Key Points
Epcoritamab-mediated killing of CLL cells by autologous T cells correlates with the effector-to-target ratio but not CD20 expression.
Epcoritamab efficacy is increased by concurrent use of a BTKi or venetoclax, supporting combination therapy.
Abstract
Chronic lymphocytic leukemia (CLL) is an immunosuppressive disease characterized by increased infectious morbidity and inferior antitumor activity of immunotherapies. Targeted therapy with Bruton's tyrosine kinase inhibitors (BTKis) or the Bcl-2 inhibitor venetoclax has profoundly improved treatment outcomes in CLL. To overcome or prevent drug resistance and extend the duration of response after a time-limited therapy, combination regimens are tested. Anti-CD20 antibodies that recruit cell- and complement-mediated effector functions are commonly used. Epcoritamab (GEN3013), an anti–CD3×CD20 bispecific antibody that recruits T-cell effector functions, has demonstrated potent clinical activity in patients with relapsed CD20+ B-cell non-Hodgkin lymphoma. Development of CLL therapy is ongoing. To characterize epcoritamab-mediated cytotoxicity against primary CLL cells, peripheral blood mononuclear cells from treatment-naive and BTKi-treated patients, including patients progressing on therapy, were cultured with epcoritamab alone or in combination with venetoclax. Ongoing treatment with BTKi and high effector-to-target ratios were associated with superior in vitro cytotoxicity. Cytotoxic activity was independent of CD20 expression on CLL cells and observed in samples from patients whose condition progressed while receiving BTKi. Epcoritamab induced significant T-cell expansion, activation, and differentiation into Th1 and effector memory cells in all patient samples. In patient-derived xenografts, epcoritamab reduced the blood and spleen disease burden compared with that in mice receiving a nontargeting control. In vitro, the combination of venetoclax with epcoritamab induced superior killing of CLL cells than either agent alone. These data support the investigation of epcoritamab in combination with BTKis or venetoclax to consolidate responses and target emergent drug-resistant subclones.
Introduction
Bruton tyrosine kinase inhibitors (BTKis) and the BCL-2 inhibitor venetoclax have profoundly changed the treatment landscape of chronic lymphocytic leukemia (CLL).1 Ibrutinib, the first-in-class BTKi, covalently binds to a cysteine residue (C481) in the active site, leading to sustained inhibition of BTK-dependent signaling.2-4 In addition to BTK, ibrutinib inhibits interleukin-2–inducible kinase (ITK) and TEC. Covalent BTKis that do not inhibit ITK include acalabrutinib and zanubrutinib.5,6 Although side effect profiles differ, the 3 BTKis demonstrated potent clinical efficacy.5-10 Although the depth of response tends to improve with extended therapy, minimal residual disease–negative remissions are uncommon with BTKis.11-13 The most common reasons for BTKi discontinuation are disease progression and side effects.11,14 Acquired resistance to covalent BTKis has been linked to mutations affecting the C481 residue, thereby preventing covalent binding.15-17 The BCL-2–targeting drug venetoclax is used in combination with obinutuzumab in first line and with rituximab for patients with relapsed/refractory (R/R) CLL, including patients whose conditions progressed while receiving BTKis, and has resulted in durable responses and minimal residual disease negativity.18-21 Recently, the focus has been on combination therapies that could deepen the response, shorten the duration of therapy, and prevent or overcome drug resistance.
