Key Points
Splenectomy limits RBC morphology and functionality defects according to the septin content but does not improve maturation defects.
The increased septin content in patient RBCs might result from maturation defects and could affect RBC membrane properties.
Abstract
Splenectomy improves the clinical parameters of patients with hereditary spherocytosis, but its potential benefit to red blood cell (RBC) functionality and the mechanism behind this benefit remain largely overlooked. Here, we compared 7 nonsplenectomized and 13 splenectomized patients with mutations in the β-spectrin or the ankyrin gene. We showed that hematological parameters, spherocyte abundance, osmotic fragility, intracellular calcium, and extracellular vesicle release were largely but not completely restored by splenectomy, whereas cryohemolysis was not. Affected RBCs exhibited decreases in β-spectrin and/or ankyrin contents and slight alterations in spectrin membrane distribution, depending on the mutation. These modifications were found in both splenectomized and nonsplenectomized patients and poorly correlated with RBC functionality alteration, suggesting additional impairments. Accordingly, we found an increased abundance of septins, small guanosine triphosphate–binding cytoskeletal proteins. Septins-2, -7, and -8 but not -11 were less abundant upon splenectomy and correlated with the disease severity. Septin-2 membrane association was confirmed by immunolabeling. Except for cryohemolysis, all parameters of RBC morphology and functionality correlated with septin abundance. The increased septin content might result from RBC maturation defects, as evidenced by (1) the decreased protein 4.2 and Rh-associated glycoprotein content in all patient RBCs, (2) increased endoplasmic reticulum remnants and endocytosis proteins in nonsplenectomized patients, and (3) increased lysosomal and mitochondrial remnants in splenectomized patients. Our study paves the way for a better understanding of the involvement of septins in RBC membrane biophysical properties. In addition, the lack of restoration of septin-independent cryohemolysis by splenectomy may call into question its recommendation in specific cases.
Introduction
The cytoskeleton is one of the main red blood cell (RBC) constituents involved in deformability and functionality.1 It is composed of a meshwork of spectrin (SPT) tetramers linked to the lipid bilayer by 2 nonredundant anchorage complexes based on 4.1R and ankyrin (ANK1) proteins.2 The 4.1R anchorage complexes allow for SPT horizontal linkages, whereas the ANK1 anchorage complexes ensure most of the vertical linkages between the SPT meshwork and the lipid bilayer.2 Upon RBC deformation, a transient rise of intracellular calcium activates Gardos channels, leading to cell dehydration, and favors local uncoupling between the membrane and the cytoskeleton.3 Besides a specific surface-to-volume ratio, a finely regulated cytoplasmic viscosity controlled by the intracellular hemoglobin (Hb) concentration, the adenosine triphosphate (ATP) content, and the redox state also contribute to the RBC deformation process.1,4-6 During the RBC’s normal lifespan of 120 days, the RBC membrane elasticity is strained ∼12 000 times upon its passage from the splenic cords to the venous sinuses demarcated by a discontinuous endothelium. Defective or old RBCs have difficulty managing this passage and remain blocked in the cords, where they are phagocytosed.7
This is the case in hereditary spherocytosis, a disease characterized by a loss of RBC biconcavity in favor of spherocytes, stomatocytes, acanthocytes and echinocytes and by an impairment of RBC deformability.8-10 Spherocytosis is the most common cause of chronic hemolytic anemia because of a red cell membrane defect. It is characterized by a broad spectrum of clinical severity, from mild (∼20%, nearly asymptomatic) to moderate (∼75%, possible intermittent need for transfusions) to severe (∼5%, life-threatening and transfusion-dependent anemia).8,11,12 In Northern America and Europe, it can affect 1 out of 2000 or 5000 children. Spherocytosis results in ∼75% cases from an autosomal dominant inheritance and in ∼15% from autosomal recessive inheritance. De novo mutations are rare. Mutations affect predominantly the genes encoding for ANK1, Band3, α- and β-SPT (SPTB), and 4.2R.13 Those mutations cause the weakening of the vertical linkages between the cytoskeleton and the lipid bilayer and thereby its destabilization. In consequence, there is a premature clearance of RBCs in the spleen, leading, in the worst cases, to anemia, jaundice, splenomegaly, and cholelithiasis.14,15
Besides transfusion and erythropoietin treatment,14 splenectomy represents a therapeutical intervention for hereditary spherocytosis.16 It might be the only intervention granting long-time relief from symptoms, allowing for the reduction of RBC destruction and preservation of less deformable but still functional RBCs. Thus, after splenectomy, the Hb concentration almost always increases, reticulocytes decrease, bilirubin levels return to normal, and RBCs exhibit a relatively normal life span.16 This favorable response led to the recommendation of splenectomy even for patients with spherocytosis with moderate degrees of hemolysis based on quality of life and spleen size.17,18
Nevertheless, it is largely unknown whether and to what extent splenectomy is able to improve RBC morphology and functionality as well as the RBC maturation process. Enucleation represents the final step of the RBC maturation process, resulting in the formation of a reticulocyte and the release of a pyrenocyte. The reticulocyte is then released from the bone marrow and undergoes its terminal differentiation in the circulating blood. Enucleation is a complex process involving multiple steps, including (1) cell polarization thanks to microtubules, (2) formation of a contractile actomyosin ring similar to cytokinesis but in an asymmetric manner, and (3) vesicle trafficking that creates an asymmetric protein distribution.19 Protein sorting might involve the RBC cytoskeleton proteins, as revealed by the aberrant protein sorting in mice lacking ANK or 4.1R proteins.20 Nevertheless, cytoskeletal proteins involved in cytokinesis, cell polarity, and endo and exocytosis could also contribute to this. Among such types of proteins are septins, small guanosine triphosphate (GTP)–binding proteins that can form filaments and preferentially arrange on specific curvature.21-24 Those septins have been shown to contribute to platelet morphology and functionality25 but were not previously described in RBCs, to the best of our knowledge.
Thus, several years ago, we launched a study aiming at comparing RBCs from 13 splenectomized patients, 7 nonsplenectomized patients, and 10 matching healthy donors (including 1 splenectomized) for their morphological, functional, biophysical, and biomechanical properties and their potential improvement by splenectomy. This paper focuses on RBC morphological and functional properties in relation with cytoskeleton defects, evaluated as follows: (1) for RBC morphology, optical microscopy of living RBCs and scanning electron microscopy of fixed RBCs; (2) for RBC baseline characteristics, RBC distribution width and hemi-RBC area expressed in relation to RBC volume; (3) for RBC functionality, osmotic fragility through Hb release and cryohemolysis, a fragility parameter independent of surface-to-volume ratio but dependent on molecular defect,26 extracellular vesicle (EV) release as well as intracellular calcium and ATP contents; and (4) for RBC cytoskeleton, SPT confocal imaging and transmission electron microscopy.27,28 In addition, we performed a comprehensive proteomic study, which revealed the presence of septins in diseased RBCs.
Materials and methods
Blood collection and preparation
The study (B403201316580) was approved by the medical ethics committee of the UCLouvain (Brussels, Belgium), and all donors gave written informed consent. The study includes 17 different patients, of whom, 3 were analyzed both before and after splenectomy, allowing for the comparison of a cohort of 13 splenectomized patients vs a cohort of 7 nonsplenectomized patients. These patients were analyzed, as far as it was possible, every year during their annual clinical checkups for 2 to 7 years and were compared with gender-matched healthy volunteers (6 women and 4 men aged 20-49 years old), including 1 splenectomized adult donor. Nevertheless, because patient blood samples were collected for the study during their annual hospital appointments, not all patients could be included in all types of experiments. Blood was collected by venipuncture into K+/EDTA-coated tubes. Before experiments, blood was diluted 10-fold in the adapted experimental medium (Dulbecco’s Modified Eagle Medium [Invitrogen] or 1.8 mM calcium-containing homemade medium), and RBCs were washed as described previously.27
RBC functionality alteration score
A score reflecting the extent to which the patient RBCs were affected by the disease was determined and validated as explained in supplemental Information.
