled liposomal containing small interfering RNA (siRNA) against heat shock protein 47.
Pathophysiological networks and targeted approaches for prevention and treatment of cGvHD. FDA-approved drugs are highlighted within red bubbles. Ag, antigen; AKT, Ak strain transforming; BAFF, B-cell activating factor; BAFF R, BAFF receptor; BCR, B-cell receptor; BLNK, B-cell linker; BTK, Bruton’s Tyrosine Kinase; BTK/ITK, Bruton’s Tyrosine Kinase /IL-2 Inducible T-cell Kinase; CD, cluster of differentiation; FOXP3, forkhead box P3; HSP47, heat shock protein 47; INF-γ, interferon gamma; ITK, IL-2-inducible T-cell kinase; Jakinibs, Janus kinase inhibitors; JAKs, Janus kinases; LCK, lymphocyte-specific protein tyrosine kinase; NOTCH2, Neurogenic locus notch homolog protein 2; NOTCH2 mAb, neurogenic locus notch homolog protein 2 mAb; PI3K, phosphoinositide 3-kinase; pSTAT5, phosphorylated signal transducer and activator of transcription 5; ROCK2, Rho associated coiled-coil containing protein kinase 2; STATs, signal transducers and activators of transcription; SYK, spleen associated tyrosine kinase; TCR, T-cell receptor; TGF-β, transforming growth factor beta; Th, T helper; TNF-α, tumor necrosis factor alpha; Treg, T regulatory cell; VA-lip HSP47, vitamin A coupled liposomal containing small interfering RNA (siRNA) against heat shock protein 47.
FDA-approved approaches targeting pathophysiologic mechanisms in cGvHD
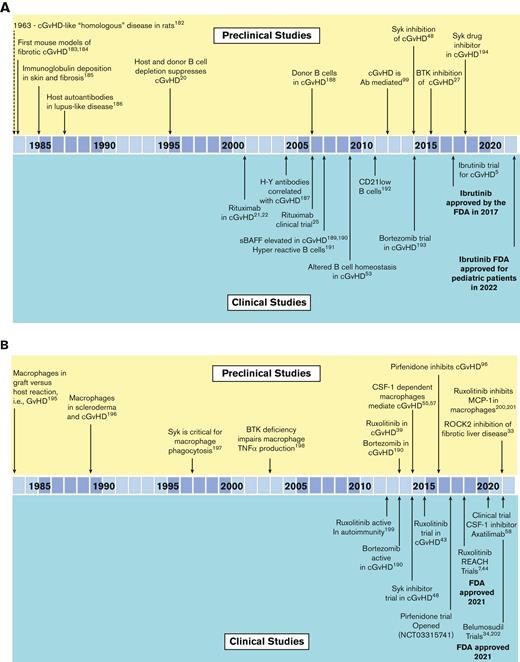
The development of the first FDA-approved therapeutic agent for cGvHD, ibrutinib, illustrates the iterative process that can exist between both preclinical models and correlative human biology studies (Figure 2A). From the first preclinical murine cGvHD studies, which established the role of B cells in cGvHD,20 the preclinical models and clinical human biomarker studies detailed the B-cell abnormalities associated with cGvHD development. This resulted in the testing of the anti-CD20 monoclonal antibody (mAb), rituximab, as a B-cell targeting strategy for cGvHD21-26 that demonstrated clinical responses in steroid-refactory cGvHD. Preclinical cGvHD mouse studies focused on inhibiting Bruton tyrosine kinase/interleukin-2 (IL-2) inducible T-cell kinase, with amelioration of disease manifestations observed in several models.27 B and T cells from patients with active and persistent cGvHD showed signs of hyperactivity that were lowered by adding ibrutinib to cell culture. Based on these aggregate results and the high unmet medical need for agents to treat patients with steroid-refractory cGvHD, a single-arm open-label study ibrutinib gained FDA regulatory approval in 2017.5,28
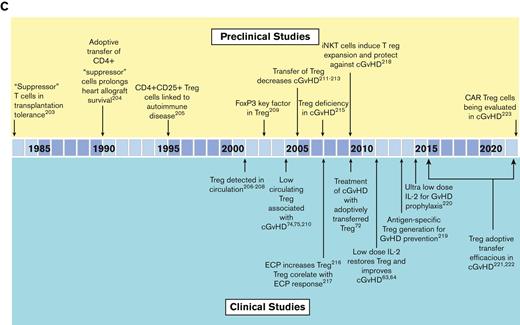
Summary of the preclinical modeling and clinical studies that led to the development of FDA-approved agents for chronic GvHD. (A) The role of B cells and targeting of B cells in cGvHD. (B) The role of macrophages and targeting of macrophage function in cGvHD. (C) The role of Tregs, in suppressing cGvHD and the development of cGvHD therapies aimed at Treg expansion/improved function. Ab, antibody; BTK, Bruton’s Tyrosine Kinase; CAR Treg, chimeric antigen receptor T regulatory cell; ECP, extracorporeal photopheresis; FoxP3, forkhead box P3; H-Y Ab, antibodies specific for male (Y) antigens; MCP-1, monocyte chemoattractant protein-1; iNKT, invariant NK T cells; ROCK2, Rho associated coiled-coil containing protein kinase 2; sBAFF, soluble B-cell activating factor; SYK, spleen sssociated tyrosine kinase; TNF-α, tumor necrosis factor alpha. Reference citations used: 5,7,20-22,25,27,33,39,43,45,48,53,55,57,58,63,64,72,74,75,96,99,182-223.
