Key Points
SAR443809 selectively inhibits the complement alternative pathway, by binding to FBb and blocking the cleavage of C3 and factor B.
The selectivity and efficacy of SAR443809 confer a robust pharmacodynamic response for the treatment of AP-mediated disorders.
Abstract
Dysregulated activation of the complement system is implicated in the onset or progression of several diseases. Most clinical-stage complement inhibitors target the inactive complement proteins present at high concentrations in plasma, which increases target-mediated drug disposition and necessitates high drug levels to sustain therapeutic inhibition. Furthermore, many efforts are aimed at inhibiting only terminal pathway activity, which leaves opsonin-mediated effector functions intact. We describe the discovery of SAR443809, a specific inhibitor of the alternative pathway C3/C5 convertase (C3bBb). SAR443809 selectively binds to the activated form of factor B (factor Bb) and inhibits alternative pathway activity by blocking the cleavage of C3, leaving the initiation of classical and lectin complement pathways unaffected. Ex vivo experiments with patient-derived paroxysmal nocturnal hemoglobinuria erythrocytes show that, although terminal pathway inhibition via C5 blockade can effectively inhibit hemolysis, proximal complement inhibition with SAR443809 inhibits both hemolysis and C3b deposition, abrogating the propensity for extravascular hemolysis. Finally, intravenous and subcutaneous administration of the antibody in nonhuman primates demonstrated sustained inhibition of complement activity for several weeks after injection. Overall, SAR443809 shows strong potential for treatment of alternative pathway-mediated disorders.
Introduction
Dysregulation or deficiency of the complement alternative pathway (AP) is implicated in several rare blood disorders and renal diseases,1,2 and more broadly plays a role in the progression of inflammatory conditions.3 Nascently-generated C3b molecules (from any of the 3 initiation pathways) bind factor B (FB) to form the proconvertase C3bB, which is cleaved by factor D (FD) to form the active convertase alternative pathway C3 convertase (C3bBb). This then cleaves C3, forming additional C3b molecules. Once cell surfaces are opsonized with a high density of C3b, convertases cleave C5, initiating terminal complement pathway (TP) activation and eventual cell lysis by the membrane attack complex (MAC). In addition, C3b and its fragments (C3b, iC3b, C3dg, C3d) themselves can participate in a host of effector functions in innate as well as adaptive immunity via interactions with their cognate complement receptors, playing key roles in cell clearance and phagocytosis, immunoediting during tissue development, memory cell generation, and influencing T-cell responses.4 The AP amplification loop is the primary driver of C3 fragment opsonization and TP activation, contributing to at least 80% of TP activity regardless of the initiation pathway.5,6 Therefore, the self-amplifying nature of the AP facilitates not only rapid immune response to imminent pathogenic threats, but also overactivation in the context of disease.
Over the last decade or so, efforts have focused on inhibiting the AP or TP, as these pathways generate the main effector molecules of complement. The first clinical success was in paroxysmal nocturnal hemoglobinuria (PNH). PNH manifests because of the absence of glycosylphosphatidylinositol-linked complement regulatory proteins CD55 and CD59 on erythrocytes, important regulators of AP amplification and TP activity. Dysregulation causes high levels of C3 fragment opsonization and MAC formation, resulting in hemolysis. Eculizumab, a monoclonal antibody against C5, represents a major milestone in PNH treatment that greatly improved the quality of life and increased the median survival for patients with PNH.7-9 However, a subset of patients treated with eculizumab patients (∼10%) exhibit breakthrough hemolysis.10 In some patients this was attributed to elevated free C5 levels, resulting in a pharmacokinetic breakthrough that can be overcome with ravulizumab, the next-generation anti-C5 inhibitor.10 In others, it was shown to be from a pharmacodynamic breakthrough because of excessive complement activation and amplification. Although intravascular hemolysis (IVH) by MAC is thought to be the primary mediator of PNH pathophysiology, extravascular hemolysis (EVH) can occur in patients under complement amplifying conditions.11-13 Several studies have now shown that upon blockade of the TP, C3 fragment opsonization is increased compared with that of untreated controls resulting in extravascular clearance and hemolysis in the liver and spleen.11 This activity is refractory to eculizumab because it results from complement activation upstream of C5.12,14 Retrospective studies of patients with PNH treated with C5 inhibitors revealed continued disease burden in many patients, including low hemoglobin levels and ongoing symptoms.15 Comorbidities in patients on C5 inhibitors may result in breakthrough hemolysis arising from complement amplifying conditions or by excessive C3b deposition and C5 cleavage-independent MAC formation,13,16,17 which can be ameliorated by inhibiting complement upstream of C5.16,18,19
Recently, pegcetacoplan, a macrocyclic C3 inhibitor, that showed improved efficacy over eculizumab, was approved by the US Food and Drug Administration for the treatment of PNH, validating C3 and proximal complement as a therapeutic target.20 Incidence of breakthrough hemolysis in patients treated with pegcetacoplan was reduced compared with that of eculizumab treatment, but not eliminated, possibly from pharmacokinetic factors and dosing.20 Other molecules specifically targeting the AP via either FB or FD are also currently in clinical development.19,21 Recent phase III data have showed that these oral small molecules targeting FD or FB have improved efficacy either when administered in combination with C522 or as a standalone therapy.23 Ultimately, both FB and FD inhibitors may prove efficacious, which would represent a major advancement in PNH treatment. However, it is important to also note the potential limitations of these targets. FB, like C3, is present at high concentrations in plasma (∼2 μM), which may require high dosing for therapeutic efficacy. Similarly, FD has a very high turnover rate,24 which could make complete and sustained pharmacological inhibition challenging. Therefore, targeting an activated component of the AP is an attractive strategy to overcome such challenges.