Isolation and analysis of EVs
EV abundance in plasma samples was analyzed via Nanoparticle Tracking Analysis with the ZetaView (Particle Metrix) as described previously.27 Data were expressed as percentage of control values.
Intracellular calcium
Proteomic analysis
RBC ghosts were prepared and samples further processed for mass spectrometry and relative quantification via tandem mass tag labeling, as described elsewhere.28 This approach was performed in 3 nonsplenectomized vs 9 splenectomized patients, and the statistical analysis was therefore adapted, as explained in supplemental Information.
Intracellular ATP
Western blotting
RBC ghosts were prepared as described previously,28 mixed with 2% Tris-buffered saline (TBS) sample buffer (0.25 M Tris-HCl, pH 6.8, 10% sodium dodecyl sulfate, 20% glycerol, and 0.005% bromophenol blue) containing 5 mM dithiothreitol and boiled for 5 minutes. Western blotting was performed as described previously,30 except that 30 μg of proteins were loaded onto 3% to 10% sodium dodecyl sulfate polyacrylamide gel, and membranes were incubated overnight with a septin-2 mouse monoclonal or a septin-7 rabbit polyclonal antibody (ProteinTech) in TBS with Tween 20 and 5% milk. After visualization, antibodies were removed from membranes using the Reblot Plus Strong Antibody Stripping Solution 10× (Merck Millipore), and membranes were incubated overnight with rabbit (Merck Millipore) or mouse (Invitrogen) glyceraldehyde-3-phosphate dehydrogenase antibody in TBS with Tween 20 and 5% milk. Data were expressed as percentages of control values.
Immunofluorescence
SPT immunolabeling was performed as described previously.27,28 For septin-2 immunolabeling, RBCs and K562 erythroleukemic cells were spread onto poly-L-lysine–coated coverslips, fixed/permeabilized in ice-cold methanol for 1 minute at −20°C and then in 3% Triton X-100 for 5 minutes and blocked by 4% bovine serum albumin and 0.05% Tween 20 in phosphate-buffered saline for 1 hour at room temperature. Cells were immunolabeled for 2 hours at room temperature with the septin-2 antibody, washed, and incubated with the Alexa Fluor 488–coupled secondary antibody for 1 hour in the dark. All coverslips were then mounted overnight with Dako on SuperFrost blades and visualized using Zeiss LSM980 (SPT and septin) or COSD confocal microscopes (SPT) with a plan-Apochromat 63× NA 1.4 oil immersion objective. The same settings for illumination were used for all samples from the same experiment. SPT data in patients were expressed as a percentage of the corresponding controls analyzed in the same conditions.
Vital imaging of ceramide, mitochondria, and lysosomes
RBCs were labeled with BODIPY-ceramide, as described,27,29,31 and with MitoTracker31 or LysoTracker (Invitrogen) for 5 minutes at 50 nM. Coverslips were then placed upside down in LabTek chambers filled with medium (still containing the LysoTracker) and directly observed with a wide-field fluorescence microscope Observer.Z1. The proportion of RBCs with MitoTracker- or LysoTracker-positive patches in control samples was subtracted from patient samples.
Further information can be found in the supplemental Material.
Results
Overview of patients included in the study
The patients presented mutations in the SPTB or ANK1 gene, as revealed via next-generation sequencing. The splenectomized cohort included 13 patients (closed and semiclosed symbols in graphs). The nonsplenectomized group (open symbols) included (1) 2 patients (P13 and P21) who were compared with splenectomized patients from the same family (P12 and P19; semiclosed symbols), (2) 3 patients (P20, P24, and P25) without other family members for comparison, and (3) 3 patients followed before and after splenectomy (P1, P7, and P13; Table 1 describes the patients baseline characteristics; supplemental Table 1 describes the cohort baseline characteristics). The patients were compared with healthy control donors, who were gender-matched in 90% of the cases.
Overview of patients included in the study
Each patient was associated with a number according to the order of inclusion in the study. Family relationship (same color), gender, year of birth, mutated gene, and year of splenectomy.
− indicates nonsplenectomized; +, splenectomized; F, female; M, male; NA, not applicable; ND, not determined.
Splenectomy reestablishes the RBC distribution width and the blood parameters but only partially improves RBC morphology and area-to–mean corpuscular volume ratio
As expected, the RBC morphology was impaired in nonsplenectomized patients, as reflected by a twofold decrease in the proportion of discocytes in favor of spherocytes and stomatocytes as well as spiculated echinocytes and acanthocytes (Figure 1A-D; see supplemental Figure 1A for RBC classification). Nonsplenectomized patients also showed increased RBC distribution width, which can be linked not only to the decrease of the hemi-RBC area and surface-to-volume ratio (Figure 1E,G) but also and, especially, to the high proportion of reticulocytes (supplemental Figure 1E) and their bigger size, compared with healthy donor RBCs. This is further supported by the direct comparison of RBCs from nonsplenectomized P7 with splenectomized P8 from the same family, showing a strong increase in RBC distribution width and reticulocyte count but a less marked effect on RBC biconcavity (Figure 1E; supplemental Figure 1B,E). All RBC morphology changes were partially restored by splenectomy, except the proportion of discocytes, which was maintained lower than in healthy control donors, mainly because of the remaining spherocyte population (Figure 1A-D,E-G). The blood content in bilirubin and Hb as well as the reticulocyte count were also impaired in spherocytosis and significantly improved because of splenectomy (supplemental Figure 1C-E). In contrast, the RBC count, the mean corpuscular volume, and the mean corpuscular Hb concentration were in the normal range for both cohorts (supplemental Figure 1F-H).
Splenectomy partially reestablishes RBC baseline characteristics as well as RBC morphology and functionality–related parameters. (A-L) RBCs from patients (P; 1 color, 1 family), either splenectomized (spl; filled circles or semifilled circles for intrafamily comparison) or not (nonspl; open circles), and RBCs from healthy controls (CTLs; light and dark blue dotted lines for child and adult donor ranges, respectively; or subtraction from P values [ΔP–CTL]) were compared for morphology (A-D), baseline characteristics (E-G), and functionality (H-L). (M-N) Based on these different parameters, a RBC alteration score was calculated. Statistics are indicated above the patient cohorts for the comparison with CTL values and above a horizontal line for comparison between the 2 patient cohorts, respectively. (A-D) RBC morphology determined by electron or light microscopy on RBCs in suspension. The relative abundance of discocytes (A), spherocytes (B), stomatocytes (C), and echinocytes (D) was evaluated and expressed as percentage of the global RBC population (mean of 1-5 independent experiments per patient; Kruskal-Wallis tests followed by Dunn post hoc for the comparison of the 3 cohorts). (E) RBC distribution width (mean of 1-7 independent measurements per patient; Mann-Whitney tests to compare the 2 patient cohorts). (F-G) Hemi-RBC membrane area and area-to–mean corpuscular volume (MCV) ratio. (F) Hemi-area of RBCs spread on poly-L-Lysine (PLL)–coated coverslips. (G) Ratio of values provided in panel F to the MCV provided in supplemental Figure 1G (mean of 4-23 independent measurements per patient for panel F; Kruskal-Wallis tests followed by Dunn post hoc for the comparison of the 3 cohorts). (H) RBC osmotic fragility determined in increasingly hypotonic media. The osmolarity required to lyse 50% of RBCs (Half maximal effective concentration [EC50]) was calculated using hemolysis curves (mean of 1-5 independent experiments per patient; Kruskal-Wallis test followed by Dunn post hoc). (I) RBC cryohemolysis (mean of 1-6 independent experiments per patient; Mann-Whitney test). (J) EV abundance in plasma samples determined by Nanoparticle tracking analysis (mean of 1-3 independent experiments per patient; Kruskal-Wallis test followed by Dunn post hoc). (K) Intracellular calcium content. RBCs were labeled with the nonfluorescent Fluo4-AM, which is transformed in RBCs into the fluorescent Fluo4 after de-esterification and interaction with calcium ions. Labeled RBCs were analyzed by fluorimetry, and data were normalized to the Hb content (mean of 1-11 independent experiments per patient; Kruskal-Wallis test followed by Dunn post hoc). (L) Intracellular ATP content determined with a kit based on the activity of the firefly luciferase in the presence of ATP and emitted light in the presence of luciferin. ATP levels were normalized to Hb (mean of 1-8 independent experiments/patient; Kruskal-Wallis test followed by Dunn post hoc. (M) RBC morphology, functionality and biological parameters considered to establish the RBC functionality alteration score. These parameters were associated with a scale ranging from 0 to 1 when the parameter was nearly unaffected for most patients or from 0 up to maximum 8 when different degrees of affection for a parameter were observed in patient cohorts. The different scores corresponding to the different parameters were then added and the sum divided by the maximal score that could have been obtained to determine the RBC global functionality alteration score for each patient. The closer the score to 1, the more affected the RBCs by the disease. (N) RBC alteration score (Kruskal-Wallis tests followed by Dunn post hoc). MCHC, mean corpuscular Hb concentration; ns, not significant; RDW, red cell distribution width.