Summary of the preclinical modeling and clinical studies that led to the development of FDA-approved agents for chronic GvHD. (A) The role of B cells and targeting of B cells in cGvHD. (B) The role of macrophages and targeting of macrophage function in cGvHD. (C) The role of Tregs, in suppressing cGvHD and the development of cGvHD therapies aimed at Treg expansion/improved function. Ab, antibody; BTK, Bruton’s Tyrosine Kinase; CAR Treg, chimeric antigen receptor T regulatory cell; ECP, extracorporeal photopheresis; FoxP3, forkhead box P3; H-Y Ab, antibodies specific for male (Y) antigens; MCP-1, monocyte chemoattractant protein-1; iNKT, invariant NK T cells; ROCK2, Rho associated coiled-coil containing protein kinase 2; sBAFF, soluble B-cell activating factor; SYK, spleen sssociated tyrosine kinase; TNF-α, tumor necrosis factor alpha. Reference citations used: 5,7,20-22,25,27,33,39,43,45,48,53,55,57,58,63,64,72,74,75,96,99,182-223.
Two additional agents have garnered FDA approval in 2021 (Figure 2B-C) and both emanated from preclinical studies. Belumosudil, a Rho associated coiled-coil containing protein kinase 2 inhibitor, tipped the balance from phosphorylated signal transducer and activator of transcription 3 to phosphorylated signal transducer and activator of transcription 5, dampened IL-17 and IL-21 production that plays a role in fibrotic manifestations in animal cGvHD models,29-34 and led to the initiation of a first-in-human and first-in-disease clinical trial culminating in FDA approval for the treatment of steroid-refractory cGvHD.6
Ruxolitinib, a janus kinase/signal transducers and activators of transcription inhibitor (Figure 1) that has known benefits in myelofibrosis35,36 and autoimmunity,37,38 was evaluated in acute GvHD animal models,39-43 followed by clinical trials, REACH-144 and REACH-2.7,45 Positive findings led to FDA approval for treatment of steroid-refractory acute GvHD. Subsequently, REACH-3, a prospective randomized phase 3 trial in steroid-refractory (or -dependent) cGvHD as compared to the best available therapy,7 led to the approval from FDA for this indication.46
Agents being evaluated for FDA approval in cGvHD treatment
Other cGvHD B-cell targeting strategies have been vetted in mouse cGvHD models (Figure 2A). Of note, increased B-cell receptor responsiveness was observed in B cells from patients with cGvHD.47 In vitro exposure of cGvHD B cells to a spleen associated tyrosine kinase inhibitor selectively induced B-cell apoptosis in cells derived from patients with cGvHD compared with those without cGvHD. In vivo testing in mouse cGvHD models resulted a consistent reduction in disease severity.48 Beyond targeting of spleen associated tyrosine kinase, positive preclinical data for inhibitors of phosphoinositide-3 kinase delta and B-cell activating factor delineated subsequent clinical investigations in patients with cGvHD5,34,49-53 with welcome promising results given modest postapproval clinical response rates, now reported with ibrutinib.54
In mice with cGvHD, tissue accumulation of monocytes and macrophages that depend upon colony stimulating factor-1 receptor (CSF-1R) signaling for generation and survival was observed55-57 (Figure 2B). Anti-CSF-1R mAb treatment was effective in multiple cGvHD models providing the impetus for initiating the ongoing clinical trial of a CSF-1R mAb, axatilimab, to treat steroid-refractory cGvHD.55 To date, axatilimab is demonstrating very promising activity58 and is now undergoing pivotal clinical trials.
