Key Points
CART-38 cells are effective against preclinical models of human AML, T-ALL, and MM.
CART-38 cells are at least as effective as current immunotherapies for these diseases.
Abstract
Many hematologic malignancies are not curable with chemotherapy and require novel therapeutic approaches. Chimeric antigen receptor (CAR) T-cell therapy is 1 such approach that involves the transfer of T cells engineered to express CARs for a specific cell-surface antigen. CD38 is a validated tumor antigen in multiple myeloma (MM) and T-cell acute lymphoblastic leukemia (T-ALL) and is also overexpressed in acute myeloid leukemia (AML). Here, we developed human CD38-redirected T cells (CART-38) as a unified approach to treat 3 different hematologic malignancies that occur across the pediatric-to-adult age spectrum. Importantly, CD38 expression on activated T cells did not impair CART-38 cells expansion or in vitro function. In xenografted mice, CART-38 mediated the rejection of AML, T-ALL, and MM cell lines and primary samples and prolonged survival. In a xenograft model of normal human hematopoiesis, CART-38 resulted in the expected reduction of hematopoietic progenitors, which warrants caution and careful monitoring of this potential toxicity when translating this new immunotherapy into the clinic. Deploying CART-38 against multiple CD38-expressing malignancies is significant because it expands the potential for this novel therapy to affect diverse patient populations.
Introduction
Chimeric antigen receptor (CAR) T-cell therapy has shown tremendous promise for the treatment of patients with relapsed or refractory (R/R) malignancies, in particular in B-lymphoid neoplasms in which the lineage antigen CD19 is an attractive target.1,2 In most cancers, however, tumor-specific antigens are not well-defined, which hampers further development of CAR–T-cell therapies. Despite recent advances in the treatment of acute myeloid leukemia (AML), the prognosis of children and adults with R/R disease remains dismal.3,4 Similarly, there are no standard-of-care immunotherapies for patients with relapsed T-cell acute lymphoid leukemia (T-ALL),5 and the outcome for children with relapsed T-ALL is also poor with overall survival <20%.6 New therapies are thus desperately required for these populations that are chemo-refractory, and immune therapies have the potential to improve outcomes with less morbidity and mortality.
Human CD38 is an ectoenzyme that catalyzes the synthesis and hydrolysis of cyclic adenosine diphoshate-ribose, leading to Ca2+ mobilization and multiple immunoregulatory functions. Signaling events downstream of CD38 include activation of NF-κB, cyclin A, and cyclin D1, and MDM2/p53,7 and recent data highlight the role of CD38 as a metabolic inhibitory counterregulatory molecule on both cancer cells and myeloid-derived suppressor cells.8,9
CD38 is a validated tumor antigen in multiple myeloma (MM),10 and preclinical data and clinical case reports also support its attractiveness as a target antigen of interest in T-ALL11,12 and AML.13 Daratumumab combinations are now being tested in clinical trials in patients with B- and T-ALL and AML (NCT03067571 and NCT03384654). Although CD38 is expressed on healthy hematopoietic progenitor cells, treatment with the anti-CD38 antibody daratumumab does not appear to cause significant cytopenias.14
Deploying a CD38-targeting CAR-T approach against multiple malignancies is significant because it increases the potential for such therapy to affect multiple patient populations simultaneously and thereby increasing the efficiency of the translational discovery pipeline. Here, we describe optimization of new CAR–T cells targeting the CD38 molecule as a single effective approach to treat 3 different hematologic malignancies across a spectrum of age ranges, including adult and pediatric AML, T-ALL, and MM and compare CD38-redirected T cells (CART-38) with other immunotherapies that are currently being tested clinically for AML, T-ALL, and MM.