CLL is characterized by defects of cellular and humoral immunity that increase infectious morbidity and mortality, decrease vaccine responses, and hamper the efficacy of immunotherapeutic approaches.22,23 Ibrutinib improves T-cell immunity by modulating T-cell differentiation, reducing the expression of inhibitory receptors, and restoring immune synapse formation.24-30 Although some of these effects were initially attributed to ITK inhibition,24,25,31,32 more recent studies with acalabrutinib, which does not inhibit ITK, have shown similar changes in the T-cell compartment.25,33,34 Improved T-cell function in patients on BTKi therapy provides a rationale for combination immunotherapy. We recently reported superior T-cell cytotoxicity of an anti–CD3×CD19 bispecific antibody (bsAb) against CLL cells in peripheral blood mononuclear cells (PBMCs) from patients being treated with ibrutinib or acalabrutinib.34,35 In a patient-derived xenograft model, the CD3×CD19 bsAb eliminated primary CLL cells, including those from patients with disease progression while receiving ibrutinib.35 Mechanistically, BTKi therapy downmodulates an immunosuppressive program in CLL cells, resulting in improved T-cell cytotoxicity.34
Epcoritamab (DuoBody-CD3xCD20, GEN3013) is a full-length human immunoglobulin G1 (IgG1) bsAb in which Fc-dependent effector functions are silenced by 3 point mutations that were selected based on functional assays.36,37 Epcoritamab’s potent activity was demonstrated ex vivo against tumor cells from patients who were treatment-naive and had R/R diffuse large B-cell lymphoma (DLBCL), follicular lymphoma, and mantle cell lymphoma.37,38 In an ongoing phase 1/2a clinical trial (NCT03625037), subcutaneous administration of epcoritamab showed efficient antitumor activity as a single agent, achieving 68% and 90% overall response rates and 45% and 50% complete responses in R/R DLBCL and follicular lymphoma, respectively.39,40 The first results from the phase 1b/2 EPCORE CLL-1 trial (NCT04623541) showed a favorable safety profile and promising antileukemic activity in patients with R/R CLL.41
To explore the possible benefits of combining epcoritamab with BTKi or venetoclax, we obtained PBMCs from treatment-naive patients with CLL and from patients treated with either ibrutinib or acalabrutinib and assessed epcoritamab-dependent activation and cytotoxicity of autologous T cells in vitro and in patient-derived xenograft (PDX) models. Combinations with venetoclax were tested in vitro.
Patients, materials, and methods
Patients and clinical samples
PBMCs were obtained from patients with CLL enrolled in phase 2 clinical trials with ibrutinib (NCT01500733) or acalabrutinib (NCT02337829), and/or in an observational study (NCT00923507), samples from treatment-naive patients were collected. These studies were approved by the institutional review board, and written informed consent was obtained in accordance with the Declaration of Helsinki. Patient characteristics are summarized in Table 1, and sample usage in supplemental Table 1. Samples from all treated patients were collected upon the initiation of BTKi therapy, irrespective of whether patients continued to respond or had progressive disease (supplemental Table 2).
Patient characteristics
| . | Treatment-naive, number or median (% or range) . | On ibrutinib, number or median (% or range) . | On acalabrutinib, number or median (% or range) . | Progressing, number or median (% or range) . |
|---|---|---|---|---|
| Age | 59 (48-77) | 67 (53-78) | 61 (50-74) | 64 (39-76) |
| Sex | ||||
| Female | 10 (50%) | 6 (50%) | 6 (40%) | 0 (0%) |
| Male | 10 (50%) | 6 (50%) | 9 (60%) | 10 (100%) |
| Prior therapy | ||||
| No | 20 (100%) | 6 (60%) | 5 (33%) | 8 (80%) |
| Yes | 0 (0%) | 4 (40%) | 10 (67%) | 2 (20%) |
| IGHV | ||||
| Mutated | 8 (40%) | 5 (42%) | 5 (33%) | 0 (0%) |
| Unmutated | 10 (50%) | 5 (42%) | 10 (67%) | 10 (100%) |