Thymus injury that accompanies cGvHD, along with cell exhaustion within target organs, leads to a deficiency of regulatory T cells (Tregs). Adoptive transfer of ex vivo expanded Tregs or in vivo expansion via low-dose IL-2 administration has clear efficacy in cGvHD, as seen in animal and clinical studies59-76 (Figure 2C). It will soon be tested in a steroid-refractory cGvHD setting through the Children’s Oncology Group in a trial that combines low-dose IL-2 with ex vivo expanded Tregs.
To summarize, numerous bench-to-bedside studies have advanced the understanding of cGvHD pathogenesis, which has resulted in the development of new therapies. Furthermore, biological pathways implicated in autoimmune diseases have also yielded therapeutic targets proven efficacious in acute and cGvHD upon bench-to-bedside evaluations.
Other available treatment strategies
Currently, available therapies to treat steroid-refractory cGvHD have been previously extensively reviewed and include extracorporeal photopheresis, hydroxychloroquine, methotrexate, bortezomib, pentamidine, etanercept, calcineurin inhibitors, sirolimus, and others.49
Influence of graft and early post alloHSCT manipulations on cGvHD
A number of interventions at the time of alloHSCT have resulted in a decreased cGvHD incidence. This illustrates that early immune modulations can impact cGvHD onset that occurs many months later and can potentially affect long-term immune reconstitution and tolerance patterns. In vivo T-cell depletion, including peritransplant alemtuzumab and antithymocyte globulin hello, decrease cGvHD occurrence,77 presumably by reducing the number of alloreactive T cells that are able to orchestrate cGvHD, while allowing B and NK cell expansion, which may not be beneficial for reducing cGvHD. Another graft cell depletion approach includes ex vivo naïve T-cell depletion, which was associated with a diminution of cGvHD,78 and is supported by results of a recent clinical trial.79
When given as prophylaxis early after donor graft infusion, post-transplant cyclophosphamide (PTCy) significanctly suppressed rodent acute and cGvHD, which was initially thought to be mediated through the in vivo depletion of alloreactive T cells, with clinical efficacy confirmed in human trials.80 With low cost, favorable tolerability, and efficacy in preventing cGvHD, PTCy is now a widely used cGvHD prophylaxis approach.81-83 Despite the clinical utility of PTCy, its exact mechanism of action in alloHSCT is not fully elucidated.84 As a result, further studies in alloHSCT animal models85,86 and clinical samples87,88 are ongoing in order to refine and optimize PTCy for alloHSCT. To date, murine models have identified several potential mechanisms of action, including reduction of alloreactive CD4+ effector T cells, impairment of function in surviving alloreactive CD4+ and CD8+ effector T cells, and early expansion of Treg cells.84
It has been found that the graft stem cell source significantly impacts later cGvHD development, with a higher cGvHD incidence after donor G-CSF mobilized peripheral blood stem cells (G-PBSC) than bone marrow (BM). One study found that low CD56bright NK cell frequency in recipients of G-PBSC is associated with higher cGvHD rates than G-BM,89 but this has not been directly compared to unstimulated BM grafts. Others observed the association between faster reconstitution of naïve and memory T-cell recovery in G-PBSC and increased cGvHD, albeit with better survival.90 In the same study, higher classical dendritic cell number in circulation of G-BM recipients was associated with higher cGvHD incidence, but again with better survival. Plerixafor mobilization has been used in place of G-CSF91 and such grafts may phenotypically resemble BM-like grafts that would be expected to lead to lower GvHD rates.92,93 However, this too has yet to be confirmed clinically by direct comparison to G-PB or unstimulated BM and murine data suggest that plerixafor may be associated with more severe sclerotic GvHD than G-PBSC.94
The use of umbilical cord blood grafts is associated with lower rates of cGvHD90; yet, there is also evidence that poor immune reconstitution after umbilical cord blood can be associated with lower circulating total Th cells, slower naïve Th cells reconstitution and increased cGvHD development.95 Whereas, in another study, circulating Tfh cells were found to be lower in frequency but more highly activated in patients with cGvHD.29 Further work is needed to delineate how impaired immune reconstitution impacts on immune tolerance and cGvHD.
