Key Points
Median platelet count increased to ≥50 × 103/μL at month 2 after starting eltrombopag and stayed at 70 × 103/μL to 100 × 103/μL thereafter.
Compliance to prescribed eltrombopag dose since the previous routine visit was ≥96.0%.
Abstract
CITE was a prospective, noninterventional study in adult patients with chronic immune thrombocytopenia treated with eltrombopag under routine clinical care in Asia-Pacific, the Middle East, and Turkey. Data to assess eltrombopag usage, compliance, and outcomes were collected from May 2017 to December 2020. Platelet response was defined as platelet count ≥50 × 103/μL in the absence of rescue medications and splenectomy. Quality of life was evaluated using the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) questionnaire. Noncompliance was defined as the number of missed doses and number of days where the patient did not follow food instructions. A total of 231 patients were enrolled; the median (range) duration of eltrombopag treatment was 484.5 (1-642) days. Compliance to prescribed eltrombopag dose since the previous routine visit was high at ≥96.0%. Baseline median platelet count was 19.0 × 103/μL, which increased to ≥50 × 103/μL at month 2 and mostly fluctuated between 70 × 103/μL and 100 × 103/μL thereafter. The median time to first platelet response was 1.05 (95% confidence interval: 0.92-1.28) months, and the median (interquartile range) maximum duration of platelet response was 193 (57-456) days. FACIT-F scores improved from a mean (standard deviation) 34.4 (12.1) at baseline to 38.5 (9.1) at month 18. Adverse events occurred in 50.9% of patients (n = 116), the most common being upper respiratory tract infection (8.3%) and headache (6.6%). These findings confirmed the effectiveness of eltrombopag treatment in routine practice and reassured that real-world compliance to eltrombopag-prescribed doses and dietary instructions in Asia-Pacific, the Middle East, and Turkey were in line with current recommendations.
Introduction
Eltrombopag is an oral thrombopoietin receptor agonist approved in several countries for the treatment of patients aged ≥1 year with primary chronic immune thrombocytopenia (cITP) lasting >6 months from diagnosis who are refractory to corticosteroids and immunoglobulins (IVIG).3 In some countries, the market authorization contains a restriction for patients with cITP to be splenectomized or where splenectomy is contraindicated. The main clinical data supporting eltrombopag approval were obtained from two phase 3 double-blind, randomized, placebo-controlled clinical trials (TRA100773B and RAISE) and a single-arm, open-label extension study (EXTEND).4-6 These studies demonstrated that eltrombopag therapy was effective in raising platelet counts, reducing bleeding, and reducing the need for concomitant ITP therapies in patients with cITP refractory to other treatments. Most of the data describing efficacy and safety for eltrombopag treatment are derived from clinical trials and may not reflect the clinical experience in the real world. Results from a retrospective Spanish study of patients with primary cITP treated with eltrombopag showed that eltrombopag was effective and generally well tolerated in unselected patients.7 However, patients treated with eltrombopag require regular monitoring of platelet count to allow for dose adjustments, as well as strict dietary restrictions to ensure optimal medicinal absorption, which may reduce compliance and affect patient outcomes. Data from observational studies aimed to assess treatment patterns and outcomes for patients with cITP being treated with eltrombopag are limited, particularly in geographies outside of the United States and Europe. In addition, differences in the recommended starting dose and dietary habits between Western and Asian patients may further affect outcomes and treatment compliance. For example, the recommended eltrombopag starting dose in adult patients of Asian descent is 25 mg once daily as opposed to 50 mg once daily for Western patients. In Asian and Middle Eastern countries, where dietary habits widely differ from the Western populations, as well as in Australia and Turkey, no data on eltrombopag treatment in routine clinical practice have been published to date.
CITE is a prospective, noninterventional study aimed to assess treatment duration, compliance, effectiveness, use, and tolerability of eltrombopag in adult patients with cITP in routine clinical practice in the Middle East, Turkey, South and East Asia, and Australia. Understanding the use of eltrombopag in real-world practices, patient compliance, and impact on clinical and patient-reported outcomes in these geographies will assist practitioners in improving patients’ management and experience. Results from the first interim analysis of 122 patients enrolled from May 2017 to September 2018 showed that eltrombopag was effective in increasing the median platelet count from baseline to month 6. Compliance to eltrombopag dosing instructions was also good and was maintained over 6 months of follow-up. There was a modest increase in the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) score from baseline to month 3, followed by a decrease in the score from month 6 to 12, which could have been attributed to the small number of patients at the respective visits.1 In the second interim analysis of 215 patients enrolled from May 2017 to May 2019 and followed up till September 2019, the median platelet count increased from baseline to month 15, whereas the number of patients with platelet response remained steady. Eltrombopag provided clinically meaningful improvements on patients’ fatigue at month 1, which were maintained for ≥1 year.2 Here, we report the final analysis of the study.
