Key Points
First comparative effectiveness study of acalabrutinib and ibrutinib in real-world patients with chronic lymphocytic leukemia.
Acalabrutinib demonstrated statistically significant longer time to discontinuation than ibrutinib.
Abstract
Novel agents, including Bruton tyrosine kinase inhibitors (BTKis), have become the standard of care for patients with chronic lymphocytic leukemia (CLL). We conducted a real-world retrospective analysis of patients with CLL treated with acalabrutinib vs ibrutinib using the Flatiron Health database. Patients with CLL were included if they initiated acalabrutinib or ibrutinib between 1 January 2018 and 28 February 2021. The primary outcome of interest was time to treatment discontinuation (TTD). Kaplan-Meier analysis was used to estimate unweighted and weighted median TTD. A weighted Cox proportional hazards model was used to compare the TTD between cohorts. Of the 2509 patients included in the analysis, 89.6% received ibrutinib, and 14.1% received acalabrutinib. TTD was not significantly different between cohorts in the unweighted analysis. After weighting, the cohorts were balanced on all baseline characteristics except cardiovascular risk factors and baseline medications use. The median (95% confidence interval [CI]) TTD was not reached (NR; 95% CI, 25.1 to NR) for the acalabrutinib cohort and was 23.4 months (95% CI, 18.1-28.7) for the ibrutinib cohort. The discontinuation rate at 12 months was 22% for the weighted acalabrutinib cohort vs 31% for the weighted ibrutinib cohort (P = .005). After additional adjustment for prior BTKi use, the acalabrutinib cohort had a 41% lower risk of discontinuation vs ibrutinib (hazard ratio, 0.59; 95% CI, 0.43-0.81; P = .001). In the largest available study comparing BTKis, patients with CLL receiving acalabrutinib demonstrated lower rates of discontinuation and a prolonged time to discontinuation vs those receiving ibrutinib.
Introduction
Bruton tyrosine kinase inhibitors (BTKis) represent a significant advancement for patients with chronic lymphocytic leukemia (CLL) or small lymphocytic leukemia (SLL) as a targeted treatment leveraging disease biology to improve outcomes. Ibrutinib was the first BTKi approved for treatment of CLL/SLL in 2014. Acalabrutinib, a second-generation BTKi, was subsequently approved for treatment of CLL/SLL in 2019. Both agents have demonstrated survival benefits compared with chemoimmunotherapy and have become standards of care for patients with CLL/SLL.
Ibrutinib is a first-generation BTKi with off-target effects including inhibition of epidermal growth factor, interleukin-2–inducible kinase, and Tec kinases, among others.1 Ibrutinib has been studied in the frontline and relapsed/refractory CLL settings, demonstrating improvement in survival compared with chemoimmunotherapy. Real-world studies and extended follow-up from clinical trials have demonstrated the association of ibrutinib therapy with high discontinuation rates because of toxicity. A real-world series of 616 patients treated with ibrutinib found that 41% had discontinued therapy, with a median follow-up time of 17 months, with toxicity being the most common reason for discontinuation in both frontline and relapsed/refractory settings.2 In RESONATE-2, 58% of patients treated with ibrutinib in the frontline setting discontinued within 8 years of follow-up, with adverse events being the most common reason for discontinuation.3
Given the toxicity profile associated with ibrutinib, the second-generation BTKi acalabrutinib was developed with the goal of increased BTK specificity and fewer off-target adverse events. In ELEVATE-RR, a study of patients with relapsed/refractory CLL with high-risk cytogenetic features (deletion of chromosome 17p or deletion of chromosome 11q), a head-to-head comparison of acalabrutinib and ibrutinib was conducted.4 The primary end point of progression-free survival (PFS) was similar between the 2 arms with a hazard ratio (HR) of 1.00 (95% confidence interval [CI], 0.79-1.27), demonstrating noninferiority. A significant difference in toxicity, however, was observed as patients treated with ibrutinib experienced higher rates of atrial fibrillation (16.0% vs 9.4%), hypertension (23.2% vs 9.4%), and bleeding events (51.3% vs 38.0%) compared with patients treated with acalabrutinib.4 Adverse events led to treatment discontinuation in 21.3% of patients treated with ibrutinib and 14.7% of patients treated with acalabrutinib.
Real-world data provide insight into the use of novel agents outside of the clinical trial setting, because baseline features and management strategies often differ between clinical practice and clinical trials. Thus, we conducted a real-world analysis of patients with CLL/SLL treated with acalabrutinib vs ibrutinib to compare outcomes in a noninterventional population. By examining the time to treatment discontinuation (TTD), we aimed to understand whether the patterns observed in ELEVATE-RR are mirrored outside of clinical trials in both frontline and relapsed/refractory settings. Furthermore, we examined discontinuation rates and toxicity profiles for both agents to better understand the occurrence of adverse events associated with both drugs in real-world settings.
Methods
Data source
This retrospective cohort study used deidentified, electronic health record (EHR) data from the Flatiron Health database from July 2017 to February 2021. The Flatiron Health EHR-derived database represents an estimated 280 community centers and academic institutions (∼75% community and ∼25% academic) with >800 geographically diverse sites of patient care and >2.6 million patients with cancer in the United States. Data are extracted from structured EHRs that are mapped to a common terminology and normalized across different source systems and unstructured information abstracted from physicians’ notes and other documents such as patient discharge summaries and radiology, pathology and biomarker reports.5 Supplemental study-specific data (eg, reasons for discontinuation) were extracted through review of unstructured data for all patients with CLL/SLL treated with acalabrutinib and a random subset of patients with CLL/SLL treated with ibrutinib.
Study population and design
Adult patients with CLL/SLL were identified from the Flatiron Health database based on physician-documented diagnosis using International Classification of Diseases, 10th Revision (ICD-10) diagnosis codes or evidence of having been treated specifically for CLL/SLL in unstructured documents. Patients were included in the study if they had initiated acalabrutinib or ibrutinib in any line of therapy on or after 1 January 2018. The date of initiation was defined as the index date. Patients were also required to have at least 2 clinical encounters on different days in the Flatiron Health database during the study period, which extended from 6 months before the index date (baseline period) to the end of follow-up (the earliest of the end of clinical activity, death, or end of data availability [ie, 28 February 2021]). Clinical activity was defined as clinical visits, start of any line of therapy, laboratory test, vital assessment, Eastern Cooperative Oncology Group (ECOG) performance status, or comorbidity diagnosis. Patients were excluded from the study if they were enrolled in a clinical trial or received an investigational drug during the study period or received concurrent treatment (other than the medications listed in supplemental Table 1) for another malignancy during the study period. Note that it was possible for patients to contribute to both the acalabrutinib and ibrutinib cohorts if they received the drugs in different lines of therapy. Additional details, including reasons for discontinuation, were manually abstracted from unstructured data for a subset of patients.
Study outcomes
TTD, the prespecified primary outcome of interest, was defined as the time from the index date to the discontinuation of the index treatment, which was marked by the advancement to a new line of therapy, as documented in structured data, a prolonged period of confirmed structured clinical activity (ie, >120 days) after the last recorded drug episode of index treatment to ensure that patients were still being followed-up but no further drug episodes or death occurred. The data of patients who did not discontinue their index treatment were censored at the last confirmed clinical activity date.
The time to next therapy or death (TTNTD) was defined as the time from the initiation of the index treatment to initiation of a new line of therapy or death. Data of patients who had not initiated a new line of therapy after their index treatment were censored at the last confirmed clinical activity date.
Reasons for discontinuation of the index treatment were captured for the last documented episode of the drug in the patient’s chart within the line of therapy of interest for a subset of patients.
Statistical analysis
Average treatment effect among the treated (ATT) weighting, also known as standardized mortality ratio weighting, was used to balance key baseline characteristics and reduce noncomparability between the acalabrutinib and ibrutinib cohorts. ATT weights were generated based on the propensity score (PS) for which a logistic regression model was used to estimate the probability of treatment with acalabrutinib as a function of age, sex, race, geographic region, year of index date, year of diagnosis with CLL/SLL, line of therapy, Rai stage, modified Quan-Charlson comorbidity index (CCI) score, atrial fibrillation, ECOG performance status, and use of anticoagulants. Patients in the acalabrutinib cohort received a weightage of 1, whereas the weightage for patients in the ibrutinib cohort were calculated as PS ÷ (1 − PS). Thus, ATT weighting made the distribution of baseline characteristics in the ibrutinib cohort similar to that for the acalabrutinib cohort.