Ongoing and future preclinical and clinical studies to identify targets for treatment and prevention of cGvHD
Preclinical models
Fibrotic cGvHD manifestations have long been recognized clinically and explored at the bench.55-57,96-99 Several pathways involving macrophage reprogramming are implicated in cGvHD, including CSF-1R55-57 as well as fibroblast differentiation with aberrant collagen production and uncontrolled fibrosis in mouse models,97,98 findings that are also seen in cGvHD clinical specimens.100 A topical collagen inhibitor has shown promising preclinical efficacy as prophylaxis and treatment for ocular98 and cutaneous cGvHD.97 The antifibrotic agent, pirfenidone, dampens inflammatory mediators and SMAD2/3 signaling, decreases fibroblast proliferation, and TGFβ-induced procollagen production with encouraging results in mouse models of lung and sclerotic skin cGvHD96 and efficacy in a clinical phase 1 study for bronchiolitis obliterans.101
Preclinical testing in rodent,102 canine,103 and nonhuman primate104-106 studies indicated that the inhibition of CD28/B7 costimulation suppressed GvHD. Positive results of a clinical trial of abatacept, CTLA4-Ig that blocks CD28/B7, combined with a calcineurin inhibitor and methotrexate, led to FDA approval for acute GvHD prevention in patients receiving HLA-matched or 1 allele mismatched donor grafts.107 Testing for steroid-refractory cGvHD is ongoing (NCT01954979).108
Bedside approaches undergoing bench interrogation include microbiota classification and modulation designed to preempt and ameliorate gut acute GvHD that often precedes cGvHD in patients.109-117 Despite differences in microbiome composition, diet, and environment between rodents and humans, overall patterns can yield generalizable principles, easing extrapolation between species. Clinical data from geographically diverse study centers pointed to distinct microbiota data sets that inform pertinent microbiome components117 and potential interventions.118 Microbiota driven systemic metabolic changes have been elucidated in clinical studies and modeled in animals, including in cGvHD.117 In a primary case-control cohort and 1 of 2 cross-sectional cohorts, lower day 100 plasma levels of microbial derived short chain fatty acids, proprionate and butyrate, were associated with cGVHD.117 An active cGvHD research area is cellular immune metabolism. Emerging laboratory-based15,119-121 and early clinical biospecimen analyses of cellular and plasma metabolites16,122 suggest that the metabolic reprogramming of immune cells represents a potential therapeutic and diagnostic target for acute and cGvHD.13,14,17,123,124
Other targets recently implicated in mouse125,126 and canine127 cGvHD models are the inducible costimulatory (ICOS) and Notch pathways. ICOS signaling blockade with anti-ICOS mAb in preclinical studies provides evidence for this treatment strategy.127,128 The Notch pathway has been identified as a driving force in cGvHD pathogenesis in murine129 and clinical49 cGvHD, with numerous inhibitors currently undergoing clinical testing in oncology, but not yet in GvHD.
Clinical annotation of cGvHD
Chronic GvHD represents 3 intertwined pathophysiologic processes involving inflammation, immune dysregulation, and fibrotic manifestations that contribute to the development of the disease.3,130 Although the inflammatory and fibrotic phases are often identifiable on physical exam and clinical evaluation, especially cutaneous and oral erythema (inflammatory) or dermal sclerosis (fibrotic), they may occur concurrently. Thus, a constellation of clinical findings may not fit neatly into a single category or continuum of cGvHD progression. Recently, machine learning and computational analyses that take into account clinically relevant patterns and organ involvement have expanded risk strata for cGvHD survival for application to decisions to test the validity and exportability of choosing and initiating the most appropriate therapies.131 To enable personalized cGvHD treatment, additional diagnostic tools, objective clinical features, imaging, and laboratory parameters are needed. Although laboratory, imaging, cellular, molecular, and metabolic data are being gathered, clinical annotation and scoring will be essential to categorize and diagnose GvHD not simply by severity, but by biological subtypes. With such data, rational therapy selection can yield the highest chance of efficacy for the individual patient.132
Biomarkers
Disease predictive biomarker panels are being tested for validation and adaptation in prospective studies of acute133-136 and chronic137-139 GvHD. The biomarkers, in addition to being clinically useful, may identify potential mechanisms and biologic targets for therapeutic interventions. Identified biomarkers can then be used to probe hypotheses for the pathophysiological basis of identified targets in animal modeling. As a general rule, biomarkers probably represent underlying biology, but may only be a surrogate with no direct association with cGvHD, primarily serving as a reproducible means to guide and optimize therapy, Table 1.
