Key Points
Transfusion during proinflammatory events increases alloimmunization in patients with sickle cell disease, which is not mitigated by HU.
High transfusion burden and HbSS and HbSβ0–thalassemia genotypes are additional risk factors for alloimmunization.
Abstract
We examined risk factors for red blood cell (RBC) alloimmunization in pediatric patients with sickle cell disease, focusing on the recipients’ inflammatory state at the time of transfusion and anti-inflammatory role of hydroxyurea (HU). Among 471 participants, 55 (11.70%) participants were alloimmunized and formed 59 alloantibodies and 17 autoantibodies with an alloimmunization rate of 0.36 alloantibodies per 100 units. Analysis of 27 participants in whom alloantibodies were formed with specificities showed 23.8% (30/126) of units transfused during a proinflammatory event resulting in alloantibody formation compared with 2.8% (27/952) of units transfused at steady state. Therefore, transfusion during proinflammatory events increased the risk for alloimmunization (odds ratio [OR], 4.22; 95% confidence interval [CI], 1.64-10.85; P = .003). Further analysis of all the 471 participants showed that alloimmunization of patients who received episodic transfusion, mostly during proinflammatory events, was not reduced with HU therapy (OR, 6.52; 95% CI, 0.85-49.77; P = .071), HU therapy duration (OR, 1.13; 95% CI, 0.997-1.28; P = .056), or HU dose (OR, 1.06; 95% CI, 0.96-1.16; P = .242). The analysis also identified high transfusion burden (OR, 1.02; 95% CI, 1.003-1.04; P = .020) and hemoglobin S (HbSS) and HbSβ0–thalassemia genotypes (OR, 11.22, 95% CI, 1.51-83.38; P = .018) as additional risk factors for alloimmunization. In conclusion, the inflammatory state of transfusion recipients affects the risk of RBC alloimmunization, which is not modified by HU therapy. Judicious use of transfusion during proinflammatory events is critical for preventing alloimmunization.
Introduction
Sickle cell disease (SCD) is one of the most common inherited diseases, affecting ∼100 000 people in the United States.1 Red blood cell (RBC) transfusion is essential for preventing and managing the acute and chronic complications of SCD. More than 50% of pediatric and 90% of adult patients with SCD receive at least one RBC transfusion in their lifetime.2 A remaining challenge of blood transfusion is RBC alloimmunization. The alloimmunization rate is as high as 1.94 alloantibodies per 100 units with ABO- and RhD-antigen matching and down to 0.40 alloantibodies per 100 units with additional Rh (C, E or C, c, E, e) and K antigen matching.3 RBC alloimmunization increases the risk of acute and delayed hemolytic transfusion reactions and hyperhemolysis syndrome, leading to severe anemia and even death.4-8 RBC alloimmunization may also result in transfusion delays, secondary to challenges in identifying compatible RBC units that lack the target antigens. Overall, alloimmunization reduces the survival rate of patients with SCD.9,10
Alloantibody formation can be attributed to multiple potential risk factors including older age, female sex, high transfusion load, presence of autoantibodies, and aging RBCs.11-14 For patients with SCD, the disparate expression of RBC antigens between blood recipients of African-descent and White donors2,15 and the high frequency of variant Rh antigens in both patients and donors of African descent16-18 contribute to the higher rate of alloimmunization compared with other populations that underwent transfusion. Transfusion during proinflammatory events also increases the risk of alloimmunization in patients with SCD.19 Hydroxyurea (HU), which is increasingly used for SCD preventive therapy, induces myelosuppression and reduces inflammation,20,21 but its effect on alloimmunization has not been investigated. Here, we conducted a single-center retrospective cohort study in pediatric patients with SCD to explore the risk factors for alloimmunization, focusing on the roles of the recipients’ proinflammatory state, HU therapy, and Rh variants in alloimmunization.
Methods
Study subjects
We conducted a single-center retrospective cohort study on RBC alloimmunization in pediatric patients with SCD who participated in the Sickle Cell Clinical Research and Intervention Program. The Sickle Cell Clinical Research and Intervention Program study is a lifetime cohort study that collects retrospective and prospective data on clinical, neurocognitive, geographical, psychosocial, and health outcomes in children, adolescents, and adults with SCD.22 This study was approved by the St. Jude Institutional Review Board and performed in accordance with the Declaration of Helsinki. Patient inclusion criteria were as follows: (1) age <19 years at the time of transfusion, (2) diagnosis of SCD of any genotype, and (3) recipient of at least 1 RBC transfusion at our center before 31 December 2017 or 1 day before the 19th birthday (whichever came first).
RBC transfusion and alloimmunization
Relevant clinical data before 31 December 2017 or 1 day before the 19th birthday of the included patients (whichever came first) were collected. The cutoff date for antibody screens was extended to 19 September 2022 because many patients who received episodic transfusion did not have follow-up screens until a later date after the end of the study. RBC units transfused to patients with SCD were prestorage leukoreduced, irradiated, and hemoglobin (Hb) S–negative. Prophylactic Rh (C and E)− and K−negative or matched RBCs were provided to patients with SCD at our center since 2002. RBCs administered also lacked any antigens against which antibodies were previously identified. Chronic transfusion was defined as requiring regularly scheduled transfusions for any indication. Transfusions before alloimmunization was defined as RBC units received at least 7 days before the first newly identified antibody. RBC antibody screens and identification were performed using tube-testing method. Here, autoantibodies with Rh specificity were classified as alloantibodies because these were likely alloantibodies associated with variant Rh antigens. The alloimmunization incidence rate was calculated as the number of alloantibodies divided by the total number of RBC units transfused by 31 December 2017 or 1 day before the 19th birthday (whichever came first).