Methods
Generation of CAR38 constructs
The pTRPE anti-CD38-41BB-CD3ζ (CAR38) plasmid DNAs were generated by cloning light-to-heavy or heavy-to-light chain orientations of antihuman CD38 single-chain variable fragments derived from antibodies Ab79,15 MOR202,16 and MOR3080 (US20100317546A1) with custom synthesis into a previously described CAR19 plasmid vector.17 A total of 6 CD38-directed CAR constructs (supplemental Figure 1) were evaluated in this preclinical study to select the optimal one for future clinical settings. Control CAR constructs were generated as previously published.17,18
Generation of CAR–T cells
Healthy donor peripheral blood mononuclear cells were stimulated and expanded in vitro using anti-CD3/CD28 Dynabeads added on the first day of culture. Peripheral blood mononuclear cells were transduced with lentiviral supernatant 1-day after stimulation at a multiplicity-of-infection of 5. T cells were grown in RPMI cell culture medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin (R10 medium) for up to 20 days and then viably cryopreserved in 90% FBS and 10% dimethyl sulfoxide for future experiments. Additional cytokines were not added. CD38-deficient CD19-targeting CAR–T cells were generated from total T cells obtained from the Human Immunology Core at the University of Pennsylvania. Ten million T cells, resuspended in 100 μL LONZA P3 nucleofector solution, were electroporated with a CRISPR ribonucleoprotein, composed of 5 μg IDT Cas9 protein and 2.5 μg CD38 guide RNA, using a LONZA 4D-Nucelofector X-Unit. Cells were electroporated using program code E0-115 and then quenched with T-cell medium (X-VIVO 15 cell medium, 5% human serum, 1% GlutaMAX, and 1% penicillin streptomycin). Cells were kept for resting overnight (37°C; 5% CO2) and then stimulated using anti-CD3/CD28 Dynabeads for 24 hours before transduction with CAR-19 lentivirus. The CD38-deficient CD19-targeting CAR–T cells (CART-19 CD38KO) were expanded in vitro as described earlier. Untransduced T cells (UTDs) and CD19-, CD33-, and CD123-targeting CAR–T cells were used as negative and positive controls, respectively, for the in vitro and in vivo experiments described later.17-20
Human leukemia cell lines and primary patient specimens
The MOLM14 cell line was originally obtained from the German collection of microogranisms and cell cultures GmbH (DSMZ), transduced with an EF1a-driven click beetle luciferase-enhanced green fluorescent protein construct, and sorted to purity (hereafter, called MOLM14-CBG-GFP). Deidentified or coded primary childhood or adult AML and T-ALL specimens were obtained from the Children’s Hospital of Philadelphia Center for Childhood Cancer Biorepository or the University of Pennsylvania Stem Cell and Xenograft core facility via institutional review board–approved research protocols in accordance with the Declaration of Helsinki. Patient-derived xenograft (PDX) models were established as previously described.11,18,21 For all in vitro functional studies, AML cells were thawed at least 12 hours before analysis and kept to rest overnight at 1 × 106 cells per mL in R10 at 37°C. For cell line xenograft and PDX model studies in vivo studies, AML, T-ALL, or MM cells were thawed, washed once in phosphate-buffered saline (PBS) and injected via the lateral tail vein.
Primary human CD34+ cells
Granulocyte colony-stimulating factor–mobilized peripheral blood samples were obtained from leftover clinical specimens in accordance with a University of Pennsylvania institutional review board–approved consent form for clinical hematopoietic stem or progenitor cell donation. These specimens were deidentified of patient health information, so age and sex information are not available for these specimens. CD34+ selection was performed using the CD34 Microbead Kit (catalog #130-046-703) obtained from Miltenyi Biotech, and purity of >95% was confirmed using flow cytometry.
Flow cytometry
Antibodies were purchased from BD Biosciences, BioLegend, or eBioscience and are listed in the supplemental Materials. Human leukemia cells were isolated from in vitro culture or from peripheral blood or spleens of PDX model animals, washed once in PBS supplemented with 2% FBS, and stained at 4°C after blockade of Fc receptors.
For cell number quantitation, Countbright beads were used per the manufacturer’s instructions (Invitrogen). In all analyses, the population of interest was gated based on forward vs side scatter characteristics followed by singlet gating. Live cells were gated using Live Dead Fixable Aqua (Invitrogen). Surface expression of the anti-CD38 CAR was detected by staining with allophycocyanin-conjugated recombinant human CD38 protein (Sino Biological) and the anti-CD123 and anti-CD33 CAR–T cells were stained with an Alexa Fluor 647-conjugated goat antimouse F(ab′)2 antibody (Jackson ImmunoResearch). Quantitative human leukemia and CART-38 analyses from animal studies were performed on a 3-laser Fortessa or a 4-laser FACSVerse flow cytometer. All data analyses were performed using FlowJo v10.6.2 or Cytobank software.
