Key Points
Venetoclax can be added to lenalidomide and rituximab without the occurrence of any dose-limiting toxicity in patients with untreated MCL.
Venetoclax and R2 used as a frontline regimen induced high rates of clinical and molecular responses in patients with untreated MCL.
Abstract
Mantle cell lymphoma (MCL) is a rare, incurable hematological malignancy with a heterogeneous presentation and clinical course. A wide variety of chemotherapy-based regimens are currently used in patients who are untreated. Over the last several years, several targeted or small-molecule therapies have shown efficacy in the relapsed/refractory setting and have since been explored in the frontline setting. Lenalidomide plus rituximab was explored in a phase 2 study of 38 patients with MCL who were untreated and ineligible to receive transplantation, in which the combination produced durable remissions. We looked to build upon this regimen by adding venetoclax to the combination. We conducted a multicenter, open-label, nonrandomized, single-arm study to evaluate this combination. We enrolled 28 unselected patients with untreated disease irrespective of age, fitness, or risk factors. Lenalidomide was dosed at 20 mg daily from days 1 to 21 of each 28-day cycle. The dose of venetoclax was determined using the time-to-event continual reassessment method. Rituximab was dosed at 375 mg/m2 weekly, starting on cycle 1, day 1 until cycle 2, day 1. No dose-limiting toxicities were noted. All patients were treated with venetoclax at the maximum tolerated dose of 400 mg daily. The most common adverse events were neutropenia and thrombocytopenia. The overall and complete response rates were 96% and 86%, respectively. In total, 86% of patients achieved minimal residual disease undetectability via next-generation sequencing. The median overall and progression-free survivals were not reached. The combination of lenalidomide, rituximab, and venetoclax is a safe and effective regimen in patients with untreated MCL. This trial was registered at www.clinicaltrials.gov as #NCT03523975.
Introduction
Mantle cell lymphoma (MCL) is a rare incurable hematological malignancy that compromises ∼5% to 6% of patients diagnosed with non-Hodgkin lymphoma.1-4 The disease is heterogeneous in terms of both presentation and clinical course.1,5-8 With improvements in supportive measures and treatment, the average life expectancy of people diagnosed with MCL has improved from between 3 and 5 years to >10 years in subsets of patients. To date, there is not a true induction standard of care for MCL, but several treatment courses are used in patients based on their age and/or fitness.9-16 Over the last several years, various targeted or small-molecule agents have shown efficacy in the relapsed/refractory (R/R) setting.17-23 Given the efficacy and tolerability of these medications several have been explored in the frontline setting. This has led the field to question the importance of cytotoxic chemotherapy during induction and consolidation of responses with autologous stem cell transplantation in young patients.
Lenalidomide is an oral immunomodulatory agent that was approved for patients with R/R MCL after 2 lines of therapy including bortezomib at a dose of 25 mg from days 1 to 21, every 28 days. It is effective as a single agent and in combination with rituximab.24-26 The combination was explored in a phase 2 study of 38 patients with MCL who had not received treatment and were ineligible for transplantation. In the study by Ruan et al., lenalidomide was administered at a dose of 20 mg from days 1 to 21, every 28 days; rituximab was given on weeks 1, 2, 3, 4, 13, 21, 29, 37, and 45 for a total of 9 doses. The study demonstrated an overall response rate (ORR) of 92% with a complete remission (CR) of 67%.25 A recent update indicated that the responses to this regimen were durable with a 5-year progression-free survival (PFS) and overall survival (OS) of 64% and 77%, respectively.24
BCL-2 is a BH3 family protein that is dysregulated in many cancers including MCL. Venetoclax is an oral small-molecule BH3 family inhibitor that is highly selective for the bcl-2 protein and binds far less avidly to the other BH3 antiapoptotic proteins.27 Venetoclax is efficacious in R/R MCL,28-30 and synergistic with other targeted agents and drugs including lenalidomide.31-33
Given the preclinical synergy previously noted,34 we sought to explore whether we could improve upon the original results of the R2 regimen in untreated MCL by combining the regimen with venetoclax. Because this combination had not previously been explored, we designed a phase 1 dose de-escalation study using the time-to-event continual reassessment method (TITE-CRM)35,36 to estimate the maximum tolerated dose (MTD) of venetoclax in combination with R2 in patients with untreated MCL (NCT03523975). Because we hypothesized that the combination would be safe at a standard dose of venetoclax (400 mg), we secondarily designed the study to evaluate the efficacy of the regimen through several secondary objectives using the Lugano criteria.37 Given the limitations of imaging to gauge response in lymphoma and data suggesting the benefits of minimal residual disease (MRD) testing, we planned to evaluate this in conjunction with radiographic imaging in the peripheral blood (PB) based on the data suggesting that clinical response correlated better with MRD detected in the PB than in the bone marrow.13
Patients and methods
Eligible patients included those who met the following criteria: age ≥18 years, diagnosed with MCL (defined as the presence of cyclin D1 expression and/or t[11;14] via fluorescence in-situ hybridization, unless the disease was morphologically consistent with MCL), had an immunohistochemistry expression of SOX11, and were untreated with any systemic regimen. Patients should have had symptoms attributable to MCL, an Eastern Cooperative Oncology Group (ECOG) performance status of ≤2, and adequate organ function. Please see supplemental Data for the full inclusion/exclusion criteria.
