Key Points
Allelic variation of KIR and HLA determines functional hierarchy of NK cell repertoires
Fine-tuned trimming of the granzyme B loading in NK cells serves as a metric of the functional state of NK cells
Abstract
The functionality of natural killer (NK) cells is tuned during education and is associated with remodeling of the lysosomal compartment. We hypothesized that genetic variation in killer cell immunoglobulin-like receptor (KIR) and HLA, which is known to influence the functional strength of NK cells, fine-tunes the payload of effector molecules stored in secretory lysosomes. To address this possibility, we performed a high-resolution analysis of KIR and HLA class I genes in 365 blood donors and linked genotypes to granzyme B loading and functional phenotypes. We found that granzyme B levels varied across individuals but were stable over time in each individual and genetically determined by allelic variation in HLA class I genes. A broad mapping of surface receptors and lysosomal effector molecules revealed that DNAM-1 and granzyme B levels served as robust metric of the functional state in NK cells. Variation in granzyme B levels at rest was tightly linked to the lytic hit and downstream killing of major histocompatibility complex–deficient target cells. Together, these data provide insights into how variation in genetically hardwired receptor pairs tunes the releasable granzyme B pool in NK cells, resulting in predictable hierarchies in global NK cell function.
Introduction
NK cells are cytotoxic mediators of immunity, able to distinguish healthy cells from infected and transformed cells through their varied repertoire of germ line–encoded activating and inhibitory receptors. Among these receptors are the killer cell immunoglobulin-like receptors (KIRs) that bind to HLA class I molecules, divided into 3 main groups based on their amino acid composition. KIR2DL3 binds to HLA-C1 (Ser77/Asn80), KIR2DL1 binds to HLA-C2 (Asn77/Lys80), and KIR3DL1 binds to HLA-A or B that carry the Bw4 motif (amino acid position 77-83).1,2,KIR and HLA are highly polymorphic genes,3 and the differences in their aminoacid sequences influence protein folding4 as well as the KIR-HLA strength of the interaction.5-9 Another important family of inhibitory receptors are the lectin-like CD94/NKG2A receptors, which recognize and bind HLA-E.10 The surface expression of HLA-E depends on the loading of peptides from the leader sequence of other HLA class I molecules, such as HLA-A or C, and specific allotypes of HLA-B.11,12
The interaction of KIR and NKG2A with their cognate ligands calibrates the intrinsic functional competence of NK cells during a process termed education. The stronger the inhibitory input, the stronger the functional ability of the NK cell.6,13-15 Thus, although these receptors efficiently shut down NK cell effector responses, the same interactions are critical to building functional competence in the NK cell repertoire during development and homeostasis.16 It is well established that NK cell education operates as a rheostat rather than an on and off switch,17,18 but the cellular and molecular mechanisms underlying the tuning of NK cell function remain poorly understood.
Several phenotypes have been associated with educated NK cells. These phenotypes include high expression of the activation/adhesion receptor DNAM-1,19,20 low levels of SHP-1,21 structural changes at the membrane,22,23 and remodeling of the secretory lysosomes with accumulation of granzyme B.24 The latter observation puts the core cytolytic machinery of the cell in the spotlight in the search for an underlying mechanism behind NK cell education.
Here, we show that the granzyme B content in NK cells is dictated by KIR and HLA polymorphisms. A relatively subtle difference in the overall granzyme B content in NK cells resulted in dramatic changes in the releasable pool of granzyme B at the immune synapse and therefore had important consequences on the cytolytic potential of the cell. Thus, the fine-tuned trimming of the granzyme B loading in NK cells follows the rheostat model,17 and serves as a metric of the functional state of NK cells across individuals having diverse KIR-HLA genetics.
Methods
Primary cells and cell lines
Peripheral blood mononuclear cells (PBMCs) were isolated from 365 anonymous healthy blood donors (Oslo University Hospital) using a density gradient medium and centrifugation (Lymphoprep, StemCell Technology). PBMCs from 91 of these donors were frozen in 10% DMSO and 90% fetal calf serum (FCS). NK cell isolation from PBMCs was performed using negative selection beads and the AutoMACS Pro separator (Miltenyi Biotech). Approval for collecting, storing, and analyzing these samples was obtained from the Regional Committees for Medical and Health Research Ethics (2018/2485 and 2018/2482). The study was performed in accordance with the Declaration of Helsinki. PBMCs from anonymous healthy blood donors containing detectable NKG2C+ adaptive NK cell populations were collected and cryopreserved at Karolinska Institutet, Sweden. The approval for collecting, storing, and analyzing these samples was obtained from the Swedish Ethical Review Authority, Sweden (Dnr 2020-05289). K562 cells were purchased from ATCC. All the cells were cultured in RPMI (Sigma Aldrich) supplemented with 2 mM of L-glutamine, 10% FCS (F7524, Sigma Aldrich), and 1% penicillin/streptomycin (Sigma Aldrich) and kept at 37°C, 5% CO2. Target cell lines were frequently tested for mycoplasma (Eurofins).
High-resolution KIR genotyping and HLA genotyping
Flow cytometry, antibodies, enzyme-linked immunosorbent assay, and fluorescence-activated cell sorting
Fresh or frozen PBMCs were stained with fluorochrome-conjugated antibodies in 96-well V-bottom plates. The cells were first stained for cell-surface epitopes, then fixed to be intracellularly stained. Samples were acquired on an LSRII or FACS Symphony-A5 (355 nm, 405 nm, 488 nm, 561 nm, and 640 nm lasers). Further details on flow cytometry analysis, the antibodies, and enzyme-linked immunosorbent assay are given in the supplemental Methods. NK cells were isolated from fresh or frozen PBMCs by enrichment, as all the other cell types were depleted (#130-092-657, Miltenyi, NK cell Isolation Kit) on the AutoMACS Pro separator or by manual isolation. Then, using a FACSAria II (BD) or MA900 (Sony) cell sorter, NK cells were sorted for the KIR-educated and -uneducated compartments based on KIR expression profiles.
Degranulation assays
Frozen PBMCs were thawed, counted, seeded in complete RPMI medium supplemented with 10% FCS and incubated overnight at 37°C and 5% CO2. For degranulation assays, PBMCs were coincubated with K562 (E:T 10:1) in 96-well plates for 6 hours at 37°C and 5% CO2 in the presence of CD107a antibody. After 1 hour of incubation, Brefeldin A (golgiPlug, #555029, BD Biosciences) 1:1000 and monensin (golgiStop; #554724, BD Biosciences) 1:1500 were added to the plate. After incubation, the cells were surface-stained, fixed (#554724, BD Cytofix/Cytoperm), and intracellularly stained for granzyme B-AF700.