Methods
Study design
The CITE study was a phase 4, multicenter, prospective, single-arm, noninterventional study conducted in 40 sites across Turkey, the Asia-Pacific, and the Middle Eastern regions (Australia [1 site], China [3 sites], Egypt [4 sites], Hong Kong [4 sites], Lebanon [4 sites], Oman [1 site], Qatar [1 site], Singapore [2 sites], South Korea [5 sites], Thailand [4 sites], Turkey [9 sites], and Vietnam [2 sites]). Dosage of eltrombopag followed the recommendations of the respective country’s label and the routine of treating physicians. Patients were followed up with for a maximum of 18 months or for 3 months following discontinuation (defined as discontinuing eltrombopag treatment for ≥3 months). Patients who discontinued eltrombopag for <3 months without receiving any new cITP treatment or dose increase for concomitant ITP medication were allowed to continue the study regularly. Data were collected at routine visits and approximately at baseline and 1, 3, 6, 12, and 18 months after enrollment. The first patient’s first documentation was on May 11, 2017; the last patient’s last documentation was on September 8, 2020. Field monitors visited the center to check the completeness of patient records, accuracy of entries, and adherence to the protocol and Good Clinical Practice. For each participating site, an ethics committee approval was obtained according to the country and local regulations.
Patients
Patients aged ≥18 years diagnosed with cITP and eligible for eltrombopag treatment based on the market authorization in the respective country and the investigator’s judgment were enrolled. Patients who started eltrombopag up to 12 weeks before study enrollment were also eligible to participate in the study. Previous discontinued treatment cycles of eltrombopag were permitted provided the period between the last dose in the previous cycle and the first dose within the CITE study was ≥12 weeks. Prior treatment with romiplostim was permitted. Patients were excluded if they had any contraindications listed in the local market authorization, had active alcohol or drug addiction, were unlikely to be available to obtain long-term follow-up information, or were participating in any interventional clinical trial at the start of the study or any noninterventional studies for the indication of ITP. No formal sample size calculations were required for this descriptive observational study, as no formal hypotheses were being tested. All patients gave written informed consent and could withdraw from the study at any time.
Outcome measures
The primary endpoints for treatment duration and compliance (adherence) were dosing modifications and temporary interruptions, treatment discontinuation, number of days with missed doses, and compliance to dosing instructions. Primary endpoints for effectiveness were platelet response (defined as platelet count ≥50 × 103/μL in the absence of splenectomy or cITP rescue medications that comprised platelet and whole blood transfusions, dose increases, or newly initiated concomitant ITP medications),8,9 maximum duration of response, bleeding incidence and severity as assessed using the descriptive summaries of the World Health Organization Bleeding Scale, reduction in concomitant cITP medication, rescue treatment, and number of transfusions. Dose reduction was defined as a reduction in concomitant medication dosage at least once between the current and the preceding visit date. For platelet count, platelet response, and maximum duration of platelet response, data were summarized by the following subgroups: age (18-49, 50-64, 65-74, and ≥75 years), gender (male/female), number of prior ITP medications, and country groups based on ethnicity (group 1: Hong Kong, Vietnam, Thailand, China, South Korea, Singapore; group 2: Egypt; group 3: Oman, Qatar, Lebanon; group 4 [Other]:Turkey, Australia). The secondary endpoints for treatment use encompassed disease history, demographics, comorbidities, concomitant medications, prior ITP treatment, reason for treatment with eltrombopag, and next-line cITP treatment after eltrombopag discontinuation. The duration of eltrombopag treatment within the study was defined as the time from the "date of first administration of study treatment" until the date of permanent discontinuation. For patients who took eltrombopag before inclusion into the study, the date of first dosing was captured retrospectively, provided these patients started eltrombopag within 12 weeks before informed consent. Quality of life (QoL) was assessed using the FACIT-F QoL questionnaire. The FACIT-F scale is a 13-item patient-reported outcomes instrument designed to assess fatigue/tiredness and its impact on daily activities and functioning with a 7-day recall period. Its validity has been established in cITP.10 Owing to changes requested by the local ethics committee, the revised version of the Vietnamese questionnaire was not recognized as valid by FACIT. Therefore, Vietnamese patients (4 patients) were not included in the analysis. Adverse events (AEs) were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 23.1 terminology. A patient with multiple occurrences of an AE was counted only once in the corresponding AE category. A patient with multiple AEs within a primary system organ class was counted only once for that class.
Statistical methods
No sensitivity analyses were conducted. All statistical analyses were purely descriptive as the study did not aim to confirm or reject predefined hypotheses and were performed using SAS version 9.2 or higher (SAS Institute, North Carolina, USA). For continuous variables, the number of available observations (N), mean, standard deviation (SD), median, minimum, maximum, and quartiles (25% and 75% percentiles) were provided. For categorical variables, the absolute and relative frequencies in each category were provided. Visits without visit dates or after discontinuation were excluded from the analysis. Patients who missed assessments or who terminated before the end of the study contributed to all analyses for which data were obtained. The safety set and full analysis set included all patients enrolled in the study who received at least 1 dose of eltrombopag within the study-relevant treatment course.
Results
Patient disposition and baseline characteristics
A total of 231 patients were enrolled in the study; of these, 98.7% (n = 228) of patients received ≥1 dose of eltrombopag within the study-relevant treatment course and were included in the analysis set. Overall, 52.8% (n = 122) of patients completed the study, whereas 47.2% (n = 109) of patients discontinued the study prematurely (41.6% [n = 96] of patients discontinued treatment permanently during the study; supplemental Figure 1). The most frequent reasons for permanent treatment discontinuation were no or insufficient effectiveness (10.4%, n = 24), patient preference (7.4%, n = 17), and sustained response (6.5%, n = 15). The most frequent reasons for premature study discontinuation were permanent treatment discontinuation (24.2%, n = 56) and lost to follow-up (10.4%, n = 24). Of the 228 patients included in the analysis set, 145 had already started eltrombopag before entering the study and 83 had started eltrombopag at or after providing informed consent.