T-cell degranulation and intracellular cytokine assays
CD107a degranulation assays and intracellular cytokine assays were performed as previously described.22 In brief, T cells were incubated with target cells at a 1:5 ratio. After staining for CAR expression, antibodies against CD107a, CD28, CD49d, and monensin were added at the time of incubation. After 4 hours, cells were harvested and stained for CD3 and viability. Cells were fixed and permeabilized (FIX & PERM Cell Fixation & Cell Permeabilization Kit, Life Technologies), and intracellular cytokine staining was then performed.
Cytotoxicity assays
Target MOLM14-CBG-GFP cells or primary AML samples were used for cytotoxicity assay as previously described.23 In brief, targets were incubated at the indicated ratios with effector T cells for 4 or 18 hours in technical duplicates. The number of residual live target cells was determined by quantifying photons emitted after addition of D-luciferin on a luminometer, and percent cytotoxicity was calculated according to the formula, (photons in negative control well − photons in experimental sample well) ÷ photons in negative control well × 100.
CART-38 self-protection assay
Carboxyfluorescein diacetate succinimidyl ester (CFSE)–labeled effector CART-38 cells were used in a CART-38 self-protection assay following a modified cytotoxicity assay protocol.23 In short, CART-38, CD38+ CART-19, and CART-19 CD38KO targets were incubated at the indicated ratios with effector CART-38 cells for 20 hours in technical duplicates. The number of residual live target cells was determined by measuring the CFSE fluorescence intensity via flow cytometry. Effector CART-38 cells were CFSE labeled using a CFSE fluorescent cell labeling kit (Abcam) following the manufacturer’s protocol.
Cell line and PDX models for in vivo studies
Nonobese diabetic severe combined immunodeficiency γchain–/– (NSG, Jackson Labs stock # 005557) and NSG mice transgenic for human interleukin-3 (IL-3), stem cell factor, and granulocyte-macrophage colony-stimulating factor (NSGS, Jackson Labs stock # 013062) were obtained from Jackson Laboratories and bred in-house for downstream experimental studies. All experiments were performed by certified personnel following the protocols approved by the Institutional Animal Care and Use Committees at Children’s Hospital of Philadelphia and University of Pennsylvania.
Schemas of xenograft model studies are delineated in the relevant figures and in “Results.” Human AML, T-ALL, MM, or healthy CD34+ hematopoietic cells were injected with 200 μL of PBS at the indicated concentrations into the tail veins of mice. Mice were conditioned with busulfan 25 mg/kg intraperitoneally 24 hours before AML cell injection. Primary leukemia and human hematopoiesis engraftments were defined as >1% human CD45+ cells in the peripheral blood via flow cytometry. Mice were euthanized per the protocol either at study termination, when moribund, or upon the development of hind limb paralysis.
Methylcellulose colony-forming unit assay
Sorted CD34+ adult granulocyte colony-stimulating factor -mobilized peripheral blood was resuspended in MethoCult Optimum (Stemcell Technologies catalog #04044) per the manufacturer’s instructions and plated in 6-well plates at 1 × 103 cells per well for 14 days. In some experiments, CD34+ cells were first cultured for 4 hours with CART-38 or control T cells. After 14 days, colonies were scored on an inverted microscope (Zeiss; 4×).
Statistical analyses
All statistical analyses and data display were performed using GraphPad Prism version 9.3.1. The number of independent experiments, biological and technical replicates, and statistical tests used are indicated in the relevant figure legends.
