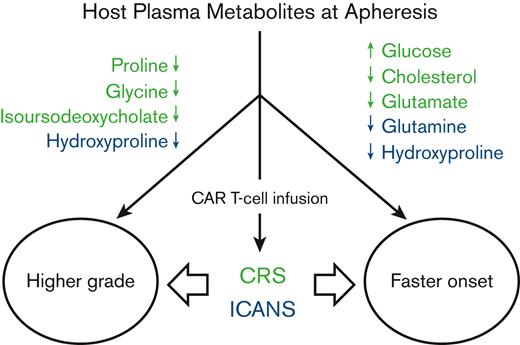
Key Points
Higher pretreatment glucose and lower cholesterol and amino acids correlate with worse and faster onset of CRS.
Lower pretreatment glutamine and hydroxyproline correlate with worse and faster onset of ICANS.
Abstract
Anti-CD19 chimeric antigen receptor (CAR) T-cell therapy is a highly effective treatment option for patients with relapsed/refractory large B-cell lymphoma. However, widespread use is deterred by the development of clinically significant acute inflammatory toxicities, including cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS), that induce significant morbidity and require close monitoring. Identification of host biochemical signatures that predict the severity and time-to-onset of CRS and ICANS may assist patient stratification to enable timely mitigation strategies. Here, we report pretreatment host metabolites that are associated with CRS and ICANS induced by axicabtagene ciloleucel or tisagenlecleucel therapy. Both untargeted metabolomics analysis and validation using targeted assays revealed a significant association between the abundance of specific pretreatment biochemical entities and an increased risk and/or onset of clinically significant CRS (q < .1) and ICANS (q < .25). Higher pretreatment levels of plasma glucose and lower levels of cholesterol and glutamate were associated with a faster onset of CRS. In contrast, low baseline levels of the amino acids proline and glycine and the secondary bile acid isoursodeoxycholate were significantly correlated with clinically significant CRS. Lower concentration of the amino acid hydroxyproline was associated with higher grade and faster onset of ICANS, whereas low glutamine was negatively correlated with faster development of ICANS. Overall, our data indicate that the pretreatment host metabolome has biomarker potential in determining the risk of clinically significant CRS and ICANS, and may be useful in risk stratification of patients before anti-CD19 CAR T-cell therapy.
Introduction
Chimeric antigen receptor (CAR) T-cell–based immunotherapy is a revolutionary approach for patients with relapsed/refractory large B-cell lymphoma (r/r LBCL) who do not respond to or are ineligible for autologous stem cell transplantation.1 In addition, this treatment is now available for second-line treatment.2 In this approach, a patient’s peripheral blood mononuclear cells are isolated, T cells are purified and engineered to express CARs, which direct T cells to a tumor antigen of interest, and infused back into the same patient after a brief period of lymphodepletion.3 Three different CAR T-cell therapies targeting the CD19 antigen are FDA-approved for the treatment of r/r LBCL: axicabtagene ciloleucel (Axi-cel),4 tisagenlecleucel (Tisa-cel),5 and lisocabtagene maraleucel.6 Despite the breakthrough nature of this type of treatment and early successes,4,5 significant limitations exist, including a lack of durable response in 60% to 70% of patients.7-9 In addition, immune-related adverse events (irAEs) limit the potential pool of recipients of CAR T-cell therapy by inducing significant morbidity, prolonged hospitalization, health care costs, and other negative downstream sequelae such as the delay or failure of patients to fully return to their pretreatment functional status.10
The most notable acute irAEs are cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS). CRS is experienced by ∼90% of patients and is characterized most commonly by fever and hemodynamic and/or respiratory instability associated with elevated serum cytokines, including interleukin-6 and others.7,8 Up to 20% of patients experience high-grade CRS that may require intensive care, such as mechanical ventilation and/or vasopressor support.7,8 ICANS occurs in ∼60% of patients, is considered high-grade in ∼30% of cases, and can result in altered mental status, seizures, and cerebral edema.7,8 The unpredictable nature of CRS and ICANS and the potential for high-grade complications limit the pool of potential patients to those without significant comorbidities or frailty. Risk factors that predict CRS and ICANS before the initiation of therapy can personalize the treatment of individual patients by enabling more informed clinical decision making, but they are limited.11-13 Therefore, a better understanding of pretreatment risk factors associated with clinically significant CRS and ICANS is needed to improve management and reduce morbidity and mortality,8,9 and this represents an important unmet clinical need.