We describe in this article the discovery of SAR443809, a humanized monoclonal immunoglobulin G4 (IgG4) antibody that selectively binds to factor Bb (FBb), the active form of FB. Our results demonstrate efficient and complete inhibition of AP in vitro, as well as sustained AP inhibition after intravenous or subcutaneous administration to nonhuman primates.
Materials and methods
Materials
Purified complement components C1s (enzyme and proenzyme), C1r, C2, C3, FB, FBb, FD, FH-depleted sera, antisera to FB, C3 and C3a, and complement assay reagents (GVB0 [gelatin veronal buffer without Ca2+ and Mg2+], Mg-EGTA, rabbit RBCs [ER]) were obtained from Complement Technology Inc, (Tyler, TX). GST-MASP-1, GST-MASP-2, and GST-C1s proenzyme were purchased from Abnova (Taipei, Taiwan). Human neutrophil elastase and thrombin were purchased from EMD Millipore (Burlington, MA). Complement preserved normal human serum (NHS) was sourced from Complement Technology Inc (Tyler, TX), BioIVT (Westbury, NY), or Innovative Research (Novi, MI). Complement preserved serum from different species was sourced from BioIVT. Whole blood of patient with PNH was prospectively collected in collaboration with Innovative Research Inc. Antibodies used for flow-cytometry analysis were from Thermo Fisher Scientific (Waltham, MA) or Invitrogen (Carlsbad, CA). All other common laboratory reagents and buffers were from either Sigma (St. Louis, MO) or Thermo Fisher Scientific.
Binding using surface plasmon resonance
Binding kinetics were determined by single-cycle kinetics on a Biacore T200 at 25°C using Series S Sensor Chip Protein A (Cytiva, Marlborough, MA) and HBS-P+ running buffer (10 mM HEPES, 150 mM NaCl, 0.05% Tween 20, pH 7.4). Forty to 150 RU of each antibody (SARXX01, SARXX02 or SAR443809) was captured on the chip followed by sample injection. Dilutions of human FB (6-500 nM), human FBb (0.4-30 nM) were sequentially injected from lowest to highest concentration, followed by a dissociation phase.
For the binding of SAR443809 to C3bBb, convertases were assembled de novo on a Series S CM5 sensor chip surface as described by Harris et al.25 Purified C3b was coupled to the chip using an amine coupling kit (Cytiva), according to manufacturer instructions. Then, 2 sequential injections of FB, FD, and C3 were performed in HBS-Ni buffer (10 mM HEPES pH 7.4, 150 mM NaCl, 1 mM NiSO4), to deposit C3b via its thioester. Sequential injections of recombinant human CD55 (R&D Systems, Minneapolis, MN) and overnight buffer flow ensured all convertases decayed and only C3b remained on the chip. Binding of SAR443809 to C3bBb was measured using single-cycle kinetics in HBS-P+ buffer containing 1 mM NiSO4, in which FB (60 μg/mL) and FD (0.5 μg/mL) were injected simultaneously, followed by ethylenediamine tetraacetic acid (EDTA) to decay C3bB complexes. Dilutions of SAR443809 (3.7-300 nM, Fab fragment) were sequentially injected from lowest to highest concentration, followed by a dissociation phase.
Data were analyzed using the Biacore T200 Evaluation Software, and ka, kd, and KD values were calculated based on a 1:1 binding model curve fit to double reference-subtracted sensorgrams.
Mass photometry
Formation of SAR443809-FB and SAR443809-FBb complexes in solution was evaluated on a Refeyn OneMP Mass Photometry system. The instrument was calibrated using bovine serum albumin (BSA; Thermo Fisher Scientific) and Apoferritin (Sigma) to calculate the linear relationship between contrast and mass. SAR443809, FB, FBb, or mixtures thereof (1:2 of drug: target) were preincubated for at least 5 minutes at 25°C, followed by dilution in buffer on the coverslip and subsequent measurement. Data were acquired at a rate of 100 frames per second for 60 seconds and analyzed using DiscoverMP software.
Complement inhibition assays
Wieslab EIA experiments
These experiments were performed using commercial Wieslab enzyme immunoassay (EIA) kits (Svar Life Science AB, Malmö, Sweden) according to manufacturer’s protocol, using either pooled NHS or normal cynomolgus monkey serum (NCS). The activity of SAR443809 was compared with that of the isotype control to assess the effect of the antibody on each of the complement pathways. The half-maximal inhibitory concentration (IC50) values were obtained from the dose-response curve fits.
In addition, the supernatant from the AP Wieslab EIA was collected and analyzed by western blotting for FB and C3a (A218, A235; Complement Technology).
AP-mediated hemolysis assay
To assess hemolysis, ERs were incubated with 10% NHS in the presence of Mg-EGTA. The red blood cells (RBCs) were prepared by washing the cells in GVB0 (B103; Complement Technology), gently mixing by inverting and centrifuging. After 3 washes, the cells were resuspended in GVB0 containing 10 mM Mg-EGTA. The antibody (SAR443809 or isotype control) was titrated into NHS and incubated with ERs for 50 minutes at 37°C with continuous shaking. Hemolysis was assessed by measuring the absorbance of the supernatant at 415 nm.
The data were normalized using the no antibody control representing maximal lysis, and IC50 values were determined from the dose-response curve fit.
Inhibition of C3b deposition on ERs
To induce C3b deposition on ERs, reactions were set up like the AP-mediated hemolysis assay described above, but 10% C5-depleted sera was used instead to prevent MAC formation and cell lysis. At the end of the incubation, the RBCs were collected and washed with cold fluorescence-activated cell sorting buffer (phosphate-buffered saline [PBS] + 0.5% BSA + 0.1% NaN3 + 5 mM EDTA) twice and then stained for C3b using a mouse anti-C3b antibody (MA1-70053; Invitrogen), followed by Alexa-488 conjugated anti-mouse IgG (A21121; Invitrogen). Serum treated unstained RBCs were used as gating controls. The data were normalized to no drug control, and IC50 was determined from the dose-response curve fit.
