Key Points
An improved pegaspargase-modified MRD and risk-oriented regimen was prospectively tested in adults aged 18-65 years with Ph– ALL/LL.
Three-year survival rates were >60% with HCT or chemotherapy, further improved by MRD negativity and age ≤40 years.
Visual Abstract
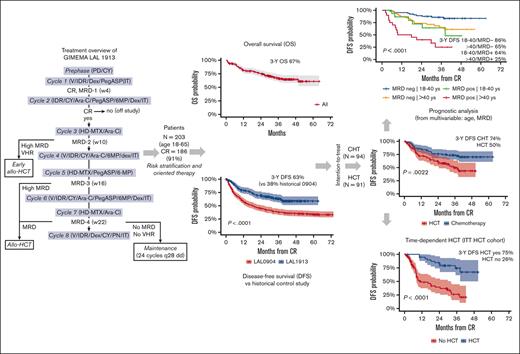
An updated risk-oriented strategy resulting in 3-year OS and DFS rates >60% in adult patients 18-65 years with Ph-negative ALL
An updated risk-oriented strategy resulting in 3-year OS and DFS rates >60% in adult patients 18-65 years with Ph-negative ALL
Abstract
Pediatric-inspired chemotherapy is the standard of care for younger adults with Philadelphia chromosome–negative acute lymphoblastic leukemia/lymphoma (Ph– ALL/LL). In LAL1913 trial, the Gruppo Italiano Malattie EMatologiche dell’Adulto added pegaspargase 2000 IU/m2 to courses 1, 2, 5, and 6 of an 8-block protocol for patients aged from 18 to 65 years, with dose reductions in patients aged >55 years. Responders were risk stratified for allogeneic hematopoietic cell transplantation (HCT) or maintenance per clinical characteristics and minimal residual disease (MRD). Of 203 study patients (median age, 39.8 years), 91% achieved a complete remission. The 3-year overall survival, event-free, and disease-free survival (DFS) rates were 66.7%, 57.7%, and 63.3%, respectively, fulfilling the primary study end point of a 2-year DFS >55%. Although based on the intention-to-treat, the DFS being 74% and 50% in the chemotherapy (n = 94) and HCT (n = 91) assignment cohorts, respectively, a time-dependent analysis proved the value of HCT in patients who were eligible (DFS HCT 70% vs no HCT 26%; P <.0001). In multivariate analysis, age and MRD were independent factors predicting DFS rates of 86% (age ≤ 40 and MRD-negative), 64%-65% (MRD-positive or age > 40) and 25% (age > 40 and MRD-positive); P < .0001. Grade ≥2 pegaspargase toxicity was mainly observed at course 1, contributing to induction death in 2 patients but was rare thereafter. This program improved outcomes of patients with Ph– ALL/LL aged up to 65 years in a multicenter national setting. This trial was registered at www.clinicaltrials.gov as #NCT02067143.
Introduction
Therapeutic success in adult Philadelphia chromosomeBCR::ABL1 rearrangement–negative acute lymphoblastic leukemia/lymphoblastic lymphoma (Ph– ALL/LL) relies on the achievement of a complete remission (CR) with deep minimal residual disease (MRD) response, followed by effective consolidation and maintenance chemotherapy or an allogeneic hematopoietic cell transplantation (HCT) in patients with high-risk (HR) features and/or MRD persistence.1,2
Data from large multi-institutional studies have proven the superiority of modern pediatric-inspired chemotherapy over conventional adult regimens.3,4 Adolescents and young adults (AYAs) aged between 18 and 35 or 45 years experience the best outcome,5-8 with survival rates between ∼65% and 70% and even higher for patients who have a favorable risk profile, including a complete MRD response.6,7,9 Toxicity from pediatric chemotherapy regimens is higher and more difficult to manage in older adults, mandating for drug dose adjustments.3,4
Pegaspargase (pegylated asparaginase) stands out as one of the most effective antileukemic drugs that has significantly contributed to improve outcomes of childhood and AYA ALL.10,11 Pegylation protects the native compound from enzymatic and immune-mediated inactivation, prolongs its half-life, and ensures a therapeutic activity lasting 2 or 3 weeks and occasionally longer. In adult patients, pegaspargase exerts its toxicity on the liver, pancreas, the blood coagulation system, and metabolism at higher rates than in children.12 Although its use was rationally advocated and is supported by expert recommendations,12,13 the risk of serious drug-related toxicity in adults can still raise concern14 and requires a careful treatment conduct.
A recent trial of the Northern Italy Leukemia Group (NILG) for Ph– ALL adopted pediatric-type elements and significantly improved the long-term outcome of patients aged from 18 to 65 years compared with that of historical controls.15 In a subsequent national trial sponsored by Gruppo Italiano Malattie EMatologiche dell’Adulto (GIMEMA), pegaspargase was incorporated into a similar chemotherapy backbone to support a postremission risk-oriented strategy with or without allogeneic HCT, driven by selected clinical characteristics and postinduction MRD.16,17 The final study analysis provides evidence in favor of this modified regimen and is presented herein.
Methods
Patients and study setting
Patients who were eligible with newly diagnosed, untreated Ph– ALL/LL and were aged from 18 to 65 years satisfied enrollment criteria and provided informed consent in accordance with the Helsinki Declaration of 1975, as revised in 2008. The GIMEMA LAL1913 protocol (trial details online, Supplement 1) was approved by the ethics committees of participating institutions (Supplemental Table 1), sponsored by GIMEMA, and clinically registered as #NCT02067143.
Diagnostics and MRD study
Diagnostic work-up and MRD study were performed centrally per standardized methods for ALL diagnosis, immunophenotypic and genetic/cytogenetic subtyping, and MRD analysis.9,15,18 For MRD, sensitive molecular probes (sensitivity of ≥10−4) recognizing patient-specific fusion genes or immunoglobulin and T-receptor gene rearrangements were generated from freshly obtained diagnostic marrow samples processed at EuroMRD-certified laboratories in Rome, Palermo, and Bergamo. The cases without suitable molecular probes were evaluated via multiparametric flow cytometry detecting leukemia-associated phenotypes.
Treatment protocol
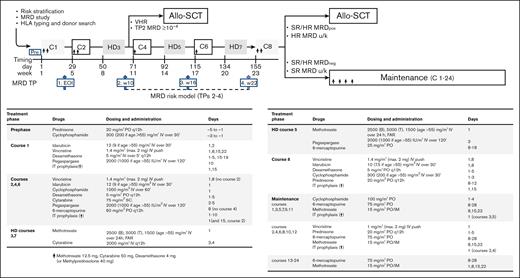
Figure 1 summarizes the treatment protocol and strategy. Pegaspargase was added at courses 1, 2, 5, and 6, and the cyclophosphamide, idarubicin and methotrexate dosage was reduced for patients aged >55 years. Pegaspargase was premedicated with hydrocortisone 100 mg and was administered, provided serum transaminase levels did not exceed 5 × the upper normal limit, bilirubin was <3 mg/dL, and amylase/lipase levels were <3 × the upper normal limit. Monitoring of serum asparaginase activity was not planned in this study. The recommended prophylaxis of pegaspargase-related coagulopathy was with antithrombin and fibrinogen cryoprecipitate when the concentrations decreased to <70% and <75 mg/dL, respectively, along with enoxaparin until the platelet count remained >50 × 109/L. Substitution with Erwinia asparaginase was recommended in case of severe allergic reaction, and L-carnitine when the direct bilirubin increase to >3 mg/dL. In support of the risk-oriented study strategy, the search for a suitable family-related or unrelated HCT donor was carried out at diagnosis in all patients; donor selection, stem cell source, HCT conditioning regimen, and graft-versus-host disease prophylaxis were per standard procedures and guidelines. Patients who were selected for but unable to undergo an allogeneic HCT went on to receive an autologous HCT or standard chemotherapy.
