Key Points
VenO is cost-effective for CLL with comorbidity and without TP53 aberrations compared with current standard treatment.
Incorporating sequential treatment lines in health economic analyses optimizes clinical decisions in sequencing the treatment for CLL.
Abstract
Several targeted treatments, such as venetoclax + obinutuzumab (VenO) and ibrutinib, have been developed to treat patients with treatment-naive chronic lymphocytic leukemia (CLL) and have been shown to improve progression-free survival compared with chlorambucil + obinutuzumab (ClbO). However, novel targeted agents are associated with a significant cost investment. The objective of this study was to investigate the cost-effectiveness of VenO compared with ClbO and ibrutinib in treatment-naive CLL without del17p/TP53 mutation in Denmark. We used a decision-analytic modeling approach to simulate hypothetical cohorts of patients with CLL from the initiation of first-line treatment to death, including the full treatment pathway and second-line therapy. VenO, ClbO, or ibrutinib was included as first-line therapy followed by either Ven + rituximab or ibrutinib. Model outcomes were expected quality-adjusted life years (QALYs), life years (LYs), and cost per patient, which were used to calculate incremental cost-effectiveness ratios (ICERs) with a willingness to pay from €23 600 to €35 600 per QALY. Compared with ClbO, VenO was associated with a QALY gain of 1.30 (1.42 LYs) over a lifetime. The incremental cost was €12 360, resulting in an ICER of €9491 per QALY gained, indicating that VenO is cost-effective. Compared with VenO, ibrutinib was associated with a QALY gain of 0.82 (1.74 LYs) but at a substantially increased incremental cost of €247 488 over a lifetime horizon. The ICER was €302 156 per QALY, indicating that ibrutinib in first-line treatment would not be considered cost-effective in Danish health care, compared with VenO. Future analyses in fit patients with CLL are needed to determine the cost-effectiveness of VenO.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common leukemia in Western countries.1 The incidence increases with age,2-4 and with an older general population and the emergence of new targeted treatment options that include combination approaches, the costs of treating CLL are estimated to rise by 300% from 2011 to 2025.5
In line with international clinical guidelines6,7 and Danish treatment guidelines8 for patients with untreated CLL without the del17P/TP53 mutation (TP53 aberrations), chemoimmunotherapy (CIT) including an anti-CD20 antibody or targeted therapy based on Bruton tyrosine kinase inhibitors and/or BCL-2 inhibitors are recommended for patients with immunoglobulin heavy chain variable region (IGHV)-mutated CLL. For patients with IGHV-unmutated CLL, targeted therapy is recommended.4,8
Follow-up results from the CLL14 clinical trial showed superior progression-free survival (PFS) with the use of the oral BCL-2 inhibitor venetoclax plus obinutuzumab (VenO) compared with the use of chlorambucil plus obinutuzumab (ClbO).2 Furthermore, recently presented preliminary results from 72 months of follow-up showed a trend of increased overall survival (OS), although it was not statistically significant.14 The iLLUMINATE study found that the Bruton tyrosine kinase inhibitors ibrutinib in combination with obinutuzumab was associated with improved PFS compared with conventional CIT with ClbO.10-13 Oral administration without additional injection treatment of CD20-antibodies makes ibrutinib a convenient treatment; however, the treatment cost is high, because it is administered until progression.11,14 The price of venetoclax is high upfront, but contrary to ibrutinib, it is administered for a fixed duration of 12 or 24 months. The shorter period of drug exposure could be associated with fewer adverse events (AEs), less risk of interactions with concomitant medications and development of resistance, and potentially lower total therapy costs than those associated with ibrutinib.2,15
Although the advent of targeted treatment regimens represents a major advance in the treatment of CLL, it is likely to significantly increase costs and affect health care budgets. Therefore, the added costs must be weighed against the potential benefits across subgroups of patients. Because treatments have different safety profiles and because patients spend different amounts of time on drugs, additional information on AEs and time on treatment must be included when assessing cost-effectiveness. Moreover, because many patients with CLL will have disease progression over time, the sequence of treatment lines is also of importance.
To our knowledge, there is no published health economic evaluation comparing VenO vs ClbO vs ibrutinib for treatment-naive CLL without TP53 aberrations that includes costs and effects of sequential lines of treatment. Therefore, the objective of this study is to assess the cost-effectiveness of VenO for patients with treatment-naive CLL and significant comorbidity or frailty without TP53 aberrations compared with ClbO and ibrutinib in a Danish setting using quality-adjusted life years (QALYs) as the main measure of effect.
Methods
Decision-analytic modeling
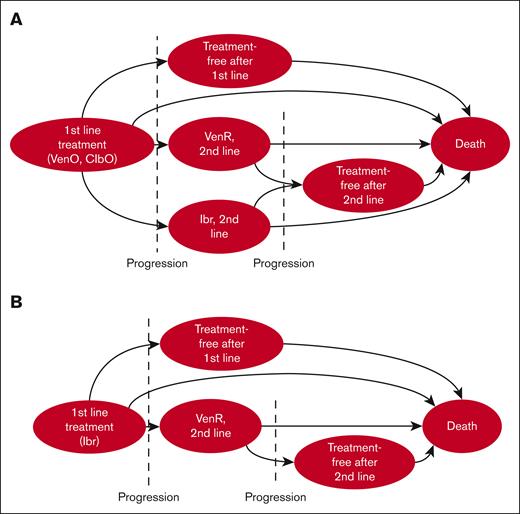
Two Markov models (Model A and B) with similar basic structures were constructed to estimate the costs, life years (LYs), and QALYs associated with the use of VenO, ibrutinib, and ClbO as the first-line treatment of CLL. Model A compares VenO vs ClbO and model B compares VenO vs ibrutinib (Figure 1).