Assessing the association of receiving transfusion during proinflammatory events with alloimmunization
For this analysis, only patients who were alloimmunized were considered. In addition, patients who were previously alloimmunized but did not form new antibodies after transferring to our center and those with only autoantibodies, antibodies of undetermined specificity, or antibodies against low-frequency antigens were excluded (supplemental Figure 1). RBC units received at least 7 days before the last newly identified alloantibody in the patients were included. Next, the units were categorized based on their proximity to the date of a newly identified alloantibody and a proinflammatory event. Proinflammatory events were defined as emergency department visits and hospitalization for acute chest syndrome (ACS), acute stroke, acute febrile illness in the absence of other SCD complications, vaso-occlusive crisis, splenic sequestration, priapism, parvovirus B19–induced transient aplastic crisis, and elective surgery, as previously described.19 Among the included RBC units, the units received during a transfusion event closest to a newly identified alloantibody were categorized as antibody-positive transfusions, and the remaining ones were categorized as antibody-negative transfusions; the units received during a transfusion event ≤8 days before or after a proinflammatory event were categorized as transfusions during proinflammatory events, and the remaining ones were categorized as transfusions at steady state. If >1 unit of RBCs were transfused during a transfusion event or clinical encounter, all units were grouped in the same category. Laboratory results of the Hb level at least 7 days before a transfused unit, and those of the absolute reticulocyte count, maximum white blood cell count, absolute neutrophil count, lactate dehydrogenase, and total bilirubin at least 7 days before and after a transfused unit were also included in the analysis. Notably, for analysis by transfusion events, the same laboratory results were used for all the transfused units in a transfusion event.
Statistical analysis
Patient characteristics were summarized based on the alloimmunization status. Continuous variables were summarized as the median and interquartile range (IQR), and categorical variables were summarized in terms of number and percentage. To investigate the association of alloimmunization and receiving transfusions during proinflammatory events, we first used generalized linear mixed models with a binomial error distribution to assess the association of alloimmunization with age at the time of transfusions for the patients who met the inclusion criteria. The remaining clinical variables were then analyzed with an adjustment for age at the time of transfusions. The models included a random intercept for subjects to account for the clustering of values from the same subject. The models were fit using the Glimmix procedure in Statistical Analysis System (SAS). To examine the roles of anti-inflammatory agent, HU and other clinical variants in alloimmunization, logistic regression models were used to assess the association of each potential risk factor with alloimmunization with an adjustment for the time of patients on the study, which we calculated as the time from which the patient received first RBC transfusion at our center to the first new antibody identified for patients who were alloimmunized or to 31 December 2017 or 1 day before the 19th birthday (whichever came first) for patients who were not alloimmunized. Risk factors with P < .1 and the time on the study were entered as candidate predictors into a multivariate logistic regression model based on a backward selection procedure. The time on the study was not forced into the final model. Transfusion load (RBC units) or transfusion frequency (chronic vs episodic transfusions) was included as a candidate predictor in the multivariate logistic regression model because of a strong correlation between the 2 variables using a multicollinearity test with a variance inflation factor <2. P values were considered significant if P <.05. All reported P values were 2-sided. Statistical analyses were performed with SAS software, version 9.4, for Windows (SAS Institute Inc, Cary, NC).
Results
Clinical characteristics of the study subjects
A total of 471 patients were included in the study (Table 1). The mean patient age at the end of the study was 12.9 ± 5.2 years. Female patients accounted for 48.6% (n = 229) of the study population. Almost all patients self-identified as Black (n = 467; 99.2%). HbSS and HbSβ0–thalassemia were the most common Hb genotypes (n = 384; 81.5%). The 471 patients received a total of 15 960 units of RBCs. The median age at first transfusion was 4.6 years (interquartile range [IQR], 1.9-8.1 years) and the median total transfusion period was 6.4 years (IQR, 3.5-10.3 years). The median total RBC exposure was 3 units per patient (IQR, 2-9 units/patient). Most patients (n = 391, 85.0%) received episodic transfusion and had a median total of 3 units per patient (IQR, 1-5 units/patient). Sixty-nine (15.0%) patients required chronic transfusion and received a median total of 143 units per patient (IQR, 70-312 units/patient). Most patients who received episodic transfusion (n = 298, 76.2%) were also treated with HU. HU was initiated at a median age of 5.7 years (IQR, 2.9-9.4 years), the median accumulative therapy duration was 4.37 years (IQR, 2.49-8.31 years), and the median maximum tolerated dose (MTD) was 29.2 mg/kg per day (IQR, 23.6-30.8 mg/kg per day). Patients who received chronic transfusion might receive HU before starting chronic transfusion, but it was very rare to have concurrent chronic transfusion and HU therapy.
