Key Points
Detection of MRD in an apheresis stem cell sample is associated with a high risk of relapse after ASCT.
Post-ASCT surveillance MRD testing of plasma samples reliably identified patients with impending relapse.
Abstract
Improved biomarkers are required to guide the optimal use of autologous stem cell transplantation (ASCT) in patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL). We hypothesized that minimal residual disease (MRD) identified using immunoglobulin high-throughput sequencing in apheresis stem cell (ASC) samples, post-ASCT peripheral blood mononuclear cell (PBMC), and plasma samples could predict relapse. We studied 159 patients with R/R DLBCL who underwent ASCT, of whom 98 had an ASC sample and 60 had post-ASCT surveillance samples. After a median post-ASCT follow-up of 60 months, the 5-year progression-free survival (PFS) was 48%. MRD was detected in of 23/98 (23%) ASC samples and was associated with very poor PFS (5-year PFS 13% vs 53%, P < .001) and inferior overall survival (52% vs 68%, P = .05). The sensitivity and specificity of ASC MRD positivity for progression and death were 36% and 93%, respectively. Positive ASC MRD remained a significant predictor of PFS in multivariable analysis (hazard ratio [HR], 3.7; P < .001). Post-ASCT surveillance MRD testing of plasma, but not PBMC samples, reliably identified patients with an impending relapse. A positive plasma MRD result was associated with inferior PFS (HR, 3.0; P = .016) in a multivariable analysis. The median lead time from MRD detection to relapse was 62 days (range, 0-518 days). In conclusion, the detection of MRD in ASC samples is associated with a very high risk of relapse, justifying alternative treatment strategies or trials of novel consolidation options in these patients. Furthermore, post-ASCT MRD monitoring may facilitate the evaluation of the early initiation of treatment at molecular relapse. This trial has been registered at www.clinicaltrials.gov as #NCT02362997.
Introduction
Autologous stem cell transplantation (ASCT) can be curative for patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL), and relapse remains common.1,2 Currently, pre-ASCT positron emission tomography (PET) is an important tool for selecting patients for ASCT; however, the sensitivity and specificity of PET in this setting are suboptimal.3-6 Better biomarkers are needed to improve patient selection for ASCT, particularly with the emergence of chimeric antigen receptor (CAR) T-cell therapy. Improved biomarkers may also be helpful in surveillance after ASCT. In the post-ASCT setting, patients typically undergo clinical examination and periodic imaging. Early detection of relapse after ASCT could help identify candidates for preemptive interventions and facilitate the clinical investigation of such strategies.
Circulating tumor DNA (ctDNA) is an emerging biomarker with promising results in DLBCL. Multiple techniques, including immunoglobulin-based high-throughput sequencing (IgHTS) and panel-based approaches, have demonstrated encouraging results. Higher levels of ctDNA at diagnosis have been linked to increased total metabolic tumor burden, higher international prognostic index, shorter diagnosis-to-treatment interval, and inferior event-free survival (EFS).7,8 The lower clearance of ctDNA following frontline treatment, salvage chemotherapy, and CAR T-cell therapy is associated with worse EFS and overall survival (OS),7,9-11 and the re-emergence of ctDNA following induction therapy is strongly correlated with relapse.9,12 To date, no studies have examined the prognostic value of HTS-based minimal residual disease (MRD) assessment in patients with DLBCL undergoing ASCT. Older studies have shown that patients with DLBCL undergoing ASCT who received a minimally contaminated stem cell product (based on cell culture, Southern analysis, or polymerase chain reaction) had significantly worse survival outcomes13-15 similar observations have been made for patients with mantle cell16-18 and follicular lymphoma.19,20 Novel MRD assays may provide a more sensitive marker of contaminated graft before ASCT, and therefore could guide more selective use of ASCT. In addition, MRD monitoring after ASCT could identify impending relapse and be used as a platform to deploy preemptive interventions after ASCT.
To characterize the prognostic value of IgHTS-based MRD testing before and after ASCT, we assembled patients with DLBCL who underwent ASCT and had apheresis stem cell (ASC) samples and/or serial plasma and peripheral blood mononuclear cell (PBMC) samples available for analysis. To maximize the sample size and obtain the best possible assessment of the prognostic value of IgHTS in this setting, we analyzed 3 different cohorts of patients with DLBCL who underwent ASCT. Here, we report the prognostic value of IgHTS in 159 patients.
Methods
Patients and samples
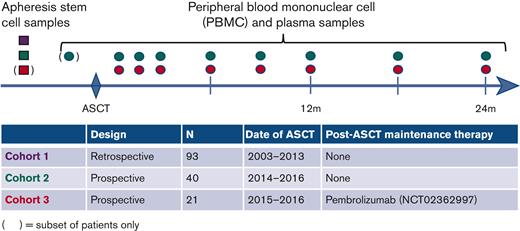
Samples were collected from 3 cohorts of patients who underwent ASCT for pathologically confirmed R/R DLBCL, transformed indolent lymphoma (TIL), or primary mediastinal large B-cell lymphoma (PMBL) (Figure 1). All eligible patients had baseline tissue samples available for tumor Ig detection. Patients in cohort 1 underwent ASCT at the Dana-Farber Cancer Institute (DFCI) from 2003 to 2013 and had an ASC sample available for analysis. Patients in cohort 2 underwent ASCT from 2014 to 2016 at DFCI and were enrolled in a prospective tissue banking study. When feasible, ASC samples were banked and serial post-ASCT peripheral blood (PB) samples (PBMC and plasma) were collected at the following time points after ASCT: 1, 2, 3, 6, 9, and 12 months and every 6 months thereafter. A subset of patients in this cohort also had pre-ASCT PB samples available for the analysis. Patients in cohort 3 underwent ASCT from 2015 to 2016 and were subsequently treated in a phase 2 trial of pembrolizumab maintenance therapy (#NCT02362997). The trial showed no benefit with pembrolizumab treatment21, and PD-1 blockade has shown limited efficacy in DLBCL in other settings22. As these patients had rigorous prospective post-ASCT sample banking, they were included and analyzed together with cohorts 1 and 2. Patients in cohort 3 had serial post-ASCT PBMC and plasma samples collected at the following time points: before initiation of pembrolizumab (generally 30-60 days post-ASCT); 10, 16, and 22 weeks after pembrolizumab initiation; and 12 and 18 months after ASCT. A subset of cohort 3 patients also had ASC samples available for the analysis. The study was conducted using samples from 2 biobanking protocols at the DFCI (both approved by the DFCI institutional review board) and a clinical trial that was institutional review board approved at each participating center (DFCI, Memorial Sloan Kettering Cancer Center, Beth Israel Deaconess Medical Center, City of Hope National Medical Center, and Massachusetts General Hospital). The study was conducted in accordance with the Declaration of Helsinki.