Individual differences such as lifestyle, dietary differences, environmental exposures, genetic variation, tumor burden, and prior treatments can influence the relative abundance of a wide range of metabolites among all individuals, including patients receiving CAR T-cell therapy.14-17 However, regardless of the basis for metabolic differences in the patient cohort, a preexisting metabolomic profile that can be predictive of CRS and ICANS has significant clinical implications. For example, it is well established that high glucose levels support higher cytokine levels18 and stronger T-cell metabolism.19 Similarly, the antiinflammatory associations of certain amino acids20-23 are well known in animal models of inflammation. Therefore, associations between the pretreatment plasma metabolome of patients with r/r LBCL and irAEs have the potential to reveal biochemical risk factors in the induction of CRS and ICANS by anti-CD19 CAR T-cell therapy. Using untargeted metabolomics and targeted validation assays of pretreatment plasma samples from patients with r/r LBCL treated with anti-CD19 CAR T cells, we identified metabolites that are significantly associated with an increased or decreased risk of severity and time-to-onset of CRS and ICANS. These findings underscore the potential of using metabolites as predictive biomarkers of CRS and ICANS in patients receiving anti-CD19 CAR T-cell therapy.
Materials and methods
Participant recruitment and collection of plasma
The collection of clinical samples was approved by the Institutional Review Board of Cleveland Clinic in accordance with guidelines for the protection of human participants according to the Declaration of Helsinki. All study participants provided written informed consent for the collection of samples and subsequent analyses. Blood samples were collected from 41 patients with r/r LBCL at the time of apheresis, before treatment with Axi-cel (n = 31) or Tisa-cel (n = 10). The sample size was determined based on sample availability, and previous metabolomics studies of drug response in complex diseases with comparable sample sizes have reported metabolite effect sizes large enough to reach statistical significance.24 Plasma and serum were collected by centrifugation at 1500g for 30 minutes and 1300g for 10 minutes, respectively, and stored at −80°C until further use.
Patient data
Clinical and demographic details of 41 patients treated with Axi-cel and Tisa-cel were collected by review of the electronic medical record and maintained in the password-protected and HIPAA-compliant Research Electronic Data Capture system. Gender, age, diagnosis, and clinical laboratory values before treatment, including blood glucose levels, were recorded. CRS and ICANS grading on each inpatient day after treatment with CAR T-cell therapy was recorded using the American Society for Transplantation and Cellular Therapy (ASTCT) consensus criteria.25 Clinical outcomes, including response to treatment and overall survival, were collected.
Metabolomics data acquisition
Plasma samples were processed at Metabolon, Inc (Morrisville, NC) using an ultra-performance liquid chromatography-tandem mass spectrometry platform, as described26 and detailed in supplemental Methods. The metabolites indicated by “∗” refer to tier 2 level identification, as defined by metabolomics standards, according to which the only reason a compound is given a tier 2 ID instead of a tier 1 ID is because the pure reference cannot be obtained, usually because the compound cannot be obtained in a stable, pure form in nature. In such cases, the fragmentation patterns, mass, and retention time would all match up in a theoretical setting.27 Therefore, there is still confidence in the identification of compounds indicated with “∗”.
Data processing and quality control
Metabolites that were absent in >20% of patient samples were excluded from analysis (n = 203 metabolites), and those with ≤20% missing were imputed with the minimum observed value across all samples. The relative abundance of each metabolite across the cohort was log transformed (supplemental Figure 1) to meet parametric assumptions. Principal component analysis identified no outlier samples among the cohort (supplemental Figure 2). Fourteen individual metabolite measurements with a median absolute deviation >7 were removed as outliers.28 A total of 808 metabolites were retained for association studies after processing and quality control analysis of 1011 named metabolites.
Metabolite associations with CRS
Association analysis of metabolites with CRS outcomes was performed using the open-source statistical analysis software, R.29 Each metabolite was tested for associations with the maximum grade of CRS using 2-sided ordinal logistic regression. Selected metabolites were tested for associations between each CRS grade using a 2-sided t test. The abundance of each metabolite was tested for associations with the time-to-onset of CRS from the time of CAR T-cell therapy initiation using a 2-sided Cox proportional hazards model, and hazard ratios (HRs) indicating a per-unit increase and confidence intervals (CIs) were determined. All P values were corrected for multiple hypothesis testing using a false discovery rate approach,30 and metabolites with both a nominal P < .05 and q < .10 were considered to be statistically significant. The cohort was stratified by treatment type, and the coefficients and CIs generated by ordinal logistic regression and Cox proportional hazard models were meta-analyzed using a random-effects model.
Metabolite associations with ICANS
Each metabolite was also tested for associations with the maximum grade of ICANS using ordinal logistic regression. Finally, the abundance of each metabolite was tested for associations with the time-to-onset of ICANS from the time of therapy initiation using a Cox proportional hazards model, and HR and CI were determined.