Study strategy and treatment elements. Allo-SCT, allogeneic stem cell transplantation; FAR, folinic acid rescue; HD, high dose; IM, intramuscular; IT, intrathecal; PO, oral; SC, subcutaneous; w, week; u/k, unknown.
Study strategy and treatment elements. Allo-SCT, allogeneic stem cell transplantation; FAR, folinic acid rescue; HD, high dose; IM, intramuscular; IT, intrathecal; PO, oral; SC, subcutaneous; w, week; u/k, unknown.
Therapy-oriented risk stratification
Study patients were risk stratified through selected risk parameters and MRD to guide the allogeneic HCT decision. The HR group included the 2 subsets of patients at very HR (VHR: white blood cell [WBC] count >100 × 109/L and/or pro/pre/mature [noncortical] T phenotype and/or poor-risk cytogenetics/genetics [Supplemental Protocol Synopsis; Table 1]), who were eligible upfront for an allogeneic HCT, regardless of MRD analysis; and patients at HR (WBC count >30 × 109/L to 100 × 109/L, pro-B phenotype, and late CR). Patients at HR and standard-risk (SR) with none of the mentioned risk factors were risk restratified per the MRD results for postremission therapy assignment. A Ph-like gene signature,19 recognized in a subsequent post hoc study,20 was not included in the prospective risk classification.
Diagnostic characteristics of study patients, with baseline risk stratification
| . | All patients (N = 203) . | B-ALL/LL (n = 139) . | T-ALL/LL (n = 64) . | P value . |
|---|---|---|---|---|
| Age (y), median (range) | 39.8 (18-65) | 41.0 (18.1-64.8) | 33.4 (18.5-65.1) | .089 |
| ≤40, n (%) | 103 (50.7) | 66 (47.5) | 37 (57.8) | .381 |
| 40-55, n (%) | 61 (30.0) | 44 (31.6) | 17 (26.5) | |
| >55, n (%) | 39 (19.2) | 29 (20.8) | 10 (15.6) | |
| Gender (male), n (%) | 118 (58.1) | 76 (54.6) | 42 (65.6) | .07 |
| Diagnosis, n (%) | ||||
| ALL | 183 (90) | 138 (99.2) | 45 (70.3) | <.001 |
| LL∗ | 20 (9.8) | 1 (0.8) | 19 (29.7) | |
| ECOG PS†, n | ||||
| 0:1:2:3:NA | 120:58:15:3:7 | 88;32;12;1 | 32;26;3;2 | |
| Hemoglobin (g/dL), median (range) | 9.5 (3.7-16.8) | 9.1 (3.7-16.2) | 11.5 (7.1-16.8) | <.000 |
| WBC count (109/L), median (range) | 7.1 (1.5-347.3) | 4.9 (1.5-347.3) | 11.2 (2.9-345.0) | .003 |
| ≤30 (%) | 159 (78.3) | 110 (79.1) | 49 (76.6) | .0367 |
| >30-100 (%) | 31 (15.27) | 24 (17.26) | 7 (10.94) | |
| >100 (%) | 13 (6.40) | 5 (3.60) | 8 (12.50) | |
| BM blasts (%), median (range) | 88.0 (0-100) | 90.0 (0-100) | 75.0 (0-100) | .0007 |
| PB blasts (%), median (range) | 41.0 (0-100) | 44.5 (0-100) | 27.0 (0-100) | .205 |
| Platelets (109/L), median (range) | 73.0 (1.2-630) | 57.0 (1.2-630) | 141.5 (7.0-476.0) | <.000 |
| Hepatomegaly, n (%) | 18 (14.9) | 14 (17.7) | 4 (10.5) | .416 |
| Splenomegaly, n (%) | 47 (28.7) | 36 (37.1) | 11 (25.0) | .157 |
| Lymphadenopathy, n (%) | 84 (45.4) | 35 (28.5) | 49 (79.0) | <.0001 |
| Mediastinal mass, n (%) | 36 (18.7) | 2 (1.55) | 34 (54.0) | <.0001 |
| CNS involvement, n (%) | 19 (9.3) | 12 (8.6) | 7 (10.9) | .635 |
| Other involved site, n | 4 | |||
| Testis/ovary (skin) | 2:2 | |||
| Immunophenotype, n (%) | ||||
| B: pro, common, pre, and undefined | 139 (68.4) | 20, 99, 10, and 9 | ||
| T: ETP, pro, pre, cortical, mature, and undefined/MPAL | 64 (31.6) | 14, 1, 10, 12, 2, and 6 | ||
| Cytogenetics/genetics, n (%) | ||||
| Normal | 57 (47.1) | 23 | 34 | |
| Adverse | 33 (27.3) | 32 | 1 | |
| t(4;11)/KMT2A::AFF4, t(11;19) | 16 | 16 | - | |
| Other‡ | 17 | 17 | 1 | |
| Nonadverse | 40 (31) | 36 | 4 | |
| t(1;19)/E2A::PBX1 | 4 | 4 | ||
| Hyperdiploid | 14 | 12 | 2 | |
| Other nonadverse | 22 | 20 | 2 | |
| Not evaluable | 73 (35.9) | 48 | 25 | |
| Ph-like signature (n = 88 studied)§ | 28 | 28 (31.8%) | ||
| Risk stratification, n (%) | ||||
| SR | 115 (56.7) | 83 (59.7) | 32‖ (50.0) | |
| HR | 20 (9.9) | 20 (14.4) | 0 | |
| VHR | 68 (33.4) | 36 (25.9) | 32 (50.0) |
| . | All patients (N = 203) . | B-ALL/LL (n = 139) . | T-ALL/LL (n = 64) . | P value . |
|---|---|---|---|---|
| Age (y), median (range) | 39.8 (18-65) | 41.0 (18.1-64.8) | 33.4 (18.5-65.1) | .089 |
| ≤40, n (%) | 103 (50.7) | 66 (47.5) | 37 (57.8) | .381 |
| 40-55, n (%) | 61 (30.0) | 44 (31.6) | 17 (26.5) | |
| >55, n (%) | 39 (19.2) | 29 (20.8) | 10 (15.6) | |
| Gender (male), n (%) | 118 (58.1) | 76 (54.6) | 42 (65.6) | .07 |
| Diagnosis, n (%) | ||||
| ALL | 183 (90) | 138 (99.2) | 45 (70.3) | <.001 |
| LL∗ | 20 (9.8) | 1 (0.8) | 19 (29.7) | |
| ECOG PS†, n | ||||
| 0:1:2:3:NA | 120:58:15:3:7 | 88;32;12;1 | 32;26;3;2 | |
| Hemoglobin (g/dL), median (range) | 9.5 (3.7-16.8) | 9.1 (3.7-16.2) | 11.5 (7.1-16.8) | <.000 |
| WBC count (109/L), median (range) | 7.1 (1.5-347.3) | 4.9 (1.5-347.3) | 11.2 (2.9-345.0) | .003 |
| ≤30 (%) | 159 (78.3) | 110 (79.1) | 49 (76.6) | .0367 |
| >30-100 (%) | 31 (15.27) | 24 (17.26) | 7 (10.94) | |
| >100 (%) | 13 (6.40) | 5 (3.60) | 8 (12.50) | |
| BM blasts (%), median (range) | 88.0 (0-100) | 90.0 (0-100) | 75.0 (0-100) | .0007 |