Median (interquartile range [IQR]) age was 48 (31.0-62.0) years, 63.2% (n = 144) were females, and the median (IQR) time since ITP diagnosis was 2.6 (0.5-8.2) years (Table 1). Overall, 59.2% (n = 135) of patients presented with ≥1 concomitant disease at baseline. The 3 most common primary system organ classes were metabolism and nutrition (25.9%, n = 59), vascular (21.5%, n = 49), and gastrointestinal disorders (10.1%, n = 23). Overall, 17.5% (n = 40) of patients received no prior ITP treatment, whereas 36.4% (n = 83), 24.1% (n = 55), 13.2% (n = 30), and 8.8% (n = 20) received 1, 2, 3, and 4 or more prior ITP treatments, respectively. The most frequent prior ITP medications were corticosteroids (46.9%, n = 107) and IVIG (20.6%, n = 47). Nearly 10% (n = 22) had received prior eltrombopag treatment, which should have been discontinued ≥12 weeks before the first dose of the study-relevant treatment course to be eligible to enter the study. The median duration of the last eltrombopag treatment cycle was 85.0 days.
Patient baseline characteristics
| . | Total (N = 228) . |
|---|---|
| Age, y | |
| Mean (SD) | 47.9 (18.3) |
| Median (IQR) | 48.0 (31.0-62.0) |
| Age categories, n (%) | |
| 18-49 y | 117 (51.3) |
| 50-64 y | 66 (28.9) |
| 65-74 y | 26 (11.4) |
| ≥75 y | 19 (8.3) |
| Gender, n(%) | |
| Male | 84 (36.8) |
| Female | 144 (63.2) |
| Race, n (%) | |
| Caucasian | 101 (44.3) |
| Black | 2 (0.9) |
| Asian | 92 (40.4) |
| Other | 33 (14.5) |
| Ethnicity, n (%) | |
| Arabic | 47 (20.6) |
| East Asian | 53 (23.2) |
| Southeast Asian | 29 (12.7) |
| South Asian | 11 (4.8) |
| Egyptian | 25 (11.0) |
| Turkish | 57 (25.0) |
| Other | 6 (2.6) |
| Time since first diagnosis, y | |
| Mean (SD) | 5.8 (7.6) |
| Median (IQR) | 2.6 (0.5-8.2) |
| Patients with thromboembolic events in the last 12 mo, n (%) | 8 (3.5) |
| Patients with prior splenectomy, n (%) | 54 (23.7) |
| Bleeding events in the last 12 mo, n (%) | 88 (38.6) |
| Mean (SD) | 1.4 (0.7) |
| Median (IQR) | 1.0 (1.0-2.0) |
| Grade 1 | 61 (26.8) |
| Grade 2 | 22 (9.6) |
| Grade 3 | 8 (3.5) |
| Grade 4 | 2 (0.9) |
| Unknown | 1 (0.4) |
| Platelet transfusions,∗ n | 47 |
| Mean (SD) | 7.0 (8.2) |
| Median (IQR) | 3.0 (1.0-10.0) |
| RBC transfusions,∗ n | 17 |
| Mean (SD) | 2.6 (1.7) |
| Median (min, max) | 2.0 (1.0, 6.0) |
| Whole blood transfusions,∗ n | 1 |
| Mean (SD) | 2.0 (n.a.) |
| Median (IQR) | 2.0 (2.0-2.0) |
| Patients with prior eltrombopag treatment,† n | 19 |
| Cycles,‡ mean (SD) | 1.1 (0.2) |
| Cycles,‡ median (IQR) | 1.0 (1.0-1.0) |
| Duration of last treatment cycle‡ in days | |
| Mean (SD) | 233.9 (410.9) |
| Median (IQR) | 85.0 (31.0-217.0) |
| Maximum daily dose (mg) of the last treatment cycle‡ | |
| Mean (SD) | 51.3 (13.1) |
| Median (IQR) | 50.0 (50.0-50.0) |
| Response, n (%) | |
| Platelet response | 2 (0.9) |
| Complete platelet response | 6 (2.6) |
| No response | 8 (3.5) |
| Missing | 3 (1.3) |
| Not applicable | 209 (91.7) |
| Prior ITP medications, n (%) | |
| 0 | 40 (17.5) |
| 1 | 83 (36.4) |
| 2 | 55 (24.1) |
| 3 | 30 (13.2) |
| ≥4 | 20 (8.8) |
| . | Total (N = 228) . |
|---|---|
| Age, y | |
| Mean (SD) | 47.9 (18.3) |
| Median (IQR) | 48.0 (31.0-62.0) |
| Age categories, n (%) | |
| 18-49 y | 117 (51.3) |
| 50-64 y | 66 (28.9) |
| 65-74 y | 26 (11.4) |
| ≥75 y | 19 (8.3) |
| Gender, n(%) | |
| Male | 84 (36.8) |
| Female | 144 (63.2) |
| Race, n (%) | |
| Caucasian | 101 (44.3) |
| Black | 2 (0.9) |
| Asian | 92 (40.4) |
| Other | 33 (14.5) |
| Ethnicity, n (%) | |
| Arabic | 47 (20.6) |
| East Asian | 53 (23.2) |
| Southeast Asian | 29 (12.7) |
| South Asian | 11 (4.8) |
| Egyptian | 25 (11.0) |
| Turkish | 57 (25.0) |
| Other | 6 (2.6) |
| Time since first diagnosis, y | |
| Mean (SD) | 5.8 (7.6) |
| Median (IQR) | 2.6 (0.5-8.2) |
| Patients with thromboembolic events in the last 12 mo, n (%) | 8 (3.5) |
| Patients with prior splenectomy, n (%) | 54 (23.7) |
| Bleeding events in the last 12 mo, n (%) | 88 (38.6) |
| Mean (SD) | 1.4 (0.7) |
| Median (IQR) | 1.0 (1.0-2.0) |
| Grade 1 | 61 (26.8) |
| Grade 2 | 22 (9.6) |
| Grade 3 | 8 (3.5) |
| Grade 4 | 2 (0.9) |
| Unknown | 1 (0.4) |
| Platelet transfusions,∗ n | 47 |