Splenectomy partially reestablishes RBC baseline characteristics as well as RBC morphology and functionality–related parameters. (A-L) RBCs from patients (P; 1 color, 1 family), either splenectomized (spl; filled circles or semifilled circles for intrafamily comparison) or not (nonspl; open circles), and RBCs from healthy controls (CTLs; light and dark blue dotted lines for child and adult donor ranges, respectively; or subtraction from P values [ΔP–CTL]) were compared for morphology (A-D), baseline characteristics (E-G), and functionality (H-L). (M-N) Based on these different parameters, a RBC alteration score was calculated. Statistics are indicated above the patient cohorts for the comparison with CTL values and above a horizontal line for comparison between the 2 patient cohorts, respectively. (A-D) RBC morphology determined by electron or light microscopy on RBCs in suspension. The relative abundance of discocytes (A), spherocytes (B), stomatocytes (C), and echinocytes (D) was evaluated and expressed as percentage of the global RBC population (mean of 1-5 independent experiments per patient; Kruskal-Wallis tests followed by Dunn post hoc for the comparison of the 3 cohorts). (E) RBC distribution width (mean of 1-7 independent measurements per patient; Mann-Whitney tests to compare the 2 patient cohorts). (F-G) Hemi-RBC membrane area and area-to–mean corpuscular volume (MCV) ratio. (F) Hemi-area of RBCs spread on poly-L-Lysine (PLL)–coated coverslips. (G) Ratio of values provided in panel F to the MCV provided in supplemental Figure 1G (mean of 4-23 independent measurements per patient for panel F; Kruskal-Wallis tests followed by Dunn post hoc for the comparison of the 3 cohorts). (H) RBC osmotic fragility determined in increasingly hypotonic media. The osmolarity required to lyse 50% of RBCs (Half maximal effective concentration [EC50]) was calculated using hemolysis curves (mean of 1-5 independent experiments per patient; Kruskal-Wallis test followed by Dunn post hoc). (I) RBC cryohemolysis (mean of 1-6 independent experiments per patient; Mann-Whitney test). (J) EV abundance in plasma samples determined by Nanoparticle tracking analysis (mean of 1-3 independent experiments per patient; Kruskal-Wallis test followed by Dunn post hoc). (K) Intracellular calcium content. RBCs were labeled with the nonfluorescent Fluo4-AM, which is transformed in RBCs into the fluorescent Fluo4 after de-esterification and interaction with calcium ions. Labeled RBCs were analyzed by fluorimetry, and data were normalized to the Hb content (mean of 1-11 independent experiments per patient; Kruskal-Wallis test followed by Dunn post hoc). (L) Intracellular ATP content determined with a kit based on the activity of the firefly luciferase in the presence of ATP and emitted light in the presence of luciferin. ATP levels were normalized to Hb (mean of 1-8 independent experiments/patient; Kruskal-Wallis test followed by Dunn post hoc. (M) RBC morphology, functionality and biological parameters considered to establish the RBC functionality alteration score. These parameters were associated with a scale ranging from 0 to 1 when the parameter was nearly unaffected for most patients or from 0 up to maximum 8 when different degrees of affection for a parameter were observed in patient cohorts. The different scores corresponding to the different parameters were then added and the sum divided by the maximal score that could have been obtained to determine the RBC global functionality alteration score for each patient. The closer the score to 1, the more affected the RBCs by the disease. (N) RBC alteration score (Kruskal-Wallis tests followed by Dunn post hoc). MCHC, mean corpuscular Hb concentration; ns, not significant; RDW, red cell distribution width.
Splenectomy decreases RBC osmotic fragility, EV release, and calcium accumulation but does not ameliorate the cryohemolysis
Because spherocytes are generally associated with increased RBC fragility and EV release,32,33 these parameters were then evaluated. The higher RBC osmotic fragility observed in the nonsplenectomized cohort was only partially corrected via splenectomy (Figure 1H), consistent with the remaining proportion of spherocytes. Cryohemolysis was also more pronounced in nonsplenectomized patients, but in contrast to osmotic fragility, it was not reestablished via splenectomy (Figure 1I). The abundance of EVs in the plasma of nonsplenectomized patients was higher than in splenectomized patients or control donors (Figure 1J). The intracellular calcium level was also heightened in nonsplenectomized patients and reduced after splenectomy (Figure 1K). This increase did not result from a limitation in intracellular ATP content, which was instead even higher before splenectomy (Figure 1L).
Splenectomy limits but does not prevent alterations in RBC morphology and functionality
Based on the before mentioned altered clinical and laboratory features affecting RBC functionality (Figure 1A-L; supplemental Figure 1C,E,H), we established an RBC alteration score ranging from 0 for nonaffected patients to 1 for the most-affected patients. Although splenectomy strongly and significantly improved this score, it did not allow for the recovery of normal RBC morphology and functionality. This was specifically reflected by a similar score between the most-affected splenectomized patients and the less-affected nonsplenectomized ones (Figure 1M,N). It should be noticed that this score did not simply reflect the abundance of reticulocytes (supplemental Figure 1I,J).
The extent of RBC alteration negatively correlates with the ANK1 content, which, in turn, correlates negatively with SPT distribution
To address the reason for the partial effectiveness of splenectomy, we performed a quantitative proteomic analysis on RBC ghosts from the 2 cohorts and the matched controls. Comparison between the SPTB- and ANK1-mutated groups indicated that the ANK1 level was, as expected, lower in the latter group, but no difference was observed for SPTB levels (Figure 2A-B). However, SPTB and ANK1 levels were similarly reduced in the nonsplenectomized and splenectomized cohorts, when compared with healthy donors (Figure 2C-D). We then evaluated the extent and distribution of SPT membrane occupation by immunolabeling and confocal microscopy while validating the data for some patients via transmission electron microscopy and including 2-week-old RBCs as a positive control for SPT cytoskeleton densification (supplemental Figure 2A). Data revealed the occasional presence of SPT-enriched patches and vesicles, reflected in an increased heterogeneity of SPT membrane distribution, particularly visible in the ANK1-mutated group, which also exhibited a denser membrane SPT coverage (Figure 2E-G; see supplemental Figure 2B for electron microscopy images). In contrast, the SPT membrane occupation and distribution in the nonsplenectomized and splenectomized cohorts were not significantly different from those in controls (Figure 2H-I). Surprisingly, no correlation was found between SPTB content and membrane distribution (data not shown). Only the ANK1 content was inversely correlated with SPT variance and alteration of RBC functionality (Figure 2J-M), leading us to suggest that other proteins could also contribute to the phenotype of spherocytosis.