Bubble diagram of the Markov model. Bubbles indicate health states and arrows indicate possible transitions between states. In each cycle, the cohort can either transition to another health state, die, or remain in the same state. (A) Transition pathways for both VenO and ClbO, and (B) pathways for ibrutinib. After progression, patients were assumed to receive either no treatment, VenR, or ibrutinib as second-line treatment, with the exception that second-line ibrutinib could not be given to patients progressing after first-line ibrutinib. Tunnel states were used for second-line treatment pathways.
Bubble diagram of the Markov model. Bubbles indicate health states and arrows indicate possible transitions between states. In each cycle, the cohort can either transition to another health state, die, or remain in the same state. (A) Transition pathways for both VenO and ClbO, and (B) pathways for ibrutinib. After progression, patients were assumed to receive either no treatment, VenR, or ibrutinib as second-line treatment, with the exception that second-line ibrutinib could not be given to patients progressing after first-line ibrutinib. Tunnel states were used for second-line treatment pathways.
The models were parameterized using the best available evidence, including a systematic literature search (supplemental Figure 1). All model assumptions and inputs are valid in accordance with Danish clinical practice. Both models were developed in TreeAge Pro Healthcare (version 2022; R2.0) following international guidelines for health economic modeling to simulate a hypothetical cohort.16,17
To predict disease evolution after the first-line treatment, patient pathways were simulated in mutually exclusive health states: treatment-free after first-line treatment, second-line treatment (VenR or ibrutinib), and death. To simplify the model, second-line treatment was assumed to occur at progression. The simulation was carried out in monthly cycles, with half-cycle correction for a 30-year period, during which all patients were expected to die.
The outputs of the model (total QALYs and LYs gained and costs over a lifetime) were used to calculate the incremental cost-effectiveness ratio (ICER), expressing the extra cost per extra QALY gained using VenO compared with either ibrutinib or ClbO. Because of the absence of an official willingness-to-pay (WTP) threshold of marginal cost per QALY in Denmark, the British National Institute for Health and Care Excellence threshold of ∼£20 000 to £30 000, which is equal to ∼€23 600 to €35 600, was used for reference (conversion rate, 1.19; 14 September 2022).
Modeling long-term outcomes
Model A was constructed to replicate OS and PFS from the recently presented, long-term, 5-year follow-up data from the CLL14 head-to-head, phase 3, randomized clinical trial for patients with treatment-naive CLL with coexisting conditions. The hypothetical cohort matched the study population from the CLL14 trial with a median age of 71 years with coexisting conditions and a cumulative illness rating score of ≥6 but was limited to the subpopulation without TP53 aberrations. Because only few patients remained alive in the last 6 months of follow-up and because of the associated increasing statistical uncertainty, these were excluded, and analyses were based on data collected until 66 months since treatment initiation.9 Lifetime extrapolation of both OS and PFS was conducted using Stata (version SE/17.0) with published extrapolation techniques.18-20 The choice of distribution was made via statistical verification using the Akaike information criterion, Bayesian information criterion, and visual inspection by Danish clinical experts. Ultimately, the lognormal distribution was chosen for OS, and the exponential distribution was chosen for PFS (supplemental Figure 2).
Second-line treatment was included using published evidence from the MURANO trial21 (VenR) and Danish real-world evidence22 (ibrutinib) to reflect Danish clinical practice. Tunnel states were used for second-line treatment to include the impact of patient history on transition probabilities, that is, to overcome the memoryless property of standard Markov models.23 PFS and OS data were extracted from the literature and extrapolated using methods identical to the estimation of first-line treatment.
Model B was constructed by applying the results from a published, peer-reviewed network meta-analysis (NMA) by Alrawashdh et al,24 as described in supplemental Figure 3. For Model B, the hypothetical cohort comprised patients with CLL who were older or with a cumulative illness rating score of ≥6,24 with studies in the NMA including but not limited to CLL11,25 RESONATE-2,26 ILLUMINATE,11 and CLL14.27 The analysis was cut off at 60 months, and the PFS was extrapolated to reflect lifetime, using the same published method as described for Model A. Extrapolation of OS was conducted with the assumption that survival rates could not exceed the survival rate of the general population, and the hazard ratio at 60 months for VenO vs ibrutinib was assumed to be constant throughout the remaining LYs. A detailed description of the methods applied are provided in supplemental Figure 3.