| Mean (SD) | 7.0 (8.2) |
| Median (IQR) | 3.0 (1.0-10.0) |
| RBC transfusions,∗ n | 17 |
| Mean (SD) | 2.6 (1.7) |
| Median (min, max) | 2.0 (1.0, 6.0) |
| Whole blood transfusions,∗ n | 1 |
| Mean (SD) | 2.0 (n.a.) |
| Median (IQR) | 2.0 (2.0-2.0) |
| Patients with prior eltrombopag treatment,† n | 19 |
| Cycles,‡ mean (SD) | 1.1 (0.2) |
| Cycles,‡ median (IQR) | 1.0 (1.0-1.0) |
| Duration of last treatment cycle‡ in days | |
| Mean (SD) | 233.9 (410.9) |
| Median (IQR) | 85.0 (31.0-217.0) |
| Maximum daily dose (mg) of the last treatment cycle‡ | |
| Mean (SD) | 51.3 (13.1) |
| Median (IQR) | 50.0 (50.0-50.0) |
| Response, n (%) | |
| Platelet response | 2 (0.9) |
| Complete platelet response | 6 (2.6) |
| No response | 8 (3.5) |
| Missing | 3 (1.3) |
| Not applicable | 209 (91.7) |
| Prior ITP medications, n (%) | |
| 0 | 40 (17.5) |
| 1 | 83 (36.4) |
| 2 | 55 (24.1) |
| 3 | 30 (13.2) |
| ≥4 | 20 (8.8) |
n.a., not applicable; RBC, red blood cell.
Number of transfusions for patients with ≥1 transfusion.
Prior eltrombopag treatment refers to patients who had received and discontinued eltrombopag previously but did not restart it ≥12 weeks before the eltrombopag cycle captured in the study, as described in “Methods.”
A cycle was defined as repeated administration(s) after interruption of ≥12 weeks.
Eltrombopag treatment
Most patients started eltrombopag because of no or insufficient effectiveness of prior treatment (51.3%, n = 117), loss of response to prior treatment (17.5%, n = 40), or platelet count fluctuation (14.5%, n = 33). The starting dose of eltrombopag was mostly either 50 mg (55.7%, n = 127) or 25 mg (40.4%, n = 92) daily. In 93.8% of patients of Asian descent, the starting dose was 25 mg daily, whereas in 76.1% of patients of non-Asian descent, the starting dose was 50 mg daily. The median (range) duration of eltrombopag treatment within the study was 484.5 (1-642) days. Overall, 73.2% (n = 167) of patients had at least 1 dose adjustment, corresponding to a total of 559 dose adjustments within the study. A total of 235 dose increases occurred in 111 patients (48.7%), and 148 dose reductions occurred in 80 patients (35.1%). Eighty-six temporary interruptions occurred in 52 patients (22.8%). The most frequent reason for dose reductions (114 reductions in 62 patients), dose increases (145 increases in 65 patients), and temporary interruptions (42 interruptions in 27 patients) was platelet count–driven dose change as per label.
Compliance to treatment
The median number of days without completely missing a dose since the previous routine visit and the median number of days compliant with dietary instructions since the previous routine visit was 28 and 28 days at month 1, 56 and 56 days at month 3, 86 and 86 days at month 6, 144 and 142 days at month 12, and 147 and 147 days at month 18, respectively. Overall, compliance to prescribed eltrombopag dose since the previous routine visit was ≥96.0% (89.3% at early discontinuation [ED] visit).
Hematologic and clinical effectiveness
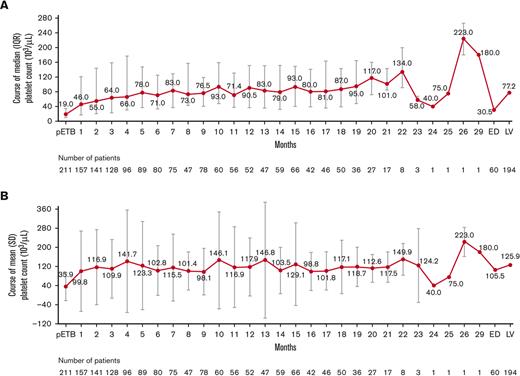
Before the start of eltrombopag, the median (range) platelet count was 19.0 × 103/μL (1.0 × 103/μL-495.0 × 103/μL), which quickly increased to ≥50 × 103/μL at month 2, with further mild increases up to month 5 (mean [SD] before starting eltrombopag was 35.9 × 103/μL [58.9 × 103/μL]). Subsequently, and up to month 20, after the start of eltrombopag, the median platelet levels mostly fluctuated between 70 × 103/μL and 100 × 103/μL (mean values 100 × 103/μL and 150 × 103/μL, respectively). From month 21 onward, <20 patients contributed to the platelet count results. The median (range) platelet count at the last visit (LV) was 77.2 × 103/μL (1.0 × 103/μL-1352.0 × 103/μL), derived from 563 laboratory measures collected from 194 patients, whereas the median (range) platelet count at the ED visit was 30.5 × 103/μL (1.0 × 103/μL-1844.0 × 103/μL), derived from 292 laboratory measures collected from 60 patients who discontinued treatment. However, fluctuating platelet count values have been observed among patients at almost all time points throughout the study course (Figure 1).