Targeted metabolite quantification
Amino acids and lipids were quantified in plasma and serum, respectively, as described in supplemental Methods. CLIA-certified assays were used for lipid profiling. Blood glucose concentrations were obtained from clinical laboratory tests performed on or immediately before the day of apheresis as part of the patients’ standard of care. Sphingomyelin was quantified in plasma, according to the manufacturer’s instructions (Abcam, MA). The concentrations were transformed using the natural log, and the log-transformed metabolite concentrations were then tested for associations with severity and time-to-onset of CRS and ICANS using ordinal logistic regression and Cox proportional hazards models, respectively. Metabolites with P < .05 were considered to have been successfully validated.
Results
Baseline patient characteristics and treatment outcomes
To identify pretreatment plasma metabolite signatures associated with CRS and ICANS, we used biospecimens and clinical data from 41 patients with r/r LBCL treated with Axi-cel (31) or Tisa-cel (10). The median age of the cohort was 61 years (range 25-77), and 61% of patients were male (Table 1). The clinical diagnosis was diffuse LBCL (DLBCL) (n = 22), DLBCL transformed from indolent non-Hodgkin lymphoma (n = 13), or primary mediastinal B-cell lymphoma (n = 6), and 51% of the patients had received prior autologous stem cell transplantation (Table 1). In 39 patients for whom response data were available, 19 (49%) responded to CAR T-cell therapy after 3 months, with 14 (36%) patients showing a complete response (Table 1).
Baseline and posttreatment clinical characteristics
| Patient clinical characteristics . | N = 41 . |
|---|---|
| Age-median (range, y) | 61 (25-77) |
| Males (%) | 61 |
| Number of prior therapies (median, range) | 3 (2-6) |
| Prior autologous stem cell transplantation (%) | 51 |
| Disease type | |
| DLBCL, no. (%) | 22 (53.7) |
| DLBCL transformed from indolent NHL, no. (%) | 13 (31.7) |
| Primary mediastinal B-cell lymphoma, no. (%) | 6 (14.6) |
| Type of therapy | |
| Axi-cel, no. (%) | 31 (75.6) |
| Tisa-cel, no. (%) | 10 (24.4) |
| CRS | |
| Grade 0, no. (%) | 10 (24.4) |
| Grade 1, no. (%) | 9 (22) |
| Grade 2-4, no. (%) | 22 (53.7) |
| Median time-to-CRS | 2 days (N = 31) |
| Median time to max. CRS | 4 days (N = 31) |
| ICANS | |
| Grade 0, no. (%) | 22 (53.7) |
| Grade 1, no. (%) | 9 (22) |
| Grade 2-4, no. (%) | 10 (24.4) |
| Median time-to-ICANS | 6 days (N = 19) |
| Median time to max. ICANS | 6 days (N = 19) |
| Treatments | |
| Use of tocilizumab, no. (%) | 23 (56) |
| Use of steroids, no. (%) | 13 (32) |
| 3-month response | Total (N = 39) |
| CR+PR, no. (%) | 19 (48.7) |
| CR, no. (%) | 14 (35.9) |
| SD, no. (%) | 2 (5.1) |
| PD, no. (%) | 13 (33.3) |
| Patient clinical characteristics . | N = 41 . |
|---|---|
| Age-median (range, y) | 61 (25-77) |
| Males (%) | 61 |
| Number of prior therapies (median, range) | 3 (2-6) |
| Prior autologous stem cell transplantation (%) | 51 |
| Disease type | |
| DLBCL, no. (%) | 22 (53.7) |
| DLBCL transformed from indolent NHL, no. (%) | 13 (31.7) |
| Primary mediastinal B-cell lymphoma, no. (%) | 6 (14.6) |
| Type of therapy | |
| Axi-cel, no. (%) | 31 (75.6) |
| Tisa-cel, no. (%) | 10 (24.4) |
| CRS | |
| Grade 0, no. (%) | 10 (24.4) |
| Grade 1, no. (%) | 9 (22) |
| Grade 2-4, no. (%) | 22 (53.7) |
| Median time-to-CRS | 2 days (N = 31) |
| Median time to max. CRS | 4 days (N = 31) |
| ICANS | |
| Grade 0, no. (%) | 22 (53.7) |
| Grade 1, no. (%) | 9 (22) |
| Grade 2-4, no. (%) | 10 (24.4) |
| Median time-to-ICANS | 6 days (N = 19) |
| Median time to max. ICANS | 6 days (N = 19) |
| Treatments | |
| Use of tocilizumab, no. (%) | 23 (56) |
| Use of steroids, no. (%) | 13 (32) |
| 3-month response | Total (N = 39) |
| CR+PR, no. (%) | 19 (48.7) |
| CR, no. (%) | 14 (35.9) |
| SD, no. (%) | 2 (5.1) |
| PD, no. (%) | 13 (33.3) |
CR, complete response; NHL, non-Hodgkin lymphoma; PD, progressive disease; PR, partial response; SD, stable disease.