Flow cytometry–based killing assay and detection of granzyme B activity
Sorted KIR-educated and -uneducated NK cells were rested overnight in RPMI supplemented with 10% FCS before being CellTrace Violet stained, according to the manufacturer’s instructions (#C34557, ThermoFisher). Then, the cells were coincubated with K562 (E:T 2:1) for 1 hour at 37°C and 5% CO2, in 50 μL of a cell permeable substrate of granzyme B (#GTL702-8, GranToxiLux Plus, OncoImmune). Then, the cells were washed and surface stained with LIVE/DEAD Fixable Near-IR stain (#L10119, Thermo Fisher Scientific) before acquisition on the LSRII flow cytometer (BD).
Fluorescence microscopy and image analysis
K562 cells and sorted educated or uneducated NK cells, previously labeled with CellTrace Violet, were mixed at the E:T ratio of 1:1 and then seeded in GranToxiLux solution (#GTL702-8, GranToxiLux Plus, OncoImmune) on a Poly-L-Ornithine (#A-004-M, Signa-Aldrich)–coated glass bottom dish. Further details regarding the imaging, including the microwell assays, are described in the supplemental Methods.
Bubble heat map and unsupervised clustering
Bubble heat map, unsupervised clustering, and visualization were performed using R version 4.2.1. Bubble heat maps were created using ggplot2 version 3.3.5. Unsupervised clustering was done using Complex Heat Map version 2.12.0 and Tidyverse version 1.3.1 packages. Pearson correlation was used.
Quantification and statistical analysis
One-way analysis of variance tests followed by Tukey multiple comparison tests were performed to determine the statistical significance of the differences between the groups. Where indicated, one-way analysis of variance tests followed by Kruskal-Wallis multiple comparison tests were performed as some groups were not following a Gaussian distribution. P > .05 was considered nonsignificant (ns), and P ≤ .05 was respectively marked. ∗∗∗∗P < .0001; ∗∗∗P < .001; ∗∗P < .05; or ∗P < .05. All the plots and the statistical analysis were performed using GraphPad Prism.
Results
Educated NK cells have high levels of granzyme B
We performed a retrospective analysis of granzyme B expression data gathered from the analysis of PBMCs from 365 healthy donors collected over 2 years. We found that KIR-educated NK cells (NKG2A–CD57–selfKIR+) expressed, on average, 1.7-fold more granzyme B than the uneducated “Nil” (NKG2A–CD57–KIR–) (Figure 1A-D). In donors carrying HLA-C1/C2 motifs, KIR2DL3+ and KIR2DL1+ NK cell subsets expressed equal levels of granzyme B (Figure 1A-C). Corroborating the results of the granzyme B screening, we analyzed the granzyme B expression in NK cells on frozen PBMCs from 91 donors derived from the original cohort. Gating on discrete NK cell subsets (supplemental Figure 1A) revealed differences in granzyme B loading, with increasing levels of granzyme B in the more mature subsets, defined by expression of NKG2A, KIR, and CD57 (supplemental Figure 1B-C).27 Further analysis of adaptive NK cells in 8 donors with large populations of NKG2C+ adaptive NK cells revealed that this subset expresses particularly high levels of granzyme B (supplemental Figure 2A-B). Notably, nearly all adaptive NK cells express self-specific KIRs.28 Nevertheless, stratification into self- and nonself KIR in adaptive NK cells revealed increased levels in educated adaptive NK cells (supplemental Figure 2B). In these experiments, we also corroborated the previous finding that DNAM-1 is expressed at higher levels in educated NK cells (supplemental Figures 1C-D and 2B-C).19 These data documented the impact of education on the granzyme B content in NK cells and revealed substantial variation in the population.
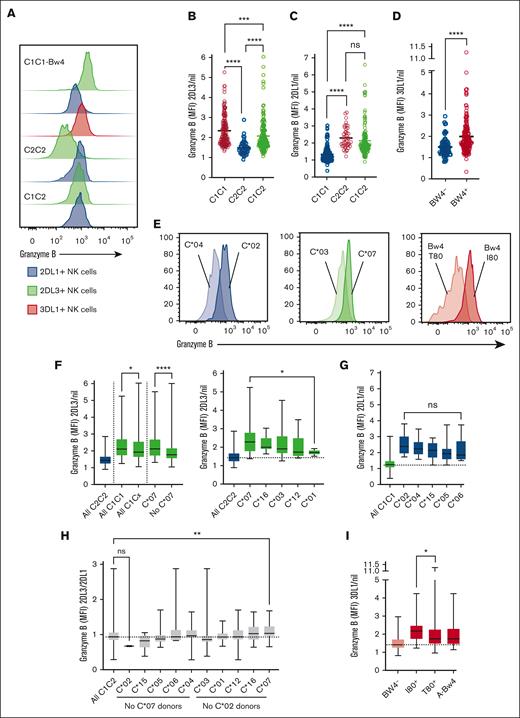
HLA class I alleles affect the granzyme B loading in NK cells. (A) Granzyme B expression in KIR2DL1+ (blue), KIR2DL3+ (green), or KIR3DL1+ (orange) NK cells from donors with different HLA-C backgrounds (one representative experiment shown). (B) Granzyme B expression in KIR2DL3+ NK cells compared with the receptor-negative (Nil) NK cells from the same donor. C1/C1 donors (n = 143), C1/C2 donors (n = 147), and C2/C2 donors (n = 43). (C) Granzyme B expression in KIR2DL1+ NK cells compared with the Nil NK cells from the same C1/C1 donors (n = 140), C1/C2 donors (n = 128), and C2/C2 donors (n = 43). (D) Granzyme B expression in KIR3DL1+ NK cells compared with the Nil NK cells from the same Bw4- donors (n = 78) and Bw4+ donors (n = 170). (E) Granzyme B expression in KIR2DL1+ (blue), KIR2DL3+ (green), or KIR3DL1+ (orange) NK cells from donors with specific HLA alleles, HLA-C∗04 or HLA-C∗02; HLA-C∗03 or HLA-C∗07; Bw4-80T or Bw4-I80, respectively. (F) Granzyme B expression in KIR2DL3+ NK cells compared with the Nil NK cells from the same donor stratified by HLA-C1 alleles. C1/C1 donors (n = 140) and C2/C2 donors (n = 43). (G) Granzyme B expression in KIR2DL1+ NK cells compared with the Nil NK cells from the same donor stratified by HLA-C2 alleles. C1/C1 donors (n = 140) and C2/C2 donors (n = 43). (H) Granzyme B expression in KIR2DL3+ NK cells compared with the KIR2DL1+ NK cells from the same donor stratified by HLA-C1 and C2 alleles. C1/C2 donors (n = 125). (I) Granzyme B expression in KIR3DL1+ NK cells compared with the Nil NK cells from the same donor stratified by Bw4 alleles. Bw4 donors (n = 179). (B-D) Self-KIR NK cells are represented in red, and the nonself KIR NK cells are represented in blue. Each dot represents 1 donor. Whiskers show the fifth to 95th percentile. Bars show the median. One-way ANOVA tests followed by Kruskal-Wallis’ multiple comparison tests were performed in panels B, C and F-I. Mann-Whitney U test was performed in panel D. ∗∗∗∗P < .0001; ∗∗∗P < .001; ∗∗P < .01; and ∗P < .05. ANOVA, analysis of variance; MFI, median fluorescence intensity.