Platelet count over time. (A) Median and (B) mean platelet counts∗ over time. ∗Pre-eltrombopag values were the values collected before the start of the current eltrombopag treatment course. Month 1 value was collected between the start of eltrombopag treatment and 1 month later, month 2 value was collected between 1 month after eltrombopag start and 2 months after eltrombopag start, and so on. Last observation carried forward was labeled as LV. pETB, pre-eltrombopag administration.
Platelet count over time. (A) Median and (B) mean platelet counts∗ over time. ∗Pre-eltrombopag values were the values collected before the start of the current eltrombopag treatment course. Month 1 value was collected between the start of eltrombopag treatment and 1 month later, month 2 value was collected between 1 month after eltrombopag start and 2 months after eltrombopag start, and so on. Last observation carried forward was labeled as LV. pETB, pre-eltrombopag administration.
A median platelet level of ≥50 × 103/μL was reached at month 1 in patients aged 18 to 49 and ≥75 years, at month 2 in patients aged 50 to 64 years, and at month 3 in patients aged 65 to 74 years. The median platelet count of ≥50 × 103/μL was reached at month 1 after starting eltrombopag in female patients and at month 4 in male patients and remained between ≥60 × 103/μL and <110 × 103/μL thereafter in both subgroups.
A median platelet count of ≥50.0 × 103/μL was reached for the first time at month 1 among patients with 0 or 1 prior ITP medication, at month 2 in patients with 2 or 3 prior ITP medications, and at month 3 after starting eltrombopag among patients with ≥4 prior ITP medications. Platelet levels remained ≥50.0 × 103/μL in patients with 0, 1, or 2 prior ITP medications but considerably dropped in patients with 3 or ≥4 prior ITP medications.
The median threshold platelet level of ≥50 × 103/μL was achieved within the first month after eltrombopag treatment in the Middle East countries (groups 2 and 3), which was earlier than in the overall study population, whereas it was reached between 2 and 3 months in Asian countries (group 1; supplemental Figure 2). The duration of response by subgroups is presented in supplemental Table 1.
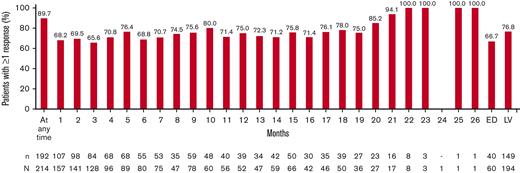
Among the 214 patients with at least 1 posttreatment measure, 89.7% (n = 192) achieved at least once a platelet count ≥50 × 103/μL in the absence of splenectomy or cITP rescue medications after the start of eltrombopag therapy. At month 1 after the start of eltrombopag, 68.2% (107/157) of patients had already achieved platelet response. At LV, 76.8% (149/194) of patients achieved ≥1 platelet response (Figure 2).
Course of platelet response∗ over time. ∗Percentage values for patients were calculated based on the respective number (n) of patients with response data available at that specific time point as denominator. A patient was defined as “with response data at that time” if ≥1 measurement was documented in the respective time window. At any time indicates the proportion of patients experiencing ≥1 platelet response at any time point since the start of eltrombopag treatment. Last observation carried forward was labeled as LV. At any time indicates the proportion of patients experiencing ≥1 platelet response at any time point since the start of eltrombopag. N, number of patients with ≥1 measurement; n, number of patients with ≥1 event.
Course of platelet response∗ over time. ∗Percentage values for patients were calculated based on the respective number (n) of patients with response data available at that specific time point as denominator. A patient was defined as “with response data at that time” if ≥1 measurement was documented in the respective time window. At any time indicates the proportion of patients experiencing ≥1 platelet response at any time point since the start of eltrombopag treatment. Last observation carried forward was labeled as LV. At any time indicates the proportion of patients experiencing ≥1 platelet response at any time point since the start of eltrombopag. N, number of patients with ≥1 measurement; n, number of patients with ≥1 event.
No notable differences were seen in response rates at any time or until LV between age groups and between male and female patients (supplemental Figure 3). Platelet response rate at any time during the study was numerically greater in patients with 1 prior ITP medication (96.1%), compared with patients without prior ITP medication (86.1%) or with 2 (88.7%), 3 (85.7%), and ≥4 (80.0%) prior ITP medications. The proportion of patients with ≥1 platelet response at any time during the study was numerically greater in the Middle East countries (groups 2, 95.0%; group 3, 94.6%) compared with other countries (group 1, 87.5%; group 4, 86.0%) (supplemental Figure 3). The maximum median duration of platelet response was 193.0 days (IQR, 57.0-456.0 days; supplemental Table 1).