The ANK1 content and the SPT membrane distribution are differentially affected by the mutation but not by splenectomy and inversely and slightly correlate together and with the RBC alteration score. RBCs from patients, either spl (filled circles or semifilled circles for intrafamily comparison) or nonspl (open circles), and RBCs from CTLs (dark blue dotted line, CTL ratio or % CTL) or a healthy spl adult donor (red dotted line) were compared for SPTB and ANK1 content (A-D) and membrane SPTB coverage and heterogeneity (E-I). Statistics are indicated above the patient cohorts for the comparison with CTL values and above a horizontal line for comparison between the 2 patient cohorts. (A-D) SPTB and ANK1 membrane association evaluated by mass spectrometry on ghosts (2 independent experiments; see Figure 3 for volcano plots). Data are presented based on either mutation (A-B) or splenectomy (C-D). (E-I) SPT occupancy and heterogeneity. RBCs were spread onto PLL-coated coverslips and SPT was immunolabeled and observed via confocal microscopy. (E) Representative images (filled arrowhead, SPT-enriched patch; open arrowhead, SPT-enriched vesicle). Scale bar, 5 μm. In panels F-I, SPT occupancy was normalized to RBC membrane area (means of 2-7 independent experiments per patient; Kruskal-Wallis tests followed by Dunn post hoc). Data are presented based on either mutation (F-G) or splenectomy (H-I). (J-K) Relations between the ANK1 levels and the SPT coverage and variance. (L-M) Relations between the RBC alteration score from Figure 1N and the ANK1 levels or the SPT variance.
The ANK1 content and the SPT membrane distribution are differentially affected by the mutation but not by splenectomy and inversely and slightly correlate together and with the RBC alteration score. RBCs from patients, either spl (filled circles or semifilled circles for intrafamily comparison) or nonspl (open circles), and RBCs from CTLs (dark blue dotted line, CTL ratio or % CTL) or a healthy spl adult donor (red dotted line) were compared for SPTB and ANK1 content (A-D) and membrane SPTB coverage and heterogeneity (E-I). Statistics are indicated above the patient cohorts for the comparison with CTL values and above a horizontal line for comparison between the 2 patient cohorts. (A-D) SPTB and ANK1 membrane association evaluated by mass spectrometry on ghosts (2 independent experiments; see Figure 3 for volcano plots). Data are presented based on either mutation (A-B) or splenectomy (C-D). (E-I) SPT occupancy and heterogeneity. RBCs were spread onto PLL-coated coverslips and SPT was immunolabeled and observed via confocal microscopy. (E) Representative images (filled arrowhead, SPT-enriched patch; open arrowhead, SPT-enriched vesicle). Scale bar, 5 μm. In panels F-I, SPT occupancy was normalized to RBC membrane area (means of 2-7 independent experiments per patient; Kruskal-Wallis tests followed by Dunn post hoc). Data are presented based on either mutation (F-G) or splenectomy (H-I). (J-K) Relations between the ANK1 levels and the SPT coverage and variance. (L-M) Relations between the RBC alteration score from Figure 1N and the ANK1 levels or the SPT variance.
Septin association with the membrane is increased in spherocytosis and partially restored upon splenectomy
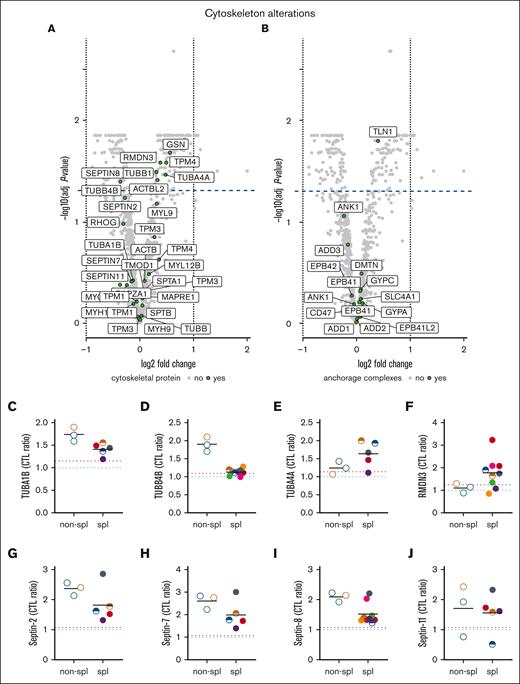
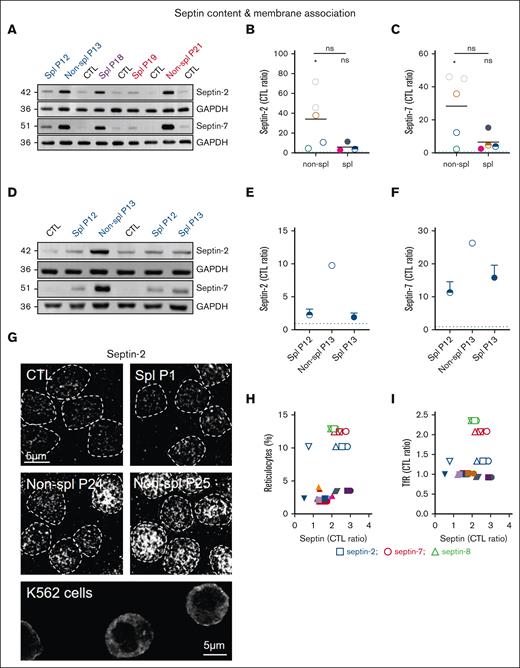
Mass spectrometry revealed the change of 2 cytoskeletal protein classes upon splenectomy, which are tubulins (TUBA and TUBB) and the regulator of microtubule dynamics protein 3 (RMDN3) as well as the small GTP-binding proteins septins. Although the effect of splenectomy on tubulin abundance was isoform-dependent, the abundance of septins was systematically decreased (Figure 3). We therefore analyzed the latter in detail. We first confirmed via western blotting the increased membrane association of septin-2 and -7 in patient RBCs (Figure 4A). Quantification even revealed a greater increase in septins than observed via proteomics (Figure 4B-C) and a clear decrease of both septins-2 and -7 upon splenectomy, as revealed by the comparison of P13 before and just after splenectomy (Figure 4D-F). Moreover, RBC immunofluorescence indicated that septin-2 formed a heterogeneous pattern with submicrometric assemblies spread over the cell surface and was clearly visible in the nonsplenectomized patients but also in splenectomized patients, although to a lower extent (Figure 4G). In contrast, the signal was barely detectable in healthy mature RBCs but substantially present in K562 erythroleukemic cells used as positive controls, confirming the presence of septins in erythroid precursors. Because the septin-2 network was not restricted to some cells of the patient and no correlation could be detected between the septin content and the reticulocyte or the transferrin receptor (TfR) content (Figures 4H-I and Figure 6K), one can reasonably exclude that septins were exclusively associated to reticulocytes.
Among cytoskeletal and anchorage proteins, tubulins and septins are modified in content upon splenectomy. RBCs from patients, either spl (filled circles or semifilled circles for intrafamily comparison) or nonspl (open circles), and RBCs from CTLs (dark blue dotted line, CTL ratio) or a healthy splenectomized adult donor (red dotted line) were assessed by differential quantitative mass spectrometry for cytoskeleton and anchorage complex protein membrane association. (A,B) Volcano plots of ghost membranes for cytoskeletal and anchorage complex proteins (extension of Figure 2A-B). Volcano plots show the log2 of the fold changes (logFC) and the adjusted P values associated with the splenectomy effect. Proteins showing a negative or a positive logFC have a lower or higher expression level in splenectomized patients, respectively. Proteins above the dotted line show a significant difference (P < .05) in the splenectomized patient cohort as compared with the nonsplenectomized one. (C-F) Tubulin α 1b (TUBA1B), TUBB4B, TUBA4A, and regulator of microtubule dynamics protein 3 (RMDN3) membrane association. (G-J) Septins-2, -7, -8, -11 ghost membrane association.