| Missing | 2 (10%) | 2 (16%) | 0 (0%) | 0 (0%) |
| FISH | ||||
| del 17p | 2 (10%) | 4 (33%) | 1 (7%) | 6 (60%) |
| Absolute lymphocyte count | 110 (10-328) | 37 (5-317) | 31 (4-146) | 12 (6-31) |
| T-cell–to–CLL cell ratio | 0.03 (0.01-0.08) | 0.5 (0.01-0.40) | 0.1 (0.01-0.56) | 0.40 (0.08-0.69) |
| Time on BTKi therapy (mo) | - | 9 (5.5-57) | 6 (5.2-48) | 50 (15-72) |
| . | Treatment-naive, number or median (% or range) . | On ibrutinib, number or median (% or range) . | On acalabrutinib, number or median (% or range) . | Progressing, number or median (% or range) . |
|---|---|---|---|---|
| Age | 59 (48-77) | 67 (53-78) | 61 (50-74) | 64 (39-76) |
| Sex | ||||
| Female | 10 (50%) | 6 (50%) | 6 (40%) | 0 (0%) |
| Male | 10 (50%) | 6 (50%) | 9 (60%) | 10 (100%) |
| Prior therapy | ||||
| No | 20 (100%) | 6 (60%) | 5 (33%) | 8 (80%) |
| Yes | 0 (0%) | 4 (40%) | 10 (67%) | 2 (20%) |
| IGHV | ||||
| Mutated | 8 (40%) | 5 (42%) | 5 (33%) | 0 (0%) |
| Unmutated | 10 (50%) | 5 (42%) | 10 (67%) | 10 (100%) |
| Missing | 2 (10%) | 2 (16%) | 0 (0%) | 0 (0%) |
| FISH | ||||
| del 17p | 2 (10%) | 4 (33%) | 1 (7%) | 6 (60%) |
| Absolute lymphocyte count | 110 (10-328) | 37 (5-317) | 31 (4-146) | 12 (6-31) |
| T-cell–to–CLL cell ratio | 0.03 (0.01-0.08) | 0.5 (0.01-0.40) | 0.1 (0.01-0.56) | 0.40 (0.08-0.69) |
| Time on BTKi therapy (mo) | - | 9 (5.5-57) | 6 (5.2-48) | 50 (15-72) |
All characteristics given apply to the time of sample collection for the study.
Patients treated with ibrutinib (n = 19) or acalabrutinib (n = 18) had completed at least 6 cycles, and 10 (37%) had completed at least 12 cycles.
Among patients with disease progression, 7 (70%) were treated with ibrutinib and 3 (30%) with acalabrutinib.
FISH, florescence in situ hybrization; IGHV, immunoglobulin heavy-chain variable region mutational status.
Bispecific antibodies
Epcoritamab (DuoBody-CD3xCD20, GEN3013) is a full-length IgG1 bsAb generated by controlled F(ab)-arm exchange of a humanized CD3 monoclonal Ab (mAb) and the human CD20 mAb 7D8. The nontargeting mAb B12 IgG1 isotype and bispecific antibodies B12×CD20 and B12×CD3 were used as controls, all of which were provided by Genmab.
In vitro cell cultures
Cryopreserved PBMCs were thawed and plated as described in the supplemental Methods. Epcoritamab, or nontargeting B12 isotype, and bsAbs B12×CD20 and B12×CD3 were added to cultures at a titrated concentration of 6.6 nM. Cells incubated at 37°C (5% CO2) were harvested after 0, 3, or 7 days. Antibody effects were normalized to viability in the B12-control condition. For combination with venetoclax, the method is detailed in supplemental Methods.
In vivo murine studies
Experiments using NOD/scid/SCID/IL2Rγnull (NSG) mice (The Jackson Laboratory, JAX strain 5557) were conducted in accordance with protocols approved by the institutional animal care and use committee. PDXs of CLL were generated as previously described.35,42 Briefly, 5 × 107 human CLL PBMCs were introduced into NSG mice via IV tail vein injection on experimental day 0. On day 2, CLL engraftment was confirmed via the flow cytometry of peripheral blood, and epcoritamab or B12 control (0.5 mg/kg) was injected intraperitoneally on days 3 and 10. CLL tumor burden was assessed in peripheral blood on days 10 and 17, and in the spleen on day 17.
Flow cytometry
Cells were stained with commercial Abs (supplemental Table 3). CLL cells were identified as CD8–CD4– or CD5+CD24+. Cell viability was assessed using the LIVE/DEAD fixable violet stain (Invitrogen). Specific lysis rates using the frequency of CLL live cells after culture with epcoritamab and B12 were calculated as follows: (B12 control viability – epcoritamab-treated viability) ÷ (B12 control viability) × 100. Effector T-cell–to–target CLL ratios (E:T) were determined based on frequencies of live cells using this formula: (CD8+% × CD4+%) ÷ %CLL. For cell immunophenotyping, the method is detailed in supplemental Methods.