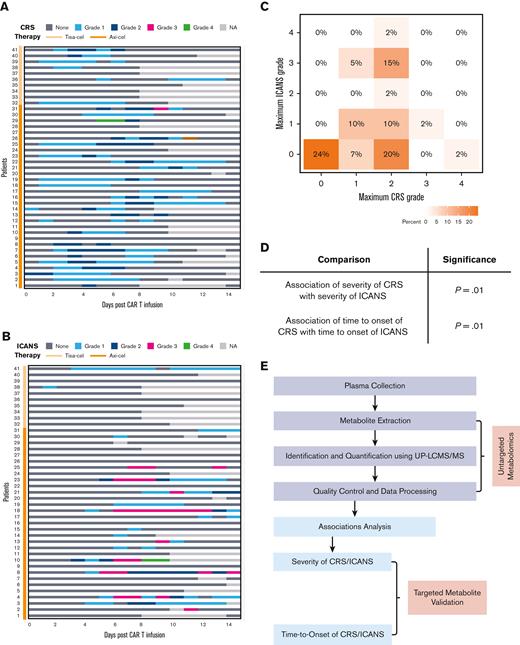
The development of CRS (Figure 1A) and ICANS (Figure 1B) in patients receiving CAR T-cell therapy is represented as a swimmer plot. For those patients who experienced CRS, the median time-to-CRS onset was 2 days, with an average duration of 5 days (range, 0-8), and patients treated with Tisa-cel exhibited a lower grade of CRS than those treated with Axi-cel (Figure 1A), as previously reported.31 For those patients who experienced ICANS, the median time-to-onset of ICANS was 6 days, with an average duration of 5.5 days (range, 0-27) (Figure 1B). In this cohort, 31 (76%) patients developed CRS and 46% developed ICANS after CAR T-cell therapy (Figure 1C; Table 1). Grade 1 CRS was reported in 9 (22%) individuals and grades 2 to 4 CRS in 22 (54%) individuals, with 56% of the patients receiving tocilizumab and 32% steroids (Figure 1C; Table 1). Grade 1 ICANS was reported in 9 (22%) and grades 2 to 4 in 10 (24%) individuals (Figure 1C; Table 1). The maximum grades of ICANS and CRS were significantly associated (P = .01), and the time-to-ICANS was significantly associated with the respective time-to-CRS (P = .01) (Figure 1D). Next, we implemented an untargeted metabolomics screen and data analysis workflow (Figure 1E) to analyze the pretreatment metabolome of Axi-cel or Tisa-cel–treated patients. Metabolites were extracted from plasma collected on the day of apheresis and identified and quantified using ultra performance liquid chromatography-tandem mass spectrometry. The association between the relative abundance of metabolites that passed quality control and CRS outcomes was examined (Figure 1E).
Association between severity, and time-to-onset of CRS with that of ICANS after CAR T-cell therapy. Kinetics of CRS (A) and ICANS (B) in each patient in the study cohort during 14 days of CAR T-cell infusion, colors indicate the grade of toxicity recorded each day. NA indicates that either the patient was discharged, assessment was declined, or grading could not be performed. (C) Maximum grade of ICANS and CRS recorded for patients after CAR T-cell infusion, color indicates the percentage of the cohort affected. (D) Associations between the outcomes (grade and time-to-onset) of CRS with those of ICANS (n = 41 patients). (E) Workflow of metabolomics platform and analysis.
Association between severity, and time-to-onset of CRS with that of ICANS after CAR T-cell therapy. Kinetics of CRS (A) and ICANS (B) in each patient in the study cohort during 14 days of CAR T-cell infusion, colors indicate the grade of toxicity recorded each day. NA indicates that either the patient was discharged, assessment was declined, or grading could not be performed. (C) Maximum grade of ICANS and CRS recorded for patients after CAR T-cell infusion, color indicates the percentage of the cohort affected. (D) Associations between the outcomes (grade and time-to-onset) of CRS with those of ICANS (n = 41 patients). (E) Workflow of metabolomics platform and analysis.