HLA class I alleles affect the granzyme B loading in NK cells. (A) Granzyme B expression in KIR2DL1+ (blue), KIR2DL3+ (green), or KIR3DL1+ (orange) NK cells from donors with different HLA-C backgrounds (one representative experiment shown). (B) Granzyme B expression in KIR2DL3+ NK cells compared with the receptor-negative (Nil) NK cells from the same donor. C1/C1 donors (n = 143), C1/C2 donors (n = 147), and C2/C2 donors (n = 43). (C) Granzyme B expression in KIR2DL1+ NK cells compared with the Nil NK cells from the same C1/C1 donors (n = 140), C1/C2 donors (n = 128), and C2/C2 donors (n = 43). (D) Granzyme B expression in KIR3DL1+ NK cells compared with the Nil NK cells from the same Bw4- donors (n = 78) and Bw4+ donors (n = 170). (E) Granzyme B expression in KIR2DL1+ (blue), KIR2DL3+ (green), or KIR3DL1+ (orange) NK cells from donors with specific HLA alleles, HLA-C∗04 or HLA-C∗02; HLA-C∗03 or HLA-C∗07; Bw4-80T or Bw4-I80, respectively. (F) Granzyme B expression in KIR2DL3+ NK cells compared with the Nil NK cells from the same donor stratified by HLA-C1 alleles. C1/C1 donors (n = 140) and C2/C2 donors (n = 43). (G) Granzyme B expression in KIR2DL1+ NK cells compared with the Nil NK cells from the same donor stratified by HLA-C2 alleles. C1/C1 donors (n = 140) and C2/C2 donors (n = 43). (H) Granzyme B expression in KIR2DL3+ NK cells compared with the KIR2DL1+ NK cells from the same donor stratified by HLA-C1 and C2 alleles. C1/C2 donors (n = 125). (I) Granzyme B expression in KIR3DL1+ NK cells compared with the Nil NK cells from the same donor stratified by Bw4 alleles. Bw4 donors (n = 179). (B-D) Self-KIR NK cells are represented in red, and the nonself KIR NK cells are represented in blue. Each dot represents 1 donor. Whiskers show the fifth to 95th percentile. Bars show the median. One-way ANOVA tests followed by Kruskal-Wallis’ multiple comparison tests were performed in panels B, C and F-I. Mann-Whitney U test was performed in panel D. ∗∗∗∗P < .0001; ∗∗∗P < .001; ∗∗P < .01; and ∗P < .05. ANOVA, analysis of variance; MFI, median fluorescence intensity.
HLA class I polymorphism dominantly influences the granzyme B content of educated NK cells
To better understand the heterogeneity in granzyme B content observed in the population, donors were genotyped for HLA class I and KIR alleles (supplemental Figure 3A-C) and then stratified based on the HLA-C alleles (supplemental Figure 3D). Past work has identified HLA-C∗07 and HLA-C∗02 as strong educators for NK cells expressing the cognate receptors KIR2DL3 and KIR2DL1, respectively.6 We found that KIR2DL3+ NK cells from HLA-C1/C1 donors bearing at least 1 HLA-C∗07 allele had significantly higher granzyme B content compared with the other donors. KIR2DL3+ NK cells from HLA-C∗01 donors had the lowest granzyme B content, whereas those from HLA-C∗03 and HLA-C∗12 donors had an intermediate phenotype among the HLA-C1/C1 donors (Figure 1E-F). KIR2DL1+ NK cells from donors having 1 or more copies of HLA-C∗02 tended to have higher granzyme B content compared with donors with other alleles, including HLA-C∗04, HLA-C∗15, HLA-C∗05, or HLA-C∗06 (Figure 1E,G). HLA-C1/C2 donors having the strong HLA-C∗07 allele showed NK cell granzyme B phenotypes that resembled those observed in HLA-C1/C1 donors, with higher granzyme B content in NK cells expressing KIR2DL3 compared with KIR2DL1, whereas the donors having HLA-C∗02 resembled those observed in HLA-C2/C2 donors (Figure 1H). These results suggest that strong HLA-C1 or HLA-C2 ligands tilt heterozygous donors toward a KIR2DL3- or KIR2DL1-dominated granzyme B phenotype, respectively. Stratification of donors based on the presence of isoleucine or threonine at position 80 of Bw4, revealed that KIR3DL1+ NK cells in donors bearing Bw4-80I expressed significantly more granzyme B than in Bw4-80T individuals (Figure 1E,I). Donors carrying only the HLA-B∗13 or A∗25 allele as the Bw4 motif were excluded from the Bw4+ population as there is evidence that these ligands do not bind to KIR3DL1 and therefore do not educate NK cells.29 Allelic variation in KIR had a relatively small impact (supplemental Figure 3D-E). For the Bw4+ donors, higher granzyme B content was found in KIR3DL1∗00501 educated NK cells compared with the other educated alleles present in the cohort (supplemental Figure 3E). This allele has been shown to be a strong receptor for Bw4.9 Finally, we found no impact of sex or age on the granzyme B loading (supplemental Figure 4A-B). Overall, these data showed that the granzyme B content of educated NK cells is dominantly influenced by allelic variation in HLA and correlates with the reported KIR affinity and educating impact of the cognate ligands.
