TO THE EDITOR:
People with African and Middle Eastern ancestry are observed to have lower peripheral absolute neutrophil counts (ANCs) than those of European or Asian descent.1-3 We now know that lower ANC values correlate with the presence of biallelic erythroid promoter single nucleotide variant in the Duffy antigen receptor for chemokines (DARC)/atypical chemokine receptor 1 gene (ACKR1) (rs2814778, NM_002036.3:c.-67T>C),4,5 which results in the lack of expression of Duffy antigens on the red blood cell. The Duffy-null (Fy[a-b-]) phenotype is found in <1% of those of European or Asian ancestry but is very common in individuals from sub-Saharan Africa (80% to 100%) and the Arabic peninsula (50% to 70%).6-10 In the United States, ∼66% of people identifying as Black or African American are expected to have the Fy(a-b-) phenotype.11 Importantly, the association between low ANC and race is completely abrogated after adjusting for the Duffy phenotype.12,13 To better reflect the biologic basis of this common variant, the recommended terminology is now “Duffy-null associated neutrophil count (DANC)”11 rather than the previously used term benign ethnic neutropenia. Furthermore, studies in adults show no difference in ANC between Black individuals with a Duffy non-null phenotype and current ANC reference ranges, but there are significantly lower ANC values among Black individuals with the Duffy-null phenotype.11
Although studies have confirmed the association between Fy(a-b-) and lower ANC, there are no robust data describing expected ANC ranges among children with the Fy(a-b-) phenotype or the lowest limit of ANC (or nadir) that can be reasonably attributed to the Fy(a-b-) phenotype alone. Because the identification and management of inborn errors of immunity, bone marrow failure disorders, and hematopoietic conditions predominantly occur in the pediatric population, it is critical to distinguish common variants from pathology with as minimally invasive and inexpensive testing as possible. This study aims to improve the understanding of ANC nadirs in a healthy pediatric population with the Fy(a-b-) phenotype.
We compiled a retrospective cohort of individuals aged ≤21 years at Michigan Medicine between 1 January 2010 and 1 July 2018. Eligible individuals were identified using the Electronic Medical Record Search Engine software and the search terms “Duffy” and “FYAB.”14 This study was exempted from review by the University of Michigan Institutional Review Board (HUM00114261). A total of 105 potential individuals were identified, and 67 patients were included after a manual review that confirmed the Fy(a-b-) phenotype via either serological testing or the Human Erythrocyte Antigen Genotyping Panel (American Red Cross National Molecular Laboratory, Philadelphia, PA) as well as the absence of medical variables that could account for neutropenia, including medications, concurrent infection, inborn errors of immunity or hematopoiesis, antineutrophil antibodies, or malignancy. ANC was measured from peripheral venous blood using GenS and LH 750 automated hematology analyzers (Beckman Coulter Inc, Brea, CA) and an XE-5000 analyzer (Sysmex Corporation, Kobe, Hyōgo Prefecture, Japan).
A cohort of age- and sex-matched controls was also identified via the search term bundle of “tonsillectomy” or “hernia” combined with “department of anesthesiology” and “absolute neutrophil count.” These term bundles were chosen to identify generally healthy children with ANCs obtained in the absence of illness. Three hundred sixty-three patients were identified. Patients were included after manual review if Asian or White were listed in the demographic information as well as the absence of medical variables that could affect neutrophil count. None of these patients had Duffy status assessed. One hundred sixty-seven patients met the criteria, and 134 age- and sex-matched non-null controls in a 2:1 ratio were included.
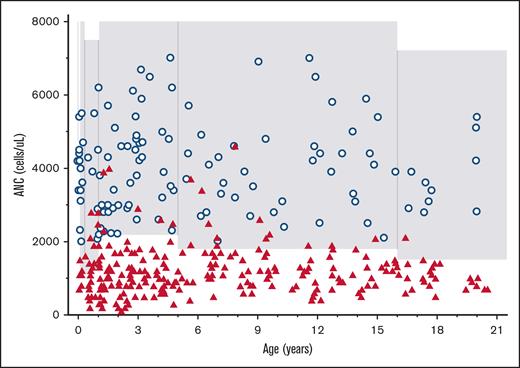
Two hundred fifty-one ANC levels were recorded in 67 unique individuals with the Fy(a-b-) phenotype (median, 4 per person; interquartile range [IQR], 2-5). The majority (n = 46 of 67 [68.7%]) of patients had at least 3 ANCs recorded, with a median interval of 1.3 years (IQR, 0.73-3.4 years) between the first and last ANC among these patients of. The median age of the Fy(a-b-) patients at the first ANC recorded was 4.5 years (IQR, 2-12; range, 0.1-20.4 years). All Fy(a-b-) ANC values and control ANC data are shown in Figure 1. The median Fy(a-b-) ANC was 1100 cells per μL (IQR, 900-1500; n = 251). Among all Fy(a-b-) ANCs of patients, 190 of 251 (75.7%) had <1500 cells per μL, 99 of 251 (39.5%) had <1000 cells per μL and 16 of 251 (6.4%) had <500 cells per μL. A total of 49 of 67 (73.1%) individuals with Fy(a-b-) had an average ANC of <1500 cells per μL. ANC nadir in Fy(a-b-) patients ranged from 100 to 2000 cells per μL (median nadir, 800 cells per μL; IQR, 600-1100 cells per μL; Figure 2). Four patients had an ANC <500 cells per μL upon subsequent analyses for up to 5 months duration.