Concomitant ITP medication
Forty-three percent of patients (n = 99) received a concomitant ITP medication at baseline, the most common being prednisolone (25.4%, n = 58) and methylprednisolone (11.0%, n = 25). Overall, a few patients reduced baseline ITP concomitant medications (13.1%; n = 13), either permanently discontinuing (8.1%, n = 8) or achieving a sustained reduction of ≥1 baseline ITP concomitant medication (8.1%, n = 8) without needing rescue medication and/or splenectomy. Fifty-six percent of patients (n = 128) received ≥1 concomitant ITP medication during the study course, and the total number of administrations was 589. The most frequently received concomitant ITP medications were corticosteroids for systemic use (33.3% of patients, n = 76, 324 medications), IVIG (11.8% of patients, n = 27, 42 medications), and other immunosuppressants (3.1% of patients, n = 7, 14 medications; all were azathioprine). Overall, 20 patients received subsequent ITP medication after discontinuation of eltrombopag. The most frequently received subsequent medication was romiplostim (6 patients).
Rescue therapy/splenectomy
During the study, 35.5% (n = 81) of patients required 422 rescue therapies, whereas 3.5% (n = 8) underwent splenectomy. The most frequent patient-based rescue therapies were newly initiated concomitant ITP medication (24.1%, n = 55), platelet transfusions, and increase in dose of concomitant ITP medication (12.7%, n = 29, each). The proportion of patients requiring any rescue therapy since their previous routine visit was the highest at month 1 (14.5%, n = 33) and decreased during the subsequent visits from month 3 (9.6%, n = 22) to month 18 (7.9%, n = 18).
Transfusions
A few patients received platelet (12.7%, n = 29) and/or whole blood transfusion (1.3%, n = 3) during the study. The median number of platelet infusions since the preceding visit was 4.0 at month 1, 1.5 at month 3, and 1.0 each at months 6, 12, and 18, and 5.0 at the ED visit.
Bleeding events
The number of patients with bleeding events remained relatively constant throughout the study, although the length of the time interval between visits increased with the study duration. The median number of bleeding events since the preceding visit was 1 each at months 1, 3, 6, 12, and 18, and at the ED visit. Overall, 47.4% (n = 108) of patients experienced ≥1 bleeding event; 8.8% (n = 20) of patients experienced ≥1 grade 3/4 bleeding event, most commonly epistaxis and abnormal vaginal bleeding. Two patients experienced grade 4 nonfatal central nervous system bleeding with neurologic signs and symptoms.
FACIT fatigue
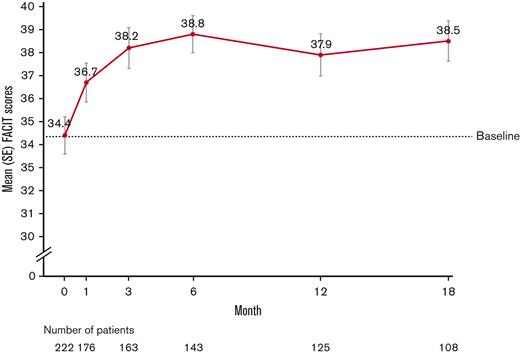
Of the 224 eligible patients (excluding the questionnaire of 4 Vietnamese patients), 99.1% (n = 222) completed the FACIT-F questionnaire at baseline, 78.6% (n = 176) at month 1, 48.7% (n = 109) at month 18, and 81.3% (n = 182) at the LV. The total FACIT-F scores improved from a mean (SD) of 34.4 (12.1) at baseline to 38.5 (9.1) at month 18 (mean [SD] change, 3.1 [11.6]) and to 37.5 (9.7) at LV (mean [SD] change, 3.0 [10.9]) (Figure 3). The increase of ≥3 points was considered clinically important.
Safety
AEs occurred in 50.9% (n = 116) of patients. The most frequently reported AEs (occurring in ≥3.0% of patients) were upper respiratory tract infection (8.3%, n = 19), headache (6.6%, n = 15), anemia (4.4%, n = 10), thrombocytopenia (4.4%, n = 10), increase in alanine aminotransferase (3.5%, n = 8), pyrexia (3.5%, n = 8), cough (3.1%, n = 7), and epistaxis (3.1%, n = 7). Most of the AEs were of grade 1 (15.8%) or grade 2 (14.5%) severity; 13.2% (n = 30) of patients experienced grade 3 AEs (occurring in ≥3 patients: anemia, thrombocytopenia, increase in alanine aminotransferase, and epistaxis), and 7.5% (n = 17) experienced grade 4 AEs (occurring in ≥3 patients: thrombocytopenia; Table 2). Serious AEs were reported in 13.2% (n = 30) of patients, including 2.6% (n = 6) serious adverse drug reactions (S[ADRs]), with 2 patients each experiencing thrombocytopenia and deep vein thrombosis, and 1 patient experiencing gingival bleeding, jaundice, presence of blood in urine, cerebral hemorrhage, cerebral infarction, and cerebral venous sinus thrombosis. Nonserious ADRs (nsADRs) were reported in 8.8% (n = 20) of patients and included headache (2.2%, n = 5), rash (0.9%, n = 2), alopecia (0.9%, n = 2), and an increase in alanine aminotransferase (0.9%, n = 2). Overall, 2 patients died because of on-treatment serious AEs (1 patient had Escherichia coli sepsis and urinary tract infection; the other patient experienced multiple organ dysfunction syndrome and fungal pneumonia); none of these were considered attributable to eltrombopag treatment. Twelve patients discontinued study treatment owing to AEs.