Application of BEST (Biomarkers EndpointS & other Tools) biomarker definitions in cGvHD∗
| Type of marker . | Definition . | Application in alloHSCT . |
|---|---|---|
| Diagnostic | A biomarker used to detect or confirm presence of disease or condition of interest or to identify individuals with a subtype of the disease. | At the onset of cGvHD or late aGvHD compared with time-matched control |
| Response | A biomarker used to show that a biological response has occurred in an individual who has been exposed to a medical product or an environmental agent. | Measured 4-8 wk after treatment initiation for cGvHD |
| Predictive | A biomarker used to identify individuals who are more likely than similar individuals without the biomarker to experience a favorable or unfavorable effect from exposure to a medical product or an environmental agent. | Measured at the onset of treatment of cGvHD |
| Prognostic | A biomarker used to identify likelihood of a clinical event, disease recurrence or progression in patients who have the disease or medical condition of interest. | Measured at the onset of treatment of cGvHD |
| Risk | A biomarker that indicates the potential for developing a disease or medical condition who does not currently have clinically apparent disease or the medical condition. | Measured before cGvHD has developed, eg, at engraftment |
| Type of marker . | Definition . | Application in alloHSCT . |
|---|---|---|
| Diagnostic | A biomarker used to detect or confirm presence of disease or condition of interest or to identify individuals with a subtype of the disease. | At the onset of cGvHD or late aGvHD compared with time-matched control |
| Response | A biomarker used to show that a biological response has occurred in an individual who has been exposed to a medical product or an environmental agent. | Measured 4-8 wk after treatment initiation for cGvHD |
| Predictive | A biomarker used to identify individuals who are more likely than similar individuals without the biomarker to experience a favorable or unfavorable effect from exposure to a medical product or an environmental agent. | Measured at the onset of treatment of cGvHD |
| Prognostic | A biomarker used to identify likelihood of a clinical event, disease recurrence or progression in patients who have the disease or medical condition of interest. | Measured at the onset of treatment of cGvHD |
| Risk | A biomarker that indicates the potential for developing a disease or medical condition who does not currently have clinically apparent disease or the medical condition. | Measured before cGvHD has developed, eg, at engraftment |
Definitions are based on the FDA-NIH Biomarker Working Group Resource224.
Diagnostic biomarkers may assist the clinician in early detection of insidious cGvHD, allowing for early intitiation of therapy or more accurate criteria for enrollment onto clinical trials. Predictive and prognostic biomarkers, usually measured at therapy intiation, may allow for biologic classification of cGvHD and aid in treatment selection most likely to engender a response to cGvHD therapy or a specific therapeutic intervention.
Risk assignment biomarkers may allow determination of whether the patient is at a high, moderate, or low risk of subsequently developing cGvHD and used in the design of preventive or preemptive trials or treatment strategies. With risk algorithms, patients may receive either a preemptive increase or decrease in therapy to avert onset of cGvHD or to decrease therapy to minimize the toxicity of immunosuppressive prophylaxis, respectively. Biomarkers not only of cGvHD, but also of operational immune tolerance, may potentially identify the safest time for a patient to stop immune suppression or predict which patient is likely not to respond to the recommended therapy.