Pretreatment metabolites associated with severity of CRS
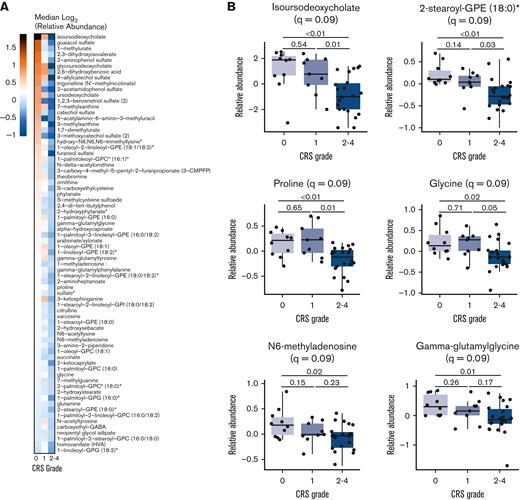
The untargeted metabolomics screen identified 1011 named biochemical entities, of which 808 metabolites that met the quality control criteria were selected for further analysis. Lipids represented 48% of the metabolites and amino acids 24%, with carbohydrates, peptides, nucleotides, xenobiotics, cofactors, vitamins, and partially characterized compounds making up the rest. Because patients exhibiting CRS with grade 2 or higher are often treated with tocilizumab,8,25 these patients were grouped together for analysis of association with clinically significant CRS. The relative abundance of 72 metabolites correlated negatively with clinically significant CRS as measured by maximum clinical grade according to ASTCT consensus criteria (Figure 2A; supplemental Table 1). However, we did not find any metabolite that was positively associated with clinically significant CRS. Both, the odds ratios for all 72 metabolites from the ordinal logistic regression and the meta-analyzed summary estimates from the treatment type–stratified cohort were less than 1, indicating that the risk of clinically significant CRS increases as the metabolite abundance decreases and that these results are not dependent on the CAR T-cell product (supplemental Figure 3; supplemental Table 3). The median metabolic abundance of these metabolites varied in the patient cohort across 3 levels of CRS severity (Figure 2A). Representative association plots include metabolites that were most statistically significant as well as biologically important based on their reported roles in inflammation (Figure 2B). The bile acid isoursodeoxycholate had the maximum difference in median metabolic abundance across the 3 severity levels, with lower relative abundance significantly associating with more clinically significant CRS (Figure 2A). Lower abundance of the lipid 2-stearoyl-GPE (18:0)∗ and amino acids proline and glycine was also significantly associated with worsening CRS severity. Among other metabolic classes, the nucleotide N6-methyladenosine and the dipeptide gamma-glutamylglycine showed a negative association with clinically significant CRS (Figure 2B).
Association of metabolites with severity of CRS. (A) Heatmap of median relative abundance of 72 metabolites (rows) significantly associated with CRS severity (P < .05, q < .10), stratified by maximum observed grade of CRS (columns). Color indicates the log2-transformed median relative abundance of each group and metabolites are ordered by their relative abundance in CRS grades 2 to 4. (B) Relative abundance of the most statistically significant metabolites that were negatively associated with maximum observed CRS grade. The ordinal logistic regression q value is displayed in the graph title and the q values from pairwise t test comparisons are displayed on the graph.
Association of metabolites with severity of CRS. (A) Heatmap of median relative abundance of 72 metabolites (rows) significantly associated with CRS severity (P < .05, q < .10), stratified by maximum observed grade of CRS (columns). Color indicates the log2-transformed median relative abundance of each group and metabolites are ordered by their relative abundance in CRS grades 2 to 4. (B) Relative abundance of the most statistically significant metabolites that were negatively associated with maximum observed CRS grade. The ordinal logistic regression q value is displayed in the graph title and the q values from pairwise t test comparisons are displayed on the graph.
Pretreatment metabolites associated with time-to-onset of CRS
Given the significant health care use of inpatient CAR T-cell therapy as well as the risks of additional complications of prolonged hospitalization, including nosocomial infection, there is significant interest in the outpatient administration of cell therapy. For patients receiving CAR T-cell therapy as an outpatient, rapid-onset CRS (<48 hours) poses significant logistic challenges, including after-hour admissions and emergency department visits, which may prevent the rapid administration of tocilizumab or other interleukin-6 inhibitors. Therefore, we next determined the relationship between pretreatment metabolite abundance and the number of days between initiation of anti-CD19 CAR T-cell therapy and CRS onset. Using the Cox proportional hazard model, 155 metabolites showed significant association with time-to-onset of CRS (Figure 3A; supplemental Table 2). HR was used to determine the direction of association with time-to-onset of CRS, with a ratio of >1 indicating a positive association and <1 indicating negative association.
Association of metabolites with time-to-CRS onset. (A) Forest plot of HRs and 95% CIs for the 155 metabolites, measured on the day of apheresis, associated with time-to-CRS onset from day of treatment initiation (P < .05, q < .10). (B) Kaplan-Meier curves for the 9 metabolites with the highest or lowest HRs display the fraction of patients who experienced CRS, stratified by median metabolite abundance. The dotted red and blue lines indicate the median time-to-CRS for each group.