Summary of overall AEs (occurring in ≥5 patients) or grade 3/4 AEs (occurring in ≥2 patients)
| Preferred term, n (%) . | Any AE . | Grade 3 . | Grade 4 . |
|---|---|---|---|
| All events | 116 (50.9) | 30 (13.2) | 17 (7.5) |
| Upper respiratory tract infection | 19 (8.3) | 1 (0.4) | 1 (0.4) |
| Headache | 15 (6.6) | 1 (0.4) | 1 (0.4) |
| Anemia | 10 (4.4) | 3 (1.3) | − |
| Thrombocytopenia | 10 (4.4) | 4 (1.8) | 5 (2.2) |
| Pyrexia | 8 (3.5) | 2 (0.9) | − |
| Alanine aminotransferase increase | 8 (3.5) | 3 (1.3) | − |
| Cough | 7 (3.1) | − | − |
| Epistaxis | 7 (3.1) | 7 (3.1) | − |
| Arthralgia | 6 (2.6) | − | − |
| Bone pain | 6 (2.6) | − | − |
| Rash | 6 (2.6) | − | − |
| Abdominal pain upper | 5 (2.2) | − | − |
| Diarrhea | 5 (2.2) | − | − |
| Nausea | 5 (2.2) | − | − |
| Urinary tract infection | 5 (2.2) | − | 1 (0.4) |
| Dizziness | 5 (2.2) | 1 (0.4) | − |
| Hypoesthesia | 5 (2.2) | − | − |
| Immune thrombocytopenia | 4 (1.8) | 1 (0.4) | 1 (0.4) |
| Gastritis | 4 (1.8) | 2 (0.9) | − |
| Gingival bleeding | 3 (1.3) | 2 (0.9) | 1 (0.4) |
| Blood bilirubin increase | 3 (1.3) | 2 (0.9) | − |
| Hemoglobin decrease | 3 (1.3) | 2 (0.9) | − |
| Vaginal hemorrhage | 3 (1.3) | 2 (0.9) | 1 (0.4) |
| Platelet count decrease | 2 (0.9) | − | 2 (0.9) |
| Hematuria | 2 (0.9) | 2 (0.9) | − |
| Preferred term, n (%) . | Any AE . | Grade 3 . | Grade 4 . |
|---|---|---|---|
| All events | 116 (50.9) | 30 (13.2) | 17 (7.5) |
| Upper respiratory tract infection | 19 (8.3) | 1 (0.4) | 1 (0.4) |
| Headache | 15 (6.6) | 1 (0.4) | 1 (0.4) |
| Anemia | 10 (4.4) | 3 (1.3) | − |
| Thrombocytopenia | 10 (4.4) | 4 (1.8) | 5 (2.2) |
| Pyrexia | 8 (3.5) | 2 (0.9) | − |
| Alanine aminotransferase increase | 8 (3.5) | 3 (1.3) | − |
| Cough | 7 (3.1) | − | − |
| Epistaxis | 7 (3.1) | 7 (3.1) | − |
| Arthralgia | 6 (2.6) | − | − |
| Bone pain | 6 (2.6) | − | − |
| Rash | 6 (2.6) | − | − |
| Abdominal pain upper | 5 (2.2) | − | − |
| Diarrhea | 5 (2.2) | − | − |
| Nausea | 5 (2.2) | − | − |
| Urinary tract infection | 5 (2.2) | − | 1 (0.4) |
| Dizziness | 5 (2.2) | 1 (0.4) | − |
| Hypoesthesia | 5 (2.2) | − | − |
| Immune thrombocytopenia | 4 (1.8) | 1 (0.4) | 1 (0.4) |
| Gastritis | 4 (1.8) | 2 (0.9) | − |
| Gingival bleeding | 3 (1.3) | 2 (0.9) | 1 (0.4) |
| Blood bilirubin increase | 3 (1.3) | 2 (0.9) | − |
| Hemoglobin decrease | 3 (1.3) | 2 (0.9) | − |
| Vaginal hemorrhage | 3 (1.3) | 2 (0.9) | 1 (0.4) |
| Platelet count decrease | 2 (0.9) | − | 2 (0.9) |
| Hematuria | 2 (0.9) | 2 (0.9) | − |
Discussion
Use of thrombopoietin receptor agonists for the treatment of patients with ITP has improved clinical outcomes by reducing bleeding risk and increasing platelet count in those patients who are refractory to first-line therapy. However, most of the data describing the efficacy and safety of eltrombopag treatment derive from randomized clinical trials, which do not always reflect outcomes in a real-world setting when a more heterogeneous population is encountered. To date, only a few studies have assessed the effectiveness and safety of eltrombopag in clinical practices; the majority of these are in Europe and the United States.7,11-15 The CITE study provided the first insight in a broad and unselected population of adult patients with ITP treated with eltrombopag across the Asia-Pacific region, the Middle East, and Turkey to support clinical decision-making in patients encountered in routine clinical practice. The study included slightly more female than male patients, in line with the general ITP population.16-18
Our findings showed that eltrombopag starting doses and the mean average daily doses were well within the dose range recommended on the product label. The number of days without missed doses and compliant with dietary instructions since the previous routine visit was largely in line with the number of days that would be expected to cover the respective time interval, thereby suggesting very good compliance. There was a high rate of dose adjustments, which points toward individualized/personalized treatment regimens that were based on different factors, of which the most frequent was “platelet count–driven as per label.” In terms of effectiveness, the median platelet count increased rapidly after the initiation of eltrombopag and remained relatively stable over time, with all postbaseline median values maintaining a platelet response of ≥50 × 103/μL. However, it should be noted that some fluctuation of platelet levels could be observed in individual patients throughout the study. Most patients experienced ≥1 platelet response at any time point since the start of therapy, particularly within the first 3 months after starting treatment, indicating early response. In addition, responses were maintained for a maximum of 6 months and were comparable among patients with ≤3 prior ITP medications.