Luminex cytokine immunoassay
Supernatant from cultured PBMCs with epcoritamab or B12 control was collected after 7 days of culture and analyzed with the Milliplex MAP Human High Sensitivity T-Cell Magnetic Bead Panel (Millipore Corporation) following the manufacturer’s protocol (supplemental Methods).
Clustering of samples based on T-cell response to bsAb
T-cell activation in response to epcoritamab after 3 days was assessed via flow cytometry using 8 markers (supplemental Table 3). Median centered data are displayed in heat maps with samples grouped based on linkage clustering using GeneCluster 3.0 (http://bonsai.hgc.jp/∼mdehoon/software/cluster/manual/TreeView).
Statistical analysis
The statistical significance between patient groups was calculated using the Mann-Whitney test. To compare the responses to different treatments in individual patient samples, Wilcoxon matched-pair signed rank test and paired t test were used. Data were analyzed with GraphPad Prism 7 software.
Results
Epcoritamab induced cytotoxicity in vitro in PBMCs from patients with CLL
We investigated epcoritamab-induced cytotoxicity in PMBCs from patients with CLL who were either treatment-naive (n = 20) or being treated with a BTKi (n = 19, ibrutinib and n = 18, acalabrutinib); 19 received a BTKi as the first-line therapy. At the time of sample collection, 10 patients had progressing disease while on therapy. Patient characteristics are summarized in Table 1, and details for patients with progressive disease are summarized in supplemental Table 2.
PBMCs were incubated in vitro for up to 7 days with epcoritamab or controls, including the nontargeting B12 IgG1 isotype and bsAbs B12×CD20 and B12×CD3. We measured cell viability after 3 and 7 days using flow cytometry and observed a significant decrease in the number of live CLL cells in the presence of epcoritamab, compared with the number of controls (supplemental Figure 1A). Because CLL cell viability was comparable for all 3 controls (supplemental Figure 1B), only the B12 isotype control was used in most of the subsequent analysis. After 3 days (Figure 1A), the median percentage (interquartile range) of live CLL cells was 67% (range, 41%-82%) in the presence of epcoritamab or B12 control, 80% (range, 53%-89%) in samples from patients who were ibrutinib-treated (P = .009), 71% (range, 63%-77%) and 79% (range, 67%-89%) in those from patients who were acalabrutinib-treated (P = .03), and 34% (range, 13%-64%) and 75% (range, 70%-76%) in samples from patients who had progressing disease while on BTKi (P = .05). In PBMCs from treatment-naive patients, CLL cell viability was higher with epcoritamab, at 77% (range, 66%-82%) compared with 67% (range, 58%-81%) with B12 control (P = .01; Figure 1A). By day 7 (Figure 1B), CLL cell viability in PBMCs from treatment-naive patients was significantly lower with epcoritamab, at 57% (range, 36%-72%), than with the control, at 74% (range, 56%-82%) (P = .03). Overall, CLL cell viability on day 7 was significantly lower with epcoritamab than with B12 control: for patients treated with ibrutinib, it was 7% (range, 1%-61%) vs 80% (range, 63%-94%) (P = .0005); for acalabrutinib-treated patients, it was 62% (range, 18%-75%) vs 85% (range, 77%-95%) (P = .0001); and for patients progressing on BTKi, it was 5% (range, 0.5%-35%) vs 72% (range, 66%-81%) (P = .02), respectively (Figure 1B).