Model inputs
A Danish, tax-funded health care sector perspective was chosen with all costs estimated in euros (€) valued in 2022. All outcomes were discounted with a 3.5% annual discounting per the Danish Guidelines for Health Economic Evaluation.28
Costs and utility values related to both first- and second-line treatment were included for all AEs grades 3 and 4 (eg, hematological and cardiac AEs) if the absolute difference was at least 2 percentage points9 (Table 1; supplemental Tables 4-6). In the clinical trials used for model inputs, AEs were not stratified based on genetic risk factors. Therefore, it was assumed that the reported AEs were representative of the subpopulation of patients with CLL without TP53 aberrations. Drug costs were obtained from the Danish Medicines Agency’s list of current medicinal product prices29 on 23 June 2022. We assumed an average total body surface area of 1.79 m2 for rituximab30 and an average body weight of 76 kg for Clb.31 Hospital-related treatment costs were identified from the Danish diagnosis-related group tariffs for 2022.32 We assumed that patients visited the hospital regularly during treatment, for example, before dose ramp-up for Ven (supplemental Table 7). Before starting second-line treatment, we assumed that patients received 1 computerized tomography of the neck, chest, and abdomen at the hospital. End-of-life costs from an unpublished Danish real-world evidence study were included.33
Input parameters for costs
| Cost description . | Cost per 28-day treatment cycle∗, € . | Item number, PPP29 . |
|---|---|---|
| Venetoclax, first line | C1: 65.54 C2: 2457.79 C3-C12: 5243.29 | 532535 |
| Venetoclax, second line | C1: 65.54 C2: 2457.70 C3-C24: 5243.29 | |
| Obinutuzumab | C1: 10 075.16 C2-C6: 3358.39 | 523596 |
| Chlorambucil | C1-C6: 110.86 | 571358 |
| Ibrutinib | 5519.29 | 143617 |
| Rituximab | C1: 1207.08 C2-C6: 1609.44 | 137019 |
| Cost description . | Cost per 28-day treatment cycle∗, € . | Item number, PPP29 . |
|---|---|---|
| Venetoclax, first line | C1: 65.54 C2: 2457.79 C3-C12: 5243.29 | 532535 |
| Venetoclax, second line | C1: 65.54 C2: 2457.70 C3-C24: 5243.29 | |
| Obinutuzumab | C1: 10 075.16 C2-C6: 3358.39 | 523596 |
| Chlorambucil | C1-C6: 110.86 | 571358 |
| Ibrutinib | 5519.29 | 143617 |
| Rituximab | C1: 1207.08 C2-C6: 1609.44 | 137019 |
| Cost description . | Cost, per unit, € . | DRG rate32 . |
|---|---|---|
| CT scan | 324.06 | 30PR06 |
| Out-patient visit | 433.47 | 17MA98 |
| End-of-life, total health care costs | 16 379.00 | 33 |
| Acute coronary syndrome | 1.50 134 | 05MA02 |
| Anemia | 3416.53 | 16MA10 |
| Arthralgia | 433.47 | 17MA98 |
| Asthenia | Not treated | — |
| Atrial fibrillation | 2284.41 | 05MA07 |
| Diarrhea | 908.07 | 06MA11 |
| Dizziness/fatigue | 433.47 | 17MA98 |
| Dyspnea | Not treated | — |
| Febrile neutropenia | 5162.37 | 16MA03 |
| Headache | 433.47 | 17MA98 |
| Hemorrhage | 5721.51 | 17MA01 |
| Hyperglycemia | 893.15 | 23MA03 + long-term admission (€293.68) |
| Hypertension | 2235.22 | 05MA11 |
| Hypogammaglobulinemia | 4989.11 | 16MA07 |
| Infections and infestations | 5376.61 | 18MA08 |
| Infusion-related reaction | 5376.61 | 18MA08 |
| Leukopenia | 433.47 | 17MA98 |
| Myalgia | 433.47 | 17MA98 |
| Neutropenia/neutrophil count decreased | 433.47 | 17MA98 |
| Pneumonia | 5385.75 | 04MA13 |
| Pyrexia | 433.47 | 17MA98 |
| Rash, maculopapular | 274.33 | 09MA98 |
| Sepsis | 6096.91 | 18MA01 |
| Thrombocytopenia | 3651.61 | 16MA09 |
| Tumor lysis syndrome | 835.35 | 10MA01 + long-term admission (€587.37) |
| Urinary tract infection | 5376.61 | 18MA08 |
| Cost description . | Cost, per unit, € . | DRG rate32 . |
|---|---|---|
| CT scan | 324.06 | 30PR06 |
| Out-patient visit | 433.47 | 17MA98 |
| End-of-life, total health care costs | 16 379.00 | 33 |
| Acute coronary syndrome | 1.50 134 | 05MA02 |
| Anemia | 3416.53 | 16MA10 |
| Arthralgia | 433.47 | 17MA98 |
| Asthenia | Not treated | — |
| Atrial fibrillation | 2284.41 | 05MA07 |
| Diarrhea | 908.07 | 06MA11 |
| Dizziness/fatigue | 433.47 | 17MA98 |
| Dyspnea | Not treated | — |
| Febrile neutropenia | 5162.37 | 16MA03 |
| Headache | 433.47 | 17MA98 |
| Hemorrhage | 5721.51 | 17MA01 |
| Hyperglycemia | 893.15 | 23MA03 + long-term admission (€293.68) |
| Hypertension | 2235.22 | 05MA11 |
| Hypogammaglobulinemia | 4989.11 | 16MA07 |
| Infections and infestations | 5376.61 | 18MA08 |
| Infusion-related reaction | 5376.61 | 18MA08 |
| Leukopenia | 433.47 | 17MA98 |
| Myalgia | 433.47 | 17MA98 |
| Neutropenia/neutrophil count decreased | 433.47 | 17MA98 |
| Pneumonia | 5385.75 | 04MA13 |
| Pyrexia | 433.47 | 17MA98 |
| Rash, maculopapular | 274.33 | 09MA98 |
| Sepsis | 6096.91 | 18MA01 |
| Thrombocytopenia | 3651.61 | 16MA09 |
| Tumor lysis syndrome | 835.35 | 10MA01 + long-term admission (€587.37) |
| Urinary tract infection | 5376.61 | 18MA08 |
CT, computed tomography; DRG, Danish diagnosis-related groups; PPP, pharmacy purchase price.