Reports from the 2020 NIH Consensus Development Project on Criteria for Clinical Trials in cGvHD offer a vision for personalized preemptive interventions for cGvHD based on clinical risk factors and biomarkers measured before symptom onset.140,141 As summarized in the report from the Etiology and Prevention Working Group of the 2020 NIH Consensus Development Project,142 the field has long recognized pretransplant clinical risk factors associated with cGvHD, though no risk assessment prognostic systems exist at this time. Progress has been made in identifying candidate biomarkers or biomarker signatures measured at specific time points after transplantation that could identify patients who have a high risk of developing cGvHD. At 3 months after alloHSCT, pediatric and adolescent patients who subsequently developed cGvHD had decreased numbers of CD21low B cells and CD56bright regulatory NK cells in the blood, increased naïve T helper cells and PD1+ naïve T cytotoxic cells, and increased plasma ST2 (IL-33 receptor) and soluble CD13 concentrations.137 At 6 months after alloHSCT, adult patients with low numbers of circulating Tregs had an increased cGvHD risk.126 For such patients, low-dose IL-2 or IL-2 mutant proteins (muteins) with decreased CD122 (IL-2Rb) affinity will favor Treg cells over NK or CD8 T cells and may ameliorate active cGvHD by expanding Treg cells in vivo. Whether the 3-month biomarkers have a causal relationship with cGvHD incidence or severity is unknown but warrants testing. Because GvHD-causing Teffectors are glycolytic, metabolic imaging with nonradioactive glucose isotopes18,119 or radioactive tracers143-146 may allow noninvasive cGvHD diagnosis, serve as an objective measurement of in situ therapeutic responses,147 and facilitate the selection of patients who are most likely to benefit from metabolic inhibitors. Multidirectional bedside-to-bench and bench-to-bedside approaches will continue to elucidate the biological understanding of cGvHD.
Clinical trial design optimization
To better understand the underlying pathophysiological cGvHD mechanisms, future trials will need to expand the scope of immunological analyses in sufficient subgroups of transplant types and cGvHD clinical presentations.148 As a field, we are receptive to new tools and sample bio-banking for incorporation into clinical studies. The careful, detailed annotation of clinical features at sample collection remains to be of critical importance. Such data should be paired with laboratory and imaging correlates and treatment responses initiated at the time of collection.149 One promising path forward is the continued accrual of prospectively collected clinical and biological data before the initiation of therapy with continued collections as interventions are implemented.150 This approach would allow determination of the clinical utility of the identified markers and guide preclinical evaluation of pathways that incorporate them. Adjusting for clinical factors is critical. As an example, recipient age, potentially associated with the onset of puberty, has a direct effect on immune biomarkers of cGvHD.151
To date, clinical trials have incorporated correlative studies based on either a hypothesized mechanism of the therapeutic intervention or broadly based assays, while omitting evaluation of known biological pathways associated with cGvHD. For blood sampling, the complexity and heterogeneity of cGvHD immunologic mechanisms and clinical factors, confounded by how samples are collected, processed, and analyzed, can explain some of the conflicting findings between clinical research groups. Logistical factors that may have a meaningful impact on such analyses include timing of collection in relation to cGvHD onset, processing, transport and storage of samples, evaluations of whole blood vs Ficoll separated peripheral blood mononuclear cells, as well as the degradation of cytokines and metabolites in plasma before evaluation.152,153 Plasma or serum samples have usually been processed immediately and frozen at the participating center, or processed in a central laboratory after overnight shipping. However, such differences may introduce variability, potentially affecting the quality of the samples. Collection processes that partially fix cells for immune phenotyping or transcriptome evaluation are more expensive but may be necessary to ensure consistency of correlative biology assay results. Plasma may need to be separated and frozen shortly after collection to preserve sample quality, which will add to shipping and storage costs for multicenter studies that typically involve downstream centralized laboratory analyses. Of note, dried blood spot, which is an inexpensive collection option, may be both low-cost and high-yield for certain analyses,154,155 for example, metabolomics. To move this field forward, it is critical that detailed descriptions of sample procurement and processing procedures are reported in publications methods sections.