Kaplan-Meier analysis was used to obtain unweighted and ATT-weighted TTD and TTNTD curves for the acalabrutinib and ibrutinib cohorts. In addition, ATT-weighted Cox proportional hazard (PH) models were used to compare TTD and TTNTD between the treatment cohorts. Baseline variables that remained imbalanced after ATT weighting (ie, with standardized differences between treatment cohorts >0.1) were included in the weighted Cox PH models to further control for residual confounding, thus allowing for a doubly robust approach. No violations of the PH assumption were detected based on the evaluation of scaled Schoenfeld residuals and the statistical test of an interaction between time and treatment. To assess potential confounding by line of therapy, the Kaplan-Meier analysis and Cox PH regression models were stratified based on the line of therapy (first line, second line, and third line or later) in a sensitivity analysis.
Among patients with abstracted reasons for discontinuation, comparisons of patient baseline characteristics, TTD, and TTNTD between the treatment cohorts were also performed as sensitivity analyses. Reasons for discontinuation of the index treatment were summarized using frequencies and percentages for these patients. SAS software (version 9.4; SAS Institute, Cary, NC) and R software (version 3.6.3; the R Foundation, Vienna, Austria) were used for statistical analyses.
Results
Study population and baseline characteristics
From January 2018 to February 2021, 2509 patients with CLL/SLL who initiated acalabrutinib or ibrutinib met the inclusion criteria and were identified for this analysis. Of those patients, 89.6% (n = 2249) received ibrutinib, and 14.1% (n = 353) received acalabrutinib (Figure 1). The acalabrutinib cohort had a median age of 73.0 years (interquartile range, 66.0-79.0), and 38.5% were female; the ibrutinib cohort had a median age of 72.0 years (interquartile range, 65.0-79.0), and 38.0% were female (Table 1).
Attrition table. (1) Patients with CLL and/or SLL were identified using ICD-9-CM codes 204.1x and ICD-10-CM codes C91.1x and C83.0x, or evidence in unstructured documents of having been treated specifically for CLL/SLL. (2) Clinical encounters included patient visits from structured data (ECOG performance status, medication administrations, medication orders, telemedicine, vitals, and laboratory reports) and abstracted treatment information (classic cytogenetics, fluorescence in situ hybridization, immunoglobulin heavy chain, immunophenotyping, and oral medications). (3) For each patient, the study period extended from 6 months prior to the initiation of acalabrutinib or ibrutinib to the end of follow-up (the earliest of end of clinical activity, death, or end of data availability [ie, 28 February 2021]). (4) Concurrent antineoplastic treatments were defined based on antineoplastic treatments other than the list of anti-CD20 monoclonal antibodies, BCL2 antagonist, and PI3K inhibitors in supplemental Table 1 and Appendix 1. (5) See supplemental Table 1 and Appendix 2 for other malignancy diagnosis codes. (6) Patients may contribute to both the acalabrutinib and ibrutinib cohorts. FISH, fluorescence in situ hybridization; IGHV, immunoglobulin heavy chain; PI3K, phosphoinositide 3-kinase; 1L, first line; 2L, second line.
Attrition table. (1) Patients with CLL and/or SLL were identified using ICD-9-CM codes 204.1x and ICD-10-CM codes C91.1x and C83.0x, or evidence in unstructured documents of having been treated specifically for CLL/SLL. (2) Clinical encounters included patient visits from structured data (ECOG performance status, medication administrations, medication orders, telemedicine, vitals, and laboratory reports) and abstracted treatment information (classic cytogenetics, fluorescence in situ hybridization, immunoglobulin heavy chain, immunophenotyping, and oral medications). (3) For each patient, the study period extended from 6 months prior to the initiation of acalabrutinib or ibrutinib to the end of follow-up (the earliest of end of clinical activity, death, or end of data availability [ie, 28 February 2021]). (4) Concurrent antineoplastic treatments were defined based on antineoplastic treatments other than the list of anti-CD20 monoclonal antibodies, BCL2 antagonist, and PI3K inhibitors in supplemental Table 1 and Appendix 1. (5) See supplemental Table 1 and Appendix 2 for other malignancy diagnosis codes. (6) Patients may contribute to both the acalabrutinib and ibrutinib cohorts. FISH, fluorescence in situ hybridization; IGHV, immunoglobulin heavy chain; PI3K, phosphoinositide 3-kinase; 1L, first line; 2L, second line.
Baseline characteristics of patients with CLL and/or SLL treated with acalabrutinib or ibrutinib
. | Original sample . | ATT-weighted sample . | ||||
|---|---|---|---|---|---|---|
| Acalabrutinib cohort . | Ibrutinib cohort . | Std. diff. . | Acalabrutinib cohort . | Ibrutinib cohort . | Std. diff . | |
| n = 353 . | n = 2249 . | . | n = 353 . | n = 364 . | . | |
| Demographic characteristics | ||||||
| Age at index date, y | ||||||
| Mean ± SD | 71.9 ± 9.2 | 70.7 ± 9.5 | 0.130∗ | 71.9 ± 9.2 | 72.3 ± 3.8 | 0.049 |
| Median (IQR) | 73.0 (66.0-79.0) | 72.0 (65.0-79.0) | 73.0 (66.0-79.0) | 73.0 (66.0-80.0) | ||
| Female, n (%) | 136 (38.5) | 855 (38.0) | 0.011 | 136 (38.5) | 141 (38.7) | 0.004 |
| Race, n (%) | ||||||
| White | 271 (76.8) | 1659 (73.8) | 0.070 | 271 (76.8) | 287 (78.8) | 0.050 |
| Black or African American | 26 (7.4) | 198 (8.8) | 0.053 | 26 (7.4) | 24 (6.7) | 0.028 |
| Other race† | 25 (7.1) | 175 (7.8) | 0.027 | 25 (7.1) | 22 (6.0) | 0.044 |
| Asian | 7 (2.0) | 27 (1.2) | 0.063 | 7 (2.0) | 5 (1.5) | 0.038 |
| Unknown | 24 (6.8) | 190 (8.4) | 0.062 | 24 (6.8) | 26 (7.0) | 0.008 |