Among cytoskeletal and anchorage proteins, tubulins and septins are modified in content upon splenectomy. RBCs from patients, either spl (filled circles or semifilled circles for intrafamily comparison) or nonspl (open circles), and RBCs from CTLs (dark blue dotted line, CTL ratio) or a healthy splenectomized adult donor (red dotted line) were assessed by differential quantitative mass spectrometry for cytoskeleton and anchorage complex protein membrane association. (A,B) Volcano plots of ghost membranes for cytoskeletal and anchorage complex proteins (extension of Figure 2A-B). Volcano plots show the log2 of the fold changes (logFC) and the adjusted P values associated with the splenectomy effect. Proteins showing a negative or a positive logFC have a lower or higher expression level in splenectomized patients, respectively. Proteins above the dotted line show a significant difference (P < .05) in the splenectomized patient cohort as compared with the nonsplenectomized one. (C-F) Tubulin α 1b (TUBA1B), TUBB4B, TUBA4A, and regulator of microtubule dynamics protein 3 (RMDN3) membrane association. (G-J) Septins-2, -7, -8, -11 ghost membrane association.
The increased septin content in spherocytosis is largely restored upon splenectomy and septin-2 associates with the RBC surface. RBCs from patients, either spl (filled circles or semifilled circles for intrafamily comparison) or nonspl (open circles), and RBCs from CTLs were compared for septin content (A-F) and membrane distribution (G). Statistics are indicated above the patient cohorts for the comparison with CTL values and above a horizontal line for comparison between the 2 patient cohorts, respectively. (A-F) Septin-2 and -7 ghost membrane association determined by western blotting. Representative western blots comparing different patients with healthy donors (A) and P13 before and after splenectomy (D). (B-C,E-F) Quantification: data were first expressed as ratio to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (loading control), then as ratio of P to CTL (Kruskal-Wallis test in panels B-C; means ± standard deviation of 1 experiment in panels E-F). (G) Septin-2 immunolabeling. Scale bar, 5 μm. RBCs were spread onto PLL-coated coverslips, fixed/permeabilized in ice-cold methanol and Triton X-100, and immunolabeled for septin-2. K562 cells were used as positive controls. Representative images of 2 independent experiments. (H-I) Correlation between septins and reticulocyte count presented in supplemental Figure 1E or Transferrin receptor (TfR) content presented at Figure 6K. Linear regressions were indicated only if r2 > 0.5.
The increased septin content in spherocytosis is largely restored upon splenectomy and septin-2 associates with the RBC surface. RBCs from patients, either spl (filled circles or semifilled circles for intrafamily comparison) or nonspl (open circles), and RBCs from CTLs were compared for septin content (A-F) and membrane distribution (G). Statistics are indicated above the patient cohorts for the comparison with CTL values and above a horizontal line for comparison between the 2 patient cohorts, respectively. (A-F) Septin-2 and -7 ghost membrane association determined by western blotting. Representative western blots comparing different patients with healthy donors (A) and P13 before and after splenectomy (D). (B-C,E-F) Quantification: data were first expressed as ratio to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (loading control), then as ratio of P to CTL (Kruskal-Wallis test in panels B-C; means ± standard deviation of 1 experiment in panels E-F). (G) Septin-2 immunolabeling. Scale bar, 5 μm. RBCs were spread onto PLL-coated coverslips, fixed/permeabilized in ice-cold methanol and Triton X-100, and immunolabeled for septin-2. K562 cells were used as positive controls. Representative images of 2 independent experiments. (H-I) Correlation between septins and reticulocyte count presented in supplemental Figure 1E or Transferrin receptor (TfR) content presented at Figure 6K. Linear regressions were indicated only if r2 > 0.5.
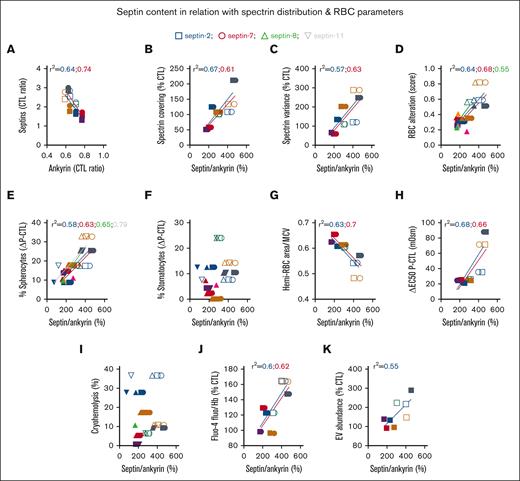
The septin/ANK1 ratio correlates with SPT covering/distribution and RBC alteration score, and different septins differentially correlate with RBC morphology and functionality parameters. (A) Correlation between septins and ANK1 content presented in Figure 3G-J and Figure 2B. (B-D) Correlation between septin/ANK1 ratios calculated from Figure 3G-J and cytoskeletal parameters presented in Figure 2F-G or the RBC alteration score from Figure 1N. (E-K) Relation of the septin/ANK1 ratio with RBC morphology (E-F), surface-to-volume ratio (G), osmotic fragility (H), cryohemolysis (I), intracellular calcium content (J), and EV release (K). Data are respectively from Figure 1B-C,G, H-K. Correlations with septin-2, squares and blue linear regressions; with septin-7, circles and red linear regressions; with septin-8, triangles and green linear regressions; and with septin-11, inverted triangles and gray linear regressions. Linear regressions were plotted only for r2 > 0.5; when 1 parameter correlates with 1 septin but not with the others, only the one correlating is indicated for the sake of clarity.
The septin/ANK1 ratio correlates with SPT covering/distribution and RBC alteration score, and different septins differentially correlate with RBC morphology and functionality parameters. (A) Correlation between septins and ANK1 content presented in Figure 3G-J and Figure 2B. (B-D) Correlation between septin/ANK1 ratios calculated from Figure 3G-J and cytoskeletal parameters presented in Figure 2F-G or the RBC alteration score from Figure 1N. (E-K) Relation of the septin/ANK1 ratio with RBC morphology (E-F), surface-to-volume ratio (G), osmotic fragility (H), cryohemolysis (I), intracellular calcium content (J), and EV release (K). Data are respectively from Figure 1B-C,G, H-K. Correlations with septin-2, squares and blue linear regressions; with septin-7, circles and red linear regressions; with septin-8, triangles and green linear regressions; and with septin-11, inverted triangles and gray linear regressions. Linear regressions were plotted only for r2 > 0.5; when 1 parameter correlates with 1 septin but not with the others, only the one correlating is indicated for the sake of clarity.