Schema of the 3 cohorts. ASC samples were collected from patients in cohort 1. Pre-ASCT ASC samples and post-ASCT surveillance plasma and PBMC samples were collected from patients in cohort 2. In addition, a subset of cohort 2 patients had 1 pre-ASCT PB sample collected. Patients in cohort 3 had post-ASCT surveillance plasma and PBMC samples collected, and a subset of patients had available pre-ASCT ASC samples. The samples are color-coded by cohort (cohort 1, purple; cohort 2, green; cohort 3, red).
Schema of the 3 cohorts. ASC samples were collected from patients in cohort 1. Pre-ASCT ASC samples and post-ASCT surveillance plasma and PBMC samples were collected from patients in cohort 2. In addition, a subset of cohort 2 patients had 1 pre-ASCT PB sample collected. Patients in cohort 3 had post-ASCT surveillance plasma and PBMC samples collected, and a subset of patients had available pre-ASCT ASC samples. The samples are color-coded by cohort (cohort 1, purple; cohort 2, green; cohort 3, red).
Double expressor lymphoma (DEL) and double-hit lymphoma (DHL) status was collected when available and defined as previously reported.23
Pre-ASCT PET analysis
Radiological reports from pre-ASCT fluoro-[18F]-deoxy-2-D-glucose (FDG) PET/CT assessments were retrospectively reviewed to determine the pre-ASCT metabolic response in all patients. Among the 98 patients with a pre-ASCT ASC sample, a pre-ASCT FDG PET/CT scan was available for review in 52 patients (53%). These FDG PET/CT scans were independently interpreted by 2 nuclear medicine radiologists (H.J. and H.P.) and assigned a Deauville score (DS) of 1 to 5. Discordant DS’s were resolved by a joint image review and consensus interpretation. In no case could a consensus be reached. The radiologists were blinded to the post-ASCT outcomes.
Autologous transplantation and post-ASCT surveillance
Stem cell mobilization, leukapheresis, conditioning chemotherapy, and supportive care were performed according to institutional standards. Radiographic assessments after ASCT were performed at the discretion of the treating physicians in cohorts 1 and 2. In cohort 3, restaging PET/CT scans were performed before the initiation of pembrolizumab, during weeks 10 and 22 of the study treatment, and again at 12 and 18 months after ASCT.
IgHTS MRD assessment
Samples were assessed for MRD using IgHTS. Samples in cohort 1 were analyzed in 2015 using the clonoSIGHT platform.24 Samples from cohorts 2 and 3 were analyzed from 2020 to 2021 using the next generation IgHTS ClonoSEQ assay (Adaptive Biotechnologies, Seattle, WA), as previously described (supplemental Methods).25 Each clonotype sequence was assigned a uniqueness score that reflects its likelihood of being detected in a healthy subject. ASC samples were collected over a median of 2 days (range 1-7) and samples from each collection were analyzed separately. The median input into the IgHTS reaction for each patient’s ASC and PBMC sample(s) were 4.26 × 106 (range, 4.22 × 104-2.62 × 107) and 2.23 × 106 cells (range, 1.92 × 105-4.55 × 106), respectively. The median input into the IgHTS reaction for plasma samples was 0.68 mL (range, 0.16-1.16 mL) which contained a median of 3.44 × 103 total genome equivalents (range, 4.46 × 102-2.86 × 105). MRD testing was not performed in real time or used to drive clinical decisions.
Statistical analysis
Progression-free survival (PFS) and OS were estimated using the Kaplan-Meier method, and differences in survival were assessed using the log-rank test. PFS was defined as the time from day 0 of ASCT to death from any cause, relapse of an aggressive or indolent lymphoma, or progression, with patients censored at the last time seen alive and PFS. OS was defined as the time from day 0 of ASCT to death from any cause with patients censored at the last time seen alive. Descriptive statistics were used to summarize the variables of interest. Univariable and multivariable analyses of PFS/OS were performed using the Cox proportional hazards model. The post-ASCT MRD assessments were treated as time-dependent covariates. Wald P values were reported as covariates. The final Cox model was selected using a penalized maximum likelihood model (LASSO), and k-fold cross-validation was performed to select a subset of predictive variables. Finally, stepwise forward/backward model selection using the Akaike information criterion was used to determine predictive variables for inclusion in the model. Nonrelapse mortality was defined as death without relapse. Deaths after relapse were defined as competing risks. Cause-specific cumulative incidence between the groups was assessed using Gray’s test. Regression models were fit for each competing risk using the method of Fine and Gray.26,27 Two-sided P values <.05 were considered significant. Hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) are reported. All analyses were performed using R v4.0 and the package glmnet v4.0-2 for the LASSO models.
Results
Patients
A total of 159 patients were accrued: 93 in cohort 1, 40 in cohort 2, and 26 in cohort 3. In total, 149 patients (94%) had sufficient DNA for the clonotypic analysis (defined as >500 ng), and among these patients, 116 had at least 1 identifiable clonotype (median, 2; range 1-7), consistent with a clonotype detection rate of 78% (overall clonotype success rate, 73%). The clonotype detection rate was higher in cohorts 2 (95%) and 3 (92%) than in cohort 1 (66%), which relied on older archival tissue samples for clonotype detection.
The clinical features and survival outcomes of patients with and without a detectable clonotype were similar (supplemental Table 1). The baseline characteristics of the 116 patients with identifiable clonotypes are summarized in Table 1. Diagnoses included DLBCL NOS (65%), TIL (28%), and PMBL (6%). Eleven patients (9%) had DHL, and 33 (28%) had DEL. Patients received a median of 2 lines of therapy (range, 2-4) before ASCT, and most patients had a complete metabolic response (CMR) (65%) or partial metabolic response (PMR) (33%) at the time of ASCT. Two patients had a history of central nervous system involvement before ASCT.