Patient demographics and clinical characteristics
| Clinical variables . | Total n = 471 (%) . | Alloimmunized, n = 55 (%)∗ . | Nonalloimmunized, n = 416 (%) . |
|---|---|---|---|
| Sex | |||
| Female | 229 (48.6%) | 28 (50.9%) | 201 (48.3%) |
| Male | 242 (51.4%) | 27 (49.1%) | 215 (51.7%) |
| Race | |||
| Black | 467 (99.2%) | 55 (100.0%) | 412 (99.0%) |
| Black and White | 1 (0.2%) | — | 1 (0.2%) |
| White | 2 (0.4%) | — | 2 (0.5%) |
| Others | 1 (0.2%) | — | 1 (0.2%) |
| Hb genotypes | |||
| SS/Sβ0 thalassemia | 384 (81.5%) | 50 (90.9%) | 334 (80.3%) |
| SC | 65 (13.8%) | 3 (5.5%) | 62 (14.9%) |
| Sβ+ thalassemia | 20 (4.2%) | 2 (3.6%) | 18 (4.3%) |
| Others | 2 (0.4%) | — | 2 (0.5%) |
| Blood transfusion | |||
| Age at first transfusion (y) | 4.6 (∼1.9-8.1) | 4.9 (∼2.7-6.8) | 4.5 (1.7-∼8.3) |
| Transfusion period (y) | |||
| Total | 6.4 (∼3.5-10.3) | 6.9 (∼4.011.9) | 6.3 (∼3.3-10.2) |
| Before alloimmunization | — | 4.1 (∼0.9-7.8) | — |
| Transfusion load (units) | |||
| Total | 3 (∼2-9) | 16 (∼3-168) | 3 (∼1-7) |
| Before alloimmunization | — | 17 (∼3-61) | — |
| Transfusion frequency | |||
| Chronic transfusion | |||
| No. of patients | 69 (15.0%) | 25 (56.8%) | 44 (10.6%) |
| Transfusion load (units) | |||
| Total | 143 (∼70-312) | 168 (∼84-293) | 138 (∼62-315) |
| Before alloimmunization | — | 55 (∼31-90) | — |
| Episodic transfusion | |||
| No. of patients | 391 (85.0%) | 19 (43.2%) | 372 (89.4%) |
| Transfusion load (units) | |||
| Total | 3 (∼1-5) | 5 (∼2-9) | 3 (∼1-5) |
| Before alloimmunization | — | 2 (∼2-5) | — |
| HU treatment | |||
| Yes | 298 (76.2%) | 18 (94.7%) | 280 (75.3%) |
| No | 93 (23.8%) | 1 (5.3%) | 92 (24.7%) |
| Age starting HU (y) | 5.7 (∼2.9-9.4) | 3.5 (∼2.1-7.2) | 5.8 (∼2.9-9.4) |
| HU therapy duration (y)† | |||
| Total | 4.37 (∼2.49-8.31) | 7.73 (∼4.84-9.56) | 4.20 (∼2.40-8.26) |
| Before alloimmunization | — | 4.50 (∼0.67-6.79) | — |
| HU MTD (mg/kg per d) | |||
| Total | 29.2 (∼23.6-30.8) | 30.0 (∼28.3-30.1) | 29.0 (∼23.0-31.0) |
| Before alloimmunization | — | 30.0 (∼28.3-30.1) | — |
| Clinical variables . | Total n = 471 (%) . | Alloimmunized, n = 55 (%)∗ . | Nonalloimmunized, n = 416 (%) . |
|---|---|---|---|
| Sex | |||
| Female | 229 (48.6%) | 28 (50.9%) | 201 (48.3%) |
| Male | 242 (51.4%) | 27 (49.1%) | 215 (51.7%) |
| Race | |||
| Black | 467 (99.2%) | 55 (100.0%) | 412 (99.0%) |
| Black and White | 1 (0.2%) | — | 1 (0.2%) |
| White | 2 (0.4%) | — | 2 (0.5%) |
| Others | 1 (0.2%) | — | 1 (0.2%) |
| Hb genotypes | |||
| SS/Sβ0 thalassemia | 384 (81.5%) | 50 (90.9%) | 334 (80.3%) |
| SC | 65 (13.8%) | 3 (5.5%) | 62 (14.9%) |
| Sβ+ thalassemia | 20 (4.2%) | 2 (3.6%) | 18 (4.3%) |
| Others | 2 (0.4%) | — | 2 (0.5%) |
| Blood transfusion | |||
| Age at first transfusion (y) | 4.6 (∼1.9-8.1) | 4.9 (∼2.7-6.8) | 4.5 (1.7-∼8.3) |
| Transfusion period (y) | |||
| Total | 6.4 (∼3.5-10.3) | 6.9 (∼4.011.9) | 6.3 (∼3.3-10.2) |
| Before alloimmunization | — | 4.1 (∼0.9-7.8) | — |
| Transfusion load (units) | |||
| Total | 3 (∼2-9) | 16 (∼3-168) | 3 (∼1-7) |
| Before alloimmunization | — | 17 (∼3-61) | — |
| Transfusion frequency | |||
| Chronic transfusion | |||
| No. of patients | 69 (15.0%) | 25 (56.8%) | 44 (10.6%) |
| Transfusion load (units) | |||
| Total | 143 (∼70-312) | 168 (∼84-293) | 138 (∼62-315) |
| Before alloimmunization | — | 55 (∼31-90) | — |
| Episodic transfusion | |||
| No. of patients | 391 (85.0%) | 19 (43.2%) | 372 (89.4%) |
| Transfusion load (units) | |||
| Total | 3 (∼1-5) | 5 (∼2-9) | 3 (∼1-5) |
| Before alloimmunization | — | 2 (∼2-5) | — |
| HU treatment | |||
| Yes | 298 (76.2%) | 18 (94.7%) | 280 (75.3%) |
| No | 93 (23.8%) | 1 (5.3%) | 92 (24.7%) |
| Age starting HU (y) | 5.7 (∼2.9-9.4) | 3.5 (∼2.1-7.2) | 5.8 (∼2.9-9.4) |
| HU therapy duration (y)† | |||
| Total | 4.37 (∼2.49-8.31) | 7.73 (∼4.84-9.56) | 4.20 (∼2.40-8.26) |
| Before alloimmunization | — | 4.50 (∼0.67-6.79) | — |
| HU MTD (mg/kg per d) | |||
| Total | 29.2 (∼23.6-30.8) | 30.0 (∼28.3-30.1) | 29.0 (∼23.0-31.0) |
| Before alloimmunization | — | 30.0 (∼28.3-30.1) | — |
Categorical variables summarized as n (%), and continuous variables summarized as median (IQR, Quartile 1 to Quartile 3).
Among 55 patients who were alloimmunized, 11 were alloimmunized before transfusion at our center. Their data about sex, race, and Hb genotypes were included for alloimmunization association analysis. Their histories of blood transfusion and HU therapy before alloimmunization were unknown so were not included in the analysis.
Included episodically transfused patients who received HU therapy only.