Inhibition of C3 cleavage in FH-depleted serum
Five percent NHS, complement factor H (FH)-depleted serum, FH-depleted serum with 100 μg/mL FH, and FH-depleted serum with 100 μg/mL SAR443809 were prepared in assay buffer from the AP Wieslab kit (Svar Life Sciences). Reactions with 100 μg/mL isotype control or 10 mM EDTA in 5% FH-depleted serum were also included. The samples were incubated at 37°C for 30 minutes, quenched with lithium dodecyl sulphate sample buffer, and western blotted to detect cleavage of C3 (A213; Complement Technology).
Inhibition of C3b deposition and hemolysis of PNH RBCs
RBCs from the blood (O+) of the patient with PNH were isolated and the percentage of CD59– cells (PNH RBC clone size) was characterized by flow cytometry using an APC-conjugated anti-CD59 antibody (Thermo Fisher, Clone OV9A2). Hemolysis of PNH RBCs was induced using acidified NHS (∼pH 6.7 using 0.2 N HCl, aNHS). PNH RBCs were incubated with 20% aNHS with Mg-EGTA for 3 hours at 37°C with or without SARXX02 or isotype control (66 μg/mL). Untreated cells, as well as reactions with EDTA, and anti-C5 mAb (A217; Quidel; at 66 μg/mL) were included as controls. To monitor the extent of C3b deposition on the RBCs, the RBCs that remained after hemolysis were collected and washed twice with 0.5% BSA in PBS. The C3b/iC3b deposited was assessed using a mouse anti-C3b/iC3b mAb (Thermo Fisher, Clone 6C9) followed by an Alexa488-conjugated anti-mouse IgG. The cells were then stained for CD59 and counted using an Accuri C6 flow cytometer.
For the dose-dependent inhibition of hemolysis, PNH RBCs were incubated with either a titration of SARXX02 or isotype control in 20% aNHS with Mg-EGTA for 3 hours at 37°C. The absorbance of supernatants was measured at 540 nm and the extent of serum induced hemolysis was normalized assuming the absorbance from hypotonic lysis as 100%. IC50 was derived from the dose-response curve fit.
SAR443809 dosing in cynomolgus monkeys
Female cynomolgous monkeys (4 animals per group) were treated with 15 mg/kg SAR443809 in PBS by IV in the cephalic vein or by subcutaneous (SC) injection in the interscapular region of the back. Control animals were treated with PBS by IV. The in-life portion of this study was performed at the test facility Altasciences Preclinical Seattle LLC (Everett, WA), an Association for Assessment and Accreditation of Laboratory Animal Care–accredited facility that was noted to be compliant with internal animal welfare policies and local and federal regulations. The ex vivo pharmacodynamic assessment was carried out in-house. Whole blood was collected at different time points and processed to complement preserved serum. The serum samples from each group were then assessed in the Wieslab AP EIA per the manufacturer’s protocol. The ex vivo activity data was normalized to the pre-dose sample of the corresponding animal, assuming the activity to be 100%. Percent activity was then plotted as a function of time to assess the pharmacology of SAR443809 over the course of the study.
Results
SAR443809 is a highly selective antibody that binds to complement factor Bb with high affinity and inhibits the activation of complement AP
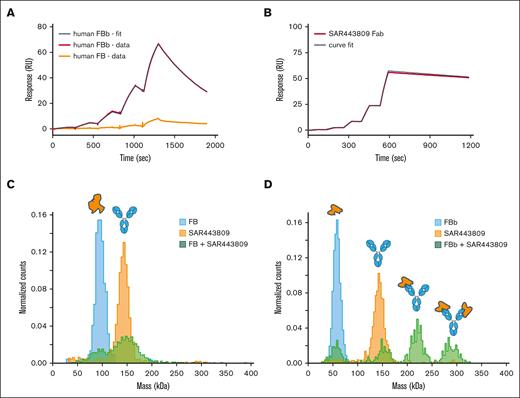
The discovery of SAR443809 is described in supplemental Figure 1. In vitro pharmacology of SAR443809 was characterized through a series of experiments using NHS and/or purified complement components. In SPR assays, SAR443809 bound to FBb with a KD of 7.3 nM, whereas little to no binding to FB was observed (Figure 1A; Table 1). This was further confirmed using solution-based mass photometry experiments (Figure 1C-D). Premixing SAR443809 with FBb resulted in the following 4 distinct populations: unbound FBb, unbound drug, FBb-drug at 1:1 stoichiometry, and FBb- drug at 2:1 stoichiometry (Figure 1D; supplemental Table 1), whereas no higher molecular weight complexes were detected with FB and SAR443809 (Figure 1C; supplemental Table 1). However, for FBb binding to translate to AP inhibition, the drug must bind to FBb on the active convertase because free FBb cannot activate AP downstream. Thus, we tested the binding of SAR443809 to active convertase by SPR, using the method described by Harris et al,25 with some modifications. SAR443809 Fab was used instead of the full-length antibody to eliminate binding artifacts because of avidity. Under these conditions, SAR443809 Fab bound to the convertase with a KD of 0.5 nM (Figure 1B; Table 1).