| PB blasts (%), median (range) | 41.0 (0-100) | 44.5 (0-100) | 27.0 (0-100) | .205 |
| Platelets (109/L), median (range) | 73.0 (1.2-630) | 57.0 (1.2-630) | 141.5 (7.0-476.0) | <.000 |
| Hepatomegaly, n (%) | 18 (14.9) | 14 (17.7) | 4 (10.5) | .416 |
| Splenomegaly, n (%) | 47 (28.7) | 36 (37.1) | 11 (25.0) | .157 |
| Lymphadenopathy, n (%) | 84 (45.4) | 35 (28.5) | 49 (79.0) | <.0001 |
| Mediastinal mass, n (%) | 36 (18.7) | 2 (1.55) | 34 (54.0) | <.0001 |
| CNS involvement, n (%) | 19 (9.3) | 12 (8.6) | 7 (10.9) | .635 |
| Other involved site, n | 4 | |||
| Testis/ovary (skin) | 2:2 | |||
| Immunophenotype, n (%) | ||||
| B: pro, common, pre, and undefined | 139 (68.4) | 20, 99, 10, and 9 | ||
| T: ETP, pro, pre, cortical, mature, and undefined/MPAL | 64 (31.6) | 14, 1, 10, 12, 2, and 6 | ||
| Cytogenetics/genetics, n (%) | ||||
| Normal | 57 (47.1) | 23 | 34 | |
| Adverse | 33 (27.3) | 32 | 1 | |
| t(4;11)/KMT2A::AFF4, t(11;19) | 16 | 16 | - | |
| Other‡ | 17 | 17 | 1 | |
| Nonadverse | 40 (31) | 36 | 4 | |
| t(1;19)/E2A::PBX1 | 4 | 4 | ||
| Hyperdiploid | 14 | 12 | 2 | |
| Other nonadverse | 22 | 20 | 2 | |
| Not evaluable | 73 (35.9) | 48 | 25 | |
| Ph-like signature (n = 88 studied)§ | 28 | 28 (31.8%) | ||
| Risk stratification, n (%) | ||||
| SR | 115 (56.7) | 83 (59.7) | 32‖ (50.0) | |
| HR | 20 (9.9) | 20 (14.4) | 0 | |
| VHR | 68 (33.4) | 36 (25.9) | 32 (50.0) |
ALL, acute lymphoblastic leukemia (BM blasts of >20%); BM, bone marrow; ECOG PS; Eastern Cooperative Oncology Group performance status; LL, lymphoblastic lymphoma (BM blasts of <20%); MPAL, mixed-phenotype acute leukemia (T cell/myeloid) (n = 1); NA, not available; NR, not reported; PB, peripheral blood.
Patients with LL (n = 20): stage I, n = 2; stage II, n = 4; stage III, n = 4; stage IV, n = 6; unreported, n = 4; and BM involvement (LL cells 5%-15%), n = 4.
ECOG PS score.
Other than t(4;11)/KMT2A rearrangement: 11q23, +8, −7, del6q, t(8;14) abnormalities, low hypodiploidy (30-39 chromosomes), near triploidy (60-78 chromosomes) or complex karyotype with ≥5 unrelated anomalies.
Ph-like signature not entered as HR/VHR baseline feature in original risk model.
Thirteen T-ALL and 19 T-LL.
MRD analysis
All patients who achieved CR underwent bone marrow MRD analysis at 4 time points (TPs), from the end of induction week 4 (EOI TP1) to weeks 10 (TP2), 16 (TP3), and 22 (TP4). In the final multipoint MRD risk model, patients with low positive (˂10−4) or negative TP2-3 and negative TP4 (or negative TP2-3 when TP4 was missing) were defined as MRD-negative (MRDneg), whereas those with TP2-3 of ≥10−4 and/or positive TP4, were defined as MRD-positive (MRDpos). EOI MRD was assessed without affecting the therapy-orienting risk model.
Risk-oriented therapy
Patients at SR or HR with TP2 MRD of ≥10−4, and all patients at VHR were eligible for an early allograft after cycle 3, because of their dismal outcome improvable via HCT.15 Patients at SR or HR who were MRDpos at TP3-4 and patients at HR without MRD study were also eligible for HCT. Patients at SR or HR who were MRDneg and patients at SR without MRD study were eligible to receive consolidation and maintenance chemotherapy. Patients with LL, often lacking MRD study and other risk parameters, normally went on to receive consolidation and maintenance, with few exceptions, reserving additional mediastinal irradiation or HCT as salvage treatment.
Response
The outcome after induction chemotherapy was CR, resistance, or death. Patients not in CR after course 2 were ethen excluded from the study. CR was defined as the disappearance of clinical and laboratory signs of ALL/LL, including extramedullary disease if previously detected; a transfusion-free status with neutrophils > 1.0 × 109/L and platelets > 100 × 109/L; and a normocellular or regenerating bone marrow with blast cell content <5%. A recurrence was defined as the reappearance of >5% marrow leukemic cells and/or an extramedullary involvement. Overall survival (OS) was calculated from diagnosis to death by any cause, disease-free survival (DFS) from CR to relapse in any site or death in CR by any cause, and event-free survival (EFS) from study entry to CR not achieved, relapse or death, whichever occurred first. Treatment-related toxicity was evaluated per Common Terminology Criteria for Adverse Events, version 4.0.
Trial objectives
The primary objective of GIMEMA LAL 1913 was DFS. Based on the 2-year DFS at 45% for the adult Ph– ALL branch of the previous GIMEMA LAL0904 study (unpublished data on file),18 it was calculated that 204 patients were required to obtain an increase in the DFS to ≥55%. The secondary objectives, analyzed in the whole cohort and based on the risk and treatment subsets, were the CR rate, OS, EFS, DFS, the cumulative incidence of relapse (CIR), the treatment-related mortality (TRM), MRD response, and serious treatment-related adverse events.