28-day treatment regimens were transformed to fit the model’s 30-day cycle length.
Quality-of-life weights for health states specific to CLL were obtained from published literature (Table 2). The progressed state was assumed to apply to all health states after the first progression. For AEs, a quality-of-life decrement was subtracted from the health state utility value for the duration of the AE.
Input parameters for utility values
| Health state . | Utility value . | Source . | |
|---|---|---|---|
| Preprogression, IV treatment | 0.67 | ||
| Preprogression, oral treatment | 0.71 | 48 | |
| Preprogression, off-treatment | 0.77 | Kosmas et al49 | |
| Progressed | 0.6 | ||
| Health state . | Utility value . | Source . | |
|---|---|---|---|
| Preprogression, IV treatment | 0.67 | ||
| Preprogression, oral treatment | 0.71 | 48 | |
| Preprogression, off-treatment | 0.77 | Kosmas et al49 | |
| Progressed | 0.6 | ||
| Adverse event . | Disutility value . | Duration, days . | Source . |
|---|---|---|---|
| Acute coronary syndrome | 0.18 | On treatment | Gencer et al50 |
| Anemia | 0.09 | 30 | Beusterien et al51 |
| Arthralgia | 0.05 | On treatment | Assumed same as nausea |
| Atrial fibrillation | 0.0557 | On treatment | Sullivan et al52 |
| Diarrhea | 0.176 | 5 | Stein et al53 |
| Dizziness/fatigue | 0.05 | 30 | Assumed same as nausea |
| Febrile neutropenia | 0.195 | 7 | Tolley et al54 |
| Headache | 0.05 | 7 | Assumed same as nausea |
| Hemorrhage | 0.131 | 14 | Wehler et al55 |
| Hyperglycemia | 0.06 | On treatment | Nafees et al56 |
| Hypertension | 0.0375 | On treatment | Sullivan et al52 |
| Hypogammaglobulinemia | 0.09 | On treatment | Assumed same as anemia |
| Infections and infestations | 0.195 | 7 | Tolley et al54 |
| Infusion-related reaction | 0.2 | 1 | Chatterjee et al31 |
| Leukopenia | 0.163 | 30 | Assumed same as neutropenia |
| Myalgia | 0.05 | On treatment | Assumed same as nausea |
| Neutropenia and neutrophil count decreased | 0.163 | 30 | Tolley et al54 |
| Pneumonia | 0.195 | 7 | Tolley et al54 |
| Pyrexia | 0.11 | 7 | Beusterien et al51 |
| Rash, maculopapular | 0.06 | 14 | Stein et al53 |
| Sepsis | 0.195 | 10 | Tolley et al54 |
| Thrombocytopenia and low platelet count | 0.108 | 30 | Tolley et al54 |
| Tumor lysis syndrome | 0.195 | 5 | Tolley et al54 |
| Urinary tract infection | 0.195 | 5 | Tolley et al54 |
| Adverse event . | Disutility value . | Duration, days . | Source . |
|---|---|---|---|
| Acute coronary syndrome | 0.18 | On treatment | Gencer et al50 |
| Anemia | 0.09 | 30 | Beusterien et al51 |
| Arthralgia | 0.05 | On treatment | Assumed same as nausea |
| Atrial fibrillation | 0.0557 | On treatment | Sullivan et al52 |
| Diarrhea | 0.176 | 5 | Stein et al53 |
| Dizziness/fatigue | 0.05 | 30 | Assumed same as nausea |
| Febrile neutropenia | 0.195 | 7 | Tolley et al54 |
| Headache | 0.05 | 7 | Assumed same as nausea |
| Hemorrhage | 0.131 | 14 | Wehler et al55 |
| Hyperglycemia | 0.06 | On treatment | Nafees et al56 |
| Hypertension | 0.0375 | On treatment | Sullivan et al52 |
| Hypogammaglobulinemia | 0.09 | On treatment | Assumed same as anemia |
| Infections and infestations | 0.195 | 7 | Tolley et al54 |
| Infusion-related reaction | 0.2 | 1 | Chatterjee et al31 |
| Leukopenia | 0.163 | 30 | Assumed same as neutropenia |
| Myalgia | 0.05 | On treatment | Assumed same as nausea |
| Neutropenia and neutrophil count decreased | 0.163 | 30 | Tolley et al54 |
| Pneumonia | 0.195 | 7 | Tolley et al54 |
| Pyrexia | 0.11 | 7 | Beusterien et al51 |
| Rash, maculopapular | 0.06 | 14 | Stein et al53 |
| Sepsis | 0.195 | 10 | Tolley et al54 |
| Thrombocytopenia and low platelet count | 0.108 | 30 | Tolley et al54 |
| Tumor lysis syndrome | 0.195 | 5 | Tolley et al54 |
| Urinary tract infection | 0.195 | 5 | Tolley et al54 |
Sensitivity analyses
Probabilistic sensitivity analyses (PSAs) with 10 000 second-order Monte Carlo simulations were performed, assigning beta distributions to transition probabilities and utility and disutility values and gamma distributions to costs. When available, the 95% confidence interval was used to reflect uncertainty, and, if unavailable, ±20% of the mean was applied. One-way deterministic sensitivity analyses were performed on all variables to test the robustness of the results (data not shown). Scenario analyses using Model A consisted of applying the same survival curve to both VenO and ClbO (same OS curve); prolonged treatment with ibrutinib until progression per assumptions from the Danish Medicines Council34 (ibrutinib until progression); exclusion of second-line treatment (only first line); and changes in the choice of second-line treatment (only VenR second line and only ibrutinib second line). For Model B, scenario analyses included the same survival probabilities for both treatments (same OS curve) and prolonged treatment with ibrutinib until progression (ibrutinib until progression). Furthermore, in Model A the price of Ven was varied in a threshold analysis to show when treatment with VenO would be less expensive than treatment with ClbO, and in Model B a threshold analysis was conducted for the price of ibrutinib.