SAR443809 binds to FBb but not FB. (A) Target binding kinetics of SAR443809. The binding affinity of SAR443809 to human FB (orange) or human FBb (red) was assessed using surface plasmon resonance (Biacore T200). The mAb was captured on a Series S Sensor Chip Protein A and the kinetic binding constants were obtained in single-cycle kinetics mode with increasing concentrations of the targets. Data were analyzed using the Biacore T200 Evaluation Software, and ka, kd, and KD values were calculated based on a 1:1 binding model curve fit to double reference-subtracted sensorgrams (black). (B) SAR443809 Fab binding to AP C3 convertase (C3bBb). The binding affinity of SAR443809 Fab to C3bBb (red) was assessed using surface plasmon resonance (Biacore T200). A small amount of C3b was amine coupled on a Series S CM5 chip, followed by natural C3b cleavage and subsequent covalent attachment to the chip via C3bBb formed on the surface (multiple injections of FB, FD, and C3). After surface preparation, C3bBb was formed via injection of FB and FD, and kinetic data were collected in single-cycle kinetics mode with increasing concentration of SAR443809 Fab. Data were analyzed using the Biacore T200 Evaluation Software, and ka, kd, and KD values were calculated based on a 1:1 binding model curve fit to double reference-subtracted sensorgrams (black). (C) SAR443809 interaction with FB in solution. The mass distribution of samples containing either SAR443809 (orange), FB (blue), or a 2:1 FB:SAR443809 mixture (green) was measured using mass photometry. Histograms for individual components reveal expected molecular weights (SAR443809-146 kDa; FB – 93 kDa). The mixture of SAR443809 and FB shows 2 peaks corresponding to the mass of the individual species (SAR443809-146 kDa; FB – 93 kDa), with no higher molecular weight species present. (D) SAR443809 interaction with FBb in solution. The mass distribution of samples containing either SAR443809 (orange), FBb (blue), or a 2:1 FBb:SAR443809 mixture (green) was measured using mass photometry. Histograms for individual components reveal expected molecular weights (SAR443809-146 kDa; FBb – 60 kDa). The mixture of SAR443809 and FBb shows 4 peaks, corresponding to free FBb (60 kDa), free SAR443809 (146 kDa), SAR443809-FBb1 complex (206 kDa), and SAR443809-FBb2 complex (266 kDa).
SAR443809 binds to FBb but not FB. (A) Target binding kinetics of SAR443809. The binding affinity of SAR443809 to human FB (orange) or human FBb (red) was assessed using surface plasmon resonance (Biacore T200). The mAb was captured on a Series S Sensor Chip Protein A and the kinetic binding constants were obtained in single-cycle kinetics mode with increasing concentrations of the targets. Data were analyzed using the Biacore T200 Evaluation Software, and ka, kd, and KD values were calculated based on a 1:1 binding model curve fit to double reference-subtracted sensorgrams (black). (B) SAR443809 Fab binding to AP C3 convertase (C3bBb). The binding affinity of SAR443809 Fab to C3bBb (red) was assessed using surface plasmon resonance (Biacore T200). A small amount of C3b was amine coupled on a Series S CM5 chip, followed by natural C3b cleavage and subsequent covalent attachment to the chip via C3bBb formed on the surface (multiple injections of FB, FD, and C3). After surface preparation, C3bBb was formed via injection of FB and FD, and kinetic data were collected in single-cycle kinetics mode with increasing concentration of SAR443809 Fab. Data were analyzed using the Biacore T200 Evaluation Software, and ka, kd, and KD values were calculated based on a 1:1 binding model curve fit to double reference-subtracted sensorgrams (black). (C) SAR443809 interaction with FB in solution. The mass distribution of samples containing either SAR443809 (orange), FB (blue), or a 2:1 FB:SAR443809 mixture (green) was measured using mass photometry. Histograms for individual components reveal expected molecular weights (SAR443809-146 kDa; FB – 93 kDa). The mixture of SAR443809 and FB shows 2 peaks corresponding to the mass of the individual species (SAR443809-146 kDa; FB – 93 kDa), with no higher molecular weight species present. (D) SAR443809 interaction with FBb in solution. The mass distribution of samples containing either SAR443809 (orange), FBb (blue), or a 2:1 FBb:SAR443809 mixture (green) was measured using mass photometry. Histograms for individual components reveal expected molecular weights (SAR443809-146 kDa; FBb – 60 kDa). The mixture of SAR443809 and FBb shows 4 peaks, corresponding to free FBb (60 kDa), free SAR443809 (146 kDa), SAR443809-FBb1 complex (206 kDa), and SAR443809-FBb2 complex (266 kDa).
Characterization of target binding and AP inhibition of anti-factor Bb monoclonal antibodies
| Antibody . | Target . | Target binding . | Functional assay IC50 (μg/mL) . | |||
|---|---|---|---|---|---|---|
| ka (M–1s–1) . | kd (s–1) . | KD (M) . | Wieslab EIA . | Hemolysis . | ||
| SARXX01 | hFBb | 1.2 × 106∗ | 1.6 × 10–3∗ | 1.4 × 10–9∗ | ND | ND |
| hFB | ND | ND | ND | |||
| SARXX02 | hFBb | 7.0 × 105 ± 1.6 × 105 | 1.0 × 10–3 ± 0.0 × 10-3 | 1.5 × 10–9 ± 0.3 × 10-9 | 3.43 (3.23-3.64) | ND |
| hFB | ND | ND | ND | |||
| SAR443809 | hFBb | 1.9 × 105 ± 0.4 × 105 | 1.4 × 10-3 | 7.3 × 10–9 ± 1.4 × 10-9 | 8.47 (6.60-10.86) | 28.45 (17.93-45.14)† |
| hFB | ND | ND | ND | |||
| cFBb | 1.4 × 105 ± 0.2 × 105 | 8.6 × 10–3 ± 0.5 × 10-3 | 6.1 × 10–8 ± 0.8 × 10-8 | 15.72 (14.41-17.14) | 84.85 (69.43-103.71)† | |
| cFB | ND | ND | ND | |||
| SAR443809 Fab | C3bBb | 2.7 × 105 | 1.8 × 10–4 | 0.5 × 10–9 | NA | NA |
| Antibody . | Target . | Target binding . | Functional assay IC50 (μg/mL) . | |||
|---|---|---|---|---|---|---|
| ka (M–1s–1) . | kd (s–1) . | KD (M) . | Wieslab EIA . | Hemolysis . | ||
| SARXX01 | hFBb | 1.2 × 106∗ | 1.6 × 10–3∗ | 1.4 × 10–9∗ | ND | ND |
| hFB | ND | ND | ND | |||
| SARXX02 | hFBb | 7.0 × 105 ± 1.6 × 105 | 1.0 × 10–3 ± 0.0 × 10-3 | 1.5 × 10–9 ± 0.3 × 10-9 | 3.43 (3.23-3.64) | ND |
| hFB | ND | ND | ND | |||
| SAR443809 | hFBb | 1.9 × 105 ± 0.4 × 105 | 1.4 × 10-3 | 7.3 × 10–9 ± 1.4 × 10-9 | 8.47 (6.60-10.86) | 28.45 (17.93-45.14)† |
| hFB | ND | ND | ND | |||
| cFBb | 1.4 × 105 ± 0.2 × 105 | 8.6 × 10–3 ± 0.5 × 10-3 | 6.1 × 10–8 ± 0.8 × 10-8 | 15.72 (14.41-17.14) | 84.85 (69.43-103.71)† | |
| cFB | ND | ND | ND | |||
| SAR443809 Fab | C3bBb | 2.7 × 105 | 1.8 × 10–4 | 0.5 × 10–9 | NA | NA |
All binding parameters represent mean ± standard deviation from 3 independent experiments. IC50 represented as geometric mean of individual runs (n = 3) and 95% confidence interval.