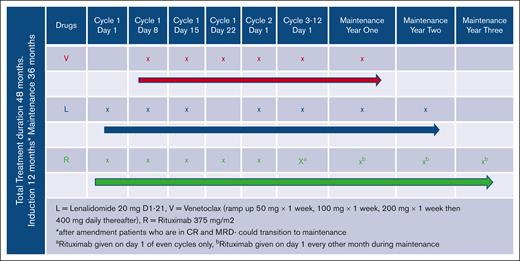
We conducted a multicenter, open-label, nonrandomized, single-arm study. The planned enrollment for the phase 1 study was 28 patients who were evaluable. All patients started induction therapy with lenalidomide 20 mg orally daily from days 1 to 21 of each 28-day cycle, with 7 days off (days 22-28). Patients were required to take aspirin 81 mg daily, unless they were already on anticoagulation for another reason. The study dose-limiting toxicity (DLT) period started with initiation of venetoclax, of which 5 dose levels were planned. The first patient was enrolled to dose level 5 (venetoclax 400 mg), with plans to dose de-escalate subsequent patients based on the frequency/occurrence of DLTs toward estimating the MTD, using the TITE-CRM model to determine the nature of de-escalation. However, regardless of dose assignment, venetoclax was started on day 8 of cycle 1 at a dose of 50 mg. Those deemed to be at a high risk for Tumor lysis syndrome (TLS) were admitted for 24 hours of observation after dosing in the outpatient setting. Venetoclax was subsequently escalated weekly (every 7 days) until a dose of 400 mg was achieved or the patient suffered a DLT. Escalation was as follows: 50 mg daily, starting on cycle 1, day 8; 100 mg daily, cycle 1, day 15; 200 mg, cycle 1, day 22; and 400 mg, cycle 2, day 1), rituximab (R) was dosed at 375 mg/m2 and given weekly starting on cycle 1, day 1 until cycle 2, day 1; this is illustrated in Figure 1. For patients at high risk/concern for infusion related reaction, rituximab was allowed to be delayed until day 15 of cycle 1. It was subsequently given every other cycle after the administration on cycle 2, day 1. It was originally planned that patients were to complete 12 induction cycles, but an amendment was approved that allowed patients to transition to maintenance after cycle 6 if they were found to be in CR in conjunction with an undetectable MRD. This was implemented because of the high frequency of patients obtaining a complete metabolic remission and undetectable MRD test at the cycle 6 response assessment. Several changes to medications occurred during maintenance. The dose of lenalidomide was reduced to 10 mg or half of the final dose administered during induction. This was continued again for 21 days with 7 days off for 24 months during maintenance. Venetoclax was given at 400 mg, or the final dose administered in induction for 12 months, and rituximab was given every other month for 36 months during maintenance. We planned for 36 months of R maintenance as standardly given after autologous stem cell transplantation (ASCT) and has demonstrated an improvement in progression-free survival (PFS), although the study was designed to forgo consolidation with ASCT in eligible patients.
Clinical end points/statistical design
The study’s coprimary objectives were to estimate the MTD among 5 dose levels of venetoclax in combination with lenalidomide (15 mg [levels 1 and 2] or 20 mg [levels 3, 4, and 5]) and rituximab (375 mg/m2 all dose levels) and to estimate the PFS. The coprimary end points included the occurrence of any DLTs, as determined using CTCAE version 4.03, and PFS. Because of the “ramp-up” period of venetoclax, which requires that a patient’s dose be titrated up from 50 mg to their assigned dose level (50 mg [levels 1 and 2], 100 mg [level 3], 200 mg [level 4], or 400 mg [level 5]), we defined the DLT window to be 42 days (6 weeks), which allows for up to 3 weeks of titration plus at least 2 additional weeks at the assigned dose level. We used the statistical model–based TITE-CRM35,36 design for the phase 1 study. Please see supplemental Data for further details on the statistical model. In brief, the design was such that each patient was assigned to the dose level for which the estimated probability of DLT was closest to, but not exceeding, 0.30, given the all-available dose toxicity data up to that point. The secondary end points included the ORR, time to best response, duration of response, MRD status by the end of induction, and adverse events (AEs) per CTCAE version 4.03. As part of exploratory end points, the relationship between potential biomarkers and clinical efficacy was also evaluated.