Epcoritamab induces a high degree of CLL cytotoxicity in vitro that is enhanced by prior treatment with BTKis. CLL cell viability was assessed in PBMCs from patients with CLL after culture with either epcoritamab or the B12 nontargeting control antibody (6.6nM): treatment-naive (TN); n = 13, triangles); ibrutinib-treated (IBR; n = 12, circles); acalabrutinib-treated (ACA; n = 14, diamonds) patients; and patients with progressing disease while receiving a BTKi (RES; n = 7). CLL cell viability (A) after 3 and (B) after 7 days in culture with B12 or epcoritamab. (C) Percentage of specific lysis of CLL cells by epcoritamab was calculated as follows: ([%B12-treated CLL viability – %epcoritamab-treated CLL viability] ÷ [%B12-treated CLL viability] × 100). Asterisks indicate statistical significance using Wilcoxon matched-pair signed rank test for comparison of different treatments applied to individual patient samples and Mann-Whitney test for the comparison of different patient groups. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
Epcoritamab induces a high degree of CLL cytotoxicity in vitro that is enhanced by prior treatment with BTKis. CLL cell viability was assessed in PBMCs from patients with CLL after culture with either epcoritamab or the B12 nontargeting control antibody (6.6nM): treatment-naive (TN); n = 13, triangles); ibrutinib-treated (IBR; n = 12, circles); acalabrutinib-treated (ACA; n = 14, diamonds) patients; and patients with progressing disease while receiving a BTKi (RES; n = 7). CLL cell viability (A) after 3 and (B) after 7 days in culture with B12 or epcoritamab. (C) Percentage of specific lysis of CLL cells by epcoritamab was calculated as follows: ([%B12-treated CLL viability – %epcoritamab-treated CLL viability] ÷ [%B12-treated CLL viability] × 100). Asterisks indicate statistical significance using Wilcoxon matched-pair signed rank test for comparison of different treatments applied to individual patient samples and Mann-Whitney test for the comparison of different patient groups. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
To compare the effect of epcoritamab between the different groups, we calculated CLL-specific lysis rates by normalizing the frequency of CLL live cells after culturing with epcoritamab to cell viability stage with the B12 control. After culturing CLL live cells for 3 days with epcoritamab, the median (interquartile range) CLL-specific lysis rate was −10% (range, −15% to –3%) in PBMCs from treatment-naive patients, compared with 7% (range, 4%-23%) for patients treated with ibrutinib (P = .0005) and 9% (range, –1 to 29) for those treated with acalabrutinib (P = .001). After 7 days, the median CLL-specific lysis rate was 29% (range, –4 to 50) for patients who were treatment-naive, 90% (range, 27%-98%) for patients who were ibrutinib-treated (P = .01), and 41% (range, 17%-81%) for patients who were acalabrutinib-treated (P = .1). There was no statistically significant difference in CLL-specific lysis between patients treated with ibrutinib or acalabrutinib (P ≥ .1) (Figure 1C). Interestingly, superior epcoritamab activity was observed in PBMCs from patients with progressing disease while on BTKis, with a CLL cell–specific lytic rate of 41% (range, 15%-85%) after 3 days and 93% (range, 56%-99%) after 7 days (P < .005 for comparison with treatment-naive patients).
In summary, epcoritamab effectively induced autologous T cells to lyse CLL cells in vitro. Compared with samples from treatment-naive patients, epcoritamab-mediated cytotoxicity was higher in PBMCs from patients being treated with a BTKi, including that for patients who had progressive disease upon therapy.
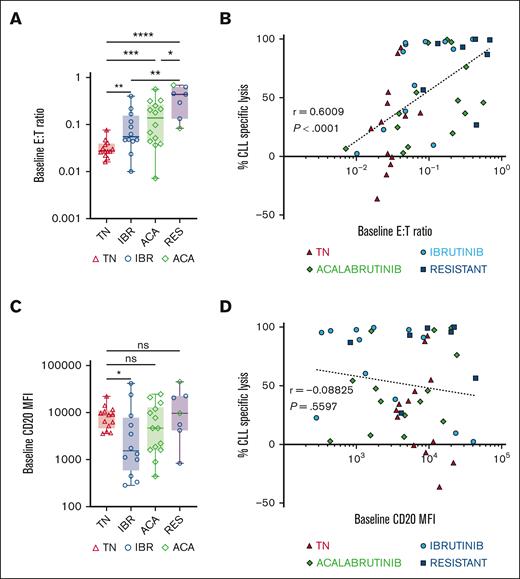
Epcoritamab-induced CLL cell lysis correlated with E:T ratio but not with CD20 expression
We evaluated the relationship between epcoritamab-induced CLL cell lysis and CD20 target antigen expression or effector T-cell frequencies in PBMCs at the start of the experiments. Baseline E:T ratios ranged from 0.02 to 0.6, with higher ratios in samples from patients treated with BTKi and patients with progressing disease while on BTKi than in samples from treatment-naive patients (Figure 2A). Baseline E:T ratios and CLL-specific lysis after 7 days of epcoritamab highly correlated (r = 0.6; P < .0001; Figure 2B) with comparable Spearman r values in samples from patients who were treatment-naive, ibrutinib-, and acalabrutinib-treated (supplemental Figure 1C). Consistently, we found a negative correlation between patients’ absolute lymphocyte counts and CLL cell lysis (r = −0.5; P < .0001; supplemental Figure 1D). Among samples from patients treated with BTKi, baseline E:T ratios highly correlated with the duration of BTKi therapy (r = 0.6; P < .0001; supplemental Figure 1E).