All healthy Fy(a-b-) ANC values and control ANC values. ANC values of healthy children with the Fy(a-b-) phenotype (red triangles; n = 251) compared with healthy controls (blue circles; n = 134) based on age. The shaded area represents the ANC institutional reference range based on age.
All healthy Fy(a-b-) ANC values and control ANC values. ANC values of healthy children with the Fy(a-b-) phenotype (red triangles; n = 251) compared with healthy controls (blue circles; n = 134) based on age. The shaded area represents the ANC institutional reference range based on age.
ANC nadir values of healthy children with the Fy(a-b-) phenotype. Dotted lines indicate the degree of neutropenia: mild- ANC, from 1000 to 1500 cells per μL; moderate- ANC, from 500 to 999 cells per μL; and severe- ANC, <500 per μL. Children younger than 5 years had an ANC as low as 100 cells per μL. Children older than 5 years had an ANC as low as 400 cells per μL but rarely had values <500 cells per μL.
ANC nadir values of healthy children with the Fy(a-b-) phenotype. Dotted lines indicate the degree of neutropenia: mild- ANC, from 1000 to 1500 cells per μL; moderate- ANC, from 500 to 999 cells per μL; and severe- ANC, <500 per μL. Children younger than 5 years had an ANC as low as 100 cells per μL. Children older than 5 years had an ANC as low as 400 cells per μL but rarely had values <500 cells per μL.
Although there is a growing understanding that individuals with the Fy(a-b-) phenotype have lower ANCs, much uncertainty remains regarding the healthy lower ANC limit for children. Previously, ANC with the Fy(a-b-) phenotype was described as >1200 cells per μL, with only occasional occurrences of patients having an ANC <1000 cells per μL.15 However, more than half of the healthy Fy(a-b-) children in this cohort had an ANC <1200 cells per μL. In fact, these children had ANCs as low as 100 cells per μL, with a median nadir of 800 cells per μL. Notably, children younger than 5 years had the most profound neutropenia at 100 cells per μL, whereas those aged ≥5-years-old rarely had an ANC <500 cells per μL. We are unaware of the physiological explanation for this discrepancy, and prospective studies are required to validate this finding. Despite these very low ANC levels and many children also completing additional tests for inborn errors of immunity or malignancy, none of these children had a history of infection or alternative pathology identified.
This study had some notable limitations. Duffy antigen typing is not a routinely obtained test. Many of the patients who had Duffy phenotype with Fy(a-b-) were given a referral to Michigan Medicine for the evaluation of neutropenia, resulting in ascertainment bias. Patients with the lowest ANC values were additionally more likely to undergo repeat ANC testing, which almost certainly skews the data toward the lower end of the possible healthy values for patients with Fy(a-b-). Follow-up to determine the risk of infection or alternative causes of low ANC was based on a retrospective chart review and limited to the patients’ contact with Michigan Medicine (range of single encounter to 7.0 years between the first and last ANC). Furthermore, we assumed that our control patients are Duffy non-null based on their listed ethnicity, but Duffy antigen typing was not clinically indicated and therefore not completed. Reassuringly, the ANCs from the control patients were within the normal ranges during this study.
This study elucidates that healthy children with Fy(a-b-) may have a very low ANC without a clearly increased risk of infection. An ANC >500 cells per μL in children is likely to be consistent with DANC in an appropriate clinical context. Furthermore, healthy children with the Fy(a-b-) variant may have a limited duration of ANC <500 cells per μL without any other apparent pathology. Although very low ANC is alarming for providers, the full context of the child must be considered. We strongly encourage providers who are presented with a child with a low ANC and no concerning history or physical findings to obtain Duffy antigen typing as an initial step in evaluation, particularly if the child is of any African or Middle Eastern ancestry. It is important to note that the Fy(a-b-) phenotype is supportive of DANC but cannot rule out other pathologies, and >1 pathology may exist simultaneously. However, we believe that identifying this phenotype in the correct clinical context could avoid unnecessary and costly workup. The next steps for clinical laboratories include the development of new Fy(a-b-) pediatric ANC reference ranges based on age, which is essential for understanding the expected range of normal for this common variant. Establishing normal ANC ranges for individuals with Fy(a-b-) will not only offer the opportunity for more equitable care by accurately reflecting states of health for these patients but also potentially help avoid unnecessary testing and referrals.
Contribution: L.E.M. and G.N. gathered the data; L.E.M. and S.-H.L. analyzed the data and made the figures; L.E.M., K.J.W., and S.-H.L. designed the research; and L.E.M., S.-H.L., G.N., T.F.M., and M.C.H. drafted and edited the manuscript.
Conflict-of-interest disclosure: S.-H.L. is a consultant for Molecular Innovations, Novi, and MI; has a chair in the education committee for Michigan Society of Pathologists; is a member of CP Certlink and Chemical Pathology Test Development; and serves in advisory committees for the American Board of Pathology. T.F.M. consults for X4 Pharmaceuticals. K.J.W. is on advisory boards for Sobi, Pharming Group, X4 Pharmaceuticals, Agios; research funding from X4 Pharmaceuticals (Local PI), Pharming Group (Project PI); and serves in professional committees as NICER Consortium Executive Chair, with sponsored testing program with Blueprint Genetics. The remaining authors declare no competing financial interests.
The current affiliation of G.N. is Division of Cancer Epidemiology and Genetics, Clinical Genetics Branch, National Cancer Institute, Bethesda, MD.
Correspondence: Lauren E. Merz, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02115; e-mail: lauren_merz@dfci.harvard.edu.
References
Author notes
Data are available on request from the corresponding author, Lauren E. Merz (lauren_merz@dfci.harvard.edu).