Assessments
All patients treated were monitored for AEs using CTCAE version 4.03. DLTs were defined as AEs that were treatment emergent and occurred since day 8 of cycle 1 (initiation of venetoclax) through cycle 2 day 28 (42 days). Response was assessed per the Lugano criteria.37 With the use of [18F] fluorodeoxyglucose positron emission tomography and computed tomography (CT) at baseline and, subsequently, after cycle 3, 6, 9, and 12 during induction. To confirm CR, a bone marrow biopsy was obtained at the conclusion of induction in all patients noted to be in remission who had marrow involvement detected during screening. CT scans alone were obtained every 6 months during maintenance. MRD testing using the clonoSEQ assay by Adaptive Technologies was performed in conjunction with the planned imaging studies. A baseline sample was obtained for patients with a suitable clone. Testing was performed after cycles 6 and 12 during induction, and thereafter yearly during maintenance. After the first 3 patients were enrolled, the study was amended to change the acquisition of MRD testing to after cycle 3, 6, 9, and 12 during induction. In select patients (enrolled at the University of Michigan only) we concurrently monitored the MRD using multicolor flow cytometry at the same time points as those in the clonoSEQ assay. BH3 profiling at baseline and during treatment was performed using a BH3 profiling functional assay. See supplemental Data for complete details regarding performance of the assay. Tissue for the BH3 profiling was obtained from a core needle biopsy taken during screening and an optional biopsy taken after cycle 1, day 15 in all patients who gave consent.
Trial conduct
The study was conducted at the University of Michigan in Ann Arbor, MI; City of Hope in Duarte, CA and the Ohio State University in Columbus, OH. Each institution’s institutional review board approved the protocol, and the study was conducted in accordance with general best practices and the Declaration of Helsinki. All participants were provided with a copy of a signed written informed consent form. The study was monitored and organized through the University of Michigan Clinical Trials Office and was governed by the US Food and Drug Administration through Investigational New Drug (IND) number 139035 (T.J.P.is a holder). The ata cutoff date was 4 June 2022.
Results
From December 2018 to September 2021, we enrolled 29 patients, of whom 28 were evaluable; 1 patient was nonevaluable after developing a pulmonary embolus before the start of the DLT period. Out of the 28 patients who were evaluable, 22 were still in active follow-up as on the last data cutoff date. Patient baseline characteristics are listed in Table 1. Per protocol, patients were required to have an indication for treatment to enroll. Based on a reverse Kaplan-Meier (KM)38 estimate of the potential follow-up, the median patient follow-up was 27.5 months. There were no DLTs noted during the study; as such, all patients were assigned and successfully escalated to the largest dose of venetoclax (400 mg daily), which was also the estimated MTD. Because of a white blood cell count of >500 000 at enrollment, 1 patient was not treated per study protocol. This patient was started on venetoclax at 20 mg instead of 50 mg venetoclax, per the discretion of the treating investigator. The patient’s dose was successfully escalated to 400 mg, but there was a longer dose escalation (1 extra week) and DLT period (49 days) because of this protocol deviation. AEs were noted in all 28 (100%) patients enrolled on study. All documented AEs are listed in Table 2. The most common AEs were neutropenia, thrombocytopenia, diarrhea, anemia, and fatigue. The most common grade ≥3 AEs were neutropenia, thrombocytopenia, anemia, lymphopenia, and leukopenia. All patients completed the planned number of cycles of induction therapy. During the study treatment holds were noted in 23 (82%) patients. The dose of lenalidomide only was reduced in 8 (38%) patients, the dose of venetoclax only was reduced in 5 (18%) patients. In 11 (39%) patients, both medications were reduced while on study. All dose reductions occurred after cycle 6 of induction. The most common reasons for dose reduction of venetoclax were thrombocytopenia and malaise. The most common reasons for dose reduction for lenalidomide were diarrhea and thrombocytopenia. Venetoclax was discontinued before planned cessation in the absence of progressive disease (PD) in 7 (25%) patients, the most common reasons being prolonged thrombocytopenia and malaise. Lenalidomide was discontinued in 6 (21%) patients because of AEs, the most common being malaise. All the discontinuations were noted after the DLT period.