Association of metabolites with time-to-CRS onset. (A) Forest plot of HRs and 95% CIs for the 155 metabolites, measured on the day of apheresis, associated with time-to-CRS onset from day of treatment initiation (P < .05, q < .10). (B) Kaplan-Meier curves for the 9 metabolites with the highest or lowest HRs display the fraction of patients who experienced CRS, stratified by median metabolite abundance. The dotted red and blue lines indicate the median time-to-CRS for each group.
Higher relative abundance of 3 carbohydrates, glucose (HR, 5.04; 95% CI, 1.02-24.9; q = .08), mannose (HR, 3.26; 95% CI, 1.18-8.99; q = .06), and 1,5-anhydroglucitol (1,5-AG) (HR, 2.66; 95% CI, 1.17-6.06; q = .06), were associated with more rapid onset of CRS (Figure 3B; supplemental Table 2). In contrast, higher levels of the remaining 152 metabolites were associated with a less rapid onset of CRS (Figure 3A; supplemental Table 2). Of the latter, several lipids, including cholesterol (HR, 0.17; 95% CI, 0.05-0.56; q = .04), 1-oleoyl-GPI (18:1) (HR, 0.57; 95% CI, 0.33-0.99; q = .08), and sphingomyelin (d18:2/23:0, d18:1/23:1, d17:1/24:1)∗ (HR, 0.2; 95% CI, 0.07-0.69; q = 0.048), as well as the amino acids glutamate (HR, 0.4; 95% CI, 0.13-1; q = 0.08) and glycine (HR, 0.3; 95% CI, 0.09-0.8; q = 0.06), had the lowest HRs (Figure 3B: supplemental Table 2), indicating a negative association between these metabolites and onset of CRS. In addition, lower relative abundance of the nucleotide N6-methyladenosine (HR, 0.28; 95% CI, 0.08-0.98; q = 0.08) was associated with faster CRS onset. These results are concordant with the summary estimates derived from the meta-analyzed cohorts stratified by CAR T-cell treatment type, indicating that the associations are not unique to 1 type of therapy (supplemental Figure 4; supplemental Table 4).
Targeted validation of metabolite associations with CRS
Next, to validate the findings of the untargeted metabolomics screen, we performed targeted, quantitative assays of a subset of metabolites that associate with the severity or time-to-onset of CRS. Among these metabolites, 3 main classes, including glucose, amino acids, and lipids, were chosen because quantitative assays are readily available for these. A metabolite was considered validated when it was significantly associated with CRS in both untargeted metabolomics and targeted assay. Glucose was validated using clinical laboratory measurements taken independently of the apheresis samples as part of standard of care. Cholesterol was selected for validation among lipids because of its biological importance in inflammatory diseases32 and because it was negatively associated with time-to-CRS onset. Among the amino acids, proline, glycine, and glutamate were selected to validate associations with severity and time-to-onset of CRS, as these have been previously reported to affect inflammation in other contexts.22,23,33,34 The concentrations were transformed using the natural log (supplemental Table 5), and the log-transformed metabolite concentrations were then tested for associations with severity and time-to-onset of CRS. Each of these 6 metabolites was successfully validated for at least 1 CRS outcome (Figure 4). Glucose and cholesterol were significantly associated with time-to-onset of CRS, both in the untargeted metabolomics screen and clinical laboratory measurement. Proline and glycine were negatively associated with CRS severity in the metabolomics screen and targeted clinical assays (P < .05), but not validated for association with time-to-onset of CRS. Glutamate was significantly associated with time-to-onset of CRS in both untargeted metabolomics analysis and targeted validation assay (P < .05). Sphingomyelin was validated for its negative association with time-to-onset of CRS at P < .1; the less significant association is likely due to the detection of all naturally occurring sphingomyelins in the targeted assay rather than just the specific forms found significant in untargeted metabolomics analysis.
Targeted analysis of metabolites validates significant associations with CRS. Association of glucose, cholesterol, proline, glycine, glutamate, and sphingomyelin concentrations with severity or time-to-onset of CRS were validated using clinical and targeted quantitative assays.
Targeted analysis of metabolites validates significant associations with CRS. Association of glucose, cholesterol, proline, glycine, glutamate, and sphingomyelin concentrations with severity or time-to-onset of CRS were validated using clinical and targeted quantitative assays.