Results
Anti-CD38 CAR–T cells exhibit antigen-specific effector functions
We designed 6 lentiviral CAR38 constructs using the scFvs of 3 different antibodies linked to 4-1BB costimulatory and CD3ζ intracellular signaling domains, as previously described for CAR19 (supplemental Figure 1).17 Previously published studies reported CAR38-mediated T-cell fratricide.24,25 As shown in Figure 1A, upon primary activation and transduction of T cells with the 6 candidate constructs there was no significant difference in numerical expansion compared with untransduced controls. CAR38 and CD38 were likely present on the same cells over time, and we noted progressive enrichment of CAR38-expressing cells during the culture (Figure 1B-C; supplemental Figure 2). This suggests that CART-38 can lyse CD38-expressing T cells that do not express the CAR and that expression of the CAR protects CART-38 from self-lysis, as was previously shown with CAR19-expressing cells.26
To further address the lack of observed CAR38-mediated T-cell fratricide and demonstrate that CAR38 expression protects CART-38 cells from self-lysis, we performed a modified cytotoxicity assay (Figure 1D). Effector CART-38 cells were labeled with CFSE and incubated with CART-38 target cells that were not CFSE labeled. CD38+ CART-19 cells served as positive control, and CART-19 cells that had undergone CRISPR-Cas9–mediated knockout of CD38 (CART-19 CD38KO) were used as negative control. Live residual CFSE-negative target cells were measured at different effector-to-target (E:T) ratios (Figure 1E,F). The results demonstrate statistically significant differences in the percentages of live remaining target cells at the different E:T ratios tested between the CD38+ CART-19 and CART-38 target cells. Notably, at higher E:T ratios, CART19 that had undergone CRISPR-based CD38 deletion were only partially protected, likely because of a residual 8% CD38 expression in the CART-19 CD38KO cells (data not shown). Taken together, these results provide evidence for CAR38-mediated protection against CART-38 self-lysis.
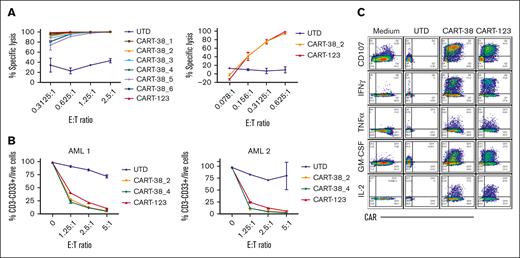
T cells transduced with each of the 6 CAR-38 constructs were first assessed for lysis of MOLM14, a human CD38+ AML cell line. All were highly potent at low E:T ratios, and T cells transduced with CAR-38 constructs 1, 2, or 4 most significantly inhibited MOLM14 proliferation compared with UTD control treatment (Figure 2A; supplemental Figure 3). CART-38_2 and CART-38_4 were then incubated with 2 different primary AML samples and similarly demonstrated strong cytotoxic activity at low E:T ratios, comparable with control anti-CD123 CART cells (CART-123) used as a positive killing control (Figure 2B; supplemental Figure 4). Finally, we confirmed the ability of the different constructs to mediate antigen-specific T-cell degranulation and effector cytokine production (Figure 2C; supplemental Figure 5). Based on data showing broad similarity in effector functions among the different constructs, we selected CART-38_2 as the optimal product to advance to more detailed testing in vivo.
CART-38 cells mediate a potent in vivo anti-AML effect
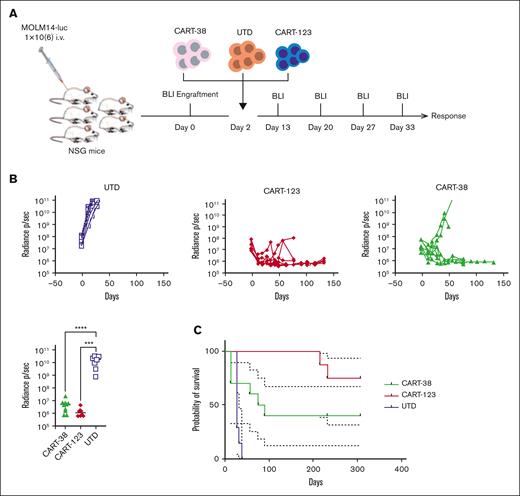
To evaluate the in vivo efficacy of CART-38_2, NSG mice were engrafted with 1 × 106 luciferase-expressing MOLM14 cells (Figure 3A). After 6 or 8 days, tumor engraftment was confirmed via bioluminescence imaging, and mice were randomized to receive a single injection of either CART-38, CART-123 (positive control), or UTD (negative control) cells. The AML burden over time was quantified via serial bioluminescence imaging. Mice treated with UTD cells succumbed quickly to disease, whereas mice treated with CART-38 or control CART-123 showed marked reduction of leukemia burden (Figure 3B). Treatment with CART-38 and with CART-123 also significantly prolonged survival compared with UTD-treated animals with a nonsignificant trend to improved survival in the CART-123 vs CART-38 cohort in this model (Figure 3C).