cFB, cynomolgus factor B; cFBb, cynomolgus factor Bb; ka, association rate constant; Fab, antibody fragment; hFB, human factor B; hFBb, human factor Bb; kd, dissociation rate constant; KD, equilibrium binding constant; NA, not applicable; ND, not determined.
N = 1; values confirmed through parallel BLI measurements.
Same data as in supplemental Table 3.
As expected from the binding results, SAR443809 showed robust, dose-dependent AP inhibition in 2 independent functional assays. The mean IC50 for the inhibition of the Wieslab AP EIA was 8.47 μg/mL (Figure 2A; Table 1) and for the inhibition of hemolysis was 28.45 μg/mL (Figure 2B; Table 1). The antibody specifically inhibited the AP and did not inhibit the classical pathway (CP) or lectin pathway (LP) (Figure 2C-D).
SAR443809 specifically inhibits AP without inhibiting CP or LP. SAR443809 and its corresponding IgG isotype control were tested in a series of assays to assess their ability to inhibit the different complement pathways. A dose-dilution series of SAR443809 (●) or IgG control antibody (○) were tested in the commercial Wieslab EIA assays for (A) AP, (C) CP, and (D) LP, in which the inhibition of MAC deposition upon pathway specific activation was assessed per manufacturer’s protocol. (B) Antibodies were also tested in AP hemolysis assay. Rabbit erythrocytes were incubated with 10% NHS containing Mg2+-EGTA in the presence of varying concentrations of either SAR443809 (●) or the isotype control (○). The extent of hemolysis was quantified using the absorbance of the released hemoglobin in the supernatant. The data reported here is a mean of 3 independent experiments ± standard deviation, for all 4 data sets.
SAR443809 specifically inhibits AP without inhibiting CP or LP. SAR443809 and its corresponding IgG isotype control were tested in a series of assays to assess their ability to inhibit the different complement pathways. A dose-dilution series of SAR443809 (●) or IgG control antibody (○) were tested in the commercial Wieslab EIA assays for (A) AP, (C) CP, and (D) LP, in which the inhibition of MAC deposition upon pathway specific activation was assessed per manufacturer’s protocol. (B) Antibodies were also tested in AP hemolysis assay. Rabbit erythrocytes were incubated with 10% NHS containing Mg2+-EGTA in the presence of varying concentrations of either SAR443809 (●) or the isotype control (○). The extent of hemolysis was quantified using the absorbance of the released hemoglobin in the supernatant. The data reported here is a mean of 3 independent experiments ± standard deviation, for all 4 data sets.
A direct enzyme-linked immunosorbent assay (ELISA) was used to assess the binding selectivity of SAR443809 against a panel of plasma serine proteases. In this assay format, the targets were adsorbed at high densities and, thus, any binding observed would encapsulate affinity as well as avidity effects. SAR443809 bound to FBb with a mean EC50 of 30.9 ng/mL (Table 2). Dose-dependent binding to FB, although measurable, was less efficient and variable between runs (Table 2; supplemental Figure 2). Although binding to GST-MASP1 and GST-MASP2 was also detected at high antibody concentrations, a follow-up experiment with a different lot of these proteins showed no binding, suggesting the initial observation may have been a reagent-specific artifact. No binding was observed for all other proteases tested (Table 2; supplemental Figure 2).
Plasma protease selectivity profile of SAR443809
| Plasma protease . | EC50 (ng/mL)∗ . |
|---|---|
| Human complement factor Bb | 30.9 (8.6-131.1) |
| Human complement factor B | 364.8 (0.01-14090938.19)† |
| Human complement C2 | No binding |
| Human Complement C2a | No binding |
| Human Complement C1s | No binding |
| Human Complement aC1s | No binding |
| Human Complement C1r | No binding |
| MASP1 | >50 μg/mL‡ |
| MASP2 | >50 μg/mL‡ |
| Human complement factor D | No binding |
| Elastase, human neutrophil | No binding |
| Thrombin, human plasma | No binding |
| Plasma protease . | EC50 (ng/mL)∗ . |
|---|---|
| Human complement factor Bb | 30.9 (8.6-131.1) |
| Human complement factor B | 364.8 (0.01-14090938.19)† |
| Human complement C2 | No binding |
| Human Complement C2a | No binding |
| Human Complement C1s | No binding |
| Human Complement aC1s | No binding |
| Human Complement C1r | No binding |
| MASP1 | >50 μg/mL‡ |
| MASP2 | >50 μg/mL‡ |
| Human complement factor D | No binding |
| Elastase, human neutrophil | No binding |
| Thrombin, human plasma | No binding |
CI, confidence interval; EC50, half-maximal effective concentration.