Epcoritamab-induced cytotoxicity in CLL PBMCs correlates with the E:T ratio but not with CD20 expression levels. (A) Comparison of baseline E:T ratio, calculated as follows: (% CD4+ and CD8+ T cells) ÷ % CLL cells, in indicated patient groups: TN (n = 13, red triangles), IBR (n = 12, blue circles), ACA (n = 14, green diamonds), and RES (n = 7). (B) Spearman correlation of baseline E:T ratios and percentage of CLL cell–specific lysis in samples from all 4 groups cultured with epcoritamab for 7 days. (C) Comparison of baseline CD20 mean fluorescence intensity (MFI) in CLL cells between different patient groups. (D) Spearman’s correlation of baseline CD20 MFI and percentage of specific lysis of CLL cells in samples from all 4 groups cultured with epcoritamab for 7 days. Asterisks indicate statistical significance using Mann-Whitney test for the comparison of different patient groups. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Epcoritamab-induced cytotoxicity in CLL PBMCs correlates with the E:T ratio but not with CD20 expression levels. (A) Comparison of baseline E:T ratio, calculated as follows: (% CD4+ and CD8+ T cells) ÷ % CLL cells, in indicated patient groups: TN (n = 13, red triangles), IBR (n = 12, blue circles), ACA (n = 14, green diamonds), and RES (n = 7). (B) Spearman correlation of baseline E:T ratios and percentage of CLL cell–specific lysis in samples from all 4 groups cultured with epcoritamab for 7 days. (C) Comparison of baseline CD20 mean fluorescence intensity (MFI) in CLL cells between different patient groups. (D) Spearman’s correlation of baseline CD20 MFI and percentage of specific lysis of CLL cells in samples from all 4 groups cultured with epcoritamab for 7 days. Asterisks indicate statistical significance using Mann-Whitney test for the comparison of different patient groups. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
A wide range of CD20 expression levels in CLL cells was observed, with patients treated with ibrutinib expressing the lowest CD20 levels compared with the treatment-naive group (P = .02; Figure 2C). There was no statistically significant difference in CD20 expression on CLL cells from patients treated with acalabrutinib, treatment-naive patients, and patients with disease progressing while receiving BTKi. Across all samples, baseline CD20 expression levels did not correlate with epcoritamab-induced cytotoxicity on day 7 (Figure 2D). There was also no correlation between CLL cell lysis and immunoglobulin heavy chain variable (IGHV) region mutational status, Rai stage, cytogenetic characteristics, or prior treatment status (data not shown).
Taken together, the E:T ratio emerged as a major determinant of epcoritamab-mediated cytotoxicity. In contrast, cytotoxic activity was comparable across the wide range of CD20 expression levels observed in these samples.
Epcoritamab enhances autologous T-cell cytotoxic effector function, activation, and proliferation
To determine the effect of epcoritamab on T-cell activation and proliferation, we quantified the frequency of CD4+ and CD8+ T cells expressing immunophenotypic markers of activation and cytotoxic potential at baseline and after 3 days of culture (supplemental Figure 2A). Compared with B12 controls, the proportion of CD4+ and CD8+ T cells expressing Ki-67, granzyme B, HLA-DR, PD-1, CTLA-4, TIM-3, and LAG-3 increased significantly after 3 days of culture with epcoritamab in all patient groups (supplemental Figure 2B). No T-cell activation or proliferation was observed with B12×CD3 (supplemental Figure 3) or B12×CD20 (not shown) control bsAbs.