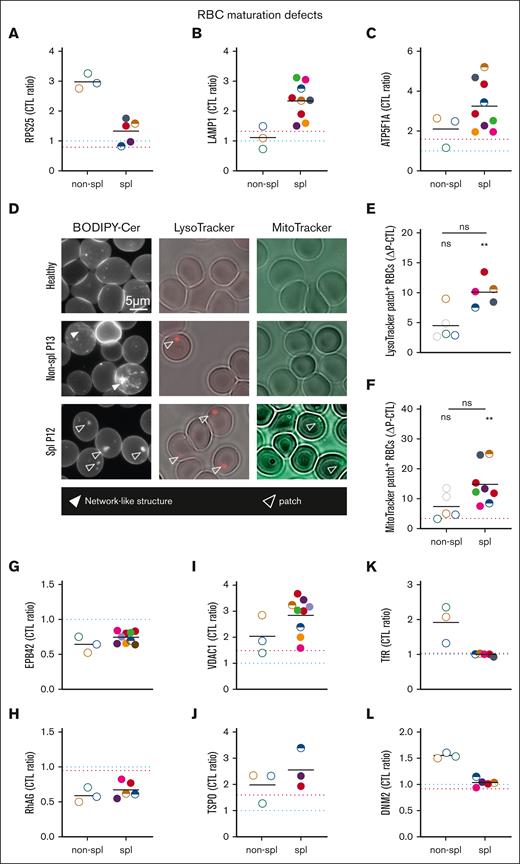
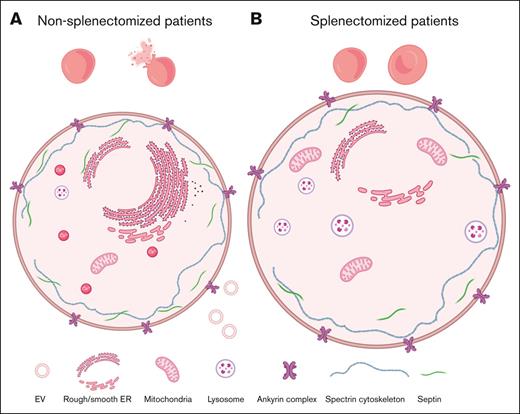
The presence of ER proteins and remnants in RBCs of nonspl patients contrasts with lysosome and mitochondria proteins and remnants in RBCs of spl patients. RBCs from patients, either spl (filled circles or semifilled circles for intrafamily comparison) or nonspl (open circles), and RBCs from CTLs (dark blue dotted line or CTL ratio or ΔP–CTL) or a healthy splenectomized donor (red dotted line) were compared for membrane association of proteins of the ER-ribosomes (A), lysosomes (B), or mitochondria (C) and for the presence of organelle remnants (D-F). Scale bar represents 5 μm in panel D. Statistics are indicated above the patient cohorts for the comparison with CTL values and above a horizontal line for comparison between the 2 patient cohorts, respectively. (A-C) Membrane ghost association of ribosomal protein S25 (RPS25), lysosomal-associated membrane protein 1 (LAMP1) and ATP synthase F1 subunit alpha (ATP5F1A), respectively, enriched in ribosomes, lysosomes, and mitochondria and determined by proteomics (statistical analysis and additional examples in supplemental Figure 3). (D-F) Organelle labeling. RBCs spread on PLL-coated coverslips were labeled with BODIPY-ceramide (Cer), LysoTracker, or MitoTracker and observed by fluorescence microscopy (LysoTracker was maintained during observation). (D) Representative images. Open and filled arrowheads, patches, and network-like structures, respectively. (E-F) Quantification of RBCs presenting LysoTracker- or MitoTracker-positive patches expressed as percentage of the total RBC population and then as ΔP–CTL (mean of 1-3 and 1-7 independent experiments perpatient in panels E and F, respectively; Kruskal-Wallis tests followed by Dunn post hoc). (G-L) Membrane ghost association of band 4.2 (EPB42), Rh-associated glycoprotein (RhAG), voltage-dependent anion channel 1 (VDAC1), translocator protein (TSPO), Transferrin receptor (TfR) and DNM2 (dynamin), respectively, involved in cytoskeleton anchorage complexes (G-H), mitophagy (I-J), and endocytosis (K-L) and determined by proteomics (for volcano plots, see supplemental Figure 3).
The presence of ER proteins and remnants in RBCs of nonspl patients contrasts with lysosome and mitochondria proteins and remnants in RBCs of spl patients. RBCs from patients, either spl (filled circles or semifilled circles for intrafamily comparison) or nonspl (open circles), and RBCs from CTLs (dark blue dotted line or CTL ratio or ΔP–CTL) or a healthy splenectomized donor (red dotted line) were compared for membrane association of proteins of the ER-ribosomes (A), lysosomes (B), or mitochondria (C) and for the presence of organelle remnants (D-F). Scale bar represents 5 μm in panel D. Statistics are indicated above the patient cohorts for the comparison with CTL values and above a horizontal line for comparison between the 2 patient cohorts, respectively. (A-C) Membrane ghost association of ribosomal protein S25 (RPS25), lysosomal-associated membrane protein 1 (LAMP1) and ATP synthase F1 subunit alpha (ATP5F1A), respectively, enriched in ribosomes, lysosomes, and mitochondria and determined by proteomics (statistical analysis and additional examples in supplemental Figure 3). (D-F) Organelle labeling. RBCs spread on PLL-coated coverslips were labeled with BODIPY-ceramide (Cer), LysoTracker, or MitoTracker and observed by fluorescence microscopy (LysoTracker was maintained during observation). (D) Representative images. Open and filled arrowheads, patches, and network-like structures, respectively. (E-F) Quantification of RBCs presenting LysoTracker- or MitoTracker-positive patches expressed as percentage of the total RBC population and then as ΔP–CTL (mean of 1-3 and 1-7 independent experiments perpatient in panels E and F, respectively; Kruskal-Wallis tests followed by Dunn post hoc). (G-L) Membrane ghost association of band 4.2 (EPB42), Rh-associated glycoprotein (RhAG), voltage-dependent anion channel 1 (VDAC1), translocator protein (TSPO), Transferrin receptor (TfR) and DNM2 (dynamin), respectively, involved in cytoskeleton anchorage complexes (G-H), mitophagy (I-J), and endocytosis (K-L) and determined by proteomics (for volcano plots, see supplemental Figure 3).
Distinct septins correlate with different RBC morphology and functionality parameters
We then asked whether septins could explain the alterations of the RBC cytoskeleton and alteration score. We found an inverse correlation between ANK1 and septin-2 and -7 contents (Figure 5A). This led us to determine septin/ANK1 ratios for further analyses and to consider both cytoskeletal protein groups. Thus, correlations of SPT coverage, SPT heterogeneity, and RBC alteration score were stronger with septin-2 and -7/ANK1 ratios than with the ANK1 content itself (compare Figures 5B-D and 2J-L). RBC alteration score also slightly correlated with septin-8/ANK1 but not with septin-11/ANK1 ratio (Figure 5D). This may be explained by the lesser decrease in membrane association of the latter septin upon splenectomy (Figure 3J), which itself may result from the differential dynamics and actin/microtubule association of septin-11 compared to the others.34
A closer look at RBC morphology and functionality–related parameters revealed that among the different septins, septin-11 showed the best correlation with spherocytes (Figure 5E). Higher septin-2 and -7/ANK1 ratios correlated well with a reduced RBC surface-to-volume ratio, increased osmotic fragility, and a rise in intracellular calcium, whereas the septin-2/ANK1 ratio correlated with EV release (Figure 5G-H,J-K). In contrast, no septin appeared to correlate with stomatocyte abundance or cryohemolysis (Figure 5F,I). All these data suggested the differential control of RBC parameters by different septins.
ER proteins decrease upon splenectomy, whereas lysosomal and mitochondrial proteins and remnants increase
We finally explored whether the septin increase could result from RBC maturation defects. In RBCs from nonsplenectomized patients, proteomic analyses revealed a particular increase in proteins implicated in protein synthesis and folding in the endoplasmic reticulum (ER) and in endocytosis (see Figure 6A for a selected ER protein; see supplemental Figure 3A-B,F-G for volcano plots and other examples) but no major differences in nuclei and secretory pathway–associated proteins (supplemental Figure 3C-D). In contrast, an increase in lysosomal and mitochondrial proteins was observed in splenectomized patients (see Figure 6B-C for selected proteins; see supplemental Figure 3B,E,H-K for volcano plots and other examples).
To determine whether splenectomy-based differential protein enrichment could result from the differential presence of ER, lysosomal, and/or mitochondrial fragments, RBCs were labeled with a fluorescent ceramide analog (enriched in the ER-Golgi and mitochondria), a LysoTracker, and a MitoTracker, respectively. In RBCs from nonsplenectomized patients, a network-like structure reminiscent of the ER was observed, but almost no LysoTracker- and MitoTracker-positive structures could be detected. The opposite was observed in splenectomized patients, who had patches enriched in LysoTracker, MitoTracker, and ceramide (Figure 6D-F). RBCs from a healthy splenectomized donor also showed a slight increase in lysosomal and mitochondrial proteins as well as in MitoTracker-positive patches, contrasting with a slight decrease in ER-ribosomal proteins (red dotted line; Figure 6).