RBC alloimmunization
Among the 471 participants, 55 were alloimmunized, including 11 patients who had been alloimmunized before receiving transfusions at our center (Table 1). A total of 76 antibodies were identified among the whole cohort, including 59 alloantibodies and 17 autoantibodies (and including 10 alloantibodies and 3 autoantibodies from the 11 patients who were previously alloimmunized; Figure 1). The time to antibody detection from the last transfusion was 110 days (IQR, 23-439 days) for patients who received episodic transfusion and 28 days (IQR, 26-32 days) for patients who received chronic transfusion. The most common antibody specificity was anti-D and anti-M (n = 6 for both, 7.9%). Notably, all the anti-Ms were identified at least 7 days after transfusions. Fifteen antibodies were against Rh antigens despite transfusion with prophylactic Rh (D, C, and E)-negative or -matched RBCs. The alloimmunization prevalence in our patient cohort was 11.7% (55/471), and the alloimmunization incidence rate was 0.36 alloantibodies per 100 transfusions (59 alloantibodies per 15 960 RBC units). Antibodies were first detected at a median age of 9.9 years (IQR, 6.0-14.0 years) after a median of 17 units of RBCs (IQR, 3-61 units; Table 1). In total, 36.2% (25/69) of chronically transfused patients were alloimmunized and had formed their first antibody after receiving a median of 55 units (IQR, 31-90 units); 4.9% (19/391) of episodically transfused patients were alloimmunized after a median of 2 units (IQR, 2-5 units). For the patients who were alloimmunized while receiving episodic transfusion, 94.7% (18/19) of the patients were managed with HU. HU was started at a median age of 3.5 years (IQR, 2.1-7.2 years); the median therapy duration was 4.50 years (IQR, 0.67-6.79 years), and the median MTD was 30.0 mg/kg per day (IQR, 28.3-30.1 mg/kg per day) before alloimmunization. Notably, the changes in HbF percentage and mean corpuscular volume from baseline to 1 and 2 years after starting HU were similar among the alloimmunized and non-alloimmunized patients, suggesting similar HU adherence across both groups (supplemental Table 1).
Antibodies identified in pediatric patients with SCD. ∗Indicates antibodies found in D- or e-positive patients showing D or e specificities, respectively. ∗∗Indicates antibodies against low-frequency antigens without determined specificity.
Antibodies identified in pediatric patients with SCD. ∗Indicates antibodies found in D- or e-positive patients showing D or e specificities, respectively. ∗∗Indicates antibodies against low-frequency antigens without determined specificity.
Assessing the effect of proinflammatory events on alloimmunization
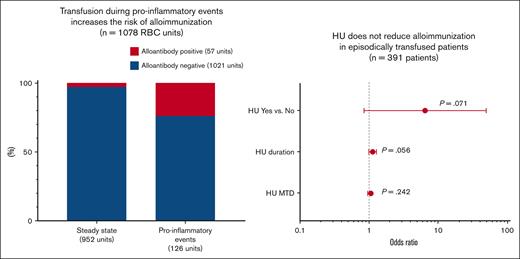
Because exposure to a foreign RBC antigen does not always lead to alloantibody formation, other factors such as recipients’ inflammatory state at the time of transfusion can contribute as well. To investigate whether RBC transfusion during proinflammatory events results in a higher risk of alloantibody formation than that at steady state, we analyzed the transfusions received by the patients who were alloimmunized with determined alloantibody specificities. Of the 55 patients who were alloimmunized, 27 patients were analyzed after excluding 11 patients who were previously alloimmunized but did not form new antibodies at our center and 17 patients with only autoantibodies, antibodies of undetermined specificity, or antibodies against low-frequency antigens (supplemental Figure 1). Patients with antibodies against low-frequency antigens only were excluded on the basis that the chances to encounter low-frequency antigens during proinflammatory events and at steady state are very low. The 27 patients received 1078 RBC units before 35 new alloantibodies were identified with a median time until antibody detection of 36 days (IQR, 28-184 days; Table 2; supplemental Table 2). Among the 1078 transfused units, 126 units were administered during a proinflammatory event, and 952 units administered at steady state; 57 units were associated with subsequent alloantibody positivity, and 1021 units were not. Specifically, 30 of the 126 (23.8%) units transfused during a proinflammatory event resulted in alloantibody production, compared with 27 of 952 (2.8%) units received at steady state. Therefore, alloantibodies were 4.22-fold likely to form after transfusions received during a proinflammatory event (95% confidence interval [CI], 1.64-10.85; P = .003). Among the proinflammatory events, parvovirus B19–induced transient aplastic crisis was associated with an increased risk of alloimmunization (odds ratio [OR], 187.76; 95% CI, 4.67-7542; P = .006), but no association was found for other SCD complications such as ACS (OR, 1.24; 95% CI, 0.05-29.12; P = .894) as reported previously.19 We did not observe a correlation between alloimmunization and the Hb level (OR, 0.79; 95% CI, 0.54-1.14; P = .203), absolute reticulocyte counts (OR, 185.88; 95% CI, 0.05-698390; P = .212), white blood cell counts (OR, 0.99; 95% CI, 0.91-1.08; P = .859), absolute neutrophil count (OR, 0.95; 95% CI, 0.84-1.09; P = .487), lactate dehydrogenase (OR, 0.99; 95% CI, 0.67-1.45; P = .943), or total bilirubin (OR, 1.15; 95% CI, 0.85-1.55; P = .368) around the time of transfusions. We also analyzed the data based on transfusion events (Table 3). Among the 583 transfusion events, 20 of 97 (20.6%) transfusion events during a proinflammatory event led to alloantibodies formation compared with 13 of 486 (2.7%) transfusion events at steady state. Thus, transfusions during proinflammatory events increased the risk of alloantibody formation up to 7.85-fold (95% CI, 2.98-20.65; P < .001). Parvovirus B19–induced transient aplastic crisis was associated with an increased risk of alloimmunization (OR, 87.86; 95% CI, 2.83-2727; P = .011), but not other complications, including ACS (OR, 1.29; 95% CI, 0.08-20.61; P = .856). We also found that the Hb level was negatively correlated to alloimmunization (OR, 0.63; 95% CI, 0.43-0.94; P = .023). The median RBC units per transfusion event were 1.00 (IQR, 1.00-2.00) for the alloantibody-positive transfusion events, 1.00 (IQR, 1.00-2.00) for the alloantibody-negative ones, and not significantly different (OR, 0.99; 95% CI, 0.60-1.62; P=.967). Thus, alloantibody formation was not due to the increased number of units transfused during the alloantibody-positive transfusion events.