Collection of samples obtained early in the course of cGvHD before the escalation of therapy138 or before first-line therapy initiation, as proposed by the recent NIH cGvHD Consensus Working Group III report,150 will further advance risk stratification at disease onset unencumbered by therapies, eventually leading to precision therapy. Few prospective cGvHD biology studies have been performedand few studies have included evaluations or sampling of tissue/target organ involvement. One attempt at addressing these concerns was the first137 of the ABLE studies performed by the Pediatric Transplant and Cellular Therapy Consortium, which enrolled patients prospectively from over 25 centers and included adjudication of clinical disease classification.156 Several open consortia studies are collecting samples and clinical data. These include Close Assessment and Testing for cGvHD, CATCH Study (NCT04188912), which collects serial samples of blood, skin and oral biopsies, tears, saliva, and fecal microbiome, starting before HSCT and every 2 months for the first year after alloHSCT. In addition, Predicting the Quality of Response to Specific Treatments in Patients with cGvHD, PQRST Study (#NCT04431479) calls for the collection of blood samples and clinical data before starting treatment for cGvHD after 1 month of therapy along with clinical data collected at 3 and 6 months after alloHSCT, if treatment has changed. Tissue immunohistopathology data emerging from trials which inlcuded pre- and posttargeted intervention organ biopsies should be of great interest (#NCT03640481). Correlative studies recommendations for future clinical trials, include standardized collection times, sample collection methodology, and suggested assays.157 Blood is likely to remain the main material that can be collected serially on trials, although lymphoid and affected organ tissue analyses would likely yield superior interrogation of upfront disease biology and changes as a result of therapies. Thus, animal models, in which target, lymphoid, and blood can be sampled and evaluated serially will continue to be used for elucidating biological responses to therapeutic interventions.
Emerging technologies for in-depth cGvHD biology interrogations in clinical and bench studies
New technologies have emerged that will allow greater insight into underlying biological processes gleaned from preclinical and clinical samples. These include new omics tools, that is, metabolomics directly measured122 or computed by in silico single cell RNAseq data,158 lipidomics, high-parameter (30-50) proteomic analyses by mass cytometry in situ or traditional flow cytometry, combinatorial proteomics (>150 parameter), discovery transcriptomics, such as cellular indexing of transcriptomes and epitopes by sequencing/RNAseq that couples DNA bar-coded antibodies directed against surface proteins with RNAseq), and epigenetic assays, such as assay for transposase-accessible chromatin using sequencing to gauge open chromatin patterns. These approaches may be applied to cell suspensions or whole tissue. For the latter, high-parameter multispectral platforms, such as mass spectrometry, Vectra, and CO-Detection by inDEXing based imaging are now feasible. As proteomics and spatial transcriptomics platforms rapidly evolve to true subcellular definition with transcriptomics, the interrogation of immune networks in tissue should be readily attainable. Single cell T-cell receptor and B-cell receptor sequencing can assess clonotypic donor-, host- and tumor-specific immune responses. Such OMICS technologies can be applied to large animal models, as recently reported for single cell RNAseq of gut-infiltrating donor T cells,159 and as a technique for in vivo T-cell clonal tracking in nonhuman primates to characterize alloreactive T-cell clones identified via mixed lymphocyte reaction in GvHD target organs after alloHSCT.160 Other advances include multiple cell labeling in situ with techniques like multiplexed ion beam imaging by time of flight of metal-tagged antibodies,161 which will be highly informative in preclinical models and in patients. When combined with single cell transcriptomics, this approach could become an especially powerful tool for studying the pathologic activation networks in human T cells.132 Logistics, such as how these data will be acquired and analyzed and financial constraints they will impose, deserve consideration. Nonetheless, as cumulative data sets expand and as understanding of how to best deploy these new technologies grows, there is an opportunity to turn empiricisms into personalized precision medicine.
Preclinical modeling gaps and suggestions for early development of the next generation cGvHD therapies
Although preclinical models with reproducible and highly penetrant multiorgan involvement have yielded insights into cGvHD biological underpinnings,162 the simulation of clinical disease is complicated by numerous variables that can impact the underlying alloimmune processes in patients. These variables include age,151,163-166 sex,4,165,167,168 obesity,169 metabolism,170 microbiome,116 preceding therapy/conditioning,4,171 primary disease,4,165 antecedent and ongoing infections,165,172-174 and thymic function,73,175-180 among others. Although few preclinical studies have been designed to incorporate these key disease initiators and modifiers, adjustments in preclinical mouse modeling can be undertaken before translation or after therapeutic agent/s have been tested, the latter through bedside-to-bench approaches. Moreover, biological assessments of pathway targeting in multiple anatomical sites over time, that is, gastrointestinal, hepatic, and pulmonary chronic GvHD162 should be undertaken in preclinical models.127,162,181 Whether a mechanism is initially identified through clinical studies or generated in preclinical GvHD models, it is best to use preclinical tools to further refine and dissect pertinent biology for successful translation into clinical trials.