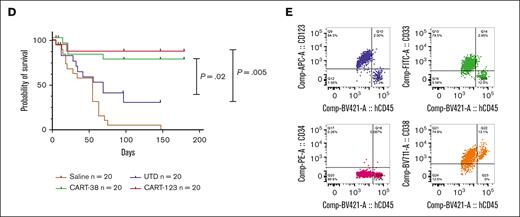
Because primary AML samples may exhibit more phenotypic diversity than immortalized cell lines, we considered it important to evaluate the ability of CART-38 to eradicate human AML in vivo in a spectrum of adult and childhood AML PDX models.27,28 Groups of NSGS mice were injected with 1 of 5 primary adult AML specimens, representing a continuum of CD38 expression levels, or 1 of 2 pediatric primary AML specimens of different genetic backgrounds (supplemental Table 1; supplemental Figure 6). Mice were treated with a single injection of 1 × 105 CART-38, CART-123,18 UTD cells, or saline (Figure 4A). We observed that both CART-38 and CART-123 treatment robustly targeted leukemia in all adult and pediatric AML PDX models tested (Figure 4B-C) and in prolonged animal survival (Figure 4D). Interestingly, in cases with residual marrow AML, there was no evidence of CD38 antigen loss (Figure 4E). We further saw that CART-38 and CART-123 treatment was generally well-tolerated with minimal animal weight loss over time as a surrogate for toxicity assessment (supplemental Figure 7).29,30
In vivo activity of CART-38 in AML patient-derived xenograft models.
In vivo activity of CART-38 in AML patient-derived xenograft models.
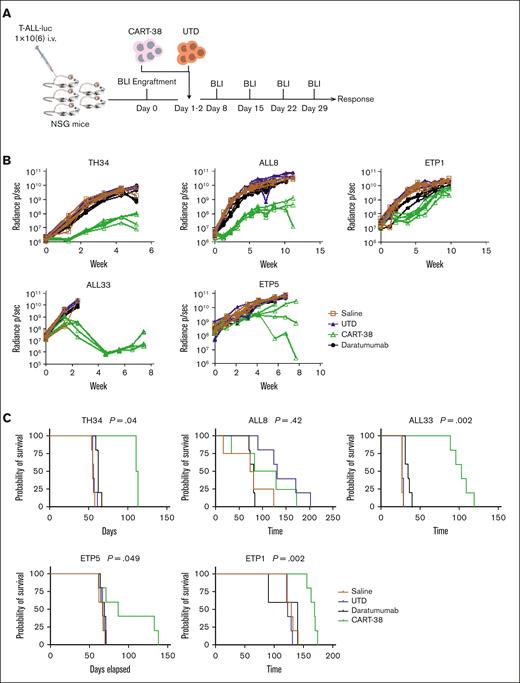
CART-38 cells mediate a potent in vivo anti-T-ALL effect
We previously reported that the anti-CD38 antibody daratumumab can mediate an antileukemic effect in preclinical models of pediatric T-ALL, leading to clinical testing of anti-CD38 monoclonal antibodies in this disease as monotherapy and combined with chemotherapy.11 Although single-agent monoclonal antibody therapy can be an efficacious treatment modality, it rarely leads to durable remissions.31 Here, we sought to extend our earlier findings to CART-38. PDX models were established from 6 pediatric patients with T-ALL (supplemental Table 1), including 3 patients with early T-cell precursor (ETP) ALL and 3 patients with non-ETP ALL. Mice were randomized to receive a single administration of either CART-38, UTD cells, or saline vehicle control. The T-ALL burden in mice was followed up with serial weekly bioluminescent imaging (Figure 5A). CART-38 treatment resulted in a variable antitumor activity against T-ALL in 5 PDX models, whereas rapid leukemic progression was observed in all UTD or daratumumab-treated control mice (Figure 5B). CART-38 also prolonged survival in 4 of 5 T-ALL PDX models tested (Figure 5C).