Statistics
Patient characteristics were summarized using crosstabulations for categorical variables, or quantiles for continuous variables. Nonparametric tests were performed for univariate comparisons among groups (χ2 and Fisher exact tests for categorical variables and Mann-Whitney and Kruskal-Wallis tests for continuous variables). Three-year OS, EFS, and DFS, with a 95% confidence interval (CI), were estimated using the Kaplan-Meier product limit estimator. Differences between survival curves were evaluated using a log-rank test. Cox regression model was used in univariate and multivariate analyses: hazard ratio and 95% CI were reported as parameter results. Simon-Makuch plot was used to assess the time-dependent effects of HCT. CIR and TRM were estimated using the cumulative incidence method, considering death in CR (CIR analysis) or relapse (TRM analysis) as a competing event. The Gray test was applied to test differences between subgroups. All tests were 2-sided, accepting P < .05 as indicative of a statistically significant difference. All analyses were performed by the GIMEMA data center following the intention-to-treat (ITT) principle using R software. Study data were collected and managed using REDCap electronic data capture tools hosted at the GIMEMA Foundation.21,22
Results
Patients
Table 1 summarizes the characteristics of the 203 patients who were evaluable. The median patient age was 39.8 years (range, 18-65 years); 19.2% were aged >55 years; 68.5% (n = 139) had B-cell ALL/LL (B-ALL/LL); and 9.8% (n = 20) had a LL with <20% marrow blasts (19 T-phenotype; P < .001). The median WBC count was higher in T-cell ALL (T-ALL; P = .003), whereas HR phenotypes were detected in 20 patients with B-ALL (pro-B) and 33 with T-ALL (early thymic precursor [ETP], n = 14; pro/pre-T, n = 11; mature-T, n = 2; and undefined T or mixed T/myeloid, n = 6). Nineteen patients (9.3%) had a central nervous system (CNS) involvement and 54% of patients with T-ALL/LL displayed an enlarged mediastinum. As for cytogenetics/genetics, 16 patients had either a KMT2A;11q23 rearrangement or a t(11;19) translocation, 17 had other adverse karyotypes, whereas a t(1;19)/TCF3:PBX1 translocation was detected in 4 and hyperdiploidy in 14. Twenty-eight of 88 patients (31.8%) who were evaluable displayed a Ph-like gene signature.20 Overall, 56.7% of study patients were at SR, 9.9% at HR, and 33.4% at VHR.
Trial disposition
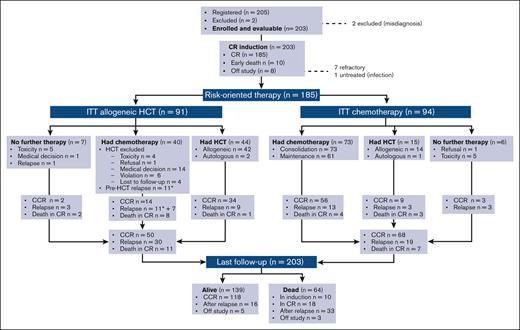
The trial database was locked on June 2021. Data from all 203 patients who enrolled between December 2014 and September 2016 were analyzed, including a patient with pneumonia at presentation who could not start chemotherapy. Figure 2 details the study flowchart. With a median follow-up of 38.7 months (range, 2.2-64.2 months) and all study subjects off therapy, 139 patients were alive (68.4%; 118 [58.1%] in CR1), whereas 64 patients had died of leukemia or treatment-related events. The main outcome figures for distinct B- and T-ALL/LL and age subsets are available in Supplemental Table 2.
Study flowchart. Patient disposition and outcome are shown overall and per risk-oriented treatment, by ITT, and as treated. Patients displaying refractory ALL after 2 treatment cycles were off study. Application of allogeneic HCT and chemotherapy based on patient age in the respective risk-oriented therapy cohorts is detailed in Supplemental Table 4; overall, patients aged from 18 to 40 years were more likely to complete the assigned chemotherapy steps, whereas HCT rates did not differ significantly across age groups. CCR, continuous first CR.
Study flowchart. Patient disposition and outcome are shown overall and per risk-oriented treatment, by ITT, and as treated. Patients displaying refractory ALL after 2 treatment cycles were off study. Application of allogeneic HCT and chemotherapy based on patient age in the respective risk-oriented therapy cohorts is detailed in Supplemental Table 4; overall, patients aged from 18 to 40 years were more likely to complete the assigned chemotherapy steps, whereas HCT rates did not differ significantly across age groups. CCR, continuous first CR.
CR induction therapy
CR was achieved after 1 (n = 173) or 2 (n = 12) induction courses in 185 patients (91.1%; 87.1% and 100% in B-ALL/LL and T-ALL/LL, respectively; P = .001). In 139 patients with B-ALL/LL, the incidence of early death and resistance was 7.2% (n = 10) and 5.7% (n = 7), respectively. Of the patients who were refractory, 4 expressed a Ph-like gene signature.
Postinduction MRD study
MRD molecular markers were available for 140 patients, whereas 24 additional patients could be monitored via flow cytometry. On total, 151 patients could be stratified per the multipoint MRD model: 114 were tested as MRDneg (75.5%), and 37 tested as MRDpos (24.5%; n = 30 with TP2 MRD ≥ 10−4). Although MRDneg rates were similar across clinical risk groups, 12 of 37 MRDpos cases expressed a Ph-like gene signature. MRD was <10−4 at EOI TP1 in 56% of 164 patients who were evaluable (43% MRDneg), and, at TP2, in 80% of the 153 patients who were evaluable (68% MRDneg), increasing further at TP3 (84%; 78% MRDneg) and TP4 (95%; 84% MRDneg), although in smaller patient groups because of early transplants or other events (Supplemental Table 3).
Comprehensive risk model and risk-oriented therapy
Per the study design, 91 patients in CR were eligible for an allogeneic HCT. This group consisted of 63 patients at VHR, 24 patients who tested MRDpos (SR, n = 23; HR, n = 1; and 19 with high TP2 MRD), and 4 patients at HR with unknown MRD. The allogeneic HCT realization rate was 46.1% (n = 42) (Supplemental Table 4), at a median time from CR of 5.1 months (range, 1.6-10.9 months). Two further patients received an autologous HCT. Pretransplantation relapse (n = 12), ineligibility because of treatment-related complications of any type and grade (n = 24), loss to follow-up (n = 4), patient refusal (n = 1), and protocol infringement (n = 6) accounted for the exclusions from allogeneic HCT.
Of the patients in CR, 94 were eligible to receive chemotherapy only. This group comprised 77 patients who tested as MRDneg (SR, n = 68; HR, n = 9) and 17 patients at SR with unknown MRD status. The treatment realization rate was 77.6%. Conversely, 14 of these patients received an allograft and 1 received an autograft, because of patient’s or physician’s preference, but mostly because of a poor tolerance to the intensive chemotherapy program.