Patient characteristics
| Characteristics . | Patients (N = 28) (%) . |
|---|---|
| Sex, n (%) | |
| Male | 18 (64) |
| Female | 10 (36) |
| Ethnicity | |
| Caucasian | 28 (100) |
| Age (y) | |
| Median | 65 (IQR, 57-69 y) |
| Range | 46-72 |
| ECOG performance status score (%) | |
| 0-1 | 22 |
| >1 | 6 |
| Ann Arbor stage III or IV, n (%) | 28 (100) |
| Bone marrow involvement | |
| Yes | 26 |
| No | 2 |
| MIPI score, n (%) category | TB |
| Low | 0 (0) |
| Intermediate | 10 (6) |
| High | 18 (88) |
| Variant, n (%) | |
| Blastoid/blastic | 4 (14) |
| Pleomorphic | 2 (7) |
| Ki-67 | |
| <30% | 8 (1 not reported at City of Hope) |
| 30% | 19 |
| p53 status | |
| Deleted | 2 (1 had concurrent mutation) |
| Mutated | 5∗ |
| Cytogenetics at diagnosis | |
| 19 | Normal |
| 8 | Abnormal |
| 1 | Unknown |
| Unfit or ineligible for high-dose chemotherapy | 3 patients 2 patients for age/fitness 1 patient concurrent medical condition |
| Characteristics . | Patients (N = 28) (%) . |
|---|---|
| Sex, n (%) | |
| Male | 18 (64) |
| Female | 10 (36) |
| Ethnicity | |
| Caucasian | 28 (100) |
| Age (y) | |
| Median | 65 (IQR, 57-69 y) |
| Range | 46-72 |
| ECOG performance status score (%) | |
| 0-1 | 22 |
| >1 | 6 |
| Ann Arbor stage III or IV, n (%) | 28 (100) |
| Bone marrow involvement | |
| Yes | 26 |
| No | 2 |
| MIPI score, n (%) category | TB |
| Low | 0 (0) |
| Intermediate | 10 (6) |
| High | 18 (88) |
| Variant, n (%) | |
| Blastoid/blastic | 4 (14) |
| Pleomorphic | 2 (7) |
| Ki-67 | |
| <30% | 8 (1 not reported at City of Hope) |
| 30% | 19 |
| p53 status | |
| Deleted | 2 (1 had concurrent mutation) |
| Mutated | 5∗ |
| Cytogenetics at diagnosis | |
| 19 | Normal |
| 8 | Abnormal |
| 1 | Unknown |
| Unfit or ineligible for high-dose chemotherapy | 3 patients 2 patients for age/fitness 1 patient concurrent medical condition |
COH; IQR, interquartile range; MIPI, Mantle Cell Lymphoma International Prognostic Index.
AEs: the percentage and number (out of 28 evaluable patients) of patients with specified AEs (any grade and grades greater than 3 only) limited to possible or greater attribution
| AE . | Any grade, % (n) . | Grade ≥3, % (n) . |
|---|---|---|
| Neutrophil count decreased | 85.7 (24) | 75 (21) |
| Platelet count decreased | 60.7 (17) | 60.7 (17) |
| Anemia | 50 (14) | 32.1 (9) |
| Febrile neutropenia | 14.3 (4) | 14.3 (4) |
| Tumor lysis syndrome | 14.3 (4) | 14.3 (4) |
| Hypokalemia | 28.6 (8) | 10.7 (3) |
| White blood cell count decreased | 21.4 (6) | 10.7 (3) |
| Lymphocyte count decreased | 14.3 (4) | 10.7 (3) |
| Diarrhea | 75 (21) | 7.1 (2) |
| Fatigue | 71.4 (20) | 7.1 (2) |
| Upper respiratory infection | 25 (7) | 3.6 (1) |
| Dysgeusia | 42.9 (12) | 0 (0) |
| Nausea | 42.9 (12) | 0 (0) |
| Headache | 39.3 (11) | 0 (0) |
| Bruising | 28.6 (8) | 0 (0) |
| Constipation | 28.6 (8) | 0 (0) |
| Pruritus | 28.6 (8) | 0 (0) |
| Abdominal pain | 25 (7) | 0 (0) |
| Maculo-papular rash | 25 (7) | 0 (0) |
| AE . | Any grade, % (n) . | Grade ≥3, % (n) . |
|---|---|---|
| Neutrophil count decreased | 85.7 (24) | 75 (21) |
| Platelet count decreased | 60.7 (17) | 60.7 (17) |
| Anemia | 50 (14) | 32.1 (9) |
| Febrile neutropenia | 14.3 (4) | 14.3 (4) |
| Tumor lysis syndrome | 14.3 (4) | 14.3 (4) |
| Hypokalemia | 28.6 (8) | 10.7 (3) |
| White blood cell count decreased | 21.4 (6) | 10.7 (3) |
| Lymphocyte count decreased | 14.3 (4) | 10.7 (3) |
| Diarrhea | 75 (21) | 7.1 (2) |
| Fatigue | 71.4 (20) | 7.1 (2) |
| Upper respiratory infection | 25 (7) | 3.6 (1) |
| Dysgeusia | 42.9 (12) | 0 (0) |
| Nausea | 42.9 (12) | 0 (0) |
| Headache | 39.3 (11) | 0 (0) |
| Bruising | 28.6 (8) | 0 (0) |
| Constipation | 28.6 (8) | 0 (0) |
| Pruritus | 28.6 (8) | 0 (0) |
| Abdominal pain | 25 (7) | 0 (0) |
| Maculo-papular rash | 25 (7) | 0 (0) |
Only AEs with an overall incidence of 25% and/or with a grade ≥3 incidence of 10% are shown.