Pretreatment metabolite associations with ICANS grade and time-to-onset
To determine if any pretreatment plasma metabolites were associated with the neurotoxicity reported in patients after anti-CD19 CAR T-cell therapy, we tested for associations between the relative abundance of each plasma metabolite and the clinical outcomes of ICANS. We did not find any metabolite that was significantly associated with the outcomes of ICANS when using q < 0.1. Because CRS and ICANS are significantly and likely causally associated with each other, we used P < .05 and q < .25 to identify associations between host metabolites and ICANS outcomes. Using these criteria, we found that a lower relative abundance of 2 metabolites, hydroxyproline and 1-stearoyl-2-oleoyl-GPI (18:0/18:1)∗, was associated with worsening ICANS severity (supplemental Figure 6; supplemental Table 5). The relative levels of 11 metabolites were each significantly associated with the time-to-onset of ICANS (supplemental Figure 7A; supplemental Table 6). Perfluorooctanoate (HR, 2.89; 95% CI, 1.36-6.11; q = .24) and taurocholate sulfate (HR, 2.59; 95% CI, 1.45-4.64; q = .21) had the highest HRs, whereas hydroxyproline (HR, 0.08; 95% CI, 0.02-0.4; q = .18) and glutamine (HR, 0.08; 95% CI, 0.013-0.45; q = .24) were among the ones with lowest HRs (supplemental Figure 7B; supplemental Table 6). Decreased levels of a single metabolite, hydroxyproline, were significantly associated with both the grade (supplemental Figure 6) and time-to-onset (supplemental Figure 7) of ICANS. To validate the findings of the untargeted metabolomics screen and quantify metabolite concentrations associated with ICANS, we performed targeted assays of a subset of these metabolites. In targeted quantitative assays, glutamine was found to be significantly associated (P < .05) with time-to-onset of ICANS (supplemental Figure 8A), and hydroxyproline was significantly associated with severity and time-to-onset of ICANS (P < .05) (supplemental Figure 8B).
Discussion
Here, we report the identification of specific pretreatment metabolites in the plasma of patients with r/r DLBCL treated with anti-CD19 CAR T-cell therapy that are associated with an increased risk of clinically significant and rapid-onset CRS requiring tocilizumab and/or hospitalization. In particular, clinicians managing outpatient treatment of patients receiving CAR T-cell therapy may benefit from improved predictive methods to avoid urgent and/or after-hours hospitalizations. Our data show that anti-CD19 CAR T-cell–induced severity and time-to-onset of ICANS were significantly associated with the respective outcomes of CRS, which is consistent with previous reports.13,35 We looked for metabolite associations that are common to both CRS and ICANS and found that 3-methylxanthine, 1-stearoyl-2-oleoyl-GPI (18:0/18:1)∗, 3-aminoisobutyrate, lactosyl-N-nervonoyl-sphingosine (d18:1/24:1)∗, (16 or 17)-methylstearate (a19:0 or i19:0), and sphingomyelin (d18:2/24:1, d18:1/24:2)∗ were significantly associated with the time-to-onset of both toxicities. These findings also point toward common host factors that may predispose the patients to developing both CRS and ICANS, although further research is needed to establish cause-and-effect relationships, and this is an area of ongoing investigation.
Untargeted metabolomics screening, as well as targeted analysis using clinical measurements revealed quantitative correlations of a variety of biochemical entities, including carbohydrates, lipids, and amino acids, with CRS. A higher abundance of plasma glucose at the time of apheresis was associated with a higher risk of rapid CRS onset. Furthermore, the hexose sugar mannose, which can be converted to glucose, was also positively correlated with the time-to-onset of CRS. These positive associations suggest that exposure of newly infused CAR T cells to a sugar-rich host environment may promote their expansion and initiate the cascade of proinflammatory cytokine production more rapidly. It is noteworthy that higher abundance of 1, 5-anhydroglucitol, a metabolite of glucose that represents glycemic variability over a 2-week period,36 was also positively associated with more rapid onset of CRS. As the measurement of glucose and 1, 5-anhydroglucitol at the time of apheresis represents a snapshot in time, future studies will examine if chronic elevation in glucose concentration, as quantified by hemoglobin A1c, predisposes patients to a more rapid onset of CRS. Overall, the positive association of circulating hexose sugars and average glycemia with faster CRS indicates that higher pretreatment levels of circulating sugars may place patients with r/r DLBCL at risk of faster CRS development upon treatment with Axi-cel or Tisa-cel. In the future, it may be possible to use circulating glucose levels as a criterion to preemptively treat such patients prophylactically with tocilizumab, siltuximab, or other cytokine pathway inhibitors.37,38 Interestingly, patients with COVID-19 who have severe disease develop cytokine storms,39 similar to the CRS observed in patients receiving CAR T-cell therapy, and an integrated analysis of cytokines and metabolites has highlighted the importance of immunometabolic programming in patients with COVID-19.40 A connection between diabetes and COVID-19 has been reported41 and potentially originates from an enhanced immune response when glucose levels are elevated.42 Similar functional interactions between metabolites and cytokines may occur in the immune environment of patients receiving CAR T-cell therapy.