Treatment results
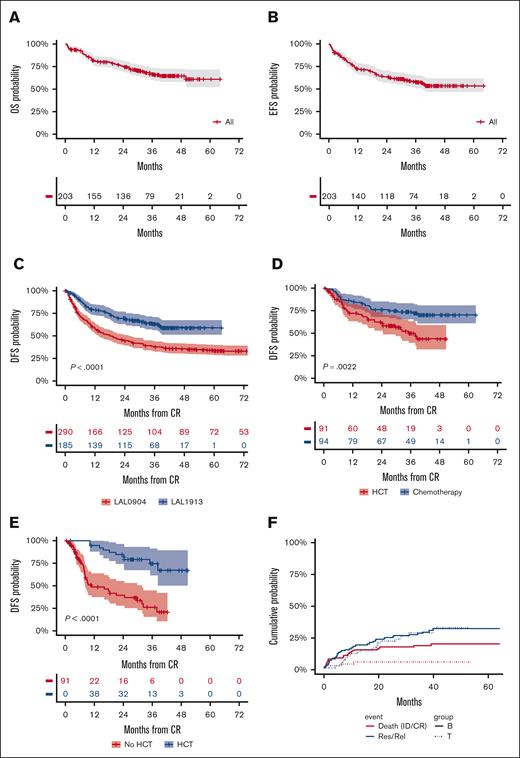
Median OS, EFS, and DFS were not achieved, with 3-year estimates projected at 66.7%, 57.7%, and 63.3%, respectively (Figure 3A-C), which by far exceeds the primary study objective of a 2-year DFS of >55%. Considering risk-oriented therapy, 3-year DFS per ITT was 74% in the chemotherapy group and 50% in the HCT group (P = .0022; Figure 3D). When the outcome was reassessed per treatment, excluding 13 patients who were unable to receive any postremission therapy, the 3-year DFS was 71% for HCT (n = 59; allogeneic, n = 56 and autologous, n = 3) and 63% for patients treated with chemotherapy (n = 113; P = .18; Supplemental Figure 1). Because patients with HCT were at a higher risk of early relapse and had a transplant at a median of 5.1 months from CR, a time-dependent analysis was performed to confirm the therapeutic benefit associated with an allotransplant, with a 75% DFS rate for the actual HCT cohort compared with 26% for patients with similar risk features without HCT (P < .0001; Figure 3E). When the same analysis was repeated based on the disease immunophenotype, HCT proved of outstanding value in T-ALL (DFS, 95%) and was also highly effective in B-ALL (Supplemental Figure 2).
Main outcome results. (A) OS: median was not reached; 3-year rate, 66.7% (95% CI, 60-74); (B) EFS: median was not reached; 3-year rate, 58% (95% CI, 51-65); (C) DFS, representing the primary study objective compared with prior GIMEMA study LAL 0904: median was not reached; 2-year rate, 70% (95% CI, 63-77) vs 45% (95% CI, 39-51); and 3-year rate, 63% (95% CI, 56-71) vs 38% (95% CI, 38-44), P < .0001; (D) 3-year DFS per ITT risk-oriented therapy: chemotherapy, 74% (95% CI. 65-83), allogeneic HCT, 50% (95% CI, 39-63), P = .0022; (E) 3-year DFS in the ITT allogeneic HCT group per time-dependent HCT realization: HCT, 75% (95% CI, 55-89) vs no HCT, 26% (95% CI, 15-45), P < .0001; (F) Cumulative incidence of TRM during induction (ID) and CR, and of resistance/relapse (Res/Rel) based on B- or T-ALL/LL diagnosis: 3-year incidence ID/CR death B-ALL/LL, 17.8% (95% CI, 11.3-24.3) vs T-ALL/LL, 4.8% (95% CI, 0-10.1), P = .0127; Res/Rel B-ALL/LL, 28.7% (95% CI, 20.9-36.6) vs T-ALL/LL, 28.1% (95% CI, 16-40.4), P = .773.
Main outcome results. (A) OS: median was not reached; 3-year rate, 66.7% (95% CI, 60-74); (B) EFS: median was not reached; 3-year rate, 58% (95% CI, 51-65); (C) DFS, representing the primary study objective compared with prior GIMEMA study LAL 0904: median was not reached; 2-year rate, 70% (95% CI, 63-77) vs 45% (95% CI, 39-51); and 3-year rate, 63% (95% CI, 56-71) vs 38% (95% CI, 38-44), P < .0001; (D) 3-year DFS per ITT risk-oriented therapy: chemotherapy, 74% (95% CI. 65-83), allogeneic HCT, 50% (95% CI, 39-63), P = .0022; (E) 3-year DFS in the ITT allogeneic HCT group per time-dependent HCT realization: HCT, 75% (95% CI, 55-89) vs no HCT, 26% (95% CI, 15-45), P < .0001; (F) Cumulative incidence of TRM during induction (ID) and CR, and of resistance/relapse (Res/Rel) based on B- or T-ALL/LL diagnosis: 3-year incidence ID/CR death B-ALL/LL, 17.8% (95% CI, 11.3-24.3) vs T-ALL/LL, 4.8% (95% CI, 0-10.1), P = .0127; Res/Rel B-ALL/LL, 28.7% (95% CI, 20.9-36.6) vs T-ALL/LL, 28.1% (95% CI, 16-40.4), P = .773.
Treatment failures
Apart from 7 patients who became refractory, 49 (26.5%) patients in CR experienced a recurrence in the bone marrow (n = 33), marrow and CNS (n = 3), CNS only (n = 4), and other extramedullary sites (n = 9). With a median observation time of 5.3 months from relapse, 16 patients were still alive. Although the cumulative incidence of resistance and relapse was comparable among the B- and T-cell subsets (P = .772), the incidence of TRM (cumulative n = 28, 13.8%) was higher in patients with B-ALL (P = .0127; Figure 3F), which led to an improved EFS in T-ALL/LL (Supplemental Figure 3). The risk of relapse was significantly higher in patients aged >40 years, whereas their remission death rate was only minimally increased (Supplemental Figure 4). Induction deaths were caused by infections in 7 patients, brain hemorrhage in 1, and pegaspargase-related toxicity in 2. Deaths in CR (n = 18) were caused by infection (n = 12), and hemorrhage, cardiovascular, renal, or gastrointestinal complications, multiorgan failure, and unspecified toxicity (1 each), and occurred during high-dose (n = 4) or standard (n = 5) consolidation, maintenance (n = 2), HCT (n = 4), or as late deaths in patients who were followed up (n = 3). Overall transplant-related mortality for the 56 patients who had allogeneic HCT in CR1 (42 in, and 14 off, protocol) was 7.1%.