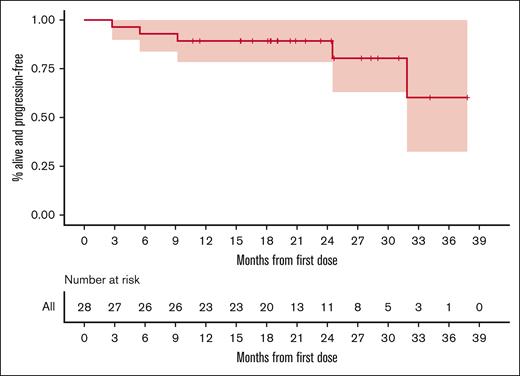
A total of 24 (86%) of the 28 patients who were evaluable completed induction therapy. Of those who failed to complete induction, 3 (11%) had PD or radiographic relapse and 1 (3%) had a biopsy-proven metastatic recurrence of nonlymphomatous cancer. The ORR of all patients who were evaluable was 96% with a CR rate of 86%. The median duration of response among patients (defined as the longest time elapsed during which partial or complete responses were recorded in consecutive assessments) was 17.8months. However, this is likely a significant underestimation of the true median duration of response, given that all but 4 patients were still considered responders at the date of data cutoff. Indeed, using the method of reverse KM38 to estimate the time to loss of response, censoring the data of those who were still considered responders at the time of their last assessment, the median duration of response was not reached. The first quartile for duration of response was 21.5 months, meaning that the estimated median duration of response was >21.5 months. All patients in CR, as stated previously, had a confirmatory bone marrow biopsy if the involvement was noted at screening. MRD testing was obtained for all patients who were evaluable. At the time of publication, 19 patients remain on study. Using KM methodology, the median PFS was not reached, (the lower bound of 95% confidence interval [CI] was 31.8 months). The estimated 2-year PFS probability was 0.89 (95% CI, 0.785-1). Figure 2 gives the entire KM-estimated distribution of PFS.
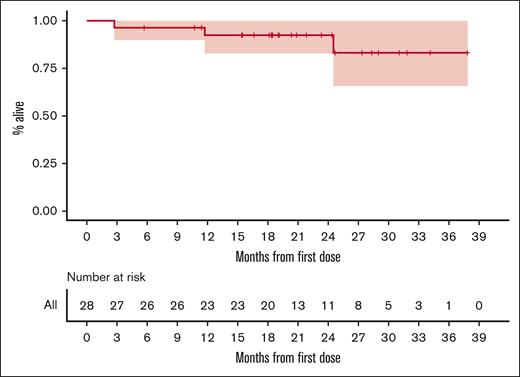
The median OS was also not reached, nor was a lower bound for the 95% CI. The estimated 2-year OS probability was 0.924 (95% CI, 0.828-1). Figure 3 gives the entire KM-estimated distribution of OS.
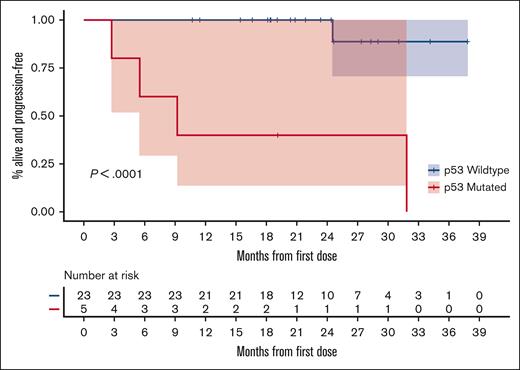
When separating patients based on the p53 mutation status, the PFS was significantly longer among those without a p53 mutation than among those with this mutation, as illustrated in Figure 4. The ORR of those without a p53 mutation was 100% with a CR of 91%. For those with a known p53 mutation, the ORR was 80% with a CR of 60%.