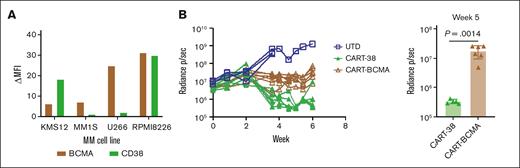
CART-38 cells mediate a potent in vivo anti-MM effect
Immunotherapeutic targets for MM have bifurcated into CD38 (monoclonal antibodies, such as daratumumab and isatuximab) and B-cell maturation antigens (BCMAs; CAR–T cells and bispecific T-cell engagers). We, therefore, sought to directly compare the activity of anti-BCMA with that of anti-CD38 CAR-Ts in vivo. We selected the human MM RPMI 8226 cell line with similar levels of CD38 and BCMA positivity (Figure 6A; supplemental Figure 9). NSG mice engrafted with the CD38+ BCMA+ cell line RPMI 8226 were then treated with CART-38 or with CART-BCMA that was previously used in a recently published clinical trial,32 and tumor burden was followed over time (Figure 6B). Disease control was superior in the CART-38–treated mice compared with the CART-BCMA–treated mice. In contrast, the activity of CART-38 against U266, a MM cell line with high expression of BCMA and minimal expression of CD38, was more modest than that of CART-BCMA, as expected (supplemental Figure 10).
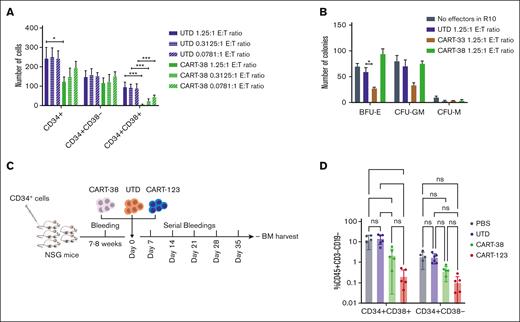
Targeting CD38 results in hematopoietic toxicity
Human CD38 is expressed on immature hematopoietic cells and is highly expressed by activated T cells, B cells, dendritic cells and natural killer cells.33 Given CD38 expression on a variety of normal hematopoietic cells,33 we hypothesized that CART-38 treatment could induce hematopoietic toxicity to CD34+CD38+ hematopoietic progenitors while sparing CD34+CD38− hematopoietic stem cells. Short-term exposure of primary human CD34+ cells to CART-38 via in vitro methylcellulose colony assays resulted in reduction of the number of CD34+CD38+ progenitor cells in a dose-dependent manner (Figure 7A). In contrast, colony formation was not markedly impaired after CART-38 treatment, suggesting that CD34+CD38– stem cells were not impaired (Figure 7B).20,34
To investigate the effect of CART-38 on hematopoiesis in vivo, NSG mice engrafted with normal human CD34+ cells from healthy bone marrow transplant donors were treated with CART-38, CART-123, or UTD human T cells (Figure 7C). As predicted based on CD38 expression on immature hematopoietic cells,35 decreased numbers of CD34+CD38+ progenitor cells were observed in the bone marrow of mice 5 weeks after CART-38 treatment. A more profound reduction of normal hematopoietic cells was detected after CART-123 treatment, as we previously reported.18 A statistically insignificant reduction in the CD34+CD38− hematopoietic stem cell compartment after CART-38 treatment was also observed (Figure 7D).