Baseline characteristics
| . | All clonotyped patients . | Cohort 1 . | Cohort 2 . | Cohort 3 . |
|---|---|---|---|---|
| N | 116 | 56 (48%) | 37 (32%) | 23 (20%) |
| Male | 69 (59%) | 31 (55%) | 23 (62%) | 15 (65%) |
| Median age at ASCT (range) | 59 (23-77) | 60 (39-77) | 59 (30-77) | 57 (23-76) |
| Diagnosis | ||||
| DLBCL NOS | 75 (65%) | 34 (61%) | 26 (70%) | 15 (65%) |
| TIL | 34 (29%) | 20 (36%) | 10 (27%) | 4 (17%) |
| PMBCL | 7 (6%) | 2 (4%) | 1 (3%) | 4 (17%) |
| DEL | ||||
| Yes | 3 (28%) | 28 (50%) | 3 (8%) | 2 (9%) |
| No | 61 (53%) | 27 (48%) | 16 (43%) | 18 (78%) |
| Not available | 22 (19%) | 1 (2%) | 18 (49%) | 3 (13%) |
| DHL | ||||
| Yes | 11 (9%) | 8 (15%) | 2 (5%) | 1 (4%) |
| No | 89 (77%) | 47 (85%) | 32 (86%) | 10 (43%) |
| Not available | 16 (14%) | 1 (2%) | 3 (8%) | 12 (52%) |
| Primary refractory | 36 (31%) | 20 (36%) | 9 (24%) | 7 (30%) |
| Lines of systemic therapy before ASCT | ||||
| 2 | 95 (82%) | 47 (84%) | 32 (86%) | 16 (70%) |
| 3 | 19 (16%) | 7 (12%) | 5 (14%) | 7 (30%) |
| 4 | 2 (2%) | 2 (4%) | 0 (0%) | 0 (0%) |
| Disease status at ASCT∗ | ||||
| CR | 75 (65%) | 33 (59%) | 28 (76%) | 14 (61%) |
| PR | 38 (33%) | 20 (36%) | 9 (24%) | 9 (39%) |
| SD | 3 (3%) | 3 (5%) | 0 (0%) | 0 (0%) |
| Conditioning chemotherapy | ||||
| BEAM | 59 (51%) | 0 (0%) | 37 (100%) | 22 (96%) |
| CBV | 54 (47%) | 54 (96%) | 0 (0%) | 0 (0%) |
| Other | 3 (3%) | 2 (4%) | 0 (0%) | 1 (4%) |
| ASC sample available | 98 (84%) | 56 (100%) | 36 (97%) | 6 (26%) |
| . | All clonotyped patients . | Cohort 1 . | Cohort 2 . | Cohort 3 . |
|---|---|---|---|---|
| N | 116 | 56 (48%) | 37 (32%) | 23 (20%) |
| Male | 69 (59%) | 31 (55%) | 23 (62%) | 15 (65%) |
| Median age at ASCT (range) | 59 (23-77) | 60 (39-77) | 59 (30-77) | 57 (23-76) |
| Diagnosis | ||||
| DLBCL NOS | 75 (65%) | 34 (61%) | 26 (70%) | 15 (65%) |
| TIL | 34 (29%) | 20 (36%) | 10 (27%) | 4 (17%) |
| PMBCL | 7 (6%) | 2 (4%) | 1 (3%) | 4 (17%) |
| DEL | ||||
| Yes | 3 (28%) | 28 (50%) | 3 (8%) | 2 (9%) |
| No | 61 (53%) | 27 (48%) | 16 (43%) | 18 (78%) |
| Not available | 22 (19%) | 1 (2%) | 18 (49%) | 3 (13%) |
| DHL | ||||
| Yes | 11 (9%) | 8 (15%) | 2 (5%) | 1 (4%) |
| No | 89 (77%) | 47 (85%) | 32 (86%) | 10 (43%) |
| Not available | 16 (14%) | 1 (2%) | 3 (8%) | 12 (52%) |
| Primary refractory | 36 (31%) | 20 (36%) | 9 (24%) | 7 (30%) |
| Lines of systemic therapy before ASCT | ||||
| 2 | 95 (82%) | 47 (84%) | 32 (86%) | 16 (70%) |
| 3 | 19 (16%) | 7 (12%) | 5 (14%) | 7 (30%) |
| 4 | 2 (2%) | 2 (4%) | 0 (0%) | 0 (0%) |
| Disease status at ASCT∗ | ||||
| CR | 75 (65%) | 33 (59%) | 28 (76%) | 14 (61%) |
| PR | 38 (33%) | 20 (36%) | 9 (24%) | 9 (39%) |
| SD | 3 (3%) | 3 (5%) | 0 (0%) | 0 (0%) |
| Conditioning chemotherapy | ||||
| BEAM | 59 (51%) | 0 (0%) | 37 (100%) | 22 (96%) |
| CBV | 54 (47%) | 54 (96%) | 0 (0%) | 0 (0%) |
| Other | 3 (3%) | 2 (4%) | 0 (0%) | 1 (4%) |
| ASC sample available | 98 (84%) | 56 (100%) | 36 (97%) | 6 (26%) |
BEAM, BCNU, etoposide, cytrabine, melphalan; PR, partial response; SD, stable disease.
115/116 (99%) had a PET scan for response assessment before ACST.
Among survivors, the median post-ASCT follow-up was 60.2 months (range, 1.7-185.0) for all cohorts, 100.6 months (range, 18.7-185.0) in cohort 1, 57.8 months (range, 12.6-72.9) in cohort 2, and 35.6 months (range, 1.7-60.0) in cohort 3. Fifty-five patients relapsed, including 3 with biopsy-proven indolent lymphoma. Five-year PFS and OS rates were 47.7% (95% CI, 40.2-56.5) and 65.9% (95% CI, 58.5-74.2), respectively, with no significant differences in PFS or OS across the 3 cohorts.
Pre-ASCT samples
Ninety-eight patients (84%) had 1 or more pre-ASCT ASC samples available for the analysis. Twenty-three patients (23%) had detectable MRD at a median frequency of 1.87 counts per million (cpm) (range 0.05-304). There was a trend toward more frequent MRD positivity in patients with TIL (11/31, 35%) than in patients with DLBCL (12/64, 19%) or PMBL (0/3, 0%) (P = .15). Notably, MRD was detected at similar rates among patients who achieved a CMR or PMR before ASCT (21% vs 29%, P = .46) and among patients with R/R lymphoma (25% vs 20%, P = .80).