KM estimates of PFS based on the presence or absence of p53 mutation.
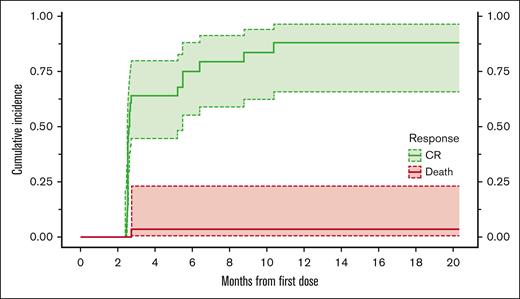
We also calculated the distribution of time to complete response. Because death is considered a competing event, we calculated the Aalen-Johansen–based estimator of the cumulative incidence for complete response.38 At 6 months, the estimated cumulative incidence of patients obtaining a complete metabolic response was 0.75 (95% CI, 0.552-0.88), as illustrated in Figure 5.
Aalen-Johansen estimates of cumulative incidence of the time to CR or death.
At the date of data cutoff, 9 patients had discontinued the study drug; 4 of whom had done so because of disease progression, and these 4 patients were noted to have a p53 mutation. A total of 4 other patients discontinued the study therapy for other medical reasons, including recurrence of melanoma, adenocarcinoma of unknown primary, and myelodysplastic syndrome (1 patient later found to have a germ line p53 mutation, and 1 patient had an extensive history of radiation for squamous cell carcinoma); two of the 4 patients died during the study because of a secondary cancer; 1 patient stopped therapy after completing induction because of treatment intolerance.
All patients who were evaluable were noted to have a detectable clone at baseline for MRD monitoring. Twenty-four (86%) patients were noted to have an undetectable MRD test using next-generation sequencing during study participation; 57% of patients obtained an undetectable test by month 3, this number increased to 75% by month 6. MRD analysis with flow cytometry noted a high concordance with the results that we obtained using next-generation sequencing. To date, only 1 of 21 patients who obtained an MRD undetectable test has had a clinical relapse. This was a patient with a known p53 mutation. At the time of relapse, the patient was found to have a new lesion when an unscheduled CT was performed. An unscheduled MRD test was also performed at that time, which noted detectable clones in the PB. Upon MRD testing, 1 patient who had a CR to therapy was found to have a new clone. This patient subsequently relapsed within 3 months of this evaluation. On repeat MRD testing, the patient was noted to have increased copies of all noted clones, with the new clone detected at month 3 becoming the predominant clone. Two patients had radiographic assessments suggestive of PD, with undetectable MRD test results. Both of these patients had resolution of FDG avidity on follow-up scans supporting continued remission; both remain on study. This provides additional support for MRD testing as a compliment to radiographic imaging.
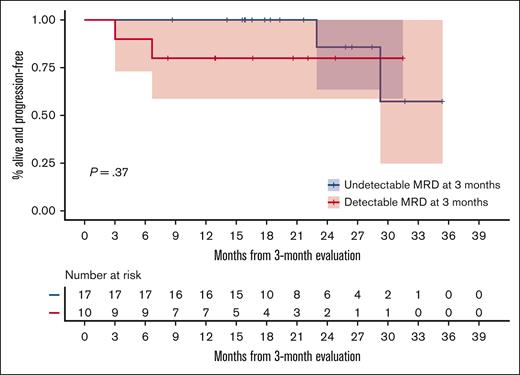
We estimated the conditional PFS distribution starting at 3 months since the first dose for the 27 patients who were alive and free of progression, stratified based on the MRD status measured at 3 months. The median conditional PFS was not reached in either MRD group. The estimated 2-year conditional PFS probabilities were .857 (95% CI, 0.633-1) and .8 (95% CI, 0.587-1) for the MRD− and MRD+ groups, respectively. Figure 6 gives the entire KM-estimated conditional distribution of PFS.
PFS based on MRD status. KM estimates of conditional PFS among patients who were alive and free of progression at 3 months since the first dose, stratified based on the MRD status at 3 months.
PFS based on MRD status. KM estimates of conditional PFS among patients who were alive and free of progression at 3 months since the first dose, stratified based on the MRD status at 3 months.