Granzyme B loading is genetically hardwired and stable over time
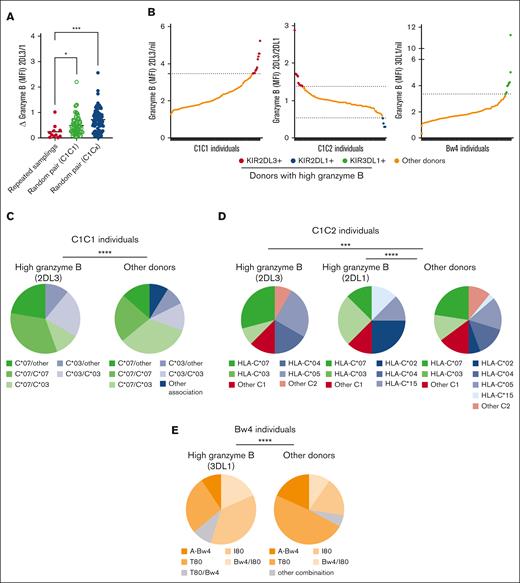
Next, we tracked the granzyme B content of NK cells in individuals sampled at multiple time points, with an average of 7.5 months between the 2 blood donations. The granzyme B loading of NK cells appeared stable in repeated samplings compared with samplings from random donor pairs (Figure 2A). To decipher the genotype-phenotype link in terms of granzyme B loading of educated NK cells, we mapped the dominant HLA-A, -B, and -C alleles in donors showing consistently high levels of granzyme B in their NK cell repertoire (statistical outliers), with all other donors serving as a reference population (Figure 2B-E). We found that the HLA-C allele distribution differed significantly, with a bias toward strong educating HLA-C alleles in KIR2DL3+ and KIR2DL1+ educated NK cells, respectively. Hence, among KIR2DL3+ granzyme Bhigh donors in HLA-C1/C1 individuals, we found a significant enrichment for HLA-C∗07 homozygosity, and all had at least 1 copy of either HLA-C∗03 or HLA-C∗07 (Figure 2C). Among HLA-C1/C2 donors, 1 fraction showed high levels of granzyme B in their KIR2DL3+ NK cell subset and the other fraction in their KIR2DL1+ NK cell subset. As could be predicted from the observation of HLA-C mediated tilting of the educated NK cell repertoire (Figure 1H), the KIR2DL3+ granzyme Bhigh donors showed a bias for HLA-C∗07 alleles, whereas the KIR2DL1+ granzyme B high donors showed a bias for HLA-C∗02 (Figure 2D). Finally, donors with exceptionally high levels of granzyme B in their KIR3DL1+ compartment showed a bias for the Bw4-I80 allele (Figure 2E). These results suggested a genetically hardwired instruction of granzyme B loading at the subset level through KIR-HLA interactions in which strong KIR ligands shaped the education profile of the donor NK cells.
Granzyme B loading is genetically hardwired and stable over time. (A) Dot plot representing the delta of granzyme B loading in either repeated samplings (sampling 1 vs sampling 2) or random pairing, calculated as the difference in granzyme B content between 2 randomly selected donors (donor pair) with the indicated genotype. (B) Granzyme B content of educated NK cells/Nil NK cells is shown for each donor and stratified based on the HLA-C background, either C1/C1, C1/C2 or Bw4. Each dot represents 1 donor, and bars show the median. Identification of donors with very high granzyme B content in their NK cells based on the interquartile range rule (outliers in red, blue or green). (C) Allelic distribution in KIR2DL3 granzyme Bhigh donors (n = 9) compared with other C1C1 donors (n = 148). (D) Allelic distribution KIR2DL3+ and KIR2DL1+ granzyme Bhigh donors (n = 12 and n = 4, respectively) among C1/C2 donors, compared with other C1/C2 donors (n = 110). (E) Allelic distribution in KIR3DL1+ granzyme Bhigh donors (n = 11) among Bw4+ donors compared with other Bw4+ donors (n = 159). One-way ANOVA tests followed by Kruskal-Wallis multiple comparison tests were performed. (D-F) The pie charts represent the frequencies of donors with specific HLA-C or Bw4 alleles in the outlier population and in the general population. The outliers are identified with the interquartile range formula. Χ tests were performed. ∗∗∗∗P < .0001; ∗∗∗P < .001; ∗∗P <.01; and ∗P < .05.
Granzyme B loading is genetically hardwired and stable over time. (A) Dot plot representing the delta of granzyme B loading in either repeated samplings (sampling 1 vs sampling 2) or random pairing, calculated as the difference in granzyme B content between 2 randomly selected donors (donor pair) with the indicated genotype. (B) Granzyme B content of educated NK cells/Nil NK cells is shown for each donor and stratified based on the HLA-C background, either C1/C1, C1/C2 or Bw4. Each dot represents 1 donor, and bars show the median. Identification of donors with very high granzyme B content in their NK cells based on the interquartile range rule (outliers in red, blue or green). (C) Allelic distribution in KIR2DL3 granzyme Bhigh donors (n = 9) compared with other C1C1 donors (n = 148). (D) Allelic distribution KIR2DL3+ and KIR2DL1+ granzyme Bhigh donors (n = 12 and n = 4, respectively) among C1/C2 donors, compared with other C1/C2 donors (n = 110). (E) Allelic distribution in KIR3DL1+ granzyme Bhigh donors (n = 11) among Bw4+ donors compared with other Bw4+ donors (n = 159). One-way ANOVA tests followed by Kruskal-Wallis multiple comparison tests were performed. (D-F) The pie charts represent the frequencies of donors with specific HLA-C or Bw4 alleles in the outlier population and in the general population. The outliers are identified with the interquartile range formula. Χ tests were performed. ∗∗∗∗P < .0001; ∗∗∗P < .001; ∗∗P <.01; and ∗P < .05.