Transfusion under proinflammatory conditions increases the risk of alloimmunization (based on RBC units)
| Clinical variables . | Alloantibody-positive transfusions, n = 57 units . | Alloantibody-negative transfusions, n = 1021 units . | OR (95% CI)∗ . | P value . |
|---|---|---|---|---|
| Proinflammatory events | ||||
| Yes | 30 (52.6%) | 96 (9.4%) | 4.22 (1.64, 10.85) | .003 |
| No | 27 (47.4%) | 925 (90.6%) | — | — |
| Aplastic crisis | ||||
| Yes | 4 (7.0%) | 1 (0.1%) | 187.76 (4.67, 7542) | .006 |
| No | 53 (93.0%) | 1020 (99.9%) | — | — |
| Laboratory tests | ||||
| Hb (g/dL) | 9.90 (8.10∼10.40) | 10.60 (9.50∼11.30) | 0.79 (0.54, 1.14) | .203 |
| Absolute reticulocyte count (106/μL)† | 0.26 (0.15∼0.34) | 0.19 (0.15∼0.25) | 185.88 (0.05, 698390) | .212 |
| WBC (103/μL)‡ | 11.10 (6.30∼15.15) | 8.30 (6.30∼11.90) | 0.99 (0.91, 1.08) | .859 |
| ANC ((/μL)§ | 6350 (3350∼8100) | 3600 (2300∼6800) | 0.95 (0.84, 1.09) | .487 |
| LDH (unit/L) | 472 (384∼517) | 415 (356∼520) | 0.99 (0.67, 1.45) | .943 |
| Total bilirubin (mg/dL) | 4.20 (2.50∼6.40) | 2.30 (1.50∼3.80) | 1.15 (0.85, 1.55) | .368 |
| Clinical variables . | Alloantibody-positive transfusions, n = 57 units . | Alloantibody-negative transfusions, n = 1021 units . | OR (95% CI)∗ . | P value . |
|---|---|---|---|---|
| Proinflammatory events | ||||
| Yes | 30 (52.6%) | 96 (9.4%) | 4.22 (1.64, 10.85) | .003 |
| No | 27 (47.4%) | 925 (90.6%) | — | — |
| Aplastic crisis | ||||
| Yes | 4 (7.0%) | 1 (0.1%) | 187.76 (4.67, 7542) | .006 |
| No | 53 (93.0%) | 1020 (99.9%) | — | — |
| Laboratory tests | ||||
| Hb (g/dL) | 9.90 (8.10∼10.40) | 10.60 (9.50∼11.30) | 0.79 (0.54, 1.14) | .203 |
| Absolute reticulocyte count (106/μL)† | 0.26 (0.15∼0.34) | 0.19 (0.15∼0.25) | 185.88 (0.05, 698390) | .212 |
| WBC (103/μL)‡ | 11.10 (6.30∼15.15) | 8.30 (6.30∼11.90) | 0.99 (0.91, 1.08) | .859 |
| ANC ((/μL)§ | 6350 (3350∼8100) | 3600 (2300∼6800) | 0.95 (0.84, 1.09) | .487 |
| LDH (unit/L) | 472 (384∼517) | 415 (356∼520) | 0.99 (0.67, 1.45) | .943 |
| Total bilirubin (mg/dL) | 4.20 (2.50∼6.40) | 2.30 (1.50∼3.80) | 1.15 (0.85, 1.55) | .368 |
Categorical variables summarized as n (%). Continuous variables summarized as median (IQR, from Quartile 1 to Quartile 3). Indicated in bold, P < 0.05 is considered significant. ANC, absolute neutrophil count; LDH, lactate dehydrogenase; WBC, white blood cell.
The analyses included transfusions (units) up to 7 days before all newly identified alloantibodies. Since antibody-positive transfusions usually occurred at a later time than antibody-negative transfusions for an individual patient (OR by RBC units: 1.31, 95% CI: 1.11–1.56, P = 0.002), the association analyses were adjusted for patient age at the time of transfusions.
Exclude absolute reticulocyte counts during aplastic crisis.
The maximum WBC counts during 7 days before and after the indicated transfused unit/transfusion event.
OR for ANC was based on a 1000-unit increase. The ORs for the remaining laboratory results were based on a 1-unit increase.
Transfusion under proinflammatory conditions increases the risk of alloimmunization (based on transfusion events)
| Clinical variables . | Alloantibody-positive transfusions, n = 33 events . | Alloantibody-negative transfusions, n = 550 events . | OR (95% CI)∗ . | P value . |
|---|---|---|---|---|
| Proinflammatory events | ||||
| Yes | 20 (60.6%) | 77 (14.0%) | 7.85 (2.98, 20.65) | <.001 |
| No | 13 (39.4%) | 473 (86.0%) | — | — |
| Aplastic crisis | ||||
| Yes | 3 (9.1%) | 1 (0.2%) | 87.86 (2.83, 2727) | .011 |
| No | 30 (90.9%) | 549 (99.8%) | — | — |
| Laboratory tests | ||||
| Hb (g/dL) | 9.10 (∼7.80-10.40) | 9.80 (∼9.10-10.80) | 0.63 (0.43, 0.94) | .023 |
| Absolute reticulocyte count (106/μL)† | 0.26 (∼0.15-0.34) | 0.21 (∼0.17-0.28) | 146.43 (0.01, 2644816) | .317 |
| WBC (103/μL)‡ | 12.40 (∼9.90-16.45) | 10.60 (∼6.90-14.20) | 1.03 (0.94, 1.12) | .517 |
| ANC ((/μL)§ | 7400 (∼5800-8500) | 5748 (∼2800-8200) | 1.01 (0.88, 1.16) | .869 |
| LDH (unit/L) | 495 (∼409-653) | 460 (∼367-544) | 1.29 (0.94, 1.79) | .118 |
| Total bilirubin (mg/dL) | 3.50 (∼1.50-5.50) | 2.80 (∼1.60-3.80) | 1.01 (0.73, 1.40) | .951 |
| Clinical variables . | Alloantibody-positive transfusions, n = 33 events . | Alloantibody-negative transfusions, n = 550 events . | OR (95% CI)∗ . | P value . |
|---|---|---|---|---|
| Proinflammatory events | ||||
| Yes | 20 (60.6%) | 77 (14.0%) | 7.85 (2.98, 20.65) | <.001 |
| No | 13 (39.4%) | 473 (86.0%) | — | — |
| Aplastic crisis | ||||
| Yes | 3 (9.1%) | 1 (0.2%) | 87.86 (2.83, 2727) | .011 |
| No | 30 (90.9%) | 549 (99.8%) | — | — |
| Laboratory tests | ||||
| Hb (g/dL) | 9.10 (∼7.80-10.40) | 9.80 (∼9.10-10.80) | 0.63 (0.43, 0.94) | .023 |
| Absolute reticulocyte count (106/μL)† | 0.26 (∼0.15-0.34) | 0.21 (∼0.17-0.28) | 146.43 (0.01, 2644816) | .317 |
| WBC (103/μL)‡ | 12.40 (∼9.90-16.45) | 10.60 (∼6.90-14.20) | 1.03 (0.94, 1.12) | .517 |
| ANC ((/μL)§ | 7400 (∼5800-8500) | 5748 (∼2800-8200) | 1.01 (0.88, 1.16) | .869 |
| LDH (unit/L) | 495 (∼409-653) | 460 (∼367-544) | 1.29 (0.94, 1.79) | .118 |
| Total bilirubin (mg/dL) | 3.50 (∼1.50-5.50) | 2.80 (∼1.60-3.80) | 1.01 (0.73, 1.40) | .951 |
Categorical variables summarized as n (%). Continuous variables summarized as median (IQR, from Quartile 1 to Quartile 3). Indicated in bold, P < 0.05 is considered significant.