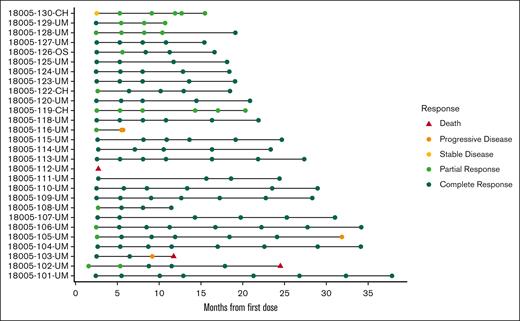
The responses for all enrolled patients are illustrated in Figure 7.
Swimmers’ plot giving the timing and nature of each patient’s response assessment.
Swimmers’ plot giving the timing and nature of each patient’s response assessment.
We were able to obtain samples from 17 patients for BH3 profiling, as described in the supplemental Data. Because of tissue availability and cell viability after processing, the baseline mitochondrial profiling was determined on 15 samples, and we were only able to obtain 7 paired samples. Most of the baseline samples showed initial significant dependency on BCL-2, as evidenced via the high mitochondrial depolarization when samples were exposed to the BH3 peptide Bad (supplemental Figure). A few baseline samples suggested a possible codependency with MCL-1, as evidenced via the depolarization induced by the BH3 peptide MS1, but we did not notice any clinical difference in outcomes compared with outcomes in those noted only to be dependent on BCL-2. With regard to the on-treatment BH3 functional profiling, most samples did not show significant changes in their dependency on Bcl-2, whereas some samples indicated an increasing dependency on MCL-1, without any noted clinical significance (Figure 7). In 1 patient BH3 profiling correlated with clinical response to venetoclax. The patient had little to no mitochondrial depolarization induced by Bad at baseline. We noted a higher sensitivity to apoptotic priming by BH3 peptides in the day-15 sample compared with the baseline sample, but this was for MS1.
Discussion
Given the noted efficacy of the R2 regimen, together with the durability reported with long-term follow-up, we sought to evaluate whether the addition of the BCL-2 inhibitor, venetoclax, would be safe, and, secondarily, whether the agent could improve outcomes sufficiently to allow use of the regimen in an unselected patient population. From this study, we were able to successfully combine R2 with venetoclax without the occurrence of any DLTs. Overall, the regimen was tolerable, with no responding patients stopping therapy during induction. As expected, we did notice high rates of cytopenias, including neutropenia and thrombocytopenia. Despite the high frequency of cytopenias, we did not notice high rates of neutropenic fever, infections, or bleeding events. Although the neutropenia was anticipated, we did notice higher than expected rates of thrombocytopenia, highlighting the issues that arise at times when combining medications, irrespective of any clinical synergistic activity. We did work to limit the exposure to these agents when we designed the protocol. The study was designed to give patients 1 year of induction with all 3 drugs, followed by a maintenance treatment equivalent in duration to the standard postautologous stem cell transplantation maintenance. During the maintenance phase, the dose of lenalidomide was reduced, but, more importantly, the oral agents were given for a finite period, with all responding patients completely discontinuing the treatment by the end of the third year. Moreover, we made changes to the length of induction based on the MRD results after noting some late toxicities. Our frequent testing for MRD during induction allowed for the modification of the protocol to allow patients to transition to maintenance sooner than originally planned. Because we noted that a large portion of the patients were in a complete metabolic remission with an undetectable MRD result, we felt comfortable amending the protocol to allow those patients to move on to maintenance. This allowed us to maximize the response to the agents during induction while reducing any cumulative toxicity that could come from the combination. With respect to the treatment response, our study allowed for the enrollment of patients who are at higher risk compared with those in the original R2 study. Patients with blastoid/pleomorphic histologies were included, and most of the enrolled patients had a Ki-67 > 30% and were classified as being at high risk based on the Mantle Cell Lymphoma International Prognostic Index-C (MIPI-C) score. Despite this, we observed that the combination of drugs was highly effective in this patient population. We noted a higher ORR and complete response rate (96% vs 92% and 86% vs 64%, respectively) compared with those in the doublet treatment.24,25 Our study is also one of the few to have consistent MRD monitoring despite participation of multiple institutions, reaffirming that MRD monitoring is feasible at a large scale. With respect to MRD testing, we documented a high rate (86%) of MRD undetectability in the PB in all our patients, compared with 80% in the original study. An important caveat is that we noted long-term undetectable rates in 24 of 28 enrolled patients, whereas in the doublet study, the 80% undetectable rate was among only 10 of the 38 enrolled patients. Furthermore, we noted an improvement in time to best response with the addition of venetoclax. We noted that 75% of enrolled patients had obtained a CR by 6 months. The median time to CR was noted to be 11 months in the doublet therapy. To date, we have not noted disease progression in any patient with wild-type p53 at diagnosis. Of the 5 patients noted to have p53 mutation at enrollment, 4 have discontinued the study because of either lack of response or disease relapse. The longest remission in this patient group was 21.5 months; the only patient with a p53 mutation who remains on the study currently has a treatment duration of only 16.6 months. The study is not sufficiently powered to evaluate a difference between those with and without a p53 mutation but this regimen is unlikely to overcome the poor prognostic outcomes associated with p53 mutations based on results noted in ∼20% of enrolled patients noted to harbor this alteration.