Granzyme B loading as a metric of the global education status
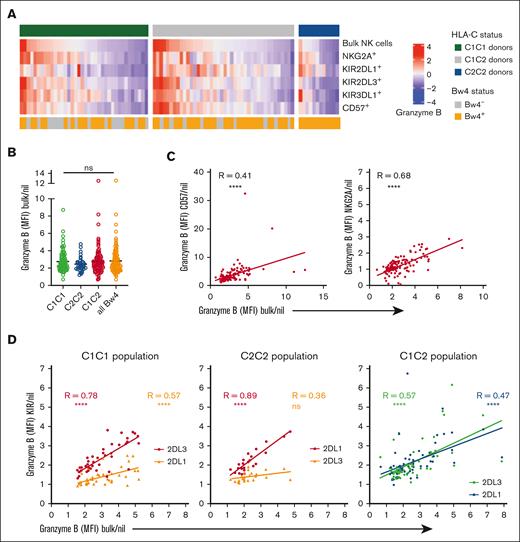
Given the correlation between strong HLA ligands and granzyme B loading at the subset level, we next asked how this affected the overall granzyme B levels in the NK cell compartment. At the bulk level, the average granzyme B content was nearly identical among donors with different HLA-C/Bw4 backgrounds, albeit with large variation within each of the genotypes (Figure 3A-B). Although different NK cell subsets can influence the total granzyme B loading, NK cells were stratified into 5 subsets of interest: single-positive NKG2A (spNKG2A+) NK cells (CD57–KIR–spNKG2A+), sp2DL1+ NK cells (CD57–NKG2A–spKIR2DL1+), sp2DL3+ NK cells (CD57–NKG2A–spKIR2DL3+), sp3DL1+ NK cells (CD57–NKG2A–spKIR3DL1+) and CD57+ NK cells (NKG2A–KIR–). We found that the levels of granzyme B relative to receptor-negative (Nil) NK cells in each of these subsets correlated significantly with the overall granzyme B loading (Figure 3A,C-D). Overall, these results showed that KIR education was a main determinant of the granzyme B pool at both the subset and global levels, suggesting that granzyme B loading can be used as a metric of global education status.
Differences in granzyme B loading at the bulk level can be explained by the differences observed in the educated population of NK cells. (A) Heatmap representing the granzyme B expression (MFI) using z-score normalization. C1/C1 donors (n = 37), C1/C2 donors (n = 42), and C2/C2 donors (n = 12). (B) Granzyme B expression in the bulk NK population were compared with receptor-negative (Nil) NK cells from the same donor and stratified by the HLA class I background of the donors. (C) Correlation analyses between granzyme B expression in the bulk NK population with the granzyme B loading in the CD57+ subset and NKG2A+ subset of NK cells. (D) Correlation analysis between granzyme B expression in the bulk NK population and granzyme B levels in the educated NK cells. Each dot represents 1 donor. R is the Pearson coefficient. One-way ANOVA tests followed by Kruskal-Wallis multiple comparison tests were performed in panel B. ∗∗∗∗P < .0001; ∗∗∗P < .001; ∗∗ P < .01; and ∗P < .05.
Differences in granzyme B loading at the bulk level can be explained by the differences observed in the educated population of NK cells. (A) Heatmap representing the granzyme B expression (MFI) using z-score normalization. C1/C1 donors (n = 37), C1/C2 donors (n = 42), and C2/C2 donors (n = 12). (B) Granzyme B expression in the bulk NK population were compared with receptor-negative (Nil) NK cells from the same donor and stratified by the HLA class I background of the donors. (C) Correlation analyses between granzyme B expression in the bulk NK population with the granzyme B loading in the CD57+ subset and NKG2A+ subset of NK cells. (D) Correlation analysis between granzyme B expression in the bulk NK population and granzyme B levels in the educated NK cells. Each dot represents 1 donor. R is the Pearson coefficient. One-way ANOVA tests followed by Kruskal-Wallis multiple comparison tests were performed in panel B. ∗∗∗∗P < .0001; ∗∗∗P < .001; ∗∗ P < .01; and ∗P < .05.
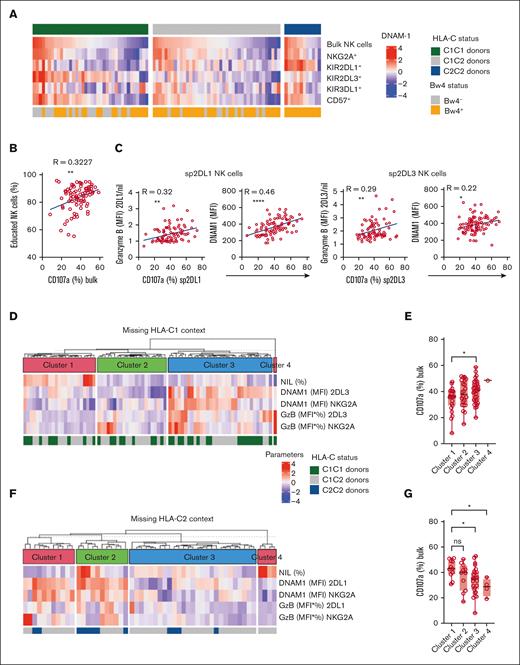
A refined phenotypic metric of NK cell function based on DNAM-1 and granzyme B levels
In addition to granzyme B, other proteins stored in secretory vesicles, including perforin, correlate with NK cell education.24 Furthermore, DNAM-1 is a sensitive marker of educated NK cells.19 To develop a more precise metric of NK cell education and to link the phenotype to functional responses, we performed an extended phenotyping and functional assessment of NK cells from cryopreserved vials of PBMC from the main cohort of PBMCs. Using a master mix of antibodies, this strategy allowed us to perform a comparative analysis of data obtained in samples freshly stained over the course of several years with data from well-controlled experiments performed on batches of frozen samples over a few days. Although the freeze thawing procedure attenuated the differences in granzyme B levels between educated and uneducated NK cells, we found a correlation and preserved the hierarchy of granzyme B levels between donors (supplemental Figure 4C). We also observed higher expression of DNAM-1 on KIR-educated NK cells and on more mature subsets, following the same trend as granzyme B (Figure 4A; supplemental Figures 1C-E and 3).
A refined phenotypic metric based on DNAM-1 and granzyme B levels. (A) Heatmap representing the DNAM-1 expression (MFI), using z-score normalization. C1/C1 donors (n = 37), C1/C2 donors (n = 42), and C2/C2 donors (n = 12). (B) Correlation between the percentage of educated NK cells and degranulation rates. (C) Correlation between DNAM-1 expression and granzyme B content in KIR2DL3+ or KIR2DL1+ NK cells with the degranulation rate of the same subset of NK cells. Negative correlation between KIR expression intensity and degranulation rate. (D) Unsupervised K-means clustering of the donors in the missing HLA-C1 context, based on DNAM-1 expression, granzyme B content and the size of the different educated subsets (n = 80). (E) Summary graph of the degranulation response (CD107a) in clusters from 1 to 4. (F) Unsupervised K-means clustering of the donors in the missing HLA-C2 context, based on DNAM-1 expression, granzyme B content, and the size of the different educated subsets (n = 54). (G) Summary graph of the degranulation response (CD107a) in clusters from 1 to 4. Each dot represents 1 donor. R is the Pearson coefficient. One-way ANOVA tests followed by Tukey multiple comparison tests were performed in panels D and E. ∗∗∗∗P < .0001; ∗∗∗P < .001; ∗∗P < .01; and ∗P < .05.