Results
Base-case analysis
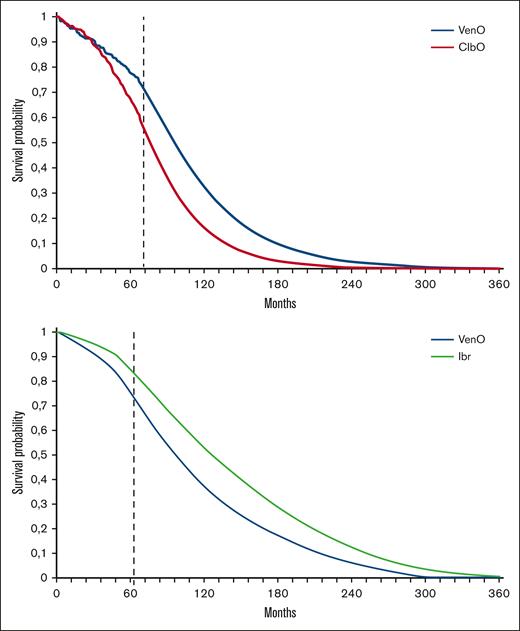
In the Model A cohort, the use of first-line VenO treatment was associated with an improvement of 1.23 QALYs, compared with ClbO, at an additional cost of €12 045 (Table 3). The projection of median OS showed an average remaining life expectancy (LYs) of 7.54 and 6.12 LYs for VenO and ClbO, respectively (Figure 2A). The ICER for VenO was €9807 per QALY gained, relative to that for ClbO. Based on this, VenO was cost-effective, assuming a WTP threshold between €23 600 and €35 600 per QALY.
Main results of cost-effectiveness analysis of Model A
| First-line treatment analysis . | VenO . | ClbO . | Incremental . | ICER . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost, € . | QALY . | Life y . | Cost, € . | QALY . | Life y . | Cost, € . | QALY . | Life y . | (€/QALY) . | |
| Base case | 194 612 | 5.20 | 7.54 | 182 567 | 3.97 | 6.12 | 12 045 | 1.23 | 1.42 | 9807 |
| Same OS curve | 194 612 | 5.20 | 7.54 | 182 567 | 4.68 | 7.54 | 12 045 | 0.52 | 0 | 23 163 |
| Ibrutinib until progression | 206 191 | 5.19 | 7.54 | 200 221 | 3.96 | 6.12 | 5970 | 1.23 | 1.42 | 4841 |
| Only first line | 109 821 | 5.75 | 8.32 | 53 525 | 4.48 | 6.76 | 56 296 | 1.27 | 1.56 | 44 265 |
| Only ibrutinib in second line | 213 373 | 4.98 | 7.18 | 211 161 | 3.64 | 5.58 | 1648 | 1.34 | 1.60 | 2212 |
| Only VenR in second line | 175 850 | 5.42 | 7.89 | 153 972 | 4.30 | 6.66 | 21 878 | 1.11 | 1.23 | 19 644 |
| First-line treatment analysis . | VenO . | ClbO . | Incremental . | ICER . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost, € . | QALY . | Life y . | Cost, € . | QALY . | Life y . | Cost, € . | QALY . | Life y . | (€/QALY) . | |
| Base case | 194 612 | 5.20 | 7.54 | 182 567 | 3.97 | 6.12 | 12 045 | 1.23 | 1.42 | 9807 |
| Same OS curve | 194 612 | 5.20 | 7.54 | 182 567 | 4.68 | 7.54 | 12 045 | 0.52 | 0 | 23 163 |
| Ibrutinib until progression | 206 191 | 5.19 | 7.54 | 200 221 | 3.96 | 6.12 | 5970 | 1.23 | 1.42 | 4841 |
| Only first line | 109 821 | 5.75 | 8.32 | 53 525 | 4.48 | 6.76 | 56 296 | 1.27 | 1.56 | 44 265 |
| Only ibrutinib in second line | 213 373 | 4.98 | 7.18 | 211 161 | 3.64 | 5.58 | 1648 | 1.34 | 1.60 | 2212 |
| Only VenR in second line | 175 850 | 5.42 | 7.89 | 153 972 | 4.30 | 6.66 | 21 878 | 1.11 | 1.23 | 19 644 |
Survival curves. OS curves as estimated in the Markov model for both Model A and Model B. Estimated survival curves include treatment effects of both first- and second-line treatment. (A) VenO and ClbO9 and subsequent second-line treatment.21,22 (B) VenO and ibrutinib (Ibr) based on the NMA24 and subsequent therapy.21,22 The dashed line indicates the change from data extracted directly from clinical trial (A) or NMA (B) to parametric extrapolation.