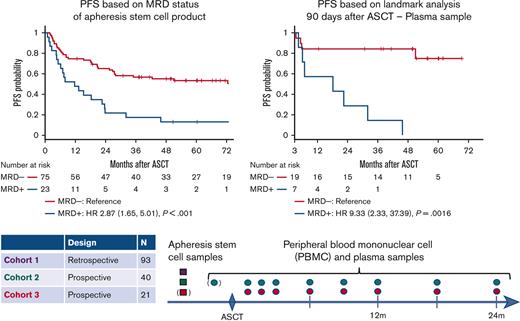
The presence of MRD in an ASC sample was associated with significantly inferior PFS (5-year PFS 13% vs 53%; HR, 2.9; 95% CI, 1.7-5.0; P < .001) (Figure 2A). MRD was a significant predictor of PFS in cohort 1, which used the older ClonoSIGHT IgHTS platform (HR, 2.9; P = .01), and in cohorts 2 to 3, which used the newer ClonoSEQ IgHTS assay (HR, 4.2; P = .001). Inferior outcomes for MRD-positive patients were driven by higher rates of relapse (5-year cumulative incidence of relapse 83% vs 38%; HR, 3.2; 95% CI, 1.8-5.6; P < .001), whereas 5-year nonrelapse mortality was similar (4% vs 9%; HR, 0.4; 95% CI, 0.05-3.3; P = .40) (Figure 2B). Among patients who relapsed, the median time to relapse was similar for MRD-positive and MRD-negative patients (7.9 vs 7.7 months, P = .9). Inferior PFS was observed in MRD-positive patients with DLBCL (HR, 4.43; P < .001), but not in TIL (HR, 1.36; P = .50). (3/3 PMBL ASC samples were MRD-negative, and all 3 patients were relapse-free). Five-year OS for MRD-positive and MRD-negative patients was 52% and 68%, respectively (HR, 2.0; 95% CI, 1.01-4.0; P = .05) (Figure 2C).
Outcomes based MRD status. (A) PFS based on ASC sample MRD status, (B) CIR/nonrelapse mortality based on ASC sample MRD status, (C) OS based on ASC sample MRD status, (D) PFS according to 90-day post-ASCT landmark analysis based on plasma MRD status. (Patients were included in the landmark analysis if they were alive and relapse-free 90 days after ASCT, and they had a plasma sample available for MRD testing 90 [±30] days after ASCT). CIR, cumulative incidence of relapse.
Outcomes based MRD status. (A) PFS based on ASC sample MRD status, (B) CIR/nonrelapse mortality based on ASC sample MRD status, (C) OS based on ASC sample MRD status, (D) PFS according to 90-day post-ASCT landmark analysis based on plasma MRD status. (Patients were included in the landmark analysis if they were alive and relapse-free 90 days after ASCT, and they had a plasma sample available for MRD testing 90 [±30] days after ASCT). CIR, cumulative incidence of relapse.
The sensitivity and specificity of the ASC MRD for progression or death were 36% (95% CI, 23-50) and 93% (95% CI, 80-98), respectively. Testing characteristics were more favorable for patients in cohorts 2 to 3, who were evaluated using the newer ClonoSEQ IgHTS assay (sensitivity, 57%; specificity, 91%), than for patients in cohort 1 evaluated using the older ClonoSIGHT IgHTS platform (sensitivity, 23%; specificity, 95%). The ASC MRD is a more specific marker of progression/death than PET (supplemental Table 2). Higher levels of MRD appear to be associated with an increased risk of relapse. Among the 23 patients with MRD-positive ASC samples, 16 (70%) had MRD detected at ≥1 cpm. All 16 patients subsequently relapsed or died, whereas of 3/7 (43%) patients with MRD detected at <1 cpm were alive and disease-free after 4.2, 5.0, and 11.3 years of follow-up. Among MRD-positive patients, the quantity of detectable MRD in ASC samples was not associated with the time to relapse (data not shown).
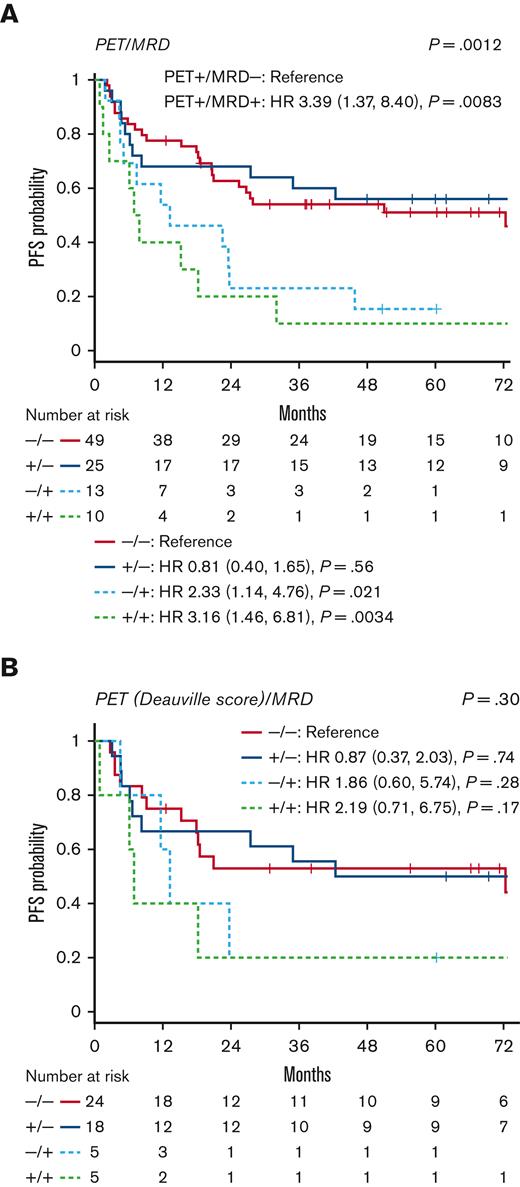
In a multivariable analysis adjusted for key clinical and biological variables, ASC MRD positivity (HR, 3.7; P < .001) remained a significant predictor of PFS. Other factors significantly associated with PFS included DEL (HR, 3.1; P < .001) and DHL (HR, 3.5; P = .009) (Table 2). DEL (HR, 3.6; P = .001) and DHL (HR, 3.9; P = .01) were also significant predictors of inferior OS, whereas ASC MRD was associated with a trend toward inferior OS (HR, 2.1; P = .07) (Table 3). The PFS of patients in a CMR on pre-ASCT PET was numerically higher than that of PET-positive patients, but the difference was not statistically significant based on the initial interpretation at the time of ASCT (HR, 1.33; P = .19) or retrospective DS interpretation (HR, 1.18; P = .67) (supplemental Table 3; supplemental Figure 1). When patients were divided into 4 groups based on pre-ASCT PET and ASC MRD status, MRD appeared to be the key driver of outcomes, with similar results for PET-positive and PET-negative patients with the same MRD status (Figure 3).