A refined phenotypic metric based on DNAM-1 and granzyme B levels. (A) Heatmap representing the DNAM-1 expression (MFI), using z-score normalization. C1/C1 donors (n = 37), C1/C2 donors (n = 42), and C2/C2 donors (n = 12). (B) Correlation between the percentage of educated NK cells and degranulation rates. (C) Correlation between DNAM-1 expression and granzyme B content in KIR2DL3+ or KIR2DL1+ NK cells with the degranulation rate of the same subset of NK cells. Negative correlation between KIR expression intensity and degranulation rate. (D) Unsupervised K-means clustering of the donors in the missing HLA-C1 context, based on DNAM-1 expression, granzyme B content and the size of the different educated subsets (n = 80). (E) Summary graph of the degranulation response (CD107a) in clusters from 1 to 4. (F) Unsupervised K-means clustering of the donors in the missing HLA-C2 context, based on DNAM-1 expression, granzyme B content, and the size of the different educated subsets (n = 54). (G) Summary graph of the degranulation response (CD107a) in clusters from 1 to 4. Each dot represents 1 donor. R is the Pearson coefficient. One-way ANOVA tests followed by Tukey multiple comparison tests were performed in panels D and E. ∗∗∗∗P < .0001; ∗∗∗P < .001; ∗∗P < .01; and ∗P < .05.
To investigate the functional relevance of granzyme B and DNAM-1 expression variability, we challenged PBMCs with K562 cells and monitored degranulation responses by assessing mobilization of CD107a to the membrane. As expected, we observed a correlation between the size of the educated repertoire and global degranulation responses (Figure 4B). Moreover, we found a positive correlation at the subset level between the granzyme B content and DNAM-1 expression in sp2DL1+ and sp2DL3+ NK cells and their respective degranulation responses (Figure 4C). To link education phenotypes to global functional responsiveness in a missing-self context, we used k-means clustering to perform an unbiased stratification of donors into 4 clusters based on granzyme B content and DNAM-1 expression on different subsets of NK cells (sp2DL3+ NK cells, sp2DL1+ NK cells, and spNKG2A+ NK cells), even considering the size of the respective subset (Figure 4D-E). For missing HLA-C1 (strongly educated KIR2DL3 repertoires), we found that clusters 3 and 4 grouped the donors that had educated NK cell repertoires with high levels of granzyme B and DNAM-1 in the KIR2DL3+ subset, whereas lower levels were observed for donors in clusters 1 and 2 (Figure 4D). In functional assays, we found that NK cells from donors in cluster 3 degranulated significantly more than those in cluster 1 (Figure 4E). Similar results were obtained when scoring the repertoires for missing HLA-C2 (strongly educated 2DL1 repertoires) reactivity (Figure 4F-G). Hence, the impact of specific KIR and HLA interactions at the subset level, combined with the subset composition, translated into predictable functional hierarchies that classified donors into those with strong and weak NK cell responses.
Educated NK cells have a more potent lytic hit
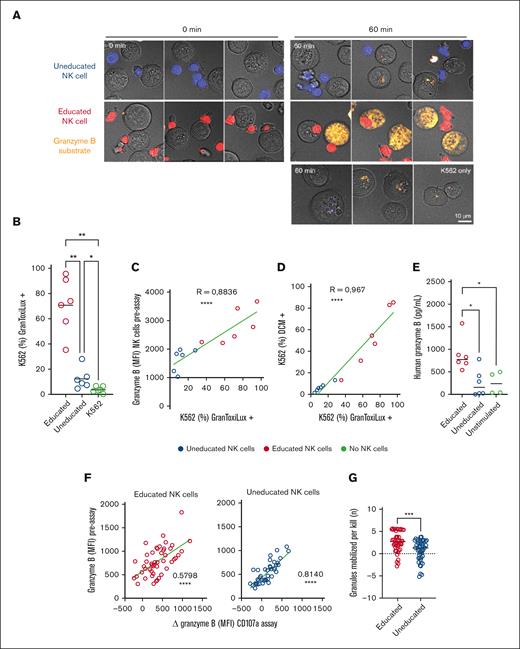
To investigate the physiological impact of differential granzyme B loading, we monitored granzyme B release and target cell killing. Following a 6-hour challenge with K562 target cells, the average level of granzyme B was significantly reduced in educated CD107a+ NK cells, whereas this level was largely unchanged in uneducated CD107a+ NK cells (supplemental Figure 5A). This suggested that uneducated NK cells were capable of mobilizing CD107a to the cell surface but with limited release of granzyme B. We, therefore, monitored the uptake and activity of granzyme B from sorted resting educated and uneducated NK cells upon challenge with K562 target cells loaded with a fluorescent granzyme B-specific substrate. After 1 hour of coincubation with educated NK cells, 70% of the K562 cells showed evidence of active granzyme B cleavage, which correlated with subsequent cell death (Figure 5A-D). In contrast, K562 cells exposed to the uneducated NK cells showed limited granzyme B uptake/activity and remained viable (Figure 5A-D). The difference in the released granzyme B was also observed in a cell-free stimulation assay probing reverse ADCC capacity, where educated NK cells released higher amounts of granzyme B into the supernatant after stimulation by a plate-bound agonistic anti-CD16 monoclonal antibody (Figure 5E).
Educated NK cells contain more granzyme B than uneducated. (A) Imaging of CTV-labeled sorted educated or uneducated NK cells (red and blue respectively) coincubated with K562 for 0 or 60 min and stained for GranToxiLux plus (orange). (B) Bar graph representing percentage of K562 positive for GranToxiLux after 1 hour challenge with either educated or uneducated NK cells (E:T) ratio of (2:1), or no NK cells. (C) Correlation between granzyme B content of NK cells measured preassay correlates with the percentage of K562 positive for GranToxiLux. (D) Correlation of GranToxiLux positive K562 cells and DCM+ cells. (E) Soluble granzyme B measured in the supernatant after stimulation of NK cells by CD16 crosslinking compared with unstimulated NK cells as baseline. (F) Correlation between granzyme B content of NK cells measured preassay with the amount of granzyme B lost by NK cells during the degranulation assay using K562 (n = 49). (G) Number of granules mobilized per kill in educated and uneducated NK cells (n = 49). (B-G) Self- KIR NK cells are represented in red and the nonself KIR NK cells in blue. Whiskers show the fifth to 95th percentiles. Bars show the median. One-way ANOVA tests followed by Tukey multiple comparison tests were performed in panels B and E. R is the Pearson coefficient in panels C, D, and F. Unpaired t test was performed in panel G. ∗∗∗∗P < .0001; ∗∗∗P < .001; ∗∗P < .01; and ∗P < .05. CTV, CellTrace Violet; DCM, dead cell marker.