Discussion
We report the preclinical development and evaluation of CD38-redirected CAR–T-cell (CART-38) immunotherapy across a range of adult and pediatric AML, pediatric T-ALL, and MM models. We developed potent T cells genetically engineered with a CAR construct that recognizes CD38 and is activated via CD3ζ and 4-1BB signals and observed their potent in vitro effector functions against cell line and primary samples, including specific killing at low effector-to-target ratios, degranulation, robust effector cytokine production, and proliferation. We further saw robust in vivo activity of our preferred CART-38 with the elimination of leukemia proliferation and prolonged survival of PDX models of AML, T-ALL, and MM, including those with diminished CD38 expression.
Daratumumab, a recombinant monoclonal anti-CD38 antibody, is approved for patients with MM and has been shown to be both clinically safe and effective. However, in most cases the response is transient.31 Others have reported the preclinical suitability of anti-CD38–modified T cells in MM,34,36 and there is an ongoing multicenter clinical study to evaluate the safety and efficacy of anti-CD38 CAR–T cells in patients with R/R MM (NCT03464916).
Yoshida et al recently reported the feasibility of anti-CD38 CAR-T therapy for human AML.37 Our results are notable when compared with the results by this group, which showed that enhancement of CD38 expression on AML cells with alltransretinoic acid treatment was prerequisite to achieve optimal CART-38 cytotoxic activity. In addition, these investigators reported that supplementing T-cell expansion with an anti-CD38 antibody was imperative to suppress CART-38–mediated fratricide.24 Conversely, we observed high transduction efficiencies of 6 different CD38 CAR constructs into T cells in our studies without clear evidence of CART-38–mediated fratricide in vitro or in vivo. Others have similarly effectively expanded CART38 cells.34,36 These observed disparities among studies could be related to a number of variables, including retroviral vs lentiviral CAR vector design, differences in costimulatory domains, different CD38-specific scFvs, and distinct T-cell transduction and expansion strategies. In a first clinical report, 6 patients with relapsed AML after allogeneic stem cell transplantation were treated with autologous CART-38 with acceptable rates of toxicity and with preliminary evidence of antileukemic activity.32 The leukemic stem cell compartment in AML has been classically described as CD34+CD38–,38 in which case one might expect CART-38 to select for the bulk AML population and leave unscathed AML LSCs that could contribute to subsequent leukemia relapse. However, this immunophenotype has been challenged by more recent observations.39,40 The clinical use of CART-38 in patients with AML may help to shed light on this question. Our group recently showed that targeting CD38 with daratumumab was effective against T-ALL in human PDX models and proposed CD38 as a novel target also in the treatment of T-ALL.11 Since then, others have reported similar results.12,13 Additionally, recent case reports have corroborated these findings in patients with relapsed T-ALL, providing further rationale for new cellular immunotherapeutic targeting of CD38 in T-ALL.41,42 Daratumumab in combination with chemotherapy was recently studied in children and young adults with relapsed T-ALL in an early phase trial (NCT03384654). Preliminary results appear encouraging with an overall response rate (complete remission and complete remission with incomplete count recovery) of 83.3% in children and 60% in young adults with relapsed T-ALL.43 We demonstrate, here, that CART-38 manifests remarkable potency against both ETP and non-ETP T-ALL PDX models and expands the potential treatment options for patients with otherwise limited therapeutic choices with potentially superior efficacy to that of daratumumab.
In these studies, we have compared our CART-38 cells with other CAR-T products, such as CART-123 in AML and CART-BCMA in MM, and acknowledge the limitations that arise from comparing constructs based on different scFvs. However, we consider the use of the other CAR-Ts as controls and have included these comparisons to provide some context with other CAR-Ts that are being tested in patients with AML and MM.