Our study has several limitations because of the study design. This was not a randomized controlled study, and, as such, we are not able to completely assess the true benefits of adding venetoclax to R2. The current study is currently too immature to estimate long-term efficacy of the regimen or finite maintenance therapy in a regimen devoid of cytotoxic chemotherapy. Given the incurable nature of MCL, this is needed to determine whether the addition of venetoclax to R2 is truly beneficial and whether it is possible to achieve a prolonged disease-free interval without constant exposure to targeted agents in those who do not receive cytoreductive chemotherapy. Such a study is especially necessary, given the long-term PFS reported with R2 alone in this patient population. We also sought to fully evaluate the benefit of BH3 profiling as a predictive test for using BH3 mimetics. Because of the onset of the COVID-19 pandemic, we did not obtain on-treatment biopsies from 2 patients and were unable to perform BH3 profiling on the last 11 enrolled patients because of research laboratory closure and suspension of elective procedures.
In conclusion, the regimen of R2 + venetoclax was demonstrated to be safe and effective in patients with untreated MCL. We have, to date, noted continued undetectable MRD results in all patients with wild-type p53 who have achieved this benchmark despite constant removal of drugs during maintenance. This has subsequently translated into durable clinical remissions for those patients. At the time of publication, 19 patients remain on study, with 20 patients still in remission. Although longer follow-up is needed, the results obtained thus far in those without p53 mutations suggest that durable responses can be achieved without the addition of cytotoxic agents in patients with MCL, irrespective of age or fitness. Given the encouraging data from this study, we have planned to expand the study to better correlate response to this regimen with other currently used modern regimens.
Acknowledgments
The funding and supply of venetoclax for the study was provided by AbbVie; lenalidomide was provided by BMS. Additional funding was provided by the University of Michigan Rogel Cancer Center.
Authorship
Contribution: T.J.P. and P.B. designed research, performed research, analyzed data, and wrote the paper; K.K., M.K., T.L.M., and V.N. performed research; and D.B., R.T., Z.N.-C., R.A.W., S.A.C., Y.H.K., M.S.K., K.M., A.F.H., L.P., and A.V.D. performed research and edited the paper.
Conflict-of-interest disclosure: T.J.P. provides consulting for AbbVie, ADC Therapeutics, AstraZeneca, BMS, Bayer, BeiGene, Eli Lilly, Epizyme, Genentech, Genmab, Gilead, Incyte, Pharmacyclics, Regeneron, Seattle Genetics, and Xencor; is a member of a scientific committee for Epizyme, Genentech, Genmab, and Merck; conducts research for Bayer, BMS, and AbbVie. D.B. provides consulting for Kite/Gilead, Seagen, and Nurix Therapeutics; and conducts research for Novartis, and Nurix Therapeutics. A.F.H. provides consulting for Bristol Myers Squibb, Genentech, Merck, Seattle Genetics, AstraZeneca, Karyopharm, ADC Therapeutics, Takeda, Tubulis, Regeneron, Genmab, Pfizer, Caribou, Adicet Bio, and AbbVie; and conducts research for Bristol Myers Squibb, Genentech, Merck, Seattle Genetics, KiTe Pharma, Gilead Science, AstraZeneca, and ADC Therapeutics. L.P. provides consulting for Seattle Genetics, Pfizer, Roche, and Novartis. A.V.D. conducts research for Leukemia & Lymphoma Society, National Cancer Institute, AbbVie, AstraZeneca, Bayer Oncology, Bristol Meyers Squibb, Cyclacel, MEI Pharma, Nurix, TG Therapeutics, and Takeda Oncology; provides consulting for AbbVie, AstraZeneca, Bayer Oncology, BeiGene, Bristol Meyers Squibb, Genentech, GenMab, Incyte, Lilly Oncology, Nurix, Oncovalent, Pharmacyclics, and TG Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Tycel J. Phillips, City of Hope National Medical Center, 1500 East Duarte Rd, Duarte, CA 91010; e-mail: tphillips@coh.org.
References
Author notes
Data are available on request from the corresponding author, Tycel J. Phillips (tphillips@coh.org).
Individual participant data will not be shared. The study protocol is included as a data supplement available with the online version of this article.
The full-text version of this article contains a data supplement.