Conclusion
New, effective, and well-tolerated targeted therapies for cGvHD have been developed and FDA-approved, but they continue to be used empirically and deployed once the lack of response to systemic corticosteroids and/or calcineurin inhibitors is established. Furthermore, rate of complete and or durable responses are unsatisfactory and choice of therapy for refractory cases remains largerly empiric. There is a gap between clinical manifestations of cGvHD and understanding of biological and molecular features of disease. Closing this gap would allow biological stratification of disease, precise assessment of treatment responses, and will ultimately lead to the development of personalized therapies guided by the detection of the active pathways in cGvHD initiation, maintenance, and progression. Powerful analytical tools and technologies are becoming increasingly available and should be used in cGvHD to decipher in greater depth its biological puzzles. Evaluation of responses by objective laboratory or imaging criteria, and selection of subsequent therapies based on reassessment of disease may be realized. To achieve these goals, cross talk between preclinical and clinical investigators is essential, including the current NIH consensus and other groups that include patient advocates and industry stakeholders. New hybrid collaborative networks may need to be formed for faster and more effective means of propelling novel agents through clinical trials. This effort seeks to maximize an exchange of ideas, opinions, and expertise to continue coordinating clinical development of the next generation of personalized therapies for patients with cGvHD.
Acknowledgments
Supported in part by National Institutes of Health (NIH) grants P01 HL158505, P01 AI056299, R37 AI34495, R01 HL155114, and R01 HL11879, a CIHR Team grant, a CIHR Foundation grant, a CIHR operating grant, and also by the Center for Cancer Research, National Cancer Institute, NIH.
Dedicated to the memory of John A. Hansen.
Authorship
Contribution: All authors contributed to the writing of this review after B.R.B. received the invitation to submit.
Conflict-of-interest disclosure: G.S. receives renumeration for serving on the advisory board of Equillium Incyte, Novartis, and PharmaCyclic. G.R.H. has consulted for Generon Corporation, NapaJen Pharma, iTeos Therapeutics, and Neoleukin Therapeutics, and has received research funding from Compass Therapeutics, Syndax Pharmaceuticals, Applied Molecular Transport, Serplus Technology, Heat Biologics, Laevoroc Oncology, and iTeos Therapeutics. S.J.L. has consulted for Mallinckrodt, Equillium, and Kadmon; has received research funding from Amgen, AstraZeneca, Incyte, Kadmon, Novartis, Pfizer, Syndax, and Takeda; serves on the steering committee for Incyte; and has received drug supply from Janssen. J.R. receives research funding from Amgen, Equillium, Kite/Gilead and Novartis; serves on Data Safety Monitoring Committees for AvroBio and scientific advisory boards for Akron Biotech, Clade Therapeutics, Garuda Therapeutics, LifeVault Bio, Novartis, Rheos Medicines, Talaris Therapeutics, and TScan Therapeutics. S.P. holds a patent on “Biomarkers and assays to detect chronic graft-versus-host disease” (US Patent # 10571478 B2). L.L. holds a patent with WindMiL therapeutics; receives grant/research/clinical trial support from Genentech; and is a consultant/advisory board member for Gilead Sciences, Rubius Therapeutics, Precision Biosciences, and Talaris Therapeutics. P.J.M. receives research funding from AltruBio; renumeration as an adviser to Mallinckrodt, Mesoblast, Rigel, Talaris; renumeration as a member of the Data and Safety Monitoring Board for Pfizer, Pediatric Transplantation and Cellular Therapy Consortium; and has received honoraria from Janssen, Mount Sinai School of Medicine, Therakos, Florida Department of Health, and Deutsche Knochenmarkspenderdatei. S.Z.P. receives research support from the Center for Cancer Research at the National Cancer Institute through the National Institutes of Health Intramural Research Program, including Clinical Research Development Agreements with Celgene, Actelion, Eli Lilly, Pharmacyclics and Kadmon. B.R.B. receives renumeration as an adviser to Magenta Therapeutics, BlueRock Therapeutics, Childrens’ Cancer Research Fund, and KidsFirst Fund, and is a cofounder of Tmunity Therapeutics.
Correspondence: Nataliya Prokopenko Buxbaum, Department of Pediatrics, Roswell Park Comprehensive Cancer Center, Elm and Carlton St, Buffalo, NY 14263; e-mail: nataliya.buxbaum@roswellpark.org.
References
Author notes
∗S.Z.P., K.R.S., and B.R.B. contributed equally to this study.