Survival curves. OS curves as estimated in the Markov model for both Model A and Model B. Estimated survival curves include treatment effects of both first- and second-line treatment. (A) VenO and ClbO9 and subsequent second-line treatment.21,22 (B) VenO and ibrutinib (Ibr) based on the NMA24 and subsequent therapy.21,22 The dashed line indicates the change from data extracted directly from clinical trial (A) or NMA (B) to parametric extrapolation.
In Model B, the use of first-line ibrutinib treatment, compared with VenO, was associated with a QALY gain of 0.67 LYs but at an extra cost of €156 167 (Table 4). The projection of median OS showed 7.57 and 9.13 LYs for VenO and ibrutinib, respectively (Figure 2B). The ICER for ibrutinib, compared with VenO, was €232 473 per QALY. Based on this, ibrutinib was not cost-effective compared with VenO, assuming the same threshold value.
Main results of cost-effectiveness analysis of Model B
| First-line treatment analysis . | Ibrutinib . | VenO . | Incremental . | ICER . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost, € . | QALY . | Life y . | Cost, € . | QALY . | Life y . | Cost, € . | QALY . | Life y . | (€/QALY) . | |
| Base case | 392 743 | 5.62 | 9.13 | 236 576 | 4.95 | 7.57 | 156 167 | 0.67 | 1.56 | 232 473 |
| Same OS curve | 392 743 | 5.62 | 9.13 | 236 576 | 5.73 | 7.57 | 156 167 | −0.11 | 0 | −1 419 700 |
| Ibrutinib until progression | 801 587 | 5.06 | 9.13 | 253 346 | 4.94 | 7.57 | 548 241 | 0.12 | 1.56 | 4 410 969 |
| Only first line | 307 442 | 6.87 | 11.12 | 109 187 | 6.60 | 10.11 | 198 255 | 0.27 | 1.01 | 739 012 |
| First-line treatment analysis . | Ibrutinib . | VenO . | Incremental . | ICER . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost, € . | QALY . | Life y . | Cost, € . | QALY . | Life y . | Cost, € . | QALY . | Life y . | (€/QALY) . | |
| Base case | 392 743 | 5.62 | 9.13 | 236 576 | 4.95 | 7.57 | 156 167 | 0.67 | 1.56 | 232 473 |
| Same OS curve | 392 743 | 5.62 | 9.13 | 236 576 | 5.73 | 7.57 | 156 167 | −0.11 | 0 | −1 419 700 |
| Ibrutinib until progression | 801 587 | 5.06 | 9.13 | 253 346 | 4.94 | 7.57 | 548 241 | 0.12 | 1.56 | 4 410 969 |
| Only first line | 307 442 | 6.87 | 11.12 | 109 187 | 6.60 | 10.11 | 198 255 | 0.27 | 1.01 | 739 012 |
Deterministic sensitivity analyses
In Model A, the results were sensitive to the inclusion of second-line treatment, showing an ICER of €44 265 per QALY gained for VenO if second-line treatment was excluded (Table 3). The deterministic analysis assuming the same OS curve for VenO and ClbO increased the ICER to €23 163 per QALY, that is, above the lower cost-effectiveness threshold value but under the upper limit. Reducing the price of Ven showed that a 28% discount would result in the selection of VenO for first-line treatment over ClbO (ie, better and cheaper total treatment pathways). The results were not sensitive to price reductions for Clb; even a 90% price reduction did not alter the results. Prolonging treatment with ibrutinib until progressive disease favored VenO with a decreased ICER of €4801 per QALY, reflecting the impact of the price of ibrutinib. Upon exclusion of 1 of the 2 possible second-line treatments, VenO remained cost-effective compared with the threshold.
In Model B, the sensitivity analysis showed that ibrutinib not being cost-effective was a robust result. Applying the same survival curve for both treatments meant that VenO was both more effective (ie, higher QALY gain) and less expensive with a negative ICER of € −1 419 700 per QALY gained compared with first-line ibrutinib treatment. VenO remained cost-effective upon adjusting the time to discontinuation of ibrutinib from 3 years to the start of progression. The results were not sensitive to price reductions of ibrutinib either; even a 90% price reduction did not alter the conclusion, mainly because total treatment costs decreased in both arms. In the VenO arm, half of the patients who received second-line treatment received ibrutinib; therefore, a reduced price of ibrutinib affects the total costs of both the ibrutinib and the VenO arms of Model B.