Multivariable Analysis of pre-ASCT variables for PFS
| PFS . | |||||
|---|---|---|---|---|---|
| . | Term . | Univariable HR . | Univariable P value . | Multivariable HR . | Multivariable P value . |
| ASC MRD-positive | |||||
| Yes | 2.87 | <.001 | 3.73 | <.001 | |
| Male | |||||
| Male | 1.19 | .44 | |||
| Age (60+) | |||||
| Yes | 1.57 | .04 | 1.30 | .41 | |
| Diagnosis | |||||
| PMBCL | 0.62 | .41 | |||
| TIL | 1.68 | .03 | |||
| Primary refractory | |||||
| Yes | 0.76 | .25 | |||
| Chemo lines | |||||
| 3-4 | 1.43 | .17 | |||
| Pre-ASCT CMR | |||||
| Yes | 0.75 | .19 | |||
| Pre-ASCT DS of 1-3∗ | |||||
| Yes | 0.85 | .67 | |||
| Conditioning | |||||
| CBV | 1.16 | .52 | |||
| Other | 1.10 | .89 | |||
| DHL | |||||
| Yes | 1.75 | .09 | 3.51 | .01 | |
| DEL | |||||
| Yes | 2.10 | .002 | 3.07 | <.001 | |
| PFS . | |||||
|---|---|---|---|---|---|
| . | Term . | Univariable HR . | Univariable P value . | Multivariable HR . | Multivariable P value . |
| ASC MRD-positive | |||||
| Yes | 2.87 | <.001 | 3.73 | <.001 | |
| Male | |||||
| Male | 1.19 | .44 | |||
| Age (60+) | |||||
| Yes | 1.57 | .04 | 1.30 | .41 | |
| Diagnosis | |||||
| PMBCL | 0.62 | .41 | |||
| TIL | 1.68 | .03 | |||
| Primary refractory | |||||
| Yes | 0.76 | .25 | |||
| Chemo lines | |||||
| 3-4 | 1.43 | .17 | |||
| Pre-ASCT CMR | |||||
| Yes | 0.75 | .19 | |||
| Pre-ASCT DS of 1-3∗ | |||||
| Yes | 0.85 | .67 | |||
| Conditioning | |||||
| CBV | 1.16 | .52 | |||
| Other | 1.10 | .89 | |||
| DHL | |||||
| Yes | 1.75 | .09 | 3.51 | .01 | |
| DEL | |||||
| Yes | 2.10 | .002 | 3.07 | <.001 | |
A subset of patients (53/98) had PET imaging available for retrospective assessment using the Deauville scoring system.
Multivariable analysis of pre-ASCT variables for OS
| OS . | |||||
|---|---|---|---|---|---|
| . | Term . | Univariable HR . | Univariable P value . | Multivariable HR . | Multivariable P value . |
| ASC MRD-positive | |||||
| Yes | 2.00 | .05 | 2.14 | .07 | |
| Male | |||||
| Male | 1.19 | .55 | |||
| Age (60+) | |||||
| Yes | 1.41 | .21 | |||
| Diagnosis | |||||
| PMBCL | 0.69 | .60 | |||
| TIL | 1.81 | .03 | |||
| Primary refractory | |||||
| Yes | 0.65 | .16 | |||
| Chemo lines | |||||
| 3-4 | 0.60 | .21 | |||
| Pre-ASCT CMR | |||||
| Yes | 0.84 | .53 | |||
| Pre-ASCT DS of 1-3∗ | |||||
| Yes | 1.09 | .85 | |||
| Conditioning | |||||
| CBV | 1.66 | .10 | |||
| Other | 0.00 | 1.00 | |||
| DHL | |||||
| Yes | 2.24 | .03 | 3.88 | .01 | |
| DEL | |||||
| Yes | 2.59 | .002 | 3.61 | .001 | |
| OS . | |||||
|---|---|---|---|---|---|
| . | Term . | Univariable HR . | Univariable P value . | Multivariable HR . | Multivariable P value . |
| ASC MRD-positive | |||||
| Yes | 2.00 | .05 | 2.14 | .07 | |
| Male | |||||
| Male | 1.19 | .55 | |||
| Age (60+) | |||||
| Yes | 1.41 | .21 | |||
| Diagnosis | |||||
| PMBCL | 0.69 | .60 | |||
| TIL | 1.81 | .03 | |||
| Primary refractory | |||||
| Yes | 0.65 | .16 | |||
| Chemo lines | |||||
| 3-4 | 0.60 | .21 | |||
| Pre-ASCT CMR | |||||
| Yes | 0.84 | .53 | |||
| Pre-ASCT DS of 1-3∗ | |||||
| Yes | 1.09 | .85 | |||
| Conditioning | |||||
| CBV | 1.66 | .10 | |||
| Other | 0.00 | 1.00 | |||
| DHL | |||||
| Yes | 2.24 | .03 | 3.88 | .01 | |
| DEL | |||||
| Yes | 2.59 | .002 | 3.61 | .001 | |
A subset of patients (53/98) had PET imaging available for retrospective assessment using the Deauville scoring system.
PFS based on pre-ASCT PET and MRD status. Patients were divided into 4 groups based on their pre-ASCT PET and ASC MRD status. (A) PFS according to the initial PET interpretation and MRD, (B) PFS according to retrospective PET analysis using the DS and MRD (subgroup of 52 patients).
PFS based on pre-ASCT PET and MRD status. Patients were divided into 4 groups based on their pre-ASCT PET and ASC MRD status. (A) PFS according to the initial PET interpretation and MRD, (B) PFS according to retrospective PET analysis using the DS and MRD (subgroup of 52 patients).
Pre-ASCT PB samples
Thirteen patients (35%) from cohort 2 had 1 plasma sample and 1 PBMC sample collected at a single time after salvage chemotherapy and before ASCT. In an exploratory analysis of this small subgroup, MRD was detected in 2 of 13 patients (15%) (in both plasma and PBMCs), and both patients relapsed (Figure 4). Among the 12 patients with both pre-ASCT PB and pre-ASCT ASC samples available for analysis (drawn a median of 19 days apart [range, 11-47]), the results were concordant for 11/12 patients. The single patient with a discordant result (C2-34) had a negative result from PB and a positive result (0.76 cpm) from an ASC sample 16 days later. This patient relapsed 4.5 months after ASCT.