The untargeted metabolomics screen revealed that the amino acids proline and glycine were inversely associated with the severity and time-to-onset of CRS. The association of proline and glycine was successfully validated with the severity of CRS using clinical assays, but not with the time-to-onset of CRS. Regardless, the negative correlation between these amino acids and clinically significant CRS is consistent with their previously described anti-inflammatory function.22,23 Furthermore, bile acid metabolism is altered in various animal models of inflammation, including chronic cholestasis, wherein secondary bile acids exhibit anti-inflammatory effects.43 We found that lower circulating levels of isoursodeoxycholate were associated with more clinically significant CRS in patients treated with anti-CD19 CAR T cells. Primary bile acids such as cholic acid and chenodeoxycholic acid have a cholesterol backbone, are synthesized in the liver, conjugated with glycine, and stored in the gallbladder.44 In response to food intake, the conjugated bile acids are transported to the intestine, where the activity of the gut microbiota converts them to secondary bile acids, such as ursodeoxycholic acid and isoursodeoxycholic acid.44 A negative correlation of cholesterol, glycine, and secondary bile acids with CRS outcomes reported in this study suggests that alterations in bile acid metabolism may increase the risk of clinically significant CRS in patients receiving anti-CD19 CAR T-cell therapy. In addition, the association of higher levels of circulating sphingomyelins, a class of sphingolipids, with a slower onset of CRS is also consistent with the anti-inflammatory effects of dietary sphingomyelin.45
The interconvertible amino acids proline and hydroxyproline20 are known to have anti-inflammatory effects in animals21,22 and were inversely associated with CRS and ICANS, respectively. Glycine can be synthesized from hydroxyproline20 and was inversely associated with the severity of CRS. An anti-inflammatory role for glycine has been previously reported in various proinflammatory diseases, including arthritis23 and acute pancreatitis,46 suggesting that these amino acids may form an anti-inflammatory biomarker axis in patients with r/r LBCL. Previous reports have shown a positive correlation between ICANS and post–CAR T-cell cerebrospinal fluid metabolites such as glutamate and quinolinic acid.13 Lower concentrations of glutamate were observed in patients with a more rapid onset of CRS. Glutamine is converted to glutamate by glutaminase, and its inhibition for short durations may improve CAR T-cell function,34 offering a potential mechanism for the association of low levels of glutamate and glutamine with increased CRS and ICANS, respectively. Even though individual metabolite associations with ICANS were only significant at q < .25, they still have the potential to jointly contribute and will be tested in future predictive modeling studies.
Taken together, our study demonstrates that the pretreatment host plasma metabolome has the potential to predict the severity and time-to-onset of CRS and ICANS in patients receiving anti-CD19 CAR T cells. Our findings pave the path to the development of clinical assays that may be used to measure these metabolites before anti-CD19 CAR T-cell therapy to stratify patients at risk of clinically significant CRS and ICANS. Furthermore, our results suggest that metabolite-based targeted clinical interventions may be developed in the future to mitigate the risk of toxicities associated with anti-CD19 CAR T-cell therapy in advance of treatment.
Acknowledgments
The authors thank the patients who consented to participate in this study, as well as the clinical nurses and coordinators. The authors also acknowledge assistance from the Translational Research Core and the Proteomics and Metabolomics Core at the Cleveland Clinic.
Funding for this study was provided by VeloSano Bike to Cure, Cleveland Clinic Center of Excellence in Lymphoid Malignancies Research, and Taussig Cancer Institute.
Authorship
Contribution: A.J. and M.S.P. collected and processed the blood samples; A.J., C.E.H., A.M., A.F., G.K., D.M.R, B.T.H., and N.G. analyzed the data; A.J., C.E.H., and N.G. wrote the manuscript; B.T.H. and N.G. provided funding support; and N.G. conceptualized and supervised the study.
Conflict-of-interest disclosure: N.G., B.T.H., D.M.R., A.J., C.E.H., and G.K. have intellectual property related to the detection of CAR T-cell–associated toxicities; D.M.R. has stock and other ownership interests in Interpares Biomedicine and Clariifi and intellectual property related to the detection of liver cancer; B.T.H. has received research funding from Takeda; consultancy, honoraria, research funding from Genentech, Karyopharm, Celgene, AbbVie, Pharmacyclics, BeiGene, AstraZeneca, Kite, a Gilead Company, and Bristol Myers Squibb; and consultancy and honoraria from Novartis, though not used for this study.
Correspondence: Neetu Gupta, 9500 Euclid Ave, NE40, Cleveland, OH 44195; e-mail: guptan@ccf.org.
References
Author notes
∗A.J. and C.E.H. contributed equally to this study.
Data are available on request from the corresponding author, Neetu Gupta (guptan@ccf.org).
The full-text version of this article contains a data supplement.