The analyses included transfusions (events) up to 7 days before all newly identified alloantibodies. Because antibody-positive transfusions usually occurred at a later time than antibody-negative transfusions for an individual patient (OR based transfusion events, 1.20; 95% CI, 1.02-1.41; P = .028), the association analyses were adjusted for patient age at the time of transfusions.
Exclude absolute reticulocyte counts during aplastic crisis.
The maximum WBC counts 7 days before and after the indicated transfused unit per transfusion event.
OR for ANC was based on a 1000-unit increase. The ORs for the remaining laboratory results were based on a 1-unit increase.
Determining the effects of HU therapy and other risk factors on alloimmunization
Because recipient inflammatory state at the time of transfusion influenced the risk of alloimmunization, we examined the roles of anti-inflammatory agent HU and other clinical variables in alloimmunization (Table 4). For this analysis, the entire study cohort of 471 patients was included. For the 11 patients who had been previously alloimmunized before transfusion at our center, only their sex, race, and Hb genotype were included in the analysis. Logistic regression analysis demonstrated that for episodically transfused patients who received transfusion mostly during proinflammatory events, neither did the HU receipt affect their likelihood of alloimmunization (OR, 6.52; 95% CI, 0.85-49.77; P = .071), nor the HU duration (OR, 1.13; 95% CI, 0.997-1.28; P = .056), nor the HU MTD (OR, 1.06; 95% CI, 0.96-1.16; P = .242). Instead, the analysis identified HbSS and HbSβ0–thalassemia genotypes (OR, 12.14; 95% CI, 1.64-89.86; P = .015), high transfusion load (OR, 1.02; 95% CI, 1.01-1.04; P = .007), and frequent RBC exposure or chronic transfusion (OR, 17.64; 95% CI, 8.19-38.00; P < .001) as the risk factors for alloimmunization. Because chronic transfusion usually results in high transfusion load, we performed multivariate logistic analyses with transfusion load and transfusion frequency, respectively. The multivariate logistic analysis including transfusion load showed that the HbSS and HbSβ0–thalassemia genotypes (OR, 11.22; 95% CI, 1.51-83.38; P = .018) and high transfusion load (OR, 1.02; 95% CI, 1.003-1.04; P = .020) were significantly associated with alloimmunization. The multivariate logistic analysis including transfusion frequency identified chronic transfusion (OR, 14.19; 95% CI, 6.54-30.80; P < .0001) as a significant risk factor for alloimmunization. Hb genotypes were no longer significantly associated with alloimmunization (OR, 5.84; 95% CI, 0.76-45.67; P= .089).
Risk factors associated with alloimmunization in patients with SCD
| Clinical variables . | Logistic regression analysis∗ . | Multivariate logistic regression analysis using transfusion load . | Multivariate logistic regression analysis using transfusion frequency . | |||
|---|---|---|---|---|---|---|
| Adjusted OR (95% CI) . | P value . | Adjusted OR (95% CI) . | P value . | Adjusted OR (95% CI) . | P value . | |
| All patients | ||||||
| Age at first transfusion (y) | 0.96 (0.90, 1.03) | .31 | — | — | — | — |
| Sex, male vs female | 0.90 (0.48, 1.69) | .75 | — | — | — | — |
| Hb genotype, SS/Sβ0 thalassemia vs Others† | 12.14 (1.64, 89.86) | .015 | 11.22 (1.51, 83.38) | .018 | 5.84 (0.76, 45.67) | .089 |
| Transfusion load (units)‡ | 1.02 (1.01, 1.04) | .007 | 1.02 (1.003, 1.04) | .020 | — | — |
| Transfusion frequency, Chronic vs Episodic | 17.64 (8.19, 38.00) | < .001 | — | — | 14.19 (6.54, 30.80) | < .0001 |
| Patients who received episodic transfusion | ||||||
| HU yes/no | 6.52 (0.85, 49.77) | .071 | — | — | — | — |
| HU therapy duration | 1.13 (0.997, 1.28) | .056 | — | — | — | — |
| HU MTD (mg/kg per d) | 1.06 (0.96, 1.16) | .242 | — | — | — | — |
| Clinical variables . | Logistic regression analysis∗ . | Multivariate logistic regression analysis using transfusion load . | Multivariate logistic regression analysis using transfusion frequency . | |||
|---|---|---|---|---|---|---|
| Adjusted OR (95% CI) . | P value . | Adjusted OR (95% CI) . | P value . | Adjusted OR (95% CI) . | P value . | |
| All patients | ||||||
| Age at first transfusion (y) | 0.96 (0.90, 1.03) | .31 | — | — | — | — |
| Sex, male vs female | 0.90 (0.48, 1.69) | .75 | — | — | — | — |
| Hb genotype, SS/Sβ0 thalassemia vs Others† | 12.14 (1.64, 89.86) | .015 | 11.22 (1.51, 83.38) | .018 | 5.84 (0.76, 45.67) | .089 |
| Transfusion load (units)‡ | 1.02 (1.01, 1.04) | .007 | 1.02 (1.003, 1.04) | .020 | — | — |
| Transfusion frequency, Chronic vs Episodic | 17.64 (8.19, 38.00) | < .001 | — | — | 14.19 (6.54, 30.80) | < .0001 |
| Patients who received episodic transfusion | ||||||
| HU yes/no | 6.52 (0.85, 49.77) | .071 | — | — | — | — |
| HU therapy duration | 1.13 (0.997, 1.28) | .056 | — | — | — | — |
| HU MTD (mg/kg per d) | 1.06 (0.96, 1.16) | .242 | — | — | — | — |
Indicated in bold, P < 0.05 is considered significant.