Prognostic analysis
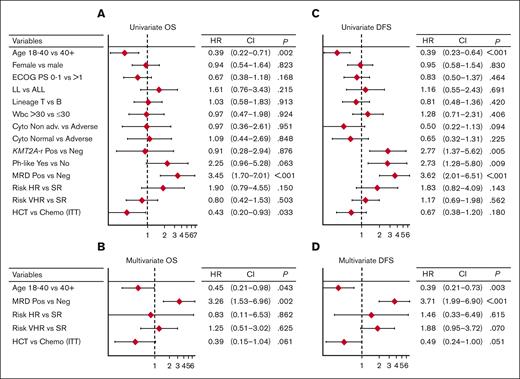
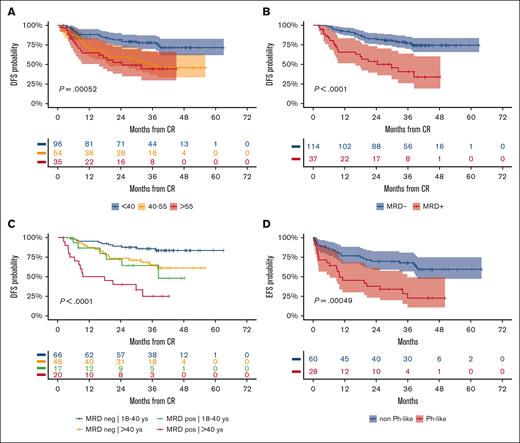
Figure 4 represents the forest plots from univariate and multivariate prognostic analysis for OS and DFS. In multivariate analysis, age ≤40 years and MRD negativity predicted a significantly better DFS (Figure 5A-B). Notably, the distribution of biologic risk factors did not differ across age groups (Supplemental Table 5), and achieving negative or low (<10−4) MRD at EOI TP1 or TP2 was equally favorable (Supplemental Figure 5). Because of their independent prognostic role, patient age and MRD risk class were entered in a combined analysis, through which the 3-year DFS was 86% in patients aged from 18 to 40 years who tested as MRDneg, 64% or 65% in those who were older or with persistent MRD, and 25% in those who displayed both risk factors (Figure 5C). When the posttransplant outcome was analyzed based on MRD, patients who tested as MRDpos appeared to benefit greatly from HCT, whereas the difference was less marked in patients who tested as MRDneg (Supplemental Figure 6). As for genetics and cytogenetics, the worse outcome of Ph-like ALL20 was confirmed at a longer follow-up, with a 3-year EFS at 23% compared with 68% for non–Ph-like B-ALL (Figure 5D). The results were superimposable for ALL or LL (Supplemental Figure 7) and for ETP and non-ETP T-ALL (data not shown). Finally, DFS was unaffected by clinical risk class, OS was marginally worse in patients at HR, and those with CNS involvement had a significantly increased relapse risk (Supplemental Figure 8).
Prognostic analysis 1. Forest plots from univariate and multivariate prognostic analysis for OS (A-B) and DFS (C-D), including major risk factors, MRD results, and risk-oriented therapy. The multivariate model analysis was performed on data from 149 patients with no missing values. All covariates were evaluated in univariate models and all relevant variables with univariate association within P < .15 were considered in the multivariate models. To compare the prognostic ability of multivariate models with the contribution of each variable, the Akaike information criterion was used to compare the models’ goodness of fit with the data. The final model includes the actual therapy received and not ITT therapy. The collinearity between treatment received and risk classification was evaluated using an interaction term into the multivariate model (resulting nonsignificant). Cyto, cytogenetics/genetics; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio.
Prognostic analysis 1. Forest plots from univariate and multivariate prognostic analysis for OS (A-B) and DFS (C-D), including major risk factors, MRD results, and risk-oriented therapy. The multivariate model analysis was performed on data from 149 patients with no missing values. All covariates were evaluated in univariate models and all relevant variables with univariate association within P < .15 were considered in the multivariate models. To compare the prognostic ability of multivariate models with the contribution of each variable, the Akaike information criterion was used to compare the models’ goodness of fit with the data. The final model includes the actual therapy received and not ITT therapy. The collinearity between treatment received and risk classification was evaluated using an interaction term into the multivariate model (resulting nonsignificant). Cyto, cytogenetics/genetics; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio.
Prognostic analysis 2. (A) 3-year DFS per patient age (years): ≤40, 78% (95% CI, 70-87); 41 to 55, 49% (95% CI, 37-66); > 55, 44% (95% CI, 30-66); P = .00052. (B) 3-year DFS per MRD risk model (n = 151 evaluable): MRDneg, 77% (95% CI, 70-86), MRDpos, 41% (95% CI, 26-64), P < .0001). (C) 3-year DFS per patient age (years) group and MRD risk model interactions: 18 to 40/MRDneg, 86% (95% CI, 78-95) vs >40/MRDpos, 65% (95% CI, 52-82) vs 18 to 40/MRDpos, 64% (95% CI, 43-95) vs >40/MRDpos, 25% (95% CI, 11-59); P < .0001. (D) 3-year EFS per Ph-like ALL gene signature in 88 patients with B-ALL who were evaluable: Ph-like, 23% (95% CI, 10-49) vs non–Ph-like, 68% (95% CI, 57-81). P = .00049.
Prognostic analysis 2. (A) 3-year DFS per patient age (years): ≤40, 78% (95% CI, 70-87); 41 to 55, 49% (95% CI, 37-66); > 55, 44% (95% CI, 30-66); P = .00052. (B) 3-year DFS per MRD risk model (n = 151 evaluable): MRDneg, 77% (95% CI, 70-86), MRDpos, 41% (95% CI, 26-64), P < .0001). (C) 3-year DFS per patient age (years) group and MRD risk model interactions: 18 to 40/MRDneg, 86% (95% CI, 78-95) vs >40/MRDpos, 65% (95% CI, 52-82) vs 18 to 40/MRDpos, 64% (95% CI, 43-95) vs >40/MRDpos, 25% (95% CI, 11-59); P < .0001. (D) 3-year EFS per Ph-like ALL gene signature in 88 patients with B-ALL who were evaluable: Ph-like, 23% (95% CI, 10-49) vs non–Ph-like, 68% (95% CI, 57-81). P = .00049.
Pegaspargase therapy
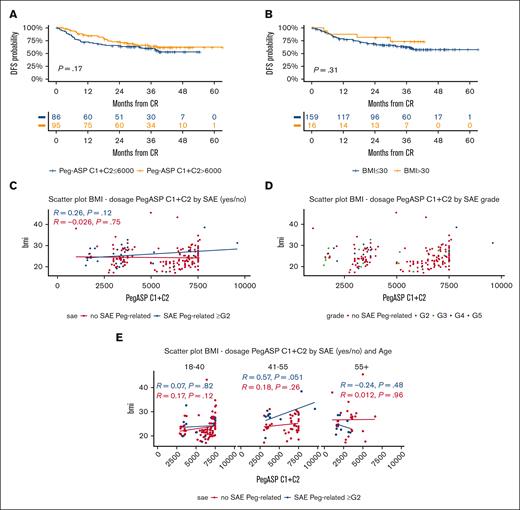
Overall, 524 total pegaspargase courses were administered, for a mean of 2.58 courses per patient (2.83 course per patient in CR), with a cumulative drug dosage of 7300 IU/m2 per patient (range, 1520-15 600) that decreased with patient age (Supplemental Table 6). The proportion of patients having a full or attenuated dose of pegaspargase ranged from 92.1% to 69% during the 4 courses, reflecting the combined effects of toxicity at first/prior drug exposure, the general treatment compliance, or an early transition to HCT. Most pegaspargase was delivered at courses 1 and 2 (n = 341, 65% of total courses), for a median patient dose of 6000 IU/m2 (range, 1000-9600), that is, 82% of the total treatment dose. A higher dose exerted a marginally positive effect on DFS, whereas a high body mass index (BMI) did not worsen outcome (Figure 6A-B).