Moreover, decreased membrane association of protein 4.2 and Rh-associated glycoprotein, both shown to be degraded before enucleation upon severe ANK1 deficiency,35 was found in all patients (Figure 6G-H). In contrast, translocator protein and voltage-dependent anion channels, which play an active role in mitophagy throughout human erythropoiesis,36 were particularly increased in splenectomized patients. This contrasted with increased TfR and dynamin 2 in nonsplenectomized patients (Figure 6K-L). These data suggested RBC maturation defects, potentially explaining the increased septin abundance.
Discussion
Main findings
Although other studies have focused on morphological and, less frequently, functional alterations of RBCs in hereditary spherocytosis, our study combined biochemical, proteomic, and imaging approaches to investigate a series of RBC functionality–related parameters in a cohort of patients, splenectomized or not. We revealed that splenectomy limited but did not prevent alterations in RBC morphology and functionality. Moreover, septins were increased in patient RBCs and correlated with RBC alteration. This increase might have resulted from RBC maturation defects. These findings are discussed below and summarized in supplemental Figure 4.
Study limitations
To determine the effect of splenectomy on RBC morphology and functionality, patients should ideally have been compared before and after splenectomy. However, as most patients were splenectomized before the study was launched, this approach could only be applied to P1, P7, and P13 for some parameters. Therefore, an entire cohort of 4 nonsplenectomized and 10 splenectomized patients was included for comparison. Because biological and RBC fragility parameters measured in P1 and P7 before and after splenectomy showed the same evolution as those in the whole patient cohort, we were confident about the data obtained. Comparison of patient baseline characteristics indicated that the nonsplenectomized group mainly includes men and patients with ANK1 mutations, whereas the splenectomized group shows a higher proportion of SPTB mutations and a wider age range, comparatively. This does not mean that the 2 groups are not comparable. Indeed, as hereditary spherocytosis is a very heterogenous disease that can show different outcomes even in family members with the same underlying genetic defect, having perfectly balanced groups might not be an advantage and potentially not even be achievable. In addition, no significant difference in phenotypes was observed between patients with variants in ANK1 vs SPTB.37 Furthermore, despite important intragroup heterogeneity regarding gender, age, and mutations, splenectomized patients were nevertheless highly comparable for all evaluated parameters.
Differential impact of splenectomy on RBC morphology and functionality–related parameters
Mutations were detected in the ANK1 or SPTB genes, reported to account for ∼50% and ∼20% to 30% of all spherocytosis cases, respectively.38 In agreement with the fact that patients with mutations in these 2 genes are associated with moderate and severe forms of the disease,32 and because splenectomy appears to be more beneficial for these patients compared with patients with mutations of the SLC4A1 gene,33 most patients included in our study are splenectomized. In agreement with data from previous studies,17,32,39-42 the RBC distribution width, Hb levels, RBC, and reticulocyte counts were improved after splenectomy, whereas the decrease in spherocytes was only partial and concerned the most microcytic ones.
The RBC osmotic fragility was also reduced upon splenectomy, in agreement with the improved surface-to-volume ratio. In contrast, cryohemolysis was not restored. Because cryohemolysis is independent of the surface-to-volume ratio but dependent on molecular defects,26 and because the molecular defect is not restored by splenectomy, cryohemolysis may remain unchanged as well.
We also revealed that splenectomy partially restored the increased intracellular calcium levels observed in nonsplenectomized patients. The latter observation is consistent with those in previous studies,43,44 but the underlying mechanism is still debated. Some studies propose a reduced activity of the plasma membrane Ca²⁺ ATPase pump,44 whereas others suggest the contribution of the higher reticulocyte count, especially in nonsplenectomized patients.45 We showed here that the increased calcium levels were accompanied by a higher than usual intracellular ATP content, which could represent a compensation mechanism to avoid extensive calcium accumulation but also to maintain sodium and potassium homeostasis, because membrane permeability for these cations might be increased in spherocytosis.46,47
Such improvements in RBC morphology and functionality because of splenectomy could have resulted from the longer RBC lifetime induced by splenectomy, which in turn prevents stress-induced erythropoiesis.48
Septins as potential new key players in spherocytosis
As expected, splenectomy was not able to restore ANK1 and SPTB deficiencies. More surprisingly, these protein deficiencies were not accompanied by significant alterations of membrane SPT occupation or distribution. This observation might partially rely on the fact that SPT immunolabeling was performed with an antibody directed against both SPTB and SPTA, potentially hiding alterations resulting from SPTB deficiency, combined with the calculation of the mean SPT occupation for the whole cohorts whatever the mutation.
The idea of assessing the molecular defect to determine the clinical severity in hereditary spherocytosis is not new.49 We showed here that ANK1 levels were negatively correlated with the SPT variance and the RBC alteration score. It might seem a little surprising that such a correlation could be established for the whole patient cohort. However, molecular defects in SPTB are often accompanied by ANK1 deficiencies in hereditary spherocytosis.8 An even better correlation could be demonstrated between the RBC alteration and septin/ANK1 ratios. Except for the abundance of spherocytes, not all septins correlated with the same RBC parameters. Thus, septin-2 and -7 both correlated with cytoskeleton and functionality parameters, but only septin-2 correlated with EV release and septin-11 with spherocyte abundance. Those differential implications might be related to the fact that, in contrast to septins-2 and -7, septins-8 and 11 are constitutively bound to GTP and do not have a polybasic domain.21,22
To the best of our knowledge, we were the first to detect septins in mature RBCs. Nevertheless, septins have been recently described in platelets, contributing to the morphology and functionality of those enucleated cells.25 Upon splenectomy, septin abundance was reduced, as revealed by the comparison of the nonsplenectomized and splenectomized patients and confirmed in 1 patient before and after splenectomy. This observation matched with reduced levels of TUBA1B, an identified partner of septin-2 and -11 in endothelial cells,21 and with the decrease of ER and ribosomal proteins. Those septins did not simply result from the presence of reticulocytes because the septin network was detected in all RBCs in nonsplenectomized patients, whereas their reticulocyte count was <10%. Moreover, the septin content did not correlate with the proportion of reticulocytes, nor did it correlate with the TfR content, a marker of reticulocytes. Both the disease itself and splenectomy might contribute to the differential contents in ER fragments and septins. However, notice that septins-2 and -7 can also interact with mitochondria and are implicated in the endolysosomal pathway.22 Because mitochondrial and lysosomal fragments and associated proteins had increased after splenectomy; this would suggest that the decrease in septin levels resulting from the loss of ER could be counterbalanced by the increase in mitochondrial/lysosomal fragments. Whatever its origin, septin-2 was found under the RBC surface, suggesting it might be present in spherocytosis RBCs independently of organelles. Accordingly, Kim et al recently showed that septins-2 and -9 seemingly interact with the platelet cytoskeleton to control their shape.25
RBC maturation defect in spherocytosis
Proteomic and microscopy approaches revealed that RBCs from nonsplenectomized patients exhibited increased amounts of proteins associated with protein synthesis and folding, endocytosis, mitochondria but also lysosomes, although for the latter to a lesser extent when compared with RBCs from splenectomized patients. After splenectomy, only the ER-ribosomal and endocytosis proteins returned to normal values, in contrast to the mitochondrial/lysosomal ones. Moreover, we found decreased membrane association of protein 4.2 and Rh-associated glycoprotein, both previously shown to be degraded before enucleation upon severe ANK1 deficiency.35 Combined with the fact that the content of those proteins negatively correlated with the septin/ANK1 content, we therefore propose that RBCs from spherocytosis patients suffered from maturation defaults already at the erythroblast stage, at least in the more severely affected patients, and that splenectomy contributed to maturation impairment. Accordingly, translocator protein and voltage-dependent anion channels, which play active roles in mitophagy throughout human erythropoiesis,36 had especially increased in splenectomized patients. In contrast, higher membrane association of endocytosis proteins was seen in nonsplenectomized patients. Although the reticulocyte maturation mechanism is still not fully elucidated, it has been proposed that autophagy and exocytosis collaborate during reticulocyte maturation through the fusion of endosomes with autophagosomes-containing mitochondria, Golgi, or lysosomes, which would then be eliminated by exocytosis. The spleen could facilitate the release of these vesicles, as large vacuoles were described in reticulocytes from splenectomized individuals.50 Accordingly, we found RBC maturation defects also in a healthy splenectomized donor, although to a lesser extent. The absence of spleen macrophages, known to clear inclusion bodies at the RBC surface, might be responsible for the retention of organelle-enriched vesicles.51 This is in line with the observation that the organelle-associated proteins could be detected by proteomics performed on RBC ghosts, suggesting a close proximity with the plasma membrane. This is further supported by the absence of an increase in nucleus-associated proteins, even though Howell-Jolly bodies are systematically detected in RBCs after splenectomy.52 Thus, both the disease itself and splenectomy seemed to induce RBC maturation defaults. Hereditary spherocytosis is not the first anemia associated with RBC maturation failures, because RBCs with even functional mitochondria have been described in sickle cell disease.53
Conclusions
Although splenectomy represents the standard therapeutical treatment for patients with hereditary spherocytosis nowadays, it is accompanied by increased risks of infections and vascular events. In consequence, partial splenectomy or laparoscopic ligation of splenic vessels are proposed as alternatives to total splenectomy in order to maintain partial splenic activity, but long-term studies to determine clinical outcomes are lacking.54,55 We reported here, upon total spleen removal, a large but not complete restoration of RBC morphology and functionality according to septin content. However, at the same time, the RBC maturation process was strongly affected, suggesting that administration of autophagy modulators could be beneficial for splenectomized patients, as described by mTOR (mammalian target of rapamycin) inhibition for anemia in β-thalassemia.56 Moreover, the absence of restoration of septin-independent parameters upon splenectomy could question its recommendation, especially for patients showing high cryohemolysis vs low osmotic fragility. Finally, septins could represent a new contributor to the pathophysiology of hereditary spherocytosis.