Geometric mean of the EC50 of the biological replicates along with the 95% CI.
very broad 95% CI – unreliable values.
Repeat experiment with new lot of MASP1 and MASP2 showed no binding.
SAR443809 inhibits the activation of AP by preventing cleavage of C3
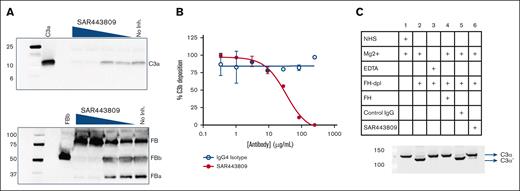
To understand the mechanism by which SAR443809 inhibits AP activation, the activated serum supernatant from the Wieslab AP EIA was western blotted for FB and C3 cleavage fragments. SAR443809 efficiently blocked the cleavage of C3 as seen by decreasing C3a levels with increasing drug concentration (Figure 3A, top). The drug also completely blocked the cleavage of FB to FBb and FBa (Figure 3A, bottom). SAR443809, but not the isotype control, also effectively blocked the deposition of C3b on the ERs in a dose-dependent manner (Figure 3B) with an apparent IC50 of 33.8 μg/mL (Figure 3B).
SAR443809 inhibits AP by blocking cleavage of C3 and FB. (A) SAR443809 inhibits the cleavage of C3 and FB in a dose-dependent manner. In a follow-up experiment to the AP Wieslab EIA, the serum supernatant containing varying amounts of SAR443809, was quenched postactivation on the EIA plate and analyzed by SDS-PAGE, and western blotted for C3a (top) and FB (bottom). (B) SAR443809 inhibits the deposition of C3b on rabbit RBCs in a dose-dependent manner. In an assay set up analogous to AP hemolysis assay, rabbit erythrocytes were incubated with 10% C5-depleted sera in the presence of varying amounts of SAR443809 or IgG4 isotype control. The C3b-deposition on the cells was assessed by flow cytometry. Data reported here is the mean of 2 independent experiments ± standard deviation. (C) SAR443809 inhibits C3 cleavage in solution in factor H depleted sera. Five percent NHS, FH-depleted serum or FH-depleted serum supplemented with FH was incubated with SAR443809. Reactions with the IgG isotype control and with 10 mM EDTA were also included as controls. The samples were quenched and analyzed by SDS-PAGE and western blotted to detect cleavage of the C3 α chain. SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis.
SAR443809 inhibits AP by blocking cleavage of C3 and FB. (A) SAR443809 inhibits the cleavage of C3 and FB in a dose-dependent manner. In a follow-up experiment to the AP Wieslab EIA, the serum supernatant containing varying amounts of SAR443809, was quenched postactivation on the EIA plate and analyzed by SDS-PAGE, and western blotted for C3a (top) and FB (bottom). (B) SAR443809 inhibits the deposition of C3b on rabbit RBCs in a dose-dependent manner. In an assay set up analogous to AP hemolysis assay, rabbit erythrocytes were incubated with 10% C5-depleted sera in the presence of varying amounts of SAR443809 or IgG4 isotype control. The C3b-deposition on the cells was assessed by flow cytometry. Data reported here is the mean of 2 independent experiments ± standard deviation. (C) SAR443809 inhibits C3 cleavage in solution in factor H depleted sera. Five percent NHS, FH-depleted serum or FH-depleted serum supplemented with FH was incubated with SAR443809. Reactions with the IgG isotype control and with 10 mM EDTA were also included as controls. The samples were quenched and analyzed by SDS-PAGE and western blotted to detect cleavage of the C3 α chain. SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis.
In addition, SAR443809 was tested for its ability to block the fluid-phase C3 cleavage using FH-depleted sera. When serum is depleted of FH, fluid-phase “tickover” proceeds in an unregulated manner, effectively consuming C3 in the process, as observed by the formation of the C3 α' chain (C3α’) upon cleavage of C3 (Figure 3C, compares lanes 1, 3 and 4 with lane 2). In these experiments, when SAR443809 was added to FH-depleted sera, the drug effectively blocked activation of C3 when compared with that of the antibody isotype control (Figure 3C, compares lane 6 with lane 5), down to levels seen for FH-sufficient sera (Figure 3C, lane 1, 4) or the EDTA control (Figure 3C, lane 3).
SARXX02, the parent molecule of SAR443809, prevents C3b deposition and hemolysis of RBCs of patients with PNH in vitro
SARXX02, the precursor of SAR443809, was tested for its ability to block C3b deposition on PNH RBCs and, thereby, also block hemolysis. In this donor, the PNH RBC clone size (CD59- cells) was found to be around 17% (Figure 4A, cells only). aNHS treatment resulted in lysis of ∼50% of the PNH RBCs which could be completely recovered with EDTA indicating an AP-mediated effect (Figure 4A). Treatment with SARXX02 or a neutralizing anti-C5 mAb retained the entire PNH RBC clone (Figure 4A), whereas the isotype control resulted in pronounced hemolysis (Figure 4A).