The in vivo hematopoietic toxicity and reduction in CD34+CD38+ myeloid progenitors with CART-38 in our preclinical studies is to be expected based on CD38 expression on immature hematopoietic cells. We observed a trend toward a reduction in CD34+CD38– hematopoetic stem cells in vivo as well after CART-38 that merits further study in future preclinical experiments and among patients. This phenomenon could be explained by a subset of HSCs and primitive progenitors being CD34+CD38low35 and, thereby, potentially targeted by CART-38. Another possibility is that the suppression of CD34+CD38− cells is mediated via cytokines from CART-38. Consistent with previous reports,34,36 this potential toxicity was not captured in our in vitro colony-forming assay experiments and highlights the importance of complementing in vitro studies with relevant in vivo xenograft models of human hematopoiesis. Although our CD34+ xenograft model is an imperfect system to model human hematopoiesis in vivo, it may be the most stringent test for hematopoietic toxicity currently available in a preclinical setting.44 Moreover, our first preclinical findings suggest caution and need for careful planning when initiating future clinical trials of CART-38. Notably, affinity optimization of anti-CD38 binders has been shown to restrict CART-38 recognition to cells with very high expression of CD38.45 This approach led to specific recognition of myeloma cells that express very bright CD38 and sparing of healthy hematopoietic cells that express CD38 at more moderate levels. We did not incorporate this strategy here because our goal was to develop a therapeutic target for acute leukemias as well as for myeloma, and we recognize that this approach would likely result in at least transient hematopoietic toxicity.
Collectively, our results suggest that CD38 is a viable target in the 3 hematologic malignancies interrogated in this investigation, AML, T-ALL, and MM, across the pediatric-to-adult age spectrum.
Acknowledgments
The authors thank Miroslaw Kozlowski and Michael Klichinsky for their technical assistance in cloning these constructs.
This work was funded by a Leukemia and Lymphoma Society (LLS) Specialized Center of Research grant (C.H.J., D.T.T., R.A., and S.G.), an LLS scholar award (S.K.T.), National Institutes of Health (NIH)/National Cancer Institute grant 1U01CA232486 (S.K.T.), the Andrew McDonough B+ Foundation (S.K.T.), the Gerdin Family Foundation (S.K.T. and R.A.), and the St. Baldrick's Foundation/Stand Up to Cancer Pediatric Dream Team (S.K.T.). Stand Up to Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research. C.D. was supported by a CIHR Fellowship award and an ASCO Young Investigator award.
Authorship
Contribution: T.G.-A., C.D., J.A.C., and K.V. designed and performed research, analyzed data, and contributed to manuscript writing; O.S., F.S., S.N.-C., J.A.C, T.L.V., and F.M. designed and performed research and analyzed data; M.C.M. designed research, analyzed data, and wrote the manuscript; C.H.J. contributed to scientific discussion and edited the manuscript. D.T.T., S.K.T., and R.A. conceptualized the study, designed and performed research, analyzed data, and wrote and edited the manuscript; and S.G. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: M.C.M. declares multiple patents related to CAR-Ts. C.H.J. is a scientific founder and has equity in Tmunity Therapeutics and Capstan Therapeutics and reports grants from Tmunity Therapeutics and is on the scientific advisory boards of BlueSphereBio, Cabaletta, Carisma, Cellares, Celldex, ImmuneSensor, Poseida, Verismo, Viracta Therapeutics, WIRB Copernicus Group, and Ziopharm Oncology. D.T.T. serves on advisory boards for Sobi, Beam Therapeutics, and Janssen; receives research funding from Beam Therapeutics and NeoImmune Tech; and has patents related to CAR-Ts. S.K.T. receives research funding from Beam Therapeutics, Gilead Sciences, Incyte Corporation, and Kura Oncology for unrelated studies; has consulted for bluebird bio; and is on the scientific advisory boards of Aleta Biotherapeutics, Kura Oncology, and Syndax Pharmaceuticals. S.G. declares multiple patents related to CAR-Ts; is a scientific founder and has equity in Carisma Therapeutics and Interius Biotherapeutics; and reports grants from Carisma Therapeutics and Interius Biotherapeutics. The remaining authors declare no competing financial interests.
Correspondence: Saar Gill, Hematology/Oncology, University of Pennsylvania, 8-101 Smilow Research Center, 3400 Civic Center Blvd, Philadelphia, PA 19104; e-mail: saargill@pennmedicine.upenn.edu.
References
Author notes
∗C.D. and J.A.C. contributed equally to this study.
Data are available on request from the corresponding author, Saar Gill (saargill@pennmedicine.upenn.edu).
The full-text version of this article contains a data supplement.