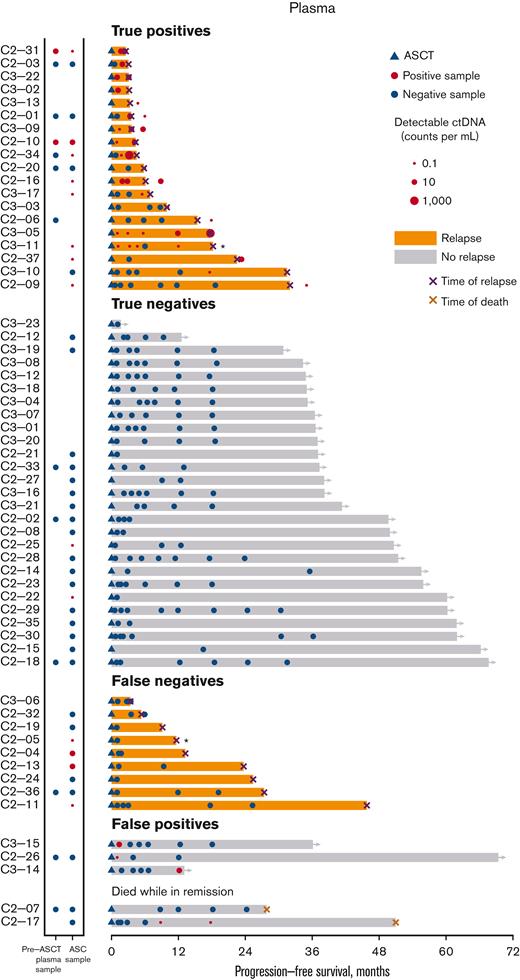
Swimmer’s plot for post-ASCT plasma MRD samples. In this swimmer’s plot, orange bars represent relapsed patients and a black X marks the time of relapse. An asterisk next to black X indicates a patient who relapsed with indolent lymphoma. Gray bars indicate patients in remission. The blue triangle marks the timing of ASCT. Red circles indicate positive MRD samples (with the size of the circle corresponding to the quantity of detectable ctDNA). Blue circles represent the MRD-negative samples. A red X indicates death without lymphoma relapse. Patients are grouped according to relapse status and MRD detection. The first group of patients (“true positives”) had MRD detected before or at the time of relapse. The second group of patients (“true negatives”) are patients in remission with only negative MRD assessments. The third group of patients (“false negatives”) relapsed without detectable MRD. (Note: most of these patients did not have a plasma sample available for testing in close proximity to clinical relapse). The fourth group of patients (“false positives”) are in remission, but each had 1 positive MRD assessment. The final group of patients died without any evidence of lymphoma relapses.
Swimmer’s plot for post-ASCT plasma MRD samples. In this swimmer’s plot, orange bars represent relapsed patients and a black X marks the time of relapse. An asterisk next to black X indicates a patient who relapsed with indolent lymphoma. Gray bars indicate patients in remission. The blue triangle marks the timing of ASCT. Red circles indicate positive MRD samples (with the size of the circle corresponding to the quantity of detectable ctDNA). Blue circles represent the MRD-negative samples. A red X indicates death without lymphoma relapse. Patients are grouped according to relapse status and MRD detection. The first group of patients (“true positives”) had MRD detected before or at the time of relapse. The second group of patients (“true negatives”) are patients in remission with only negative MRD assessments. The third group of patients (“false negatives”) relapsed without detectable MRD. (Note: most of these patients did not have a plasma sample available for testing in close proximity to clinical relapse). The fourth group of patients (“false positives”) are in remission, but each had 1 positive MRD assessment. The final group of patients died without any evidence of lymphoma relapses.
Post-ASCT surveillance samples
Sixty patients from cohort 2 (n = 37) and cohort 3 (n = 23) had a median of 3 (range, 1-8) plasma and 3.5 (range, 0-8) PBMC post-ASCT samples. Among these patients, 28 relapsed (including 2 with indolent lymphoma) and 2 died in remission.
The post-ASCT surveillance MRD results from the plasma samples are depicted as a swimmer’s plot (Figure 4). MRD was detected in 45/220 (20%) plasma surveillance samples from 23 patients, including 19 patients who subsequently relapsed (median, 2 samples/patient, range 1-5). The median lead time from the first MRD positivity to clinical relapse was 62 days (range, 0-518 days). Four patients had detectable MRD (median, 1 sample/patient; range, 1-2), but did not relapse. One patient (C2-17) had 2 MRD-positive samples, but died without lymphoma relapse after allogeneic stem cell transplantation for treatment-related leukemia. Two patients (C3-15 and C2-26) had MRD detected at a single time point early after ASCT. Multiple subsequent MRD assessments yielded negative results, and both the patients remained in remission. The final patient (C3-14) had a single positive MRD result 36 days before the last follow-up, and was in remission at that time.
Among the 28 patients who relapsed, 19 (68%) had detectable MRD before or around the time of relapse, whereas 9 (32%) did not. For 7 of these patients, there was a long interval (range, 239-624 days) from the last MRD assessment to relapse; however, 2 patients (C3-06, C2-32) had at least 1 sample available for analysis at the time of relapse, and MRD was not detected. One of these patients had an isolated recurrence in the central nervous system. The volume of plasma analyzed was similar for false negative and true positive samples (P = .87). Higher quantities of ctDNA were associated with a shorter time to relapse (P = .0031) (SF-2). A landmark analysis (performed 90 days post-ASCT) demonstrated that plasma MRD was associated with significantly worse PFS (HR, 9.3; P = .002) (Figure 2D). Detection of MRD from a post-ASCT plasma sample (as a time-dependent variable) was a significant predictor of both PFS (HR, 5.8; P < .001) and OS (HR, 5.4; P = .005) in univariable analyses and remained a significant predictor of PFS (HR, 3.0; P = .016) (ST-4), but not OS in multivariable analyses. Among patients with an MRD-positive ASC sample, MRD detected in early post-ASCT plasma samples identified patients more likely to rapidly relapse (median PFS: 5 months vs 23 months; P = .011).
Post-ASCT surveillance MRD results from the PBMC samples are depicted as a swimmer’s plot (SF-3). The quantity of MRD detected in plasma and PBMC samples was positively correlated, but the association was not statistically significant (SF-4). MRD was detected in 73/223 (33%) post-ASCT PBMC samples from 37 patients, including 21 patients who subsequently relapsed (median, 2 samples per patient; range, 1-6) and 16 patients who did not relapse (median, 2 samples per patient; range, 1-4). Detection of MRD from a post-ASCT PBMC sample (as a time-dependent variable) was not a significant predictor of PFS (HR, 2.0; P = .12) or OS (HR, 1.0; P = .94) on univariable analyses. Higher quantities of MRD from PBMC samples were observed among relapsing patients compared with patients in remission (median, 6.51 vs 1.07 cpm; P = .006). Compared with true positive results, false positives were more likely to rely on the detection of a light chain clonotypic sequence with a low uniqueness score as the only detectable clonotype (16/27 [59%] vs 7/43 [16%], P < .001). A higher threshold for MRD positivity (>2 cpm rather than >0 cpm) improved the specificity of the PBMC-based MRD testing from 80% to 96%.