Pegaspargase-related outcome and toxicity analysis. (A) 3-year DFS per the cumulative pegaspargase dose (IU/m2) administered at cycles (C) 1 and 2: ≤6000 IU/m2, 60% (95% CI, 50-72) vs >6000 IU/m2, 64% (95% CI, 55-76), that is, below or above the total median dose received at C1 and C2; the higher dose usually meant the full administration of the first 2 planned protocol doses. (B) 3-year DFS per BMI: BMI of ≤30, 63% (95% CI, 55-71) vs >30, 73% (95% CI, 53-100), P = .31. (C) Correlative analyses between BMI, cumulative pegaspargase dosing at cycles C1 and C2, and occurrence of grade (G) ≥2 severe adverse events (SAEs) in individual patients (nonsignificant P values). (D) Correlative analyses between BMI, cumulative pegaspargase dosing at C1 and C2, and SAE grading (nonsignificant P values). (E) Correlative analyses between BMI, cumulative pegaspargase dosing at C1 and 2, occurrence of SAE G ≥2, and age groups (nonsignificant P values). Scatter plots in panels C-E with linear model function to depict the correlation index.
Pegaspargase-related outcome and toxicity analysis. (A) 3-year DFS per the cumulative pegaspargase dose (IU/m2) administered at cycles (C) 1 and 2: ≤6000 IU/m2, 60% (95% CI, 50-72) vs >6000 IU/m2, 64% (95% CI, 55-76), that is, below or above the total median dose received at C1 and C2; the higher dose usually meant the full administration of the first 2 planned protocol doses. (B) 3-year DFS per BMI: BMI of ≤30, 63% (95% CI, 55-71) vs >30, 73% (95% CI, 53-100), P = .31. (C) Correlative analyses between BMI, cumulative pegaspargase dosing at cycles C1 and C2, and occurrence of grade (G) ≥2 severe adverse events (SAEs) in individual patients (nonsignificant P values). (D) Correlative analyses between BMI, cumulative pegaspargase dosing at C1 and C2, and SAE grading (nonsignificant P values). (E) Correlative analyses between BMI, cumulative pegaspargase dosing at C1 and 2, occurrence of SAE G ≥2, and age groups (nonsignificant P values). Scatter plots in panels C-E with linear model function to depict the correlation index.
Pegaspargase toxicity
Among 187 patients who had pegaspargase at cycle 1, 32 (17.2%) developed 41 episodes of grade (G) ≥2 toxicity, that affected mainly the hepatobiliary system (11.7%) and contributed to an induction death in 2 patients (1.1%, liver failure, n = 1; and cerebral bleeding, n = 1) (Supplemental Table 7). The incidence of G3-4 thrombosis and pancreatitis was low (2% and 1%, respectively), and that of severe toxic side effects decreased progressively during treatment progression, although 2 patients suffered from an anaphylactic reaction after the second and third drug exposure, respectively. Occurrence of toxicity correlated with a higher median BMI and led to subsequent dose reductions (Supplemental Table 6). However, no significant relationship was detectable between individual pegaspargase doses at cycles 1 and 2, occurrence of severe adverse events, and either patient age (apart from a trend in the group of patients aged 41 to 55 years; P = .051) or BMI (Figure 6C-E).
Discussion
Prospective MRD-based risk stratification has been used for >2 decades to guide treatment intensity in adult Ph– ALL, improving treatment results.15,23-25 In these and other studies,7,26-29 patients who tested as MRDneg fared particularly well on chemotherapy only, whereas proceeding to an allograft was a better choice for patients who displayed MRD persistence and/or other HR characteristics. As a corollary, the use of a pediatric-type chemotherapy was of further advantage across all patient and risk subsets.3,7,15
In this trial, pegaspargase was added to an already effective pediatric-based chemotherapy schedule.15 This GIMEMA study contemplated 4 total pegaspargase doses at 2000 IU/m2 in patients aged from 18 to 55 years, with selective dose reductions in patients aged >55 years because of the unacceptable toxicity observed in the original NILG trial. Because pegaspargase has not been systematically used in large pediatric-inspired adult trials3,4,25 or has been associated with controversial toxicity results in association with different dosing and chemotherapy schedules,30-32 we considered this issue worthy of investigation within our treatment program for adults aged 18 to 65 years.
The results of NILG 10/0715 were largely replicated in a wider national setting within the GIMEMA LAL1913 trial, proving the feasibility and efficacy of the pegaspargase-modified regimen. With CR and MRDneg rates of 91% and 75%, respectively, in 203 patients, half of whom were aged >40 years and nearly 20% aged >55 years, 3-year DFS, the primary study end point, was 63.3%, fulfilling the statistical design that required a 2-year DFS of >55%. The CIR was 26.5%, with nonrelapse mortality not exceeding 10%, whereas induction mortality in older patients diminished from 38.5% in the NILG study to 9.5% in this study. The age-unrelated DFS figure overlaps with that from AYA trials, such as a pegaspargase-free GIMEMA study (patient age, 18-35 years; 4-year DFS, 60.4%)9 and the pegaspargase-based CALGB 10403 study (patients age, 18-39 years; 3-year DFS, 66%),7 comparing well with other trials enrolling adult patients with an upper age range of 55 to 70 years (Supplemental Table 8;.) Focusing on AYA patients aged 18-40 years), DFS increased to 78% in our study, and was still close to 50% for older patients, which is remarkable for an adult trial with no associated immunotherapy. We argue that these globally favorable results could be ascribed to the combination of our pegaspargase-modified pediatric-type protocol (including lineage-targeted methotrexate up to 5 g/m2) with an effective MRD/risk-oriented strategy for HCT in patients at higher risk.
Pegaspargase was administered for 4 total courses as suggested by Douer et al.33 to detect a clinical benefit. Severe toxic side effects were below the ranges usually reported in adults12 and were mostly confined to CR induction, during which 2 drug-related deaths occurred, whereas pegaspargase was withdrawn or curtailed for suboptimal treatment compliance or an early shift to HCT in 25% to 30% of the patients. The factors sparing a greater drug-related toxicity could be the lower pegaspargase dose used in the GIMEMA trial (4× 2000 IU/m2) compared with other reference trials (6× 2000 IU/m2,33 7× 2000 IU/m2,307× 2500 IU/m2,7 and 11-16× 2000-2500 IU/m2,32) and the recommended toxicity management protocol with antithrombin or fibrinogen replacement and enoxaparin thromboprophylaxis, along with the evaluation of liver ultrasound scan and BMI for further drug reductions in case of hepatosteatosis and BMI of >30, respectively, plus the use of L-carnitine for a direct bilirubin rise of >3 mg/mL. These measures could make treatment safer without compromising efficacy, as demonstrated in patients with a BMI of >30, whose outlook was not worsened despite the association between higher BMI and adverse events grade ≥2. The study experience was reassessed by an ad hoc committee,14 leading to an operational pegaspargase algorithm for the subsequent GIMEMA study (Supplemental Table 9).34
Although our trial was not designed nor powered enough to address the debated issue of pegaspargase dose,35,36 we remark the high rates of MRDneg CR after the first 2 pegaspargase-based courses and the first lineage-targeted methotrexate block (80% MRD < 10−4 at week 10/TP2) and the very good DFS results achieved in AYA and patients who were MRDneg.