| Hispanic or Latino, n (%) | 9 (2.5) | 86 (3.8) | 0.073 | 9 (2.5) | 11 (2.9) | 0.021 |
| Geographic region, n (%) | ||||||
| South | 147 (41.6) | 887 (39.4) | 0.045 | 147 (41.6) | 147 (40.4) | 0.026 |
| Midwest | 55 (15.6) | 381 (16.9) | 0.037 | 55 (15.6) | 55 (15.1) | 0.013 |
| West | 69 (19.5) | 346 (15.4) | 0.110∗ | 69 (19.5) | 73 (19.9) | 0.010 |
| Northeast | 59 (16.7) | 488 (21.7) | 0.127∗ | 59 (16.7) | 60 (16.4) | 0.007 |
| Unknown | 23 (6.5) | 147 (6.5) | 0.001 | 23 (6.5) | 30 (8.2) | 0.063 |
| Year of index date, n (%) | ||||||
| 2018 | 19 (5.4) | 813 (36.1) | 0.820∗ | 19 (5.4) | 19 (5.2) | 0.009 |
| 2019 | 55 (15.6) | 913 (40.6) | 0.580∗ | 55 (15.6) | 54 (14.9) | 0.018 |
| 2020 | 247 (70.0) | 483 (21.5) | 1.114∗ | 247 (70.0) | 255 (69.8) | 0.003 |
| 2021 | 32 (9.1) | 40 (1.8) | 0.326∗ | 32 (9.1) | 37 (10.0) | 0.033 |
| Clinical characteristics | ||||||
| Year of diagnosis with CLL or SLL, n (%) | ||||||
| Before 2014 | 190 (53.8) | 1019 (45.3) | 0.171∗ | 190 (53.8) | 192 (52.8) | 0.021 |
| 2014 | 20 (5.7) | 178 (7.9) | 0.090 | 20 (5.7) | 20 (5.3) | 0.014 |
| 2015 | 31 (8.8) | 148 (6.6) | 0.083 | 31 (8.8) | 33 (9.1) | 0.010 |
| 2016 | 22 (6.2) | 185 (8.2) | 0.077 | 22 (6.2) | 25 (6.9) | 0.028 |
| 2017 | 19 (5.4) | 181 (8.0) | 0.107∗ | 19 (5.4) | 23 (6.2) | 0.035 |
| 2018 | 25 (7.1) | 263 (11.7) | 0.159∗ | 25 (7.1) | 27 (7.3) | 0.008 |
| 2019 | 22 (6.2) | 212 (9.4) | 0.119∗ | 22 (6.2) | 22 (6.0) | 0.011 |
| 2020 | 23 (6.5) | 61 (2.7) | 0.182∗ | 23 (6.5) | 23 (6.2) | 0.014 |
| 2021 | 1 (0.3) | 2 (0.1) | 0.045 | 1 (0.3) | 1 (0.3) | 0.002 |
| Time from diagnosis to index date, y | ||||||
| Mean ± SD | 7.7 ± 5.7 | 5.9 ± 5.8 | 0.308∗ | 7.7 ± 5.7 | 7.6 ± 2.6 | 0.021 |
| Median (IQR) | 7.0 (3.2-11.3) | 4.6 (1.4-8.6) | 7.0 (3.2-11.3) | 6.6 (3.1-10.2) | ||
| Line of therapy in which BTKi was received, n (%) | ||||||
| 1L | 67 (19.0) | 1211 (53.8) | 0.777∗ | 67 (19.0) | 66 (18.1) | 0.024 |
| 2L | 140 (39.7) | 714 (31.7) | 0.166∗ | 140 (39.7) | 141 (38.6) | 0.022 |
| 3L+ | 146 (41.4) | 324 (14.4) | 0.630∗ | 146 (41.4) | 158 (43.4) | 0.041 |
| Rai stage at diagnosis, n (%) | ||||||
| Stage 0 | 80 (22.7) | 533 (23.7) | 0.025 | 80 (22.7) | 92 (25.3) | 0.061 |
| Stage I | 49 (13.9) | 335 (14.9) | 0.029 | 49 (13.9) | 48 (13.1) | 0.022 |
| Stage II | 30 (8.5) | 147 (6.5) | 0.075 | 30 (8.5) | 28 (7.8) | 0.026 |
| Stage III | 20 (5.7) | 109 (4.8) | 0.037 | 20 (5.7) | 19 (5.1) | 0.026 |
| Stage IV | 32 (9.1) | 172 (7.6) | 0.051 | 32 (9.1) | 33 (9.2) | 0.004 |
| Unknown | 142 (40.2) | 953 (42.4) | 0.044 | 142 (40.2) | 144 (39.5) | 0.014 |
| Modified Quan-CCI score | ||||||
| Mean ± SD | 0.1 ± 0.4 | 0.1 ± 0.4 | 0.008 | 0.1 ± 0.4 | 0.1 ± 0.1 | 0.017 |
| Median (IQR) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | ||
| Cardiovascular risk factors, n (%) | ||||||
| Hypertension | 23 (6.5) | 220 (9.8) | 0.120∗ | 23 (6.5) | 30 (8.4) | 0.070 |
| Atrial fibrillation | 12 (3.4) | 36 (1.6) | 0.115∗ | 12 (3.4) | 17 (4.7) | 0.067 |
| Hypercholesterolemia | 14 (4.0) | 176 (7.8) | 0.164∗ | 14 (4.0) | 25 (6.7) | 0.123 |
| Congestive heart failure | 4 (1.1) | 19 (0.8) | 0.029 | 4 (1.1) | 4 (1.1) | 0.007 |
| Peripheral arterial disease | 1 (0.3) | 13 (0.6) | 0.045 | 1 (0.3) | 7 (2.0) | 0.162 |
| Cerebrovascular disease | 0 (0.0) | 13 (0.6) | 0.108 | 0 (0.0) | 2 (0.7) | 0.116 |
| Diabetes | 11 (3.1) | 88 (3.9) | 0.043 | 11 (3.1) | 14 (3.7) | 0.035 |
| Myocardial infarction | 1 (0.3) | 7 (0.3) | 0.005 | 1 (0.3) | 0 (0.1) | 0.053 |
| ECOG performance status, n (%) | ||||||
| 0 | 129 (36.5) | 897 (39.9) | 0.069 | 129 (36.5) | 127 (34.8) | 0.037 |
| 1 | 128 (36.3) | 661 (29.4) | 0.147∗ | 128 (36.3) | 136 (37.3) | 0.022 |
| 2+ | 40 (11.3) | 211 (9.4) | 0.064 | 40 (11.3) | 43 (11.8) | 0.014 |
| Unknown | 56 (15.9) | 480 (21.3) | 0.141∗ | 56 (15.9) | 59 (16.1) | 0.008 |
| Baseline medications, n (%) | ||||||
| Prior BTKi use | 121 (34.3) | 55 (2.4) | 0.902∗ | 121 (34.3) | 25 (6.7) | 0.726∗ |
| Anticoagulants | 3 (0.8) | 13 (0.6) | 0.032 | 3 (0.8) | 5 (1.4) | 0.055 |
| Antiplatelets | 1 (0.3) | 6 (0.3) | 0.003 | 1 (0.3) | 1 (0.1) | 0.032 |
| CYP3A inhibitors | 0 (0.0) | 0 (0.0) | - | 0 (0.0) | 0 (0.0) | - |
| CYP3A inducers | 0 (0.0) | 1 (0.0) | 0.030 | 0 (0.0) | 2 (0.7) | 0.117∗ |
. | Original sample . | ATT-weighted sample . | ||||
|---|---|---|---|---|---|---|
| Acalabrutinib cohort . | Ibrutinib cohort . | Std. diff. . | Acalabrutinib cohort . | Ibrutinib cohort . | Std. diff . | |
| n = 353 . | n = 2249 . | . | n = 353 . | n = 364 . | . | |
| Demographic characteristics | ||||||
| Age at index date, y | ||||||
| Mean ± SD | 71.9 ± 9.2 | 70.7 ± 9.5 | 0.130∗ | 71.9 ± 9.2 | 72.3 ± 3.8 | 0.049 |
| Median (IQR) | 73.0 (66.0-79.0) | 72.0 (65.0-79.0) | 73.0 (66.0-79.0) | 73.0 (66.0-80.0) | ||
| Female, n (%) | 136 (38.5) | 855 (38.0) | 0.011 | 136 (38.5) | 141 (38.7) | 0.004 |
| Race, n (%) | ||||||
| White | 271 (76.8) | 1659 (73.8) | 0.070 | 271 (76.8) | 287 (78.8) | 0.050 |
| Black or African American | 26 (7.4) | 198 (8.8) | 0.053 | 26 (7.4) | 24 (6.7) | 0.028 |
| Other race† | 25 (7.1) | 175 (7.8) | 0.027 | 25 (7.1) | 22 (6.0) | 0.044 |
| Asian | 7 (2.0) | 27 (1.2) | 0.063 | 7 (2.0) | 5 (1.5) | 0.038 |
| Unknown | 24 (6.8) | 190 (8.4) | 0.062 | 24 (6.8) | 26 (7.0) | 0.008 |
| Hispanic or Latino, n (%) | 9 (2.5) | 86 (3.8) | 0.073 | 9 (2.5) | 11 (2.9) | 0.021 |
| Geographic region, n (%) | ||||||
| South | 147 (41.6) | 887 (39.4) | 0.045 | 147 (41.6) | 147 (40.4) | 0.026 |
| Midwest | 55 (15.6) | 381 (16.9) | 0.037 | 55 (15.6) | 55 (15.1) | 0.013 |
| West | 69 (19.5) | 346 (15.4) | 0.110∗ | 69 (19.5) | 73 (19.9) | 0.010 |
| Northeast | 59 (16.7) | 488 (21.7) | 0.127∗ | 59 (16.7) | 60 (16.4) | 0.007 |
| Unknown | 23 (6.5) | 147 (6.5) | 0.001 | 23 (6.5) | 30 (8.2) | 0.063 |
| Year of index date, n (%) | ||||||