SAR443809 prevents lysis of PNH patient RBCs by inhibiting the surface deposition of C3b. (A) Percent CD59- cells (PNH RBCs) observed under various treatment conditions. The RBCs from a patient with PNH were tested for the presence of CD59 using flow cytometry and the percentage of CD59- cells monitored as a function of various treatments (n = 1). (B) Percent hemolysis (calculated as a proportion of PNH RBCs, left Y-axis) and percent C3b deposition (observed on the surviving CD59– cells, right Y-axis) observed under various treatment conditions. The RBCs from the patient with PNH were dual stained for C3b and CD59 on the cell surface using flow cytometry (n = 1). Percentage of Lysis and C3b deposition were calculated using Equation 1 and Equation 2 respectively. (C) Dose-dependent inhibition of acidified serum-mediated lysis of PNH RBCs by SARXX02. A dose titration of SARXX02 (●) and its antibody isotype control (○) were carried to assess the inhibition of acidified serum-mediated hemolysis of PNH RBCs. The extent of hemolysis was measured using the absorbance of hemoglobin released from the cells upon lysis and normalized to complete lysis as observed under hypotonic conditions. The data shown are an average of 2 independent experiments ± standard deviation.
SAR443809 prevents lysis of PNH patient RBCs by inhibiting the surface deposition of C3b. (A) Percent CD59- cells (PNH RBCs) observed under various treatment conditions. The RBCs from a patient with PNH were tested for the presence of CD59 using flow cytometry and the percentage of CD59- cells monitored as a function of various treatments (n = 1). (B) Percent hemolysis (calculated as a proportion of PNH RBCs, left Y-axis) and percent C3b deposition (observed on the surviving CD59– cells, right Y-axis) observed under various treatment conditions. The RBCs from the patient with PNH were dual stained for C3b and CD59 on the cell surface using flow cytometry (n = 1). Percentage of Lysis and C3b deposition were calculated using Equation 1 and Equation 2 respectively. (C) Dose-dependent inhibition of acidified serum-mediated lysis of PNH RBCs by SARXX02. A dose titration of SARXX02 (●) and its antibody isotype control (○) were carried to assess the inhibition of acidified serum-mediated hemolysis of PNH RBCs. The extent of hemolysis was measured using the absorbance of hemoglobin released from the cells upon lysis and normalized to complete lysis as observed under hypotonic conditions. The data shown are an average of 2 independent experiments ± standard deviation.
In the same set of experiments, the extent of C3b/iC3b deposited on the surviving RBCs, as well as the percentage of lysis was computed as previously described by Ferreira et al, for each of these conditions.26 Untreated RBCs and RBCs treated with aNHS with EDTA showed little to no C3b/iC3b deposits (0.13% and 0.22% C3b/iC3b, respectively) and no lysis of RBCs was observed under these conditions (Figure 4B). In the presence of aNHS, <1% of the surviving PNH cells showed C3b/iC3b deposits, likely from the lysis of the PNH RBCs (Figure 4B). When the cells were treated with SARXX02, only 0.17% of the surviving PNH RBCs had C3b/iC3b deposits, comparable with the levels seen before serum exposure (0.13%), and the RBCs were completely protected from lysis (Figure 4B). In contrast, the control mAb resulted in a pronounced hemolysis with about 1.34% of the PNH RBCs staining for C3b/iC3b (Figure 4B). Consistent with its mechanism, the anti-C5 mAb effectively protected the PNH RBC clone from hemolysis (Figure 4A) but resulted in increased deposition of C3b on the cells (Figure 4B).
A dose-titration of SARXX02 under hemolytic conditions showed that SARXX02 effectively inhibited the AP-mediated lysis of PNH RBCs as compared with its isotype control, with an average IC50 of 24.66 μg/mL (Figure 4C).
In vivo studies in cynomolgus monkeys support subcutaneous dosing of SAR443809 with prolonged inhibition of AP
SAR443809 was found to cross-react with only cynomolgus monkey and rhesus monkey albeit with a lower potency compared with human (supplemental Figure 3; supplemental Table 3), consistent with its lower binding affinity for cynomolgus monkey FBb (Table 1; supplemental Figure 4). Thus, all in vivo pharmacological assessments were carried out in cynomolgus monkeys.
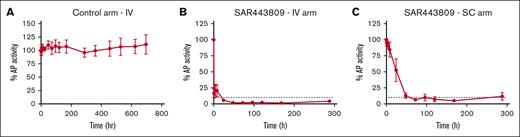
The pharmacology of the drug dosed in non-human primate was assessed ex vivo by measuring residual AP complement activity using the Wieslab EIA. The control arm of the study, dosed with PBS alone, showed no inhibition of AP complement activity (Figure 5A). In the IV arm of the study, SAR443809 resulted in a rapid inhibition of AP, achieving ≥90% inhibition at 24 hours after dose that was sustained for at least 288 hours (Figure 5B). In the SC arm, on average, the extent of AP inhibition increased gradually over time reaching ≥90% inhibition between 24- and 48-hours after dose and was sustained for at least 288 hours, similar to what was seen in the IV arm (Figure 5C). No CP inhibition was observed in any of the study groups (supplemental Figure 5).
SAR443809 demonstrates a sustained inhibition of AP activity when dosed in vivo in cynomolgus monkeys. (A) Percent AP activity measured over time in serum samples from monkeys dosed with PBS (control arm) intravenously, as measured by Wieslab EIA ex vivo. (B-C) Percent AP activity measured over time in serum samples from monkeys dosed with SAR443809 (B) intravenously or (C) subcutaneously, as measured by Wieslab EIA ex vivo. The dashed lines represent a threshold of 90% AP inhibition. Group average (n = 4) ± standard deviation for each timepoint is shown.
SAR443809 demonstrates a sustained inhibition of AP activity when dosed in vivo in cynomolgus monkeys. (A) Percent AP activity measured over time in serum samples from monkeys dosed with PBS (control arm) intravenously, as measured by Wieslab EIA ex vivo. (B-C) Percent AP activity measured over time in serum samples from monkeys dosed with SAR443809 (B) intravenously or (C) subcutaneously, as measured by Wieslab EIA ex vivo. The dashed lines represent a threshold of 90% AP inhibition. Group average (n = 4) ± standard deviation for each timepoint is shown.