The mixed risk-oriented treatment design was another peculiar study feature, reserving an allograft to all patients who were MRDpos and to patients at VHR independently of MRD, an approach used in 124 but not another25 risk-oriented trial and awaiting the final analysis of a German study that randomized patients with MRDneg with HR features to HCT or chemotherapy (#NCT02881086). More than 80% of our patients were MRD evaluable, which allowed allocation to risk/MRD-specific treatment. Based on ITT, 3-year DFS was 74% for the chemotherapy group and 50% for the HCT group, that was enriched in patients with poor prognoses. On a time-dependent analysis performed in the HCT-eligible population to counteract the negative selection bias from early study losses, DFS was 75% and 26%, with and without HCT, respectively (P < .0001), with outstanding HCT results in patients with T-ALL (DFS of 95%). Together with a rather low transplant-related mortality, these findings call into question the preparative steps to HCT, because its realization rate was 46.1% compared with 60.9% in NILG 10/0715 and 54% to 72% in other risk-oriented therapy trials.25,27,37 Therefore, a quicker donor search with a better team concertation will be essential for more patients at VHR and/or who are MRDpos who are at risk of pre-transplantation recurrence or clinical deterioration by intensive chemotherapy.
Concerning risk definitions, early risk models can be improved using continuous risk variables (WBC counts and MRD),28 integrating MRD with genetics/cytogenetics15,26,28 and improving MRD analysis, to refine the individual prognosis, better support HCT decisions, and partly forecast HCT outcome.28 High-throughput MRD study methods are useful to evaluate uncertain MRD results or patients with MRDneg who relapse (15%-20% in our study). When MRD was reassessed with digital droplet polymerase chain reaction (ddPCR) in 44 study patients, ddPCR captured 5 relapses in patients whose Reverse Quantitative PCR (RQ-PCR) was negative and no relapse in patients with negative ddPCR with positive nonquantifiable RQ-PCR.38 Another next-generation sequencing (NGS) study reanalyzed samples from 86 patients with low-level RQ-PCR MRD, separating 2 distinct NGS MRDpos and NGS MRDneg prognostic groups.39
Improving risk-oriented strategies involves team-work skills in diagnostics, application of intensive chemotherapy, MRD monitoring, HCT planning and timely execution, and long-term patient management. The present pegaspargase-modified risk-oriented nationwide phase 2 trial involved 51 study sites and fulfilled its objectives. The study had some limitations: first, too many patients who were eligible did not have access to HCT. Although partly explained by early relapse or toxicity, this underlines the importance of adhering to HCT allocation and expediting all related procedures. Second, pegaspargase was not applied very intensively, and the lack of therapeutic drug monitoring prevented us from using Erwinase in cases of silent drug inactivation, which occurs less frequently using the pegylated compound. Other studies reported significant therapeutic effects with pegaspargase doses and serum concentrations lower than common standards,35,36,40 a body of evidence in line with our study results. Third, this protocol, designed in 2013, was devoid of immunotherapy, which is currently indicated.2,41 Although this was a time effect of protocol conception, rituximab, blinatumomab, and/or inotuzumab ozogamicin are now recommended for untreated CD20/19/22+ B-ALL. These agents are enabling further therapeutic progress in any risk category and in both AYA/adults34,42,43 and older patients with very poor prognosis,44-48 who are typically less tolerant to chemotherapy and are poor candidates for HCT.
Acknowledgments
This work was supported by Associazione Italiana Ricerca sul Cancro (AIRC), Special 5×1000 Program Metastases (21198), Milan, Italy (R.F.), and Bandi di Ricerca 2021 (RM12117A8A9BCE3E) (S.C.). Study drug (pegaspargase) and research grant kindly provided by Sigma-Tau to the GIMEMA Foundation (agreement signed on 14 March 2014).
Authorship
Contribution: R.B. designed and led the study, analyzed the data, and wrote the manuscript; R.F. was a coprincipal investigator, designed and conducted the study, and edited the manuscript; S.C. conducted the study and correlative analysis (MRD and Ph-like signature), analyzed the data, and edited the manuscript; A.R. designed and conducted the study and analyzed the data; I.D.S., O.S., A.S., M.M., L.E., M.S.D.P., A.G., and A.V. performed research, conducted correlative analysis, and analyzed the data (cytogenetics, genetics, and MRD); A.M.S., E.A., L.M., E.B., P.Z., F.D.R., G.M., D.M., N.F., M. Bocchia, P.D.F., M. Bonifacio, A.C., V.C., P.D.B., G.L., E.M., S.T. and M.O. conducted the study and analyzed the data; F.P., A.P. and P.F. provided the statistical design and analyzed the data.
Conflict-of-interest disclosure: R.B. served on the speaker’s bureau and advisory board for Amgen, Incyte, Servier, Novartis, and Kite Pharma/ Gilead. S.C. served on advisory board for Amgen, Incyte, Kite Pharma/Gilead, and AbbVie. E.B. served as consultant for Janssen, as an advisory board member for AbbVie, and received travel grants from Incyte. G.M. received research support form Novartis, Bristol Myers Squibb, Amgen, Pfizer, Genzyme, and Celgene; served as consultant for Novartis, Bristol Myers Squibb, Amgen, Pfizer, Ariad Pharma, and Roche; served as speaker for Novartis, Bristol Myers Squibb, Amgen, Pfizer, Ariad Pharma, Genzyme, Celgene, Ariad, and Glaxo; and received honoraria from Novartis, Bristol Myers Squibb, Amgen, Pfizer, Genzyme, Celgene, and Ariad. N.F. served as advisory board member for, and received travel grants from, Amgen, Pfizer, AbbVie, and Incyte. M. Bocchia served as consultant and advisory board member for Incyte, Novartis, AstraZeneca, Janssen, and AbbVie. P.D.F. received fees as consultant from Jazz and Medac. M. Bonifacio received honoraria from BMS, Pfizer, and Incyte. A.C. received personal fees from AbbVie, Amgen, Gilead, Jazz, Pfizer, and Incyte. A.P. served as consultant for Takeda. A.R. served as consultant and on the speaker’s bureau for Amgen, Omeros, Novartis, Astellas, Jazz Pharmaceuticals, AbbVie, Janssen, Pfizer, Incyte, and Kite/Gilead. R.F. served on the speaker’s bureau for Amgen, Novartis, and AstraZeneca. The remaining authors declare no competing financial interests.
Correspondence: Renato Bassan, UOC Ematologia, Ospedale dell’Angelo, Via G. Paccagnella 11, 30174 Venezia, Italy; e-mail: bassanre@gmail.com.
References
Author notes
The complete GIMEMA LAL 1913 protocol is available on request from the corresponding author, Renato Bassan (bassanre@gmail.com), or from GIMEMA Central Office (gimema@gimema.it).
Study datasets are retained at GIMEMA Central Office (gimema@gimema.it).
Original study data, deidentified individual participant data, and full-study protocol are availalable through GIMEMA Foundation at www.gimema.it.
The full-text version of this article contains a data supplement.