| 2018 | 19 (5.4) | 813 (36.1) | 0.820∗ | 19 (5.4) | 19 (5.2) | 0.009 |
| 2019 | 55 (15.6) | 913 (40.6) | 0.580∗ | 55 (15.6) | 54 (14.9) | 0.018 |
| 2020 | 247 (70.0) | 483 (21.5) | 1.114∗ | 247 (70.0) | 255 (69.8) | 0.003 |
| 2021 | 32 (9.1) | 40 (1.8) | 0.326∗ | 32 (9.1) | 37 (10.0) | 0.033 |
| Clinical characteristics | ||||||
| Year of diagnosis with CLL or SLL, n (%) | ||||||
| Before 2014 | 190 (53.8) | 1019 (45.3) | 0.171∗ | 190 (53.8) | 192 (52.8) | 0.021 |
| 2014 | 20 (5.7) | 178 (7.9) | 0.090 | 20 (5.7) | 20 (5.3) | 0.014 |
| 2015 | 31 (8.8) | 148 (6.6) | 0.083 | 31 (8.8) | 33 (9.1) | 0.010 |
| 2016 | 22 (6.2) | 185 (8.2) | 0.077 | 22 (6.2) | 25 (6.9) | 0.028 |
| 2017 | 19 (5.4) | 181 (8.0) | 0.107∗ | 19 (5.4) | 23 (6.2) | 0.035 |
| 2018 | 25 (7.1) | 263 (11.7) | 0.159∗ | 25 (7.1) | 27 (7.3) | 0.008 |
| 2019 | 22 (6.2) | 212 (9.4) | 0.119∗ | 22 (6.2) | 22 (6.0) | 0.011 |
| 2020 | 23 (6.5) | 61 (2.7) | 0.182∗ | 23 (6.5) | 23 (6.2) | 0.014 |
| 2021 | 1 (0.3) | 2 (0.1) | 0.045 | 1 (0.3) | 1 (0.3) | 0.002 |
| Time from diagnosis to index date, y | ||||||
| Mean ± SD | 7.7 ± 5.7 | 5.9 ± 5.8 | 0.308∗ | 7.7 ± 5.7 | 7.6 ± 2.6 | 0.021 |
| Median (IQR) | 7.0 (3.2-11.3) | 4.6 (1.4-8.6) | 7.0 (3.2-11.3) | 6.6 (3.1-10.2) | ||
| Line of therapy in which BTKi was received, n (%) | ||||||
| 1L | 67 (19.0) | 1211 (53.8) | 0.777∗ | 67 (19.0) | 66 (18.1) | 0.024 |
| 2L | 140 (39.7) | 714 (31.7) | 0.166∗ | 140 (39.7) | 141 (38.6) | 0.022 |
| 3L+ | 146 (41.4) | 324 (14.4) | 0.630∗ | 146 (41.4) | 158 (43.4) | 0.041 |
| Rai stage at diagnosis, n (%) | ||||||
| Stage 0 | 80 (22.7) | 533 (23.7) | 0.025 | 80 (22.7) | 92 (25.3) | 0.061 |
| Stage I | 49 (13.9) | 335 (14.9) | 0.029 | 49 (13.9) | 48 (13.1) | 0.022 |
| Stage II | 30 (8.5) | 147 (6.5) | 0.075 | 30 (8.5) | 28 (7.8) | 0.026 |
| Stage III | 20 (5.7) | 109 (4.8) | 0.037 | 20 (5.7) | 19 (5.1) | 0.026 |
| Stage IV | 32 (9.1) | 172 (7.6) | 0.051 | 32 (9.1) | 33 (9.2) | 0.004 |
| Unknown | 142 (40.2) | 953 (42.4) | 0.044 | 142 (40.2) | 144 (39.5) | 0.014 |
| Modified Quan-CCI score | ||||||
| Mean ± SD | 0.1 ± 0.4 | 0.1 ± 0.4 | 0.008 | 0.1 ± 0.4 | 0.1 ± 0.1 | 0.017 |
| Median (IQR) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | ||
| Cardiovascular risk factors, n (%) | ||||||
| Hypertension | 23 (6.5) | 220 (9.8) | 0.120∗ | 23 (6.5) | 30 (8.4) | 0.070 |
| Atrial fibrillation | 12 (3.4) | 36 (1.6) | 0.115∗ | 12 (3.4) | 17 (4.7) | 0.067 |
| Hypercholesterolemia | 14 (4.0) | 176 (7.8) | 0.164∗ | 14 (4.0) | 25 (6.7) | 0.123 |
| Congestive heart failure | 4 (1.1) | 19 (0.8) | 0.029 | 4 (1.1) | 4 (1.1) | 0.007 |
| Peripheral arterial disease | 1 (0.3) | 13 (0.6) | 0.045 | 1 (0.3) | 7 (2.0) | 0.162 |
| Cerebrovascular disease | 0 (0.0) | 13 (0.6) | 0.108 | 0 (0.0) | 2 (0.7) | 0.116 |
| Diabetes | 11 (3.1) | 88 (3.9) | 0.043 | 11 (3.1) | 14 (3.7) | 0.035 |
| Myocardial infarction | 1 (0.3) | 7 (0.3) | 0.005 | 1 (0.3) | 0 (0.1) | 0.053 |
| ECOG performance status, n (%) | ||||||
| 0 | 129 (36.5) | 897 (39.9) | 0.069 | 129 (36.5) | 127 (34.8) | 0.037 |
| 1 | 128 (36.3) | 661 (29.4) | 0.147∗ | 128 (36.3) | 136 (37.3) | 0.022 |
| 2+ | 40 (11.3) | 211 (9.4) | 0.064 | 40 (11.3) | 43 (11.8) | 0.014 |
| Unknown | 56 (15.9) | 480 (21.3) | 0.141∗ | 56 (15.9) | 59 (16.1) | 0.008 |
| Baseline medications, n (%) | ||||||
| Prior BTKi use | 121 (34.3) | 55 (2.4) | 0.902∗ | 121 (34.3) | 25 (6.7) | 0.726∗ |
| Anticoagulants | 3 (0.8) | 13 (0.6) | 0.032 | 3 (0.8) | 5 (1.4) | 0.055 |
| Antiplatelets | 1 (0.3) | 6 (0.3) | 0.003 | 1 (0.3) | 1 (0.1) | 0.032 |
| CYP3A inhibitors | 0 (0.0) | 0 (0.0) | - | 0 (0.0) | 0 (0.0) | - |
| CYP3A inducers | 0 (0.0) | 1 (0.0) | 0.030 | 0 (0.0) | 2 (0.7) | 0.117∗ |
IQR, interquartile range; std. diff., standardized difference; 1L, first line; 2L, second line; 3L+, third line and later.
Standardized differences >0.1 in magnitude.
Included American Indian or Alaska Native, Hawaiian or Pacific Islander, and multiracial.
Before weighting, patients were balanced across cohorts with respect to sex, race, Rai stage, modified Quan-CCI score, and select baseline medications (anticoagulants, antiplatelets, CYP3A inhibitors, and CYP3A inducers). The cohorts were not balanced for age at index, geographic region, year of index date, year of CLL/SLL diagnosis, time from diagnosis to index date, line of therapy, select cardiovascular risk factors (including atrial fibrillation), ECOG performance status, and prior BTKi use (Table 1).
After weighting, the 2 cohorts were balanced for the age at index, sex, race, geographic region, year of index date, year of CLL/SLL diagnosis, time from diagnosis to index date, line of therapy, Rai stage, atrial fibrillation, modified Quan-CCI score, ECOG performance status, and select baseline medications (anticoagulants, antiplatelets, and CYP3A inhibitors). The cohorts were not balanced for cardiovascular risk factors (hypercholesterolemia [4.0% in the acalabrutinib cohort vs 6.7% in the ibrutinib cohort], peripheral arterial disease [0.3% vs 2.0%], and cerebrovascular disease [0.0% vs 0.7%]), prior BTKi use (34.3% vs 6.7%), and CYP3A inducer use (0.0% vs 0.7%) (Table 1). Because prior BTKi use remained imbalanced between the 2 cohorts and could have a significant impact on the outcome of interest, it was included as a covariate in the Cox PH model to adjust for residual confounding. However, cardiovascular risk factors were not further adjusted for in the Cox PH model because diagnoses for comorbidities were generally not well populated in the Flatiron Health database.
Prior CLL-directed therapy received by patients in any line before their index treatment is presented in supplemental Table 2. Besides ibrutinib, rituximab monotherapy and combination therapy were common in the study population.