Acknowledgments
The authors thank A. Debue and F. Cahay (UCLouvain, Belgium) for technical assistance in next-generation and Sanger sequencing. The MASSPROT platform (UCLouvain, Belgium) is acknowledged for the access to the liquid chromatography–mass spectrometry for protein analysis. Finally, the authors thank the group of V. Havelange for providing the K562 cell line.
This work was supported by grants from UCLouvain (FSR and Actions de Recherches concertées), The F.R.S-FNRS (National Fund for Scientific Research), and Salus Sanguinis foundation.
Authorship
Contribution: A.-S.C., H.P., D.T., P.H., and C.P. designed the experiments; A.-S.C. and D.T. analyzed and interpreted the data and wrote the manuscript; B.B. and C.L. identified the patients and established the diagnosis; A.S. and M. Maja performed biochemical measurements; M.L. helped with proteomic analysis; S.P.d.R. and D.V. performed mass spectrometry measurements and data analysis; L.G. and M. Martin carried out proteomic statistical analysis; P.V.D.S. did the electron microscopy experiments; M.V. and P.B. performed next-generation sequencing analysis; and all authors reviewed the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Donatienne Tyteca, CELL Unit & PICT Imaging Platform, de Duve Institute, UCLouvain, UCL B1.75.05, Ave Hippocrate, 75, B-1200 Brussels, Belgium; e-mail: donatienne.tyteca@uclouvain.be.
References
Author notes
The mass spectrometry proteomics data have been deposited in the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository (data set identifiers PXD034588 and 10.6019/PXD034588).
Data are available on the request from the corresponding author, Donatienne Tyteca (donatienne.tyteca@uclouvain.be).
The full-text version of this article contains a data supplement.

![Splenectomy partially reestablishes RBC baseline characteristics as well as RBC morphology and functionality–related parameters. (A-L) RBCs from patients (P; 1 color, 1 family), either splenectomized (spl; filled circles or semifilled circles for intrafamily comparison) or not (nonspl; open circles), and RBCs from healthy controls (CTLs; light and dark blue dotted lines for child and adult donor ranges, respectively; or subtraction from P values [ΔP–CTL]) were compared for morphology (A-D), baseline characteristics (E-G), and functionality (H-L). (M-N) Based on these different parameters, a RBC alteration score was calculated. Statistics are indicated above the patient cohorts for the comparison with CTL values and above a horizontal line for comparison between the 2 patient cohorts, respectively. (A-D) RBC morphology determined by electron or light microscopy on RBCs in suspension. The relative abundance of discocytes (A), spherocytes (B), stomatocytes (C), and echinocytes (D) was evaluated and expressed as percentage of the global RBC population (mean of 1-5 independent experiments per patient; Kruskal-Wallis tests followed by Dunn post hoc for the comparison of the 3 cohorts). (E) RBC distribution width (mean of 1-7 independent measurements per patient; Mann-Whitney tests to compare the 2 patient cohorts). (F-G) Hemi-RBC membrane area and area-to–mean corpuscular volume (MCV) ratio. (F) Hemi-area of RBCs spread on poly-L-Lysine (PLL)–coated coverslips. (G) Ratio of values provided in panel F to the MCV provided in supplemental Figure 1G (mean of 4-23 independent measurements per patient for panel F; Kruskal-Wallis tests followed by Dunn post hoc for the comparison of the 3 cohorts). (H) RBC osmotic fragility determined in increasingly hypotonic media. The osmolarity required to lyse 50% of RBCs (Half maximal effective concentration [EC50]) was calculated using hemolysis curves (mean of 1-5 independent experiments per patient; Kruskal-Wallis test followed by Dunn post hoc). (I) RBC cryohemolysis (mean of 1-6 independent experiments per patient; Mann-Whitney test). (J) EV abundance in plasma samples determined by Nanoparticle tracking analysis (mean of 1-3 independent experiments per patient; Kruskal-Wallis test followed by Dunn post hoc). (K) Intracellular calcium content. RBCs were labeled with the nonfluorescent Fluo4-AM, which is transformed in RBCs into the fluorescent Fluo4 after de-esterification and interaction with calcium ions. Labeled RBCs were analyzed by fluorimetry, and data were normalized to the Hb content (mean of 1-11 independent experiments per patient; Kruskal-Wallis test followed by Dunn post hoc). (L) Intracellular ATP content determined with a kit based on the activity of the firefly luciferase in the presence of ATP and emitted light in the presence of luciferin. ATP levels were normalized to Hb (mean of 1-8 independent experiments/patient; Kruskal-Wallis test followed by Dunn post hoc. (M) RBC morphology, functionality and biological parameters considered to establish the RBC functionality alteration score. These parameters were associated with a scale ranging from 0 to 1 when the parameter was nearly unaffected for most patients or from 0 up to maximum 8 when different degrees of affection for a parameter were observed in patient cohorts. The different scores corresponding to the different parameters were then added and the sum divided by the maximal score that could have been obtained to determine the RBC global functionality alteration score for each patient. The closer the score to 1, the more affected the RBCs by the disease. (N) RBC alteration score (Kruskal-Wallis tests followed by Dunn post hoc). MCHC, mean corpuscular Hb concentration; ns, not significant; RDW, red cell distribution width.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/7/17/10.1182_bloodadvances.2022009114/3/m_blooda_adv-2022-009114-gr1.jpeg?Expires=1769084908&Signature=2oQeHi76~--cicEnUxNtzet5ipPWSNGNMAlJdIfufcJU9qD9H~70Rxnr-aVXvoqM1Ntj58AnYXGR8FRAUdTEzbAUcSgshdvRp1N2J9QeC5meiEbbqVi6lcM6GIZxzF0Gw6dJ0FLVWH8e7-6bQqtvskSmVMjpjJqu0~K91RecyWRhiLcih1Wf6qbeGcVpBASnNQuLy2ziKBCyRNdtgEYNi1dq3uVyjwpuW4f~JR2KWtudlvArInRxWkBXVYcu58a5vSL5MrznUWa~FkOl8XTt6vSitzGWgtaNRPFZ1FldjmZkgyRi~uUWAsKF4-sU0igQNOKJ9LfVGQEKHREAHWI1DQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)