Adjusted for time on study, which is from patients receiving the first RBC transfusion at our center to the first newly identified antibody for patients who were alloimmunized, and to 31 December 2017 or one day before patients turning 19 years old (whichever came first) for patients who were not alloimmunized.
Others include all non-HbSS/Sβ0–thalassemia genotypes.
OR was based on a 5-unit increase.
Effect of RH genotypes on Rh alloimmunization
Variant RH alleles are common in patients with SCD. Approximately 30% of our patients have whole-genome sequencing (WGS) data, which allowed us to obtain their RH genotypes using RHtyper, an automated algorithm for accurate and high throughput RH genotyping using WGS data.23 Eleven patients formed a total of 14 anti-Rh, of which 9 patients (with 10 anti-Rh) had WGS data from which RH genotypes were obtained (Table 5). One patient (#7) with RHCE∗Ce/RHCE∗ce48C formed an anti-c, although RHCE∗ce48C is a common RHCE allele in individuals of African ancestry and not associated with partial ce expression.17,23 Five patients (#1-4 with D specificity and #9 with e specificity) are heterozygous for the corresponding conventional RH alleles, and, thus, these antibodies could be autoantibodies or alloantibodies driven by Black blood donors with immunogenic Rh variants that were recognized as foreign by the patients.17 Four patients (#4, 5, 6, and 8) had anti-C or anti-E despite receiving C− or E−negative units, which might have been stimulated by donors with Rh variants as well. Given the small number of patients with anti-Rh in our cohort, we were unable to test the association of RH genotypes with Rh alloimmunization statistically.
RH genotypes of patients with Rh antibody specificities
| Patient . | Rh antibody specificity . | RH genotype . | |
|---|---|---|---|
| RHD∗ . | RHCE∗ . | ||
| 1 | D | RHD | ce48C |
| DAU3 | Ce | ||
| 2 | D | RHD | ce733G |
| DAU0 | ce48C | ||
| 3 | D | RHD | ce |
| Pseudogene/ Ψ | ce48C | ||
| 4 | D and C (big) | RHD | ce48C,105T |
| DAU0 | ce733G | ||
| 5 | C (big) | Deletion | ce733G |
| Deletion | ce | ||
| 6 | C (big) | Deletion | ce48C |
| DAU0 | ce | ||
| 7 | c (little) | RHD | Ce |
| RHD | ce48C | ||
| 8 | E | Deletion | ceTI |
| DIVa | ceAG | ||
| 9 | e (little) | RHD | cE |
| RHD | ce | ||
| Patient . | Rh antibody specificity . | RH genotype . | |
|---|---|---|---|
| RHD∗ . | RHCE∗ . | ||
| 1 | D | RHD | ce48C |
| DAU3 | Ce | ||
| 2 | D | RHD | ce733G |
| DAU0 | ce48C | ||
| 3 | D | RHD | ce |
| Pseudogene/ Ψ | ce48C | ||
| 4 | D and C (big) | RHD | ce48C,105T |
| DAU0 | ce733G | ||
| 5 | C (big) | Deletion | ce733G |
| Deletion | ce | ||
| 6 | C (big) | Deletion | ce48C |
| DAU0 | ce | ||
| 7 | c (little) | RHD | Ce |
| RHD | ce48C | ||
| 8 | E | Deletion | ceTI |
| DIVa | ceAG | ||
| 9 | e (little) | RHD | cE |
| RHD | ce | ||
Discussion
In this retrospective cohort study, we examined RBC alloimmunization and its associated risk factors in pediatric patients with SCD who received Rh− and K–negative or matched RBCs. The alloimmunization prevalence was 11.7% and the alloimmunization rate was 0.36 alloantibodies per 100 transfusions. We found that receiving transfusions during proinflammatory events increased the risk of alloimmunization. HU did not reduce the likelihood of alloimmunization for episodically transfused patients who typically require transfusion during proinflammatory events. The risk of alloimmunization was also higher for patients with HbSS and HbSβ0–thalassemia genotypes and those with a high transfusion burden. High transfusion burden is associated with a higher likelihood of encountering a foreign donor RBC antigen. Therefore, alloimmunization is determined by foreign RBC antigen exposure as well as the context under which a foreign RBC antigen is exposed.
Receiving transfusions during an inflammatory state promotes alloimmunization in murine models.24 In human studies, increased alloimmunization occurs in inflammation-associated conditions, including autoimmune diseases, febrile nonhemolytic transfusion reactions, and infections.25-28 Fasano et al examined 52 alloimmunized patients with SCD and found that transfusions during proinflammatory events increased the risk of alloantibody formation by 8.9-fold.19 We analyzed 27 alloimmunized patients with SCD and found that transfusion during proinflammatory events was associated with a 4.22-fold higher risk for alloimmunization, albeit slightly lower than the previously reported. Notably, Fasano et al used varied levels of RBC antigen matching in their study, and up to 28% of transfused RBC units were matched for ABO and RhD antigens only. The majority of our patients received Rh (C and E)− and K–negative or matched units, which likely decreased the overall risk of alloimmunization, even during proinflammatory events. Fasano et al also found that among complications of SCD, ACS (OR, 16.7; 95% CI, 10.7-26.4; P < .001) was strongly associated with alloimmunization. We did not find ACS affecting the risk of alloimmunization. Instead, we identified that transient aplastic crisis promoted alloimmunization. However, because of the low case number of aplastic crisis in our study, further studies are warranted.