PSAs
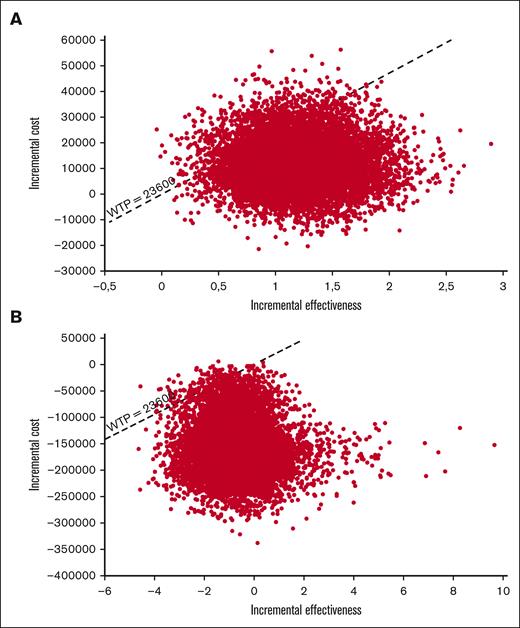
Cost-effectiveness acceptability curves were generated for both models (supplemental Figure 8). The PSA showed an 80% probability of VenO being cost-effective in Model A (Figure 3A), whereas there was a 10% likelihood that VenO provided a higher QALY gain at a lower cost than ClbO. In Model B, the probability of VenO being cost-effective was >98%, which was mainly driven by a large incremental cost between the 2 treatment strategies (Figure 3B). The PSA showed a 25% probability that VenO would provide a higher QALY gain at a lower total cost than ibrutinib.
Incremental cost-effectiveness scatterplot. Results from the PSAs with 10 000 second-order, stochastic Monte Carlo simulations shown in an ICE scatterplot for both models. (A) VenO vs ClbO, (B) VenO vs ibrutinib.
Incremental cost-effectiveness scatterplot. Results from the PSAs with 10 000 second-order, stochastic Monte Carlo simulations shown in an ICE scatterplot for both models. (A) VenO vs ClbO, (B) VenO vs ibrutinib.
Discussion
To our knowledge, this is the first study to investigate the cost-effectiveness of first-line VenO, ClbO, and ibrutinib treatment for CLL with significant comorbidity and without TP53 aberrations while also including costs and effects of second-line treatment. Furthermore, to our knowledge, this is the first study to investigate the cost-effectiveness in a European public health care setting. Compared with ClbO, VenO was associated with a QALY gain of 1.23 over a lifetime and increased average remaining life expectancy of 1.42 LYs. The incremental cost was €12 045 resulting in an ICER of €9807 per QALY gained; therefore, VenO was cost-effective at an assumed Danish WTP threshold of between €23 600 and 35 600 per QALY. Compared with VenO, ibrutinib was associated with a QALY gain of 0.67 (1.56 LYs) and a substantially increased incremental cost of €156 167 over a lifetime horizon. The ICER was €232 743 per QALY, indicating that, compared with VenO, ibrutinib in first-line treatment would not be considered cost-effective in a Danish health care setting.
Sensitivity analyses showed that the outcomes through Model A were sensitive to changes in the purchase price of Ven and the inclusion of any second-line treatment. Model B was not sensitive to the changes made in the deterministic sensitivity analyses, showing a robust economic result. Therefore, the main drivers for VenO being cost-effective compared with ClbO, despite its substantially higher upfront cost, were the inclusion of costs and effects of the expected second-line treatment, including the difference in PFS. This resulted in a larger proportion of the ClbO cohort receiving second-line treatment earlier than those in the VenO cohort. The main reason for ibrutinib not being cost-effective despite the substantially lower upfront cost of ibrutinib compared with that of VenO, which includes additional costs because of the ramp-up, is the high costs of continuous treatment with ibrutinib.
The results are based on updated, best available evidence; Model A, in particular, is informed by the phase 3, randomized, clinical CLL-14 trial directly comparing VenO and ClbO. The Markov model inputs were largely derived from CLL-14 trial, and we were able to reproduce estimations of OS and PFS with great accuracy. However, there is no head-to-head clinical trial comparing VenO and ibrutinib. NMA constitutes the best available evidence for Model B comparing VenO and ibrutinib in patients with treatment-naive CLL and significant comorbidity or frailty without TP53 aberrations. Consequently, data from the iLLUMINATE trial10 was used to inform the probabilities of AEs after treatment with ibrutinibO and not ibrutinib monotherapy. Furthermore, the NMA used for OS and PFS in Model B did not distinguish between CLL with or without TP53 aberrations, which is also reflected in the estimation of LYs gained, with VenO accruing 7.54 LYs in Model A and 8.00 LYs in Model B. Several studies have shown different treatment effects depending on TP53 aberrational status.2,9,35-37 Therefore, PFS and OS are expected to be longer for CLL without TP53 aberrations compared with the nonstratified analysis in the NMA.
This analysis did not distinguish CLL patients based on the IGHV mutational status. Several studies have demonstrated that patients with IGHV-unmutated CLL have the best survival benefit upon targeted treatment compared with CIT. Meanwhile, the difference in QALYs across treatments is partly attributable to differences in LYs. Further studies are needed to investigate whether these findings apply to patients with IGHV-mutated status, for whom the LYs are expected to be longer upon CIT compared with for those with IGHV-unmutated CLL. Therefore, the findings of this study should primarily be applied to patients with IGHV-unmutated CLL.38,39 Moreover, because the patient population associated with the results from this study comprises older patients who are rather frail with significant comorbidity, these data cannot be extrapolated directly to patients who are fit. Consequently, analysis of the cost-effectiveness for such populations based on the GAIA/CLL13 clinical trial40,41 is warranted.