The lymphoma subtype (DLBCL vs TIL) did not affect the quantity of MRD identified in the plasma (P = .21) or PBMC samples (P = .19) or the lead time from plasma MRD detection to relapse (P = .81).
Discussion
In this study, approximately one-quarter of the patients undergoing ASCT for DLBCL had MRD detected using IgHTS within an ASC sample. These patients had poor outcomes, with a long-term PFS of <15%. The quantification of MRD may allow for more precise identification of high-risk patients. All patients with MRD detected at a frequency of >1 cpm relapsed or died; however, this cutoff requires prospective validation. Based on the high specificity of MRD for relapse, ASCT should be avoided for ASC MRD-positive patients, particularly with the availability of alternative treatments, such as CAR.
The role of ASCT in the treatment of patients with R/R DLBCL is in flux. Recently, 2 randomized phase 3 trials demonstrated an improvement in EFS for CD19 CAR compared with salvage chemotherapy and ASCT in patients with refractory or early relapsed DLBCL.28,29 These trials implemented a uniform treatment strategy, but better outcomes may be possible with a biomarker-driven approach. Previous studies have reported excellent outcomes with ASCT for most chemosensitive patients, who have traditionally been selected based on pre-ASCT PET,3-6 but can also be identified using MRD. In our study, ASC MRD-negative patients had a 2-year PFS of ∼65%, with more than half still in remission after 5 years. These results compare favorably to outcomes observed with CAR in the second-line setting,28,29 however it is possible that outcomes with CAR may also be better among patients with a minimal disease burden.29 In clinical practice, many patients require bridging treatment before CAR. Based on our results, a clinical trial using MRD status to select either CAR or ASCT (for patients who are MRD-negative after second line bridging therapy) should be considered.
The specificity of MRD detection in ASC samples was very high, but the sensitivity was relatively low, even when using the newest generation IgHTS assay. Therefore, novel approaches are required to improve the sensitivity of MRD testing. Pre-ASCT MRD could be combined with other prognostic tools to improve clinical decision making, as proposed for newly diagnosed DLBCL.30 Currently, pre-ASCT PET is the primary prognostic tool used to assess suitability for ASCT; however, PET-positivity does not preclude favorable outcomes with ASCT. In our study, pre-ASCT PET was not a significant predictor of PFS, which was likely driven by the relatively favorable outcomes among PET-positive patients (5-year PFS, 39%). These results are similar to those of several recent studies that suggested that a substantial subset of clinically selected patients who achieve PMR on pre-ASCT PET have durable remissions.3-6,31 Further studies are necessary to determine whether MRD and PET could be combined as a composite biomarker to guide therapy in this setting.
MRD testing of ASC samples requires mobilization and collection of stem cells, which are typically only performed for patients who have already been selected for ASCT. It would be feasible to collect stem cells and delay high dose chemotherapy until an MRD result is obtained. However, our analysis preliminarily suggests that MRD testing from PB may provide results similar to those from an ASC product. Because pre-ASCT PB MRD testing was performed for only a small subset of patients, these results require validation.
Detection of ctDNA in post-ASCT plasma samples was closely associated with impending relapse.
Nearly all patients had detectable MRD in plasma samples collected within a few months of relapse and very few false positives were observed. These results suggest that serial MRD testing could serve as a platform for preemptive therapeutic intervention The median lead-time of ∼2 months in our study is shorter than that observed in previous HTS-based MRD studies in DLBCL (∼3-6 months),9,12 which may reflect less frequent sample collection in our study, particularly among nontrial patients. More frequent blood draws may be necessary to provide sufficient time for the initiation of investigational treatments before clinically or radiographically apparent relapse.
We found that MRD testing from PBMC samples had inferior performance characteristics compared with plasma-based testing, as reported previously for DLBCL.10 MRD testing from PBMC samples was associated with frequent false positives. False positive results are often based on very low levels of detectable ctDNA that approach the limit of detection of the IgHTS assay. The detection of a less unique light chain clonotypic sequence (rather than a heavy chain clonotypic sequence or multiple clonotypic sequences) also increases the likelihood of a false positive result. Although the incidence of false positives among PBMC samples could be reduced by adopting a higher MRD threshold, our results suggest that plasma-based testing should be prioritized in future DLBCL studies.
Notably, the prognostic value of MRD may differ depending on the lymphoma subtype. ASC MRD status was not prognostic among patients with TIL, driven at least in part by higher rates of relapse among patients with MRD-negative TIL. Interestingly, the higher relapse incidence could not be entirely explained by the more frequent recurrent indolent lymphoma. In contrast, the performance of PB surveillance testing in patients with TIL appeared to be similar to that in patients with DLBCL, but our study was not fully powered for this comparison. Given their distinct clinical behavior, it is not surprising that MRD may vary between TIL and DLBCL, and these differences should be investigated in future studies.
Our study had several limitations. We included 3 patient cohorts to broaden our sample size and allow for the best possible assessment of the prognostic value of the IgHTS. We acknowledge that there are important differences between the cohorts, including the time period of ASCT, IgHTS assay used, receipt of post-ASCT maintenance therapy, and the schedule of sample collection. However, despite the assay differences, both generations of the IgHTS assay showed a significant association between the ASC MRD status and PFS. Based on the negative results of a phase 2 pembrolizumab maintenance trial,21 we opted to include patients treated with pembrolizumab in our study. We observed no significant difference in the outcomes between the pembrolizumab and contemporaneous cohorts. Furthermore, our study was not optimally designed to compare the prognostic value of MRD with that of pre- or post-ASCT imaging. Pre-ASCT PET images were available only for retrospective review using DS for a subset of patients, and the differential use of post-ASCT surveillance imaging limited the comparison to MRD after ASCT. Additional studies are necessary to thoroughly evaluate the prognostic interplay between imaging and MRD in the peri-ASCT setting. Although we were able to collect DEL and DHL status for most patients, additional biological information (ie, cell-of-origin) was not available. Finally, many patients relapsed without recent samples available for MRD analysis, which may have led to an underestimation of sensitivity and lead time. Our experience suggests that more frequent surveillance is necessary, particularly in interventional trials. Considering all these limitations, we were still able to detect a significant prognostic impact for MRD testing before or after ASCT, which may be related to the size of our assembled cohort.