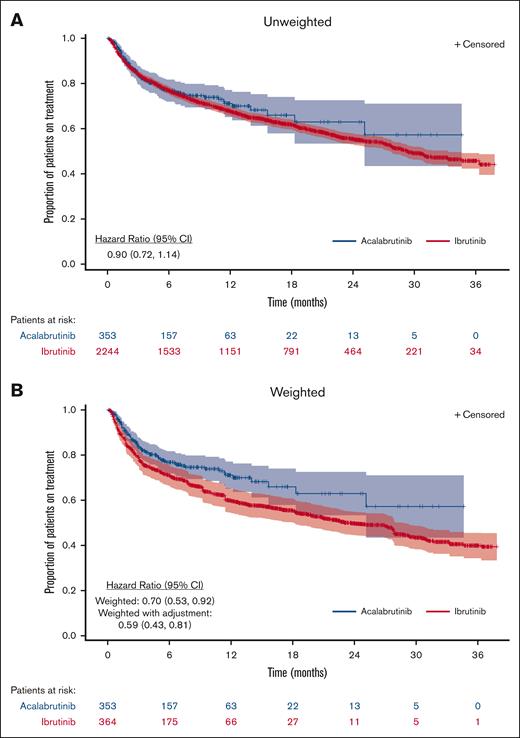
TTD
With a median follow-up of 7.1 months in the acalabrutinib cohort and 17.5 months in the ibrutinib cohort, the median TTD was not reached (NR) (95% CI, 25.1 to NR) for the unweighted acalabrutinib cohort and was 29.3 months (95% CI, 27.7-33.2) for the unweighted ibrutinib cohort (Figure 2). After ATT weighting, the median follow-up time periods for the acalabrutinib and ibrutinib cohorts were 7.1 and 7.6 months, respectively, and the median TTD was NR (95% CI, 25.1 to NR) for the acalabrutinib cohort and 23.4 months (95% CI, 18.1-28.7) for the ibrutinib cohort. The discontinuation rate at 12 months was 22% for the weighted acalabrutinib cohort vs 31% for the weighted ibrutinib cohort (P = .005; Table 2). Acalabrutinib had a numerically lower rate of discontinuation vs ibrutinib in the unweighted analysis, but the results were not statistically significant (HR, 0.90; 95% CI, 0.72-1.14; Table 2). The discontinuation rate was significantly lower for acalabrutinib vs ibrutinib after weighting (HR, 0.70; 95% CI, 0.53-0.92) and after additional adjustment for prior BTKi use (HR, 0.59; 95% CI, 0.43-0.81). Subgroup analysis based on the line of therapy and a sensitivity analysis accounting for discontinuation events derived from unstructured data demonstrated a consistent trend of improved TTD for acalabrutinib vs ibrutinib but did not meet statistical significance (supplemental Tables 3 and 4).
Kaplan-Meier curves of TTD for patients with CLL and/or SLL treated with acalabrutinib or ibrutinib.
Kaplan-Meier curves of TTD for patients with CLL and/or SLL treated with acalabrutinib or ibrutinib.
TTD for patients with CLL and/or SLL treated with acalabrutinib or ibrutinib
| . | Number of patients∗ . | Total events, n (%) . | Events at 3 mo, n (%) . | Events at 6 mo, n (%) . | Events at 12 mo, n (%) . | Events at 18 mo, n (%) . | Median TTD, mo (95% CI) . | Mean TTD†, mo (95% CI) . | HR (95% CI) . |
|---|---|---|---|---|---|---|---|---|---|
| Unweighted‡ | |||||||||
| All patients receiving acalabrutinib | 353 | 82 (23.2) | 52 (14.7) | 69 (19.5) | 77 (21.8) | 80 (22.7) | NR (25.1-NR) | 23.6 (21.3-26.0) | 0.90 (0.72-1.14) |
| All patients receiving ibrutinib | 2244 | 878 (39.1) | 339 (15.1) | 492 (21.9) | 669 (29.8) | 757 (33.7) | 29.3 (27.7-33.2) | 23.6 (22.9-24.3) | ref. |
| ATT weighted§ | |||||||||
| All patients receiving acalabrutinib | 353 | 82 (23.2) | 52 (14.7) | 69 (19.5) | 77 (21.8) | 80 (22.7) | NR (25.1-NR) | 23.6 (21.3-26.0) | 0.70 (0.53-0.92) |
| All patients receiving ibrutinib | 364 | 119 (32.7) | 69 (19.0) | 92 (25.3) | 112 (30.8) | 115 (31.6) | 23.4 (18.1-28.7) | 21.4 (19.8-23.1) | ref. |
| ATT weighted with additional adjustment|| | |||||||||
| All patients receiving acalabrutinib | 353 | 82 (23.2) | 52 (14.7) | 69 (19.5) | 77 (21.8) | 80 (22.7) | NR (25.1-NR) | 23.6 (21.3-26.0) | 0.59 (0.43-0.81) |
| All patients receiving ibrutinib | 364 | 119 (32.7) | 69 (19.0) | 92 (25.3) | 112 (30.8) | 115 (31.6) | 23.4 (18.1-28.7) | 21.4 (19.8-23.1) | ref. |
| . | Number of patients∗ . | Total events, n (%) . | Events at 3 mo, n (%) . | Events at 6 mo, n (%) . | Events at 12 mo, n (%) . | Events at 18 mo, n (%) . | Median TTD, mo (95% CI) . | Mean TTD†, mo (95% CI) . | HR (95% CI) . |
|---|---|---|---|---|---|---|---|---|---|
| Unweighted‡ | |||||||||
| All patients receiving acalabrutinib | 353 | 82 (23.2) | 52 (14.7) | 69 (19.5) | 77 (21.8) | 80 (22.7) | NR (25.1-NR) | 23.6 (21.3-26.0) | 0.90 (0.72-1.14) |
| All patients receiving ibrutinib | 2244 | 878 (39.1) | 339 (15.1) | 492 (21.9) | 669 (29.8) | 757 (33.7) | 29.3 (27.7-33.2) | 23.6 (22.9-24.3) | ref. |
| ATT weighted§ | |||||||||
| All patients receiving acalabrutinib | 353 | 82 (23.2) | 52 (14.7) | 69 (19.5) | 77 (21.8) | 80 (22.7) | NR (25.1-NR) | 23.6 (21.3-26.0) | 0.70 (0.53-0.92) |
| All patients receiving ibrutinib | 364 | 119 (32.7) | 69 (19.0) | 92 (25.3) | 112 (30.8) | 115 (31.6) | 23.4 (18.1-28.7) | 21.4 (19.8-23.1) | ref. |
| ATT weighted with additional adjustment|| | |||||||||
| All patients receiving acalabrutinib | 353 | 82 (23.2) | 52 (14.7) | 69 (19.5) | 77 (21.8) | 80 (22.7) | NR (25.1-NR) | 23.6 (21.3-26.0) | 0.59 (0.43-0.81) |
| All patients receiving ibrutinib | 364 | 119 (32.7) | 69 (19.0) | 92 (25.3) | 112 (30.8) | 115 (31.6) | 23.4 (18.1-28.7) | 21.4 (19.8-23.1) | ref. |
ref., reference group.
Five patients in the ibrutinib cohort had a treatment discontinuation date that was the same as the index date (ie, initiation of ibrutinib) and were removed from the analysis.
Mean TTD was calculated as the area under the Kaplan-Meier curve until the end of follow-up.
Median follow-up time for the acalabrutinib and ibrutinib cohorts was 7.1 and 17.5 months, respectively, among the overall study population. Mean (min, max) follow-up time for the acalabrutinib and ibrutinib cohorts was 8.6 (0.1, 34.7) months and 17.8 (0.2, 37.9) months, respectively.
The following characteristics were adjusted for using ATT weights: age, sex, race, geographic region, year of ibrutinib or acalabrutinib initiation, year of CLL/SLL diagnosis, line of therapy, Rai stage, modified Quan-CCI score, atrial fibrillation, ECOG performance status, and use of anticoagulants. Median follow-up time for the ATT-weighted acalabrutinib and ibrutinib cohorts was 7.1 and 7.6 months, respectively, among the overall study population. Mean (min, max) follow-up time for the ATT-weighted acalabrutinib and ibrutinib cohorts was 8.6 (0.1, 34.7) months and 9.1 (0.02, 37.7) months, respectively.
Prior BTKi use was further controlled for in a doubly robust Cox PH model.