SCD is an inflammatory disease, partly because of chronic hemolysis.29 Upon hemolysis, Hb is released from RBCs and oxidized into heme, which induces sterile inflammation via the toll-like receptor–4 and inflammasome signaling. The inflammatory milieu in SCD is characterized by activated neutrophils, monocytes, and lymphocytes as well as increased cytokines and chemokines in the plasma. The inflammation is further escalated during acute complications of SCD, such as ACS and vaso-occlusive crisis because of increased hemolysis and potentially concomitant infections.30-32 In addition, patients who receive alloimunization demonstrate abnormalities in monocyte or macrophage function, T-cell differentiation, and B-cell maturation and activation.24,33,34 Therefore, the augmented inflammatory milieu present during SCD acute complications may exacerbate the already abnormal immune response of those patients, facilitating RBC alloantigen recognition and subsequent alloantibody formation.
Most patients with SCD are treated with HU at our center. HU, a ribonucleotide reductase inhibitor, blocks cellular proliferation by arresting cell cycle at the G1-S interface leading to myelosuppression. Patients receiving HU have lower numbers of neutrophils, monocytes, lymphocytes, and natural killer cells.35 HU also reduces neutrophil adhesion and monocyte inflammatory potential, resulting in decreased plasma levels of various cytokines and chemokines.21,36-38 Despite these anti-inflammatory effects, our study found that HU therapy did not decrease alloimmunization in patients who received episodic transfusion, and the reasons can be multifactorial. HU improves but does not correct the SCD-associated inflammation. Patients with SCD treated with HU still have higher levels of cytokines and chemokines than age and race–matched healthy controls.21,36 Episodically transfused patients usually receive transfusions to manage acute SCD complications, and HU has been reported insufficient to dampen the further augmented inflammation associated with the acute complications.31 Furthermore, HU inhibits T-cell proliferation but does not affect T-cell activation, as evidenced by effective immunization to various vaccines despite HU use.39,40 Although HU may not reduce the risk of alloimmunization directly, HU may decrease alloimmunization indirectly by lowering the overall donor exposure because it minimizes acute SCD-related events requiring transfusion.41-43 Given the benefits of HU in preventing and treating acute SCD complications,41-44 HU treatment should be continued during episodes of complications.
Our finding that high transfusion load is associated with alloimmunization is consistent with previous studies.12,45-47 Distinct from prior studies, we found high transfusion load was a significant risk factor yet with a relatively low OR (OR: 1.02; 95% CI: 1.003-1.04; P < .020). This is likely explained by our use of transfused units up to the first alloimmunization event, whereas prior studies used total transfused units regardless of the timing of antibody formation. The rationale for defining transfusion load as units transfused before the first alloimmunization event is that (1) antigen matching strategy is the same for all patients before the formation of the first antibody and that (2) matching for additional antigens beyond routine Rh and K antigens can potentially affect future exposure to antigens for which antibodies are not formed yet.48 Overall, our findings suggest that high transfusion load is critical for alloimmunization, but other patient intrinsic factors, such as Hb genotypes, contribute as well.
There are limitations to our study. Firstly, patients who received episodic transfusion were not routinely screened for antibodies after each transfusion, consistent with reports by other groups.49,50 Although we extended the timeframe for antibody screens, 145 episodically transfused patients did not have a follow-up antibody test after their last transfusion, with a total of 478 units received during the study period. Based on our alloimmunization rate of 0.36 alloantibodies per 100 units transfused, we might have missed 2 alloantibodies among those 478 units for which no follow-up antibody test was performed (478 × 0.36 alloantibodies per 100 RBC units). In addition, a recent study showed that the alloimmunization rate among episodically transfused patients who were screened 2 or 6 months after transfusions was 0.58 alloantibodies per 100 RBC units transfused.49 When applying the rate to our patients’ results, 3 more alloantibodies (478 × 0.58 alloantibodies per 100 RBC units) could have been potentially identified. Because the numbers of missed alloantibodies are small, our findings would not be affected substantially. Secondly, although most patients received transfusions exclusively from our center, some patients received transfusions at other centers, and these exposures were not captured in our analysis. However, in our experience, the number of the outside transfusions was very low. Thirdly, in determining the association of alloimmunization with transfusion during proinflammatory events, our analysis would be strengthened by knowledge of the donor RBC antigen phenotypes. Ideally, only transfused RBC units expressing an antigen corresponding to the formed antibody should be considered as exposure (eg, include only S antigen positive RBC units that were transfused into a patient who formed anti-S). However, RBC units are not routinely phenotyped beyond Rh (D, C, and E) and K antigens. We assumed that RBC units positive for an antigen were equally distributed among the alloantibody-positive and alloantibody-negative transfusions, thus included all the units, regardless of RBC phenotypes.
In summary, our results indicate that transfusion during proinflammatory events increases the risk of alloimmunization, and HU therapy does not reduce alloimmunization. Therefore, judicious use of transfusion, especially during proinflammatory events, should be exercised to prevent alloimmunization in patients with SCD.
Acknowledgments
The authors thank the SCCRIP study investigators Ti-Cheng Chang and Gang Wu from St. Jude for providing WGS data and RH genotypes, respectively.
The study was supported by a National Blood Foundation Early-Stage Investigator’s Award (Y.Z.), the American Lebanese Syrian Associated Charities (Y.Z.), and the National Institutes of Health/National Heart, Lung, and Blood Institute (HL147879-01; S.T.C.).
Authorship
Contribution: Y.Z., J.S.H., and S.T.C. designed the study; P.-L.C., M.B., M.R., and J.Y. collected the clinical data; J.M.G. and G.K. designed and performed statistical analyses; and Y.Z., J.M.G., G.K., J.S.H., and S.T.C. wrote the manuscript.
Conflict-of-interest disclosure: J.S.H. receives consulting fees from CVS Pharmacy, Global Blood Therapeutics, and Forma Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Yan Zheng, Department of Pathology, St. Jude Children’s Research Hospital, 262 Danny Thomas Pl, MS342, Memphis, TN 38105; e-mail: yan.zheng@stjude.org.
References
Author notes
The data that support the findings of this study are available upon reasonable request from the corresponding author, Yan Zheng (yan.zheng@stjude.org).
The full-text version of this article contains a data supplement.