Educated NK cells contain more granzyme B than uneducated. (A) Imaging of CTV-labeled sorted educated or uneducated NK cells (red and blue respectively) coincubated with K562 for 0 or 60 min and stained for GranToxiLux plus (orange). (B) Bar graph representing percentage of K562 positive for GranToxiLux after 1 hour challenge with either educated or uneducated NK cells (E:T) ratio of (2:1), or no NK cells. (C) Correlation between granzyme B content of NK cells measured preassay correlates with the percentage of K562 positive for GranToxiLux. (D) Correlation of GranToxiLux positive K562 cells and DCM+ cells. (E) Soluble granzyme B measured in the supernatant after stimulation of NK cells by CD16 crosslinking compared with unstimulated NK cells as baseline. (F) Correlation between granzyme B content of NK cells measured preassay with the amount of granzyme B lost by NK cells during the degranulation assay using K562 (n = 49). (G) Number of granules mobilized per kill in educated and uneducated NK cells (n = 49). (B-G) Self- KIR NK cells are represented in red and the nonself KIR NK cells in blue. Whiskers show the fifth to 95th percentiles. Bars show the median. One-way ANOVA tests followed by Tukey multiple comparison tests were performed in panels B and E. R is the Pearson coefficient in panels C, D, and F. Unpaired t test was performed in panel G. ∗∗∗∗P < .0001; ∗∗∗P < .001; ∗∗P < .01; and ∗P < .05. CTV, CellTrace Violet; DCM, dead cell marker.
Finally, we set out to estimate the released quanta, defined as the total amount of granzyme B released from 1 single cell, as an indirect measure of the potency of the lytic hit. We found a correlation between the pre-existing levels of granzyme B and the drop in granzyme B after degranulation (Figure 5F). We, then, measured the average number of kills by placing the educated and uneducated NK cells in a single-cell microchip readout30 and calculated the number of granules mobilized per killing event (Figure 5G; supplemental Figure 5B-C), corresponding to an average release of 4.2fg and 2.6fg of granzyme B from educated and uneducated NK cells, respectively. Thus, educated NK cells had a 1.6-fold larger lytic hit than uneducated NK cells. Furthermore, in agreement with previous literature,31 we found that educated NK cells formed significantly more conjugates with K562 target cells than uneducated NK cells (supplemental Figure 5E). Hence, the qualitative difference in polarized granzyme B release between educated and uneducated NK cells was amplified by the upstream difference in the ability to form conjugates, explaining the difference in killing activity (Figure 5D).
Discussion
The NK cell repertoire is diversified through variation in KIR gene content, copy number, and extensive polymorphism.32,33 It is well established that the strength of the interaction between a given KIR and its cognate HLA ligand tunes the functionality of the NK cell subset expressing the same KIR.5,6 Furthermore, the functional capacity of the NK cell compartment varies between individuals and has been linked to decreased risk for cancer in long-term epidemiological studies.34 Here, we took advantage of the recent discovery that NK cell education shapes the lysosomal compartment and the granzyme B content in NK cells to develop a phenotypic metric of NK cell function that reflects education at the subset and repertoire levels. The granzyme B content of NK cells from a given individual was remarkably stable over time, and interindividual variation reflected significant differences in the releasable granzyme B quanta with a profound effect on the lytic hit.
During NK cell education, the intrinsic function of NK cells is dynamically calibrated against the major histocompatibility complex environment.35-37 Education is not an on or off switch but rather a continuum, or a tunable rheostat, that helps to maintain a functional and yet self-tolerant repertoire.17 Although the exact mechanism underlying NK cell education remains elusive, several unique phenotypic traits have been reported to distinguish educated from uneducated NK cells. These include increased metabolic activity,38 lower surface density of self-KIR,39,40 and intracellular SHP-1,21 and higher levels of DNAM-119 and granzyme B.24 Intriguingly, neither of these changes are due to stable changes at the epigenetic or transcriptome level but rather appear to be consequences of physical cell-cell interactions that trim surface receptors, signaling molecules, membrane organization, and shape the lysosomal compartment.16 We recently found that educated NK cells have a unique organization of the lysosomal compartment, with a subset of dense core secretory lysosomes converging at the centrosome and filled with a high density of granzyme B.24 This observation suggests that inhibitory receptors binding to self-HLA class I shape the interior of the cell and tune the cytolytic quanta stored in vesicular compartments in a manner that is independent from acute transcriptional regulation.24
Here, we analyzed granzyme B content in distinct NK cell subsets in 365 healthy donors and found that educated NK cells express on average 1.7-fold more granzyme B than uneducated NK cells. The detailed mapping of the granzyme B levels in a large cohort of individuals typed for KIR and HLA at the allele level was combined with a broad phenotypic and functional analysis. By monitoring sorted educated and uneducated NK cells in live cell imaging, we found that educated NK cells released a significantly larger amount of granzyme B during target cell interactions. The immune synapse formation is critical for the cytotoxic events, which require the transfer of the lytic granules’ content to the target cells.41 Educated NK cells provide stronger binding to the target and more efficient lysosomal polarization,24,31 allowing heightened function. Altogether, the increased ability to form stable immune conjugates (quantitative effect) as well as the release of larger granzyme B quanta (qualitative effect) resulted in a significant difference in target cell killing. Thus, the difference in function between educated and uneducated NK cells may previously have been underestimated by simply monitoring the percent of degranulating cells because it appears as if uneducated NK cells shoot blanks and fail to deliver a lytic hit.
It remains an open question to what extent granzyme B loading influences ADCC responses and the responses of cytokine-activated and chimeric antigen receptor–engineered NK cells. We have shown before that education is an inherited state in dividing NK cells,42 but that the gap between educated and uneducated NK cells decreases with cell division. We have also previously shown that chimeric antigen receptor signaling taps into the intrinsic potential of the educated NK cell subset.43 In contrast, there is evidence that strong signals through CD16 may override education and that the educated state does not have an impact on granzyme B delivery.44 Here, by monitoring the net release of granzyme B from educated and uneducated NK cells following stimulation by anti-CD16 in a reverse ADCC assay, we found that the baseline levels of granzyme B combined with the degree of responsiveness had a dramatic impact on granzyme B release. Thus, we believe the findings are relevant for ADCC responses, in particular in a resting NK cell repertoire.