Improvement of platelet count is the primary endpoint in randomized trials; however, the primary goal of ITP therapy is to lower the risk of bleeding episodes. In clinical trials, improved platelet responses translated into reduced bleeding and the use of rescue medication.19 In this study, most bleeding events were of mild severity, albeit 19 patients experienced grade 3/4 bleeding episodes. Approximately a third of the patients required rescue therapy, and only a minority of patients used splenectomy as a treatment option, in line with the availability of medicinal alternatives and current American Society of Hematology guidelines recommending the use of splenectomy as second-line treatment only with ITP persistence.20 A few patients required platelet, red blood cell, and/or whole blood transfusions during the study.
Unexplained fatigue is a common symptom in cITP and has a negative impact on patients’ QoL. Improvements in fatigue symptoms in this patient population, as captured by the FACIT-F questionnaire, were above the 3-point threshold for meaningfulness,21 suggesting that the use of eltrombopag and the associated increase in platelet count may have reflected in the improvement of fatigue in a substantial proportion of patients.
The reported nsADRs were largely consistent with the results of previous clinical trials.4-6 The SADRs were either associated with the study disease ITP, bleeding events, or thromboembolic events, or pointed to possible liver dysfunction (jaundice). No new safety signals were observed.
This study has some limitations inherent to the nature of observational studies, including the fact that study centers were selected based on the availability of the study population and the centers’ qualification to perform clinical studies. Another limitation was the risk of selection/ascertainment bias and lack of a control group, which may affect interpretation of the causality between treatment and outcomes. As the study documentation was performed during routine visits, it was anticipated that the proposed study schedule would have minimal potential for bias. Generally, the robustness of data could be affected owing to low sample size, drop out occurrence, particularly at later time points, and missing values. Furthermore, when patients were assigned to specific subgroups for the analyses, these were not always balanced regarding the number of patients included. Thus, some subgroups had a low number of patients, which hampered the comparability and generalizability of the results. Finally, underreporting of low-grade AEs often occurs in real-world studies.
In conclusion, this study provided valuable insights on eltrombopag treatment characteristics, effectiveness, and safety in an unselected population of adult patients with cITP from Asia-Pacific, the Middle East, and Turkey, complementing current findings from randomized clinical trials.
Acknowledgments
The authors thank the patients and physicians for participating in the study. They also thank Sabrina Giavara of Novartis UK Ltd and Vijay Kadasi of Novartis Healthcare Private Ltd for support with medical writing. This study was funded by Novartis Pharma AG. The authors received no financial support for the research, authorship, and/or publication of this article.
Authorship
Contribution: R.S.M.W. and M.G. contributed to the conception, design, or planning as well as the acquisition and analysis of the data and the interpretation of the results; A.E., P.A., R.Y., S.I., J.A.F., and J.H.J. contributed to the acquisition and analysis of the data and the interpretation of the results; X.W. and M.O. contributed to the acquisition and analysis of the data; İ.Y., M.A.Y., P.T., S.S.Y., M.M., and M.R. contributed to the interpretations of the results; and all authors contributed to critically reviewing or revising the manuscript for important intellectual content.
Conflict-of-interest disclosure: R.S.M.W. received speaking engagements fees, consulting fees, lecture fees, advisory board fees, and research grants from Amgen, Astellas Pharma, Alexion, Bristol Myers Squibb, GlaxoSmithKline, Janssen Pharmaceutical, Novartis, Pfizer, Roche, and Sanofi, and a research grant from Gilead. İ.Y. received consulting fees from Novartis. S.-S.Y. received consulting fees from Amgen, Astellas Pharma, Celgene, Chugai Pharmaceutical, Janssen Pharmaceutical, and Takeda; consulting fees, lecture fees, and advisory board fees from Novartis; and grant support from Kyowa Kirin, Roche–Genentech, and Yuhan Pharmaceutical. A.E. received lecture fees and advisory board fees from Amgen, Novartis, Roche, Takeda, AbbVie, Pfizer, and Sanofi. R.Y. received speaking engagement fees, consulting fees, and/or advisory board fees from Bayer, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi, and Takeda. S.I. was employed by Aerotek and contracted by Novartis Pharma AG at time of manuscript development. M.G., M.R., and J.A.F. are employees of Novartis Pharma AG. The remaining authors declare no competing financial interests.
Correspondence: Raymond Siu Ming Wong, Sir Y.K. Pao Centre for Cancer and Department of Medicine & Therapeutics, The Chinese University of Hong Kong, Hong Kong; e-mail: raymondwong@cuhk.edu.hk.
References
Author notes
Novartis is committed to sharing with qualified external researchers access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. This trial data availability is according to the criteria and process described on www.clinicalstudydatarequest.com
The full-text version of this article contains a data supplement.