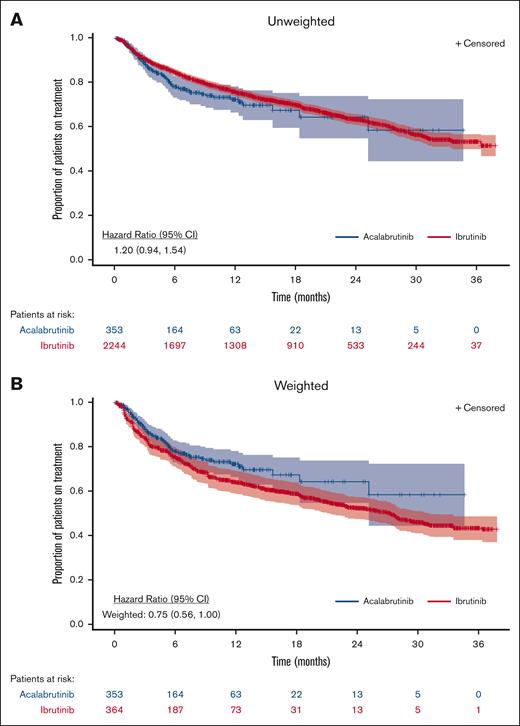
TTNTD
In the unweighted analysis, TTNTD was NR for either cohort (HR, 1.20; 95% CI, 0.94-1.54; Figure 3; Table 3). TTNTD was not significantly different between acalabrutinib and ibrutinib in the unweighted analysis. In the ATT-weighted analysis, the median TTNTD was NR (95% CI, 25.2-NR) for the acalabrutinib cohort and 27.3 months (95% CI, 21.3-31.2) for the ibrutinib cohort (HR, 0.75; 95% CI, 0.56-1.00). This trend favoring acalabrutinib did not reach statistical significance. Additional adjustment for prior BTKi use further lowered the HR to 0.62 (95% CI, 0.43-0.88), which was statistically significant. Subgroup analysis based on the line of therapy and a sensitivity analysis accounting for discontinuation events derived from unstructured data demonstrated a consistent nonstatistically significant trend of improved TTNTD for acalabrutinib vs ibrutinib (supplemental Tables 5 and 6).
Kaplan-Meier curves of TTNTD for patients with CLL and/or SLL treated with acalabrutinib or ibrutinib.
Kaplan-Meier curves of TTNTD for patients with CLL and/or SLL treated with acalabrutinib or ibrutinib.
TTNTD for patients with CLL and/or SLL treated with acalabrutinib or ibrutinib
| . | Number of patients∗ . | Total events, n (%) . | Events at 3 mo, n (%) . | Events at 6 mo, n (%) . | Events at 12 mo, n (%) . | Events at 18 mo, n (%) . | Median TTNTD, mo (95% CI) . | Mean TTNTD†, mo (95% CI) . | HR (95% CI) . |
|---|---|---|---|---|---|---|---|---|---|
| Unweighted‡ | |||||||||
| All patients on acalabrutinib | 353 | 76 (21.5) | 35 (9.9) | 62 (17.6) | 71 (20.1) | 74 (21.0) | NR (25.2-NR) | 24.2 (21.8-26.5) | 1.20 (0.94-1.54) |
| All patients on ibrutinib | 2244 | 711 (31.7) | 200 (8.9) | 326 (14.5) | 497 (22.1) | 585 (26.1) | NR (36.4-NR) | 26.5 (25.8-27.1) | ref. |
| ATT weighted§ | |||||||||
| All patients on acalabrutinib | 353 | 76 (21.5) | 35 (9.9) | 62 (17.6) | 71 (20.1) | 74 (21.0) | NR (25.2-NR) | 24.2 (21.8-26.5) | 0.75 (0.56-1.00) |
| All patients on ibrutinib | 364 | 106 (29.1) | 52 (14.3) | 76 (20.9) | 98 (26.9) | 102 (28.0) | 27.3 (21.3-31.2) | 22.7 (21.2-24.3) | ref. |
| ATT weighted with additional adjustmentǁ | |||||||||
| All patients on acalabrutinib | 353 | 76 (21.5) | 35 (9.9) | 62 (17.6) | 71 (20.1) | 74 (21.0) | NR (25.2-NR) | 24.2 (21.8-26.5) | 0.62 (0.43-0.88) |
| All patients on ibrutinib | 364 | 106 (29.1) | 52 (14.3) | 76 (20.9) | 98 (26.9) | 102 (28.0) | 27.3 (21.3-31.2) | 22.7 (21.2-24.3) | ref. |
| . | Number of patients∗ . | Total events, n (%) . | Events at 3 mo, n (%) . | Events at 6 mo, n (%) . | Events at 12 mo, n (%) . | Events at 18 mo, n (%) . | Median TTNTD, mo (95% CI) . | Mean TTNTD†, mo (95% CI) . | HR (95% CI) . |
|---|---|---|---|---|---|---|---|---|---|
| Unweighted‡ | |||||||||
| All patients on acalabrutinib | 353 | 76 (21.5) | 35 (9.9) | 62 (17.6) | 71 (20.1) | 74 (21.0) | NR (25.2-NR) | 24.2 (21.8-26.5) | 1.20 (0.94-1.54) |
| All patients on ibrutinib | 2244 | 711 (31.7) | 200 (8.9) | 326 (14.5) | 497 (22.1) | 585 (26.1) | NR (36.4-NR) | 26.5 (25.8-27.1) | ref. |
| ATT weighted§ | |||||||||
| All patients on acalabrutinib | 353 | 76 (21.5) | 35 (9.9) | 62 (17.6) | 71 (20.1) | 74 (21.0) | NR (25.2-NR) | 24.2 (21.8-26.5) | 0.75 (0.56-1.00) |
| All patients on ibrutinib | 364 | 106 (29.1) | 52 (14.3) | 76 (20.9) | 98 (26.9) | 102 (28.0) | 27.3 (21.3-31.2) | 22.7 (21.2-24.3) | ref. |
| ATT weighted with additional adjustmentǁ | |||||||||
| All patients on acalabrutinib | 353 | 76 (21.5) | 35 (9.9) | 62 (17.6) | 71 (20.1) | 74 (21.0) | NR (25.2-NR) | 24.2 (21.8-26.5) | 0.62 (0.43-0.88) |
| All patients on ibrutinib | 364 | 106 (29.1) | 52 (14.3) | 76 (20.9) | 98 (26.9) | 102 (28.0) | 27.3 (21.3-31.2) | 22.7 (21.2-24.3) | ref. |
ref., reference group.
TTNTD was defined as the time from the initiation of the index treatment to initiation of a new line of therapy or death due to any reason. Data of patients who had not initiated a new line of therapy after their index treatment were censored at the last confirmed clinical activity date based on the available data (ie, structured data and unstructured data through chart abstraction).
Five patients in the ibrutinib cohort had a treatment discontinuation date that was the same as the index date (ie, initiation of ibrutinib) and were removed from the analysis.
Mean TTNTD was calculated as the area under the Kaplan-Meier curve until the end of follow-up.
Median follow-up time for the acalabrutinib and ibrutinib cohorts was 7.1 and 17.5 months, respectively, among the overall study population. Mean (min, max) follow-up time for the acalabrutinib and ibrutinib cohorts was 8.6 (0.1, 34.7) months and 17.8 (0.2, 37.9) months, respectively.
The following characteristics were adjusted for using ATT weights: age, sex, race, geographic region, year of ibrutinib or acalabrutinib initiation, year of CLL/SLL diagnosis, line of therapy, Rai stage, modified Quan-CCI score, atrial fibrillation, ECOG performance status, and use of anticoagulants. The median follow-up time for the ATT-weighted acalabrutinib and ibrutinib cohorts was 7.1 and 7.6 months, respectively, among the overall study population. Mean (minimum, maximum) follow-up time for the ATT-weighted acalabrutinib and ibrutinib cohorts was 8.6 (0.1, 34.7) months and 9.1 (0.02, 37.7) months, respectively.
Prior BTKi use was further controlled for in a doubly robust Cox PH model.
Reasons for discontinuation
In the subset of patients for whom reasons for discontinuation of the index treatment were assessed (acalabrutinib, n = 212; ibrutinib, n = 194), 25% of patients (n = 54) treated with acalabrutinib and 41% of patients (n = 79) treated with ibrutinib discontinued treatment during the study period (Table 4). The most common reason for discontinuation in both cohorts was toxicity; among the discontinuation reasons, 50% were due to toxicity in those treated with acalabrutinib, and 47% were due to toxicity in those treated with ibrutinib.