Discussion
This study describes the identification and characterization of SAR443809, a humanized IgG4 mAb being developed for the treatment of complement AP-mediated disorders. SAR443809 inhibits AP activity blocking AP tickover and amplification, while leaving CP and LP activity intact. Furthermore, it is selective for FBb. Although no complex formation with FB was seen with solution-based methods like size-exclusion chromatography and mass photometry, or by SPR when the target is in solution, variable dose-dependent binding to FB was observed by ELISA albeit being 10- to 20-fold lower than that of FBb. We hypothesize that this may be driven by either contaminating levels of FBb in the FB preparation or a conformational change upon FB adsorption onto the ELISA plate, amplified by the avid binding format. SAR443809 shows little to no binding to other plasma proteases, including several other enzymes of the complement system. Such a selective binding and inhibition profile may mitigate safety concerns associated with inhibition of all downstream complement activity (eg, by inhibition of complement C3 or C5) or any off-target effects.
SARXX02, the precursor molecule of SAR443809, was assessed for its ability to inhibit AP-mediated deposition of C3b on PNH RBCs and their lysis, thereof. SARXX02 blocked the hemolysis of the PNH RBCs effectively in a dose-dependent manner, achieving near-complete inhibition. It also prevented the deposition of C3b on the PNH RBCs very effectively. Although inhibition of TP using a C5-neutralizing antibody prevented lysis of the PNH RBCs, it did not protect these cells from deposition of C3b on their cell surface. In fact, blocking C5 increased C3b deposition compared with that of NHS alone, consistent with what has been reported for patients with PNH on anti-C5 therapy, which could further exacerbate EVH.11 Thus, SAR443809 has the potential for preventing both IVH and EVH in patients with PNH. Recent approval of pegcetacoplan, a peptide inhibitor of C3 for PNH, addresses EVH, in addition to IVH. But C3 is present at very high concentrations in blood, necessitating high drug doses and/or frequent dosing. This also increases the chances for a pharmacologic breakthrough under certain circumstances. Moreover, inhibition of C3 shuts down all 3 complement pathways and, therefore, may increase infection risk. Although current clinical data show an overall favorable safety profile for pegcetacoplan, it will be important to monitor infection risk in these patient populations. In addition to pegcetacoplan, small molecule inhibitors of FB (iptacopan) and FD (danicopan) are also able to inhibit both IVH and EVH19,21 and have demonstrated superior clinical efficacy over C5 inhibition in clinical trials.22,23 Although oral administration is a major benefit of these therapies, they are typically dosed multiple times a day, so long-term patient compliance and pharmacologic breakthrough events remain a risk. Overall, these recent clinical successes lend support to proximal, and AP complement inhibition in PNH.
In vivo, SAR443809 was amenable to both IV and SC administration, as seen by pronounced and sustained ex vivo AP inhibition. In addition, SAR443809 has the unique advantage of targeting an activated complement component. FBb is only generated when the AP is active, thus FBb levels in blood are considerably lower compared with that of FB, even in disorders with chronic AP activation like PNH. Selective binding to FBb is expected to result in much lower target-mediated clearance of the antibody and may help localize the antibody to cell surfaces, on which much of FBb and C3bBb are formed. Hence, the drug can be maintained at a steady concentration for a sustained inhibition for prolonged periods, reducing the chances for any pharmacological breakthrough.
One important consideration for the development of complement inhibitors is the associated increased risk of infections with encapsulated bacteria.28,29 Deficiency of FB, albeit extremely rare, is associated with increased risk for recurrent infections with Neisseria or other encapsulated bacteria.28 Although pharmacologic inhibition of activated FB is not equivalent to complete deficiency of the protein and/or function, the associated risk of infection may be similar. Hence, vaccination for patients being treated with SAR443809 will be necessary as it is for patients on other complement-targeted therapeutics. Studies have demonstrated, for example, that opsonophagocytosis of Neisseria by granulocytes depends on antibody-mediated activation of the CP, and that serum bactericidal activity depends on the TP.29 Because SAR443809 selectively inhibits the AP without inhibiting the classical and lectin pathways, the protection elicited by antibodies through vaccination should be at least partially preserved. Nevertheless, the safety of SAR443809 will need to be monitored closely, especially given the contribution of the amplification loop in protecting against infections.
In conclusion, SAR443809 is an effective and selective inhibitor of complement AP. It is currently in phase 1 clinical study.
Acknowledgments
The authors thank all the members of the Complement Research Laboratory and other facilities within Sanofi that supported SAR443809 throughout its various stages of development, starting from early discovery to clinical phase 1 studies.
This research was funded by Sanofi.
Authorship
Contribution: S.P., S.M., and G.P. conceived the project and supervised a portion of the study; N.L., V.R., and M.S. supervised another portion of the study; N.L., V.R., and R.G. conceived experiments and authored the manuscript; and R.G., S.N., S.J., K.K., J.C., T.B., and C.M. contributed to the experiments of the study.
Conflict-of-interest disclosure: V.R., N.L., R.G., S.N., and M.S. are current employees of Sanofi and may hold shares and/or stock options in the company. S.J., K.K., J.C., T.B., C.M., S.P., S.M., and G.P. are former employees of Sanofi and may hold shares and/or stock options in the company.
Correspondence: Michael Storek, Immunology & Inflammation Therapeutic Area, Sanofi, 350 Water St, Cambridge, MA 02141; e-mail: michael.storek@sanofi.com.
References
Author notes
In response to reasonable requests, noncommercially available materials and experimental protocols, that Sanofi has the right to provide, will be made available to not-for-profit or academic requesters upon completion of a material transfer agreement. Requests may be made by contacting the corresponding author.
Data are available on request from the corresponding author, Michael Storek (michael.storek@sanofi.com).
The full-text version of this article contains a data supplement.