We used a conservative approach for the treatment effect of the targeted fixed-duration treatment regimen and conducted a broad set of sensitivity analyses to examine the robustness of the results. For example, we included sensitivity analyses (same OS curve) strongly favoring ClbO for Model A and a base case favoring ibrutinib for Model B. The inclusion of a time-limited treatment with ibrutinib for 3 years based on Danish real-world evidence22 was a modest estimate for ibrutinib, lowering both total drug costs and costs and quality-of-life decrements of AEs for ibrutinib in both first- and second-line treatments. In addition, in the CLL14 trial, Clb is given for 12 months, although Danish clinical guidelines suggest treatment for 6 months. Therefore, we included the lower cost of a shorter treatment duration while still including the effects of longer exposure to Clb. However, any model is a simplification of reality, and some individual patient pathways, such as prolonged time to next treatment and the presence of severe, long-lasting AEs, might differ significantly and not be reflected in this cohort model. Furthermore, we used progression as a marker for starting second-line treatment, although there is typically an off-treatment period before the patient fulfills the International Workshop on CLL criteria for initiating treatment.42 Although this is a limitation of the model that applies to all treatment regimens in the 2 models, we believe it to have little impact on the results.
A few other health economic evaluations have been conducted comparing VenO with ClbO,31,43 with only 1 distinguishing based on TP53 status and including both costs and effects of subsequent treatment after progression.43 In the previous health economic studies, VenO was found to be cost-effective compared with ClbO and ibrutinib in both a US and a Canadian setting, which is in accordance with the findings of this study. Transferring results of economic evaluations is difficult across countries because of different payment and insurance schemes.44 However, the structure of a decision-analytic model is reasonably and commonly transferred.45 The main difference of our study is that such models use a partitioned survival model, PartSA, commonly used in oncology. The choice of a Markov model allowed us to use multiple health states to model disease progression and disentangle the effects of first-line and second-line treatment in greater detail. It enabled the inclusion of the best available evidence of both first-line and second-line treatment to inform model extrapolations of OS and PFS.46 Therefore, the main strength of our study compared with previous cost-effectiveness analyses is the ability to replicate long-term clinical trial results and forecast costs and effects that resemble clinical practice in Denmark. Because many patients with CLL receive second-line treatment, the inclusion of second-line treatment choices in decision analytical modeling can improve precision in forecasting.
Oral, targeted therapies represent a significant improvement in the treatment of CLL; however, their high cost raises concerns about affordability and budget impact. The value of targeted, fixed-duration treatments, such as VenO and the recently approved Ven + ibrutinib combination,47 resides in their ability to balance the possible benefits of treatment with the potential risks and costs. Limiting the duration of therapy results in patients with CLL being less likely to experience long-term adverse effects.2,27 With health care providers struggling with rising costs, clinical effectiveness alone is no longer the only measurement for evaluating new treatment options in many countries. The results from health economic evaluations can indicate the expected value of the new interventions in terms of economic, clinical, and quality-of-life benefits for the average patient. Going forward, there is a need for further health economic analyses to explore and support the use of precision medicine in CLL and to identify the most cost-effective treatment for patients upfront.
In conclusion, the cost-effectiveness analysis demonstrates that the use of first-line VenO for patients with CLL without TP53 aberrations at a WTP threshold of €23 600/QALY is cost-effective compared with both ClbO and ibrutinib. Further efforts are needed to assess cost-effectiveness for patients who are fit and those with IGHV-mutated CLL.
Acknowledgment
This investigator-initiated study was funded by an unrestricted research grant from AbbVie. AbbVie approved the overall design of the study before analyses were initiated. The sponsor had no influence on the final study design and methods, data collection, analysis, interpretation of results, or decision to publish. They were given the opportunity to comment on the manuscript, whereas the final version was solely based on the discretion of the authors.
Authorship
Contribution: M.S. was responsible for the first draft of the model; M.S. and L.H.E. wrote the first draft of the manuscript; C.U.N. and E.C.R. critically revised all model inputs and assumptions and contributed important intellectual content; and all authors were responsible for data acquisition, analysis, and interpretation; and all authors met the International Committee of Medical Journal Editors criteria for authorship for this article and contributed to the design and conceptualization of the model.
Conflict-of-interest disclosure: M.S. received research funding and travel grants from AbbVie; received research funding from Gilead; and is a current equity holder at Ambu. C.U.N. received consultancy fees or research funding from AbbVie, AstraZeneca, Janssen, Octapharma, CSL Behring, Takeda, BeiGene, and Genmab. L.H.E. received consultancy fees or research funding from AbbVie within this study, AstraZeneca, Boehringer Ingelheim, Gilead, GSK, Janssen, Merck, Pfizer, Radiometer, and Specsavers. E.C.R. received consultancy fees or travel grants from AbbVie, Janssen, and AstraZeneca.
Correspondence: Emelie Curovic Rotbain, Department of Hematology Rigshospitalet - Copenhagen University Hospital, Blegdamsvej 9, Bldg 5074, 2100 Copenhagen, Denmark; e-mail: emelie.hamotal.curovic.rotbain@regionh.dk.
References
Author notes
Presented in abstract form at the 64th annual meeting of the American Society of Hematology, New Orleans, LA, 12 December 2022.
The full model is available at https://github.com/NIHE-research/CLLmodel.
Data are available on request from the corresponding author, Emelie Curovic Rotbain (emelie.hamotal.curovic.rotbain@regionh.dk).
The full-text version of this article contains a data supplement.