HLA class I allelic variation tuned the granzyme B content in NK cells, providing a link between genotype and phenotype. HLA-C∗07 is a very well-conserved allele inherited from the Neanderthals45 and has a high affinity for KIR2DL3.6,7,45 Consistent with reported affinities, we found that HLA-C∗07 is a strong educator of KIR2DL3+ NK cells, resulting in significantly more granzyme B content. Similarly, HLA-C∗02 has been shown to be a strong ligand of KIR2DL1,46,47 leading to high levels of granzyme B in KIR2DL1+ NK cells. In HLA-C1/C2 heterozygous donors, the net outcome of education was either neutral or tilted toward a strong KIR2DL3+ (HLA-C∗07) or KIR2DL1+ (HLA-C∗02) compartment. Similarly, we found that the Bw4-80I allotype, described as a strong ligand of KIR3DL1,48,49 had a profound influence on the education of the KIR3DL1+ NK cells, correlating with higher granzyme B content. Allelic variation in KIR had less impact on the level of granzyme B at the subset level, although KIR gene copy number variation affects the size of the respective KIR-expressing subset32 and will therefore indirectly affect the total granzyme B loading in the NK cell repertoire. The educated state at the single cell and population levels, instructed by the genetically hardwired KIR/HLA interactions, was stable over time, suggesting there may be a way to predict the functional hierarchy of highly diverse human NK cell repertoires.
Given the tight correlation between granzyme B levels and the functional imprint of KIR/HLA interactions, we asked whether granzyme B content could serve as a global metric of the education status of the whole NK cell compartment. Because it is well established that DNAM-1 levels also reflect NK cell education in mice20 as well as in humans,19 we explored the utility of combining surface phenotypes with intracellular stores of effector molecules. An unbiased clustering of DNAM-1 and granzyme B expression intensities in key NK cell subsets, combined with information about the size of these subsets, was used to classify donors into 4 different clusters. These clusters differed in their global functional responses. Donors with high DNAM-1 and granzyme B expression showed the strongest functional responses. Therefore, the net effect of KIR/HLA-driven education and subset distribution can be used to determine the global functionality of the NK cells from a given donor. This finding is relevant in the context of the immunosurveillance properties of NK cells,34 where specific KIR- and HLA-genotypes may be more protective. In support of this, Guillamón et al have shown that NK cell education can be used as a biomarker for outcomes in patients with solid tumor.50 NK cell repertoires with a higher degree of education, quantified as the ratio of DNAM-1/inhibitory KIR expression, were associated with improved overall survival.
Immunogenetic studies of epistatic interactions between alleles of KIR and HLA suggest that some variants are associated with high or low risk for a given disease outcome.51,52 However, there is no genotype-phenotype link that explains the reactivity of the NK cell repertoire at the population level. Several studies have shown the importance of KIR and HLA genes for the risk of relapse in the context of hematopoietic stem cell transplantation in acute myeloid leukemia. However, the lack of consistency between studies has made it challenging to base donor selection criteria on KIR genotyping.53 The conflicting outcomes of KIR-HLA association studies are perhaps not very surprising because they do not consider the size of the NK cell subsets expressing the different KIRs and therefore ignore the functionality of the donor NK cell repertoire. In its simplest downstream application, the metric of NK cell education defined here could help stratify donors with an expected strong NK cell–driven missing HLA-C1 or HLA-C2 response. In this context, it is also interesting to note that DNAM-1 levels remain a metric of the educated state in the posttransplant setting and affected by both donor and recipient HLA, with a slight bias toward recipient HLA.54 A metric of NK cell education could also be useful to probe the natural variation in the ability to perform missing-self and contribute to immune surveillance against HLA class I loss variants.
Overall, our results shed further insight into the functional diversification of the resting NK cell repertoire in peripheral blood, demonstrating how the net effect of polymorphisms in KIR and HLA shapes the global functional responsiveness of the NK cell compartment through modulation of the core cytolytic machinery.
Acknowledgments
The authors are grateful for the support from The Flow Cytometry Core Facility at Oslo University Hospital, Radiumhospitalet and to Weitse Mulder for use of the GenDX software.
The project was supported by a network grant of the European Commission (H2020-MSCA-MC-ITN-765104-MATURE-NK); Camille Philippon is a fellow in the project. Other funding was received from the Research Council of Norway (project numbers 275469 and 237579), The Norwegian Cancer Society (project numbers 190386 and 223310), EU H2020-MSCA Research and Innovation programme (project number 801133), National Institutes of Health (NIH) PTE Federal award number P01CA111412, subaward number P009500901, and NIH grant R01 AI151549 (P.J.N.). S.T. received fellowships from the Science Research Foundation of Zhejiang Province (LY18H080002) and Health Commission of Zhejiang Province (2018RC003 and 2019RC031).
Authorship
Contribution: C.P., P.J.N., and K.-J.M. designed the research; C.P., S.T., D.C., K.M.K., H.N., L.B., V.S.O., P.M.L., M.S., B.Ö., J.P.G., and R.K.M. performed the research and analyzed the data; A.H., F.Z., and L.K. analyzed the data; and C.P., P.J.N., and K.-J.M. wrote the paper, with input from all authors.
Conflict-of-interest disclosure: K.-J.M. is a consultant with ownership interests at Fate Therapeutics and Vycellix; reports research funding from Fate Therapeutics, Oncopeptides, and Merck; has a royalty agreement with Fate Therapeutics through the licensing of intellectual property; and has received honoraria from Oncopeptides and Cytovia. B.Ö. is a consultant and has ownership interest at Vycellix, and receives research support from Affimed. The remaining authors declare no competing financial interests.
Correspondence: Karl-Johan Malmberg, Department of Cancer Immunology, Institute for Cancer Research, Oslo University Hospital, 0310 Oslo, Norway; e-mail: k.j.malmberg@medisin.uio.no.
References
Author notes
∗P.J.N. and K.-J.M. are joint last authors.
Data are available on request from the corresponding author, Karl-Johan Malmberg (k.j.malmberg@medisin.uio.no).
The full-text version of this article contains a data supplement.