Reasons for discontinuation in patients with CLL and/or SLL treated with acalabrutinib or ibrutinib (from unstructured data on or after 1 January 2018)
| . | Original sample . | ||
|---|---|---|---|
| Acalabrutinib cohort∗ . | Ibrutinib cohort without subsequent acalabrutinib† . | Ibrutinib cohort with subsequent acalabrutinib‡ . | |
| Total patients . | n = 212 . | n = 194 . | n = 59 . |
| Patients who discontinued treatment, n (%) . | 54 (25.5) . | 79 (40.7) . | 59 (100.0) . |
| Reasons for discontinuation§ | |||
| Toxic effect of therapy/MEOI | 27 (12.7) | 37 (19.1) | 53 (89.8) |
| Cytopenia | 9 (4.2) | 4 (2.1) | 4 (6.8) |
| Arthralgia/myalgia/arthritis | 3 (1.4) | 3 (1.5) | 12 (20.3) |
| Gastrointestinal toxicity | 3 (1.4) | 3 (1.5) | 4 (6.8) |
| Headache | 3 (1.4) | 1 (0.5) | 2 (3.4) |
| Atrial fibrillation | 3 (1.4) | 5 (2.6) | 7 (11.9) |
| Bleeding episodes | 2 (0.9) | 8 (4.1) | 8 (13.6) |
| Cardiac toxicity | 2 (0.9) | 1 (0.5) | 5 (8.5) |
| Fatigue | 2 (0.9) | 10 (5.2) | 3 (5.1) |
| Rash | 3 (1.4) | 4 (2.1) | 8 (13.6) |
| Diarrhea | 1 (0.5) | 3 (1.5) | 4 (6.8) |
| Edema | 1 (0.5) | 2 (1.0) | 5 (8.5) |
| Infection | 1 (0.5) | 2 (1.0) | 0 (0.0) |
| Pulmonary toxicity / pneumonitis | 0 (0.0) | 2 (1.0) | 1 (1.7) |
| Hypertension | 0 (0.0) | 0 (0.0) | 1 (1.7) |
| Other | 2 (0.9) | 7 (3.6) | 5 (8.5) |
| Progression | 7 (3.3) | 5 (2.6) | 3 (5.1) |
| Noncancer-related medical issue | 4 (1.9) | 5 (2.6) | 2 (3.4) |
| Insufficient response | 3 (1.4) | 4 (2.1) | 3 (5.1) |
| Planned regimen change | 2 (0.9) | 2 (1.0) | 1 (1.7) |
| Cancer-related symptoms not due to therapy | 1 (0.5) | 2 (1.0) | 0 (0.0) |
| Sufficient disease control | 0 (0.0) | 2 (1.0) | 0 (0.0) |
| Patient request | 0 (0.0) | 1 (0.5) | 0 (0.0) |
| Other | 3 (1.4) | 5 (2.6) | 0 (0.0) |
| Unknown | 8 (3.8) | 17 (8.8) | 0 (0.0) |
| . | Original sample . | ||
|---|---|---|---|
| Acalabrutinib cohort∗ . | Ibrutinib cohort without subsequent acalabrutinib† . | Ibrutinib cohort with subsequent acalabrutinib‡ . | |
| Total patients . | n = 212 . | n = 194 . | n = 59 . |
| Patients who discontinued treatment, n (%) . | 54 (25.5) . | 79 (40.7) . | 59 (100.0) . |
| Reasons for discontinuation§ | |||
| Toxic effect of therapy/MEOI | 27 (12.7) | 37 (19.1) | 53 (89.8) |
| Cytopenia | 9 (4.2) | 4 (2.1) | 4 (6.8) |
| Arthralgia/myalgia/arthritis | 3 (1.4) | 3 (1.5) | 12 (20.3) |
| Gastrointestinal toxicity | 3 (1.4) | 3 (1.5) | 4 (6.8) |
| Headache | 3 (1.4) | 1 (0.5) | 2 (3.4) |
| Atrial fibrillation | 3 (1.4) | 5 (2.6) | 7 (11.9) |
| Bleeding episodes | 2 (0.9) | 8 (4.1) | 8 (13.6) |
| Cardiac toxicity | 2 (0.9) | 1 (0.5) | 5 (8.5) |
| Fatigue | 2 (0.9) | 10 (5.2) | 3 (5.1) |
| Rash | 3 (1.4) | 4 (2.1) | 8 (13.6) |
| Diarrhea | 1 (0.5) | 3 (1.5) | 4 (6.8) |
| Edema | 1 (0.5) | 2 (1.0) | 5 (8.5) |
| Infection | 1 (0.5) | 2 (1.0) | 0 (0.0) |
| Pulmonary toxicity / pneumonitis | 0 (0.0) | 2 (1.0) | 1 (1.7) |
| Hypertension | 0 (0.0) | 0 (0.0) | 1 (1.7) |
| Other | 2 (0.9) | 7 (3.6) | 5 (8.5) |
| Progression | 7 (3.3) | 5 (2.6) | 3 (5.1) |
| Noncancer-related medical issue | 4 (1.9) | 5 (2.6) | 2 (3.4) |
| Insufficient response | 3 (1.4) | 4 (2.1) | 3 (5.1) |
| Planned regimen change | 2 (0.9) | 2 (1.0) | 1 (1.7) |
| Cancer-related symptoms not due to therapy | 1 (0.5) | 2 (1.0) | 0 (0.0) |
| Sufficient disease control | 0 (0.0) | 2 (1.0) | 0 (0.0) |
| Patient request | 0 (0.0) | 1 (0.5) | 0 (0.0) |
| Other | 3 (1.4) | 5 (2.6) | 0 (0.0) |
| Unknown | 8 (3.8) | 17 (8.8) | 0 (0.0) |
MEOI, medical events of interest.
Patients in the acalabrutinib cohort might have received ibrutinib in any line of therapy. One patient in the acalabrutinib cohort discontinued treatment because of atrial fibrillation, and 1 patient in the ibrutinib cohort discontinued treatment because of arthralgia/myalgia + headache on the index date (ie, initiation of acalabrutinib or ibrutinib). These patients were excluded from the analysis.
Patients who received both acalabrutinib monotherapy and ibrutinib monotherapy on or after 1 January 2018 were excluded from this cohort.
Patients in this cohort received ibrutinib monotherapy on or after 1 January 2018, followed by acalabrutinib monotherapy in a later line; 4 patients received acalabrutinib monotherapy before ibrutinib monotherapy, and were removed from this cohort.
Reasons for discontinuation were abstracted via a review of unstructured data. Patients might have had ≥1 reasons for discontinuation. See Appendix 5 for descriptions of each reason for discontinuation per the Flatiron Analytic Guide.
Discussion
To our knowledge, this study represents the largest available comparison of ibrutinib and acalabrutinib in patients with CLL/SLL in real-world clinical practice. Although ELEVATE-RR compared these agents prospectively in the relapsed/refractory setting, this is, to our knowledge, the first comparison of these agents across lines and specifically in the frontline setting and provides additional insights regarding activity of these agents outside of a tightly controlled clinical trial.
Although TTD was not statistically different between these real-world cohorts in the unweighted analysis, after applying ATT weighting designed to balance baseline characteristics, patients treated with acalabrutinib overall had a longer time to discontinuation than those treated with ibrutinib, with trends toward longer TTD in both the frontline and relapsed/refractory settings. Although ELEVATE-RR was not designed to demonstrate differences in PFS, these head-to-head prospective data have demonstrated differences in discontinuation rates with more patients requiring drug discontinuation when treated with ibrutinib than with acalabrutinib. Consistent with those findings, this study found a lower proportion of treatment discontinuation in patients treated with acalabrutinib than in those treated with ibrutinib (23% vs 33%, respectively, after weighting). Real-world treatment discontinuations are reportedly in the range from 15% to 43% for patients treated with ibrutinib.7 Fewer real-world studies of patients treated with acalabrutinib have been conducted; 1 small cohort (n = 69) demonstrated a discontinuation rate of 19%.8 Frontline trials as well as real-world studies of patients treated with BTKis have demonstrated that adverse events are the most common reasons for discontinuation.3,7,9-11 Our analysis also found that among the patients who discontinued, approximately half of the patients in both arms discontinued treatment because of toxicity. Thus, the observed difference in TTD may reflect, at least in part, differences in toxicity profile.