Our study is the first to analyze the prognostic value of an HTS-based MRD assay in patients with DLBCL undergoing ASCT. Detectable MRD in an ASC sample is associated with a very high risk of relapse, and alternative treatment strategies should be studied for these high-risk patients. Post-ASCT surveillance testing of plasma samples (but not PMBC samples) can reliably identify patients with impending relapse and provide a platform for pre-emptive therapeutic intervention.
Acknowledgments
P.A. and R.W.M. acknowledge the support from the Harold and Virginia Lash Grant Program. R.W.M. acknowledges the support from the American Society for Transplantation and Cellular Therapy New Investigator Award and the Lymphoma Research Foundation Clinical Investigator Career Development Award.
Authorship
Contribution: R.W.M. designed and performed the research, analyzed the data, and wrote the manuscript; R.A.R. analyzed the data and reviewed the manuscript; E.T., G.A., E.J., K.M.M., J.R.B., J.L.C., M.S.D., D.C.F., A.S.F., C.A.J., A.I.K., A.S.L., S.Y.N., O.O.O., E.M.P., H.J., H.P., P.B.D., Y.N., R.M.J., Y.-B.C., and M.A.S. performed the research and reviewed the manuscript; A.F.H. and P.A. designed the study, performed the research, analyzed the data, and reviewed the manuscript.
Conflict-of-interest disclosure: R.W.M. reports consultancy from Genmab, Adaptive Biotechnologies, Bristol Myers Squibb (BMS), AbbVie, Intellia, and Epizyme, and research funding from BMS, Merck, Genentech/Roche, and Genmab. J.R.B. reports consultancy from AbbVie, Acerta/AstraZeneca, BeiGene, BMS/Juno/Celgene, Catapult, Eli Lilly, Genentech/Roche, Hutchmed, Janssen, MEI Pharma, MorphoSys AG, Novartis, Pfizer, and Rigel; research funding from Gilead, Loxo/Lilly, SecuraBio, Sun, TG Therapeutics; reports serving on the data safety monitoring committee for Invectys. J.L.C. reports consultancy from Incyte and Karyopharm, and research funding from Bayer, AbbVie, Roche, and Merck. M.S.D. reports consultancy from AbbVie, Adaptive Biosciences, Ascentage Pharma, AstraZeneca, BeiGene, BMS, Celgene, Eli Lilly, Genentech, Janssen, MEI Pharma, Novartis, Takeda, TG Therapeutics, Verastem, and Zentalis, and research support from Ascentage Pharma, AstraZeneca, BMS, Genentech, MEI Pharma, Novartis, Pharmacyclics, Surface Oncology, TG Therapeutics, and Verastem. E.J. reports consultancy from Syros, and Takeda, and research funding from Acerta, Janssen, Novartis, and Pharmacyclics. C.A.J. reports consultancy from Kite/Gilead, Novartis, BMS/Celgene, Precision Biosciences, Nkarta, bluebird bio, Epizyme, Lonza, AbbVie, and Ipsen, and research funding from Kite/Gilead and Pfizer. H.J. reports consultancy from Advanced Accelerator Applications, Spectrum Dynamics; honoraria from Bayer Healthcare and Blue Earth Diagnostics; research funding from Siemens Healthcare, Inc, GTx, Inc, and Blue Earth Diagnostics; and royalties from Cambridge Publishing. P.B.D. reports serving on advisory boards for Kite/Gilead. Y.N. reports consultancy from Affimed and Novo Nordisk, and research funding from Novartis, Biosecura, AstraZeneca, Affimed, and Takeda. Y.-B.C. reports consultancy from Incyte, Jasper, Gamida Cell, Daiichi, Celularity, Equilium, and Actinium. M.A.S. reports consultancy from Immunitas Therapeutics and AstraZeneca, and research funding from BMS, Merck, Bayer, AbbVie, and AstraZeneca. A.F.H. reports consultancy from BMS, Genentech, Merck, Seattle Genetics, AstraZeneca, Karyopharm, ADC Therapeutics, Takeda, Tubulis, Genmab, and Regeneron, and research funding from BMS, Genentech, Merck, Seattle Genetics, KiTE Pharma, Gilead Sciences, AstraZeneca, and ADC Therapeutics. P.A. reports consultancy from Merck, BMS, Pfizer, Affimmed, Adaptive, Infinity, ADC Therapeutics, Celgene, MorphoSys, Daiichi Sankyo, Miltenyi, Tessa, GenMab, C4, Enterome, Regeneron, Epizyme, AstraZeneca, and Genentech; research funding (institutional) from Merck, BMS, Affimed, Adaptive, Roche, Tensha, Otsuka, Sigma Tau, Genentech, IGM, and Kite; and honoraria from Merck and BMS. The remaining authors declare no competing financial interests.
Correspondence: Reid Merryman, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: reid_merryman@dfci.harvard.edu.
References
Author notes
∗A.F.H. and P.A. contributed equally to this study.
Data are available on request from the corresponding author, Reid W. Merryman (reid_merryman@dfci.harvard.edu).
The full-text version of this article contains a data supplement.

![Outcomes based MRD status. (A) PFS based on ASC sample MRD status, (B) CIR/nonrelapse mortality based on ASC sample MRD status, (C) OS based on ASC sample MRD status, (D) PFS according to 90-day post-ASCT landmark analysis based on plasma MRD status. (Patients were included in the landmark analysis if they were alive and relapse-free 90 days after ASCT, and they had a plasma sample available for MRD testing 90 [±30] days after ASCT). CIR, cumulative incidence of relapse.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/7/17/10.1182_bloodadvances.2022007706/3/m_blooda_adv-2022-007706-gr2.jpeg?Expires=1769757473&Signature=C-4vJPXwctDHb7sQPTzmb1KT3cQaVuvnnH0kM1iUs3Lu-f-uW53ain3qfzVUgo2SC75YGRDqUCNocu2Q7fWO2CPAr356SDuuesWR5YU3POmIWD8YrhJ9AEdP26DFBm6DbRpcwn9V50-TzKz~bo9NOZ765gQlXgreZowhvFuO76yt0ZPe6YycpaQ41ir8i6iEVXZStGCF-02EBgVxt~XKhu~8UylAtmaoENFR27IT6IMAcJDbAQo7b5~Rku0CWu9bAmAkimf3FTXvUciVIeJLVzx0BrO56HzsDkJ62eNeb2fYhMIslojvNAIFS3AYz-WOh9QqOHM1kEtLS3CRhm3dVw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)