In addition to TTD, we also assessed TTNTD as a proxy for PFS. Given the potential for patients to have gaps between treatment regimens and the potential for progression data to be missing from EHRs, TTNTD can be a pragmatic real-world clinical outcome. In the unweighted analysis, TTNTD was NR for either cohort, although the ATT-weighted analysis showed a trend favoring acalabrutinib. In contrast to our findings, a recent abstract investigating time to next treatment for patients with CLL initiated on frontline ibrutinib or acalabrutinib found an adjusted HR of 1.89 for acalabrutinib vs ibrutinib (95% CI, 1.12-3.13; P = .016).12 The conclusions of our analysis conflict with these findings, although the analyses differ in a number of meaningful ways. The differences in methodology and the different findings make it challenging to directly compare results and underscores the importance of continued research with longer follow-up in this area.
The impact of early discontinuation of ibrutinib has been assessed in both clinical trials and real-world studies. Barr et al conducted a retrospective analysis of the RESONATE trial data assessing the clinical impact of ibrutinib adherence and found that patients missing ≥8 consecutive days of ibrutinib had a shorter median PFS vs those missing <8 days (10.9 months vs NR).13 Real-world cohort studies have demonstrated mixed results; a single-center study demonstrated shorter event-free survival in patients with dose interruptions,14 whereas a larger multicenter study did not demonstrate an impact of dose interruptions on PFS.15
In this study, there was a trend toward improved TTD with acalabrutinib in the frontline setting, although the differences between those treated with ibrutinib and those treated with acalabrutinib were not statistically significant. Further evaluation with extended follow-up and a larger sample size will be illustrative as we aim to understand the optimal approach to frontline treatment of CLL.
Unlike strictly controlled clinical trial settings, real-world evidence provides insight into how medications perform in routine clinical practice. As we aim to improve outcomes for patients receiving therapy outside of the clinical trial setting, these data suggest that patients treated with acalabrutinib have a longer time on therapy and fewer treatment discontinuations because of toxicity than those treated with ibrutinib.
This study has limitations inherent to its study design. Firstly, the Flatiron Health database uses electronic medical records that are maintained for the purpose of patient care rather than research. Therefore, data may have errors or be subject to missing information. For example, because diagnoses for comorbidities are generally not well populated in the Flatiron Health database, we did not further adjust for imbalances in cardiovascular risk factors in the Cox PH model. In addition, the Flatiron database has limited information on genetic characteristics of CLL (>90% unknown status for del17p, del11q, and immunoglobulin heavy chain mutation). Although genetic features can affect the response to treatment, it would not be anticipated that these features would be meaningfully different between the treatment cohorts. Thus, the impact of this limitation is hypothesized to be minimal on the current analysis. Secondly, the retrospective nature of the study means patients cannot be randomly assigned to treatment cohorts, so there may be important differences in patient characteristics between study arms. ATT weighting was used to balance the groups in terms of baseline characteristics, although unobserved confounders may also remain and affect the results of this study. Thirdly, we had relatively limited follow-up time periods for patients receiving acalabrutinib at the time of analysis and, therefore, lacked data maturity to assess clinical outcomes such as PFS and OS. TTNTD was used as a proxy measure in the absence of mature progression or OS data. Although limitations exist, this study provides a large real-world comparison of patients treated with either acalabrutinib or ibrutinib. After adjusting for imbalances in patient characteristics, the results suggest that, compared with patients treated with ibrutinib, patients treated with acalabrutinib have a longer time to discontinuation and a trend toward longer TTNTD.
Acknowledgments
Editorial assistance was provided by Peloton Advantage, an OPEN Health company, Parsippany, NJ.
This work was funded by AstraZeneca.
Authorship
Contribution: K.R. and M.D. conceived the study; L.Y. and H.G. provided data curation; Y.C., L.Y., and H.G. performed the formal analysis; K.R. acquired funding, managed the resources, and supervised the study; L.E.R., A.R.M., K.R., M.D., M.S.D., Y.C., L.Y., and H.G. developed the methodology; M.D., M.S.D., Y.C., L.Y., and H.G. validated study results; L.Y. and H.G. provided study visualization; L.E.R., A.R.M., K.R., and M.D. interpreted study results; K.R., M.D., Y.C., L.Y., and H.G. wrote the original draft; and all authors reviewed and edited the drafts.
Conflict-of-interest disclosure: L.E.R. has served as a consultant for AbbVie, Ascentage, AstraZeneca, BeiGene, Janssen, Loxo Oncology, Pharmacyclics, Pfizer, and TG Therapeutics; has served as a CME speaker for DAVA, Curio, PeerView, PER, Research to Practice, and Medscape; holds minority ownership interest in Abbott Laboratories; has received travel support from Loxo Oncology; and has received research funding (paid to the institution) from Pfizer, Loxo Oncology, Aptose Biosciences, and Qilu Puget Sound Biotherapeutics. K.R. is an employee and shareholder of AstraZeneca. M.D., M.S.D., Y.C., L.Y., and H.G. are employees of Analysis Group, which received research funding from AstraZeneca for this study. A.R.M. has received research support from TG Therapeutics, Pharmacyclics, AbbVie, Johnson & Johnson, Regeneron, DTRM BioPharma, Sunesis, Loxo Oncology, Adaptive, Nurix, Genentech, AZ/Acerta, and BeiGene; and has received fees for advisory, consultancy, DSMB, or speaking, from TG Therapeutics, Pharmacyclics, AbbVie, Johnson & Johnson, Regeneron, DTRM BioPharma, Sunesis, Loxo Oncology, Adaptive, Nurix, Genentech, AstraZeneca/Acerta, BeiGene, DAVA, PER, PerView, Axis, Vaniam, and Research to Practice.
Correspondence: Lindsey E. Roeker, CLL Program, Leukemia Service, Division of Hematologic Oncology, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: roekerl@mskcc.org.
References
Author notes
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org.
Data for studies not listed on Vivli could be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/.
Further details are available through the AstraZeneca Vivli member page at https://vivli.org/ourmember/astrazeneca/.
The full-text version of this article contains a data supplement.

![Attrition table. (1) Patients with CLL and/or SLL were identified using ICD-9-CM codes 204.1x and ICD-10-CM codes C91.1x and C83.0x, or evidence in unstructured documents of having been treated specifically for CLL/SLL. (2) Clinical encounters included patient visits from structured data (ECOG performance status, medication administrations, medication orders, telemedicine, vitals, and laboratory reports) and abstracted treatment information (classic cytogenetics, fluorescence in situ hybridization, immunoglobulin heavy chain, immunophenotyping, and oral medications). (3) For each patient, the study period extended from 6 months prior to the initiation of acalabrutinib or ibrutinib to the end of follow-up (the earliest of end of clinical activity, death, or end of data availability [ie, 28 February 2021]). (4) Concurrent antineoplastic treatments were defined based on antineoplastic treatments other than the list of anti-CD20 monoclonal antibodies, BCL2 antagonist, and PI3K inhibitors in supplemental Table 1 and Appendix 1. (5) See supplemental Table 1 and Appendix 2 for other malignancy diagnosis codes. (6) Patients may contribute to both the acalabrutinib and ibrutinib cohorts. FISH, fluorescence in situ hybridization; IGHV, immunoglobulin heavy chain; PI3K, phosphoinositide 3-kinase; 1L, first line; 2L, second line.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/7/16/10.1182_bloodadvances.2023009739/2/m_blooda_adv-2023-009739-gr1.jpeg?Expires=1769983166&Signature=iP0-yIbh8HVVe9smnwwgh9QnBwEi5siyhKrbERVBAgzvHpRKRU3hBCLYHgl81kcvBCoteSuMj86WtHvAHfge4-zoP6-b9i6nePNstY6e5ym6k2gUVjyWA-RjW7lEEUmc60TxrnrzWtqXSHdXsT-b4J1PH8i7PKoNrLomee5KolGXZTTbBcYcNVOQOw4SB6ZRwHYKVg3aOR59tHfZoK5TcRmClBe9-nVsOkkZHZRf8Yp0QCHaio65YFRM7Q4SxIagFkkIoXnenUop9L2rP-3idHM4wJh6vzMLbrbYmiTZ7-9pDc5B5UcPIWJOyyyecs6-c3W3UbVXhO67d0pReF1xHw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)