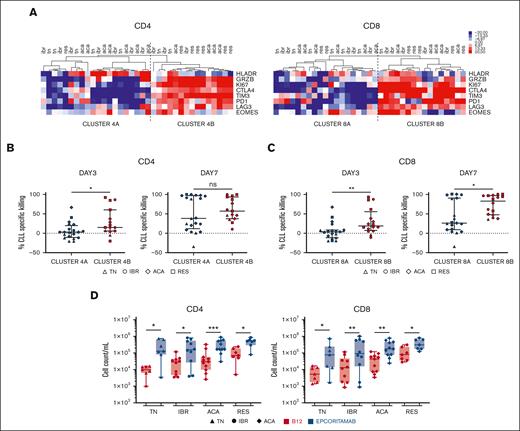
To explore the relationship between epcoritamab-induced T-cell response and cytotoxic activity, we performed hierarchical clustering of patient samples based on the frequency of CD4+ or CD8+ T cells expressing these immunophenotypic markers on day 3. Samples were divided into 2 major clusters: cluster A with relatively lower expression and cluster B with higher expression of markers indicative of activation, proliferation, and cytotoxic potential (Figure 3A). The more activated samples in B clusters, compared with those in A clusters, were associated with significantly higher cytotoxic activity on day 3 for both CD4+ (P = .02; Figure 3B) and CD8+ (P = .006; Figure 3C) T cells. On day 7, median CLL cell lysis for the activated CD8+ cluster was 82% (range, 46%-96%) compared with 25% (range, 8%-89%) for cluster A (P = .02; Figure 3C), whereas there was no statistically significant difference for the CD4+ cells. Notably, the B clusters were enriched for BTKi-treated samples (supplemental Figure 4A) and had higher median E:T ratios compared with cluster A: for CD4+, 0.29 and 0.05 and for CD8+, 0.23 and 0.04, respectively (P < .01 for both comparisons; supplemental Figure 4B). Similarly, within cluster A, greater CLL cell killing was associated with higher E:T ratios (supplemental Figure 4C).
Epcoritamab induces autologous T-cell activation and expansion. Markers of T-cell activation and cytotoxic potential were assessed via flow cytometry, in PBMCs from patients who were TN (n = 7), IBR (n = 10), or ACA (n = 11) cultured with epcoritamab or B12 control for 3 days. Samples from RES (n = 7) were also included. (A) Heatmap depicts the median centered frequencies of CD4+ and CD8+ T cells expressing the indicated markers; samples were grouped based on hierarchical clustering. Comparison of CLL-specific killing after 3 and 7 days of treatment between grouping based on (B) CD4 activation and (C) CD8 activation state. Each symbol represents 1 patient sample, and the median and interquartile range are indicated. (D) CD4+ and CD8+ T-cell counts were quantified via flow cytometry after 7 days of culture with epcoritamab (blue symbols) or B12 control (red symbols). Each symbol represents 1 patient sample. Asterisks indicate statistical significance using Wilcoxon matched-pair signed rank test for comparison of different treatments applied to individual patient samples and Mann-Whitney test for comparison of different patient groups. ∗P < .05; ∗∗P < .001; ∗∗∗P< .001.
Epcoritamab induces autologous T-cell activation and expansion. Markers of T-cell activation and cytotoxic potential were assessed via flow cytometry, in PBMCs from patients who were TN (n = 7), IBR (n = 10), or ACA (n = 11) cultured with epcoritamab or B12 control for 3 days. Samples from RES (n = 7) were also included. (A) Heatmap depicts the median centered frequencies of CD4+ and CD8+ T cells expressing the indicated markers; samples were grouped based on hierarchical clustering. Comparison of CLL-specific killing after 3 and 7 days of treatment between grouping based on (B) CD4 activation and (C) CD8 activation state. Each symbol represents 1 patient sample, and the median and interquartile range are indicated. (D) CD4+ and CD8+ T-cell counts were quantified via flow cytometry after 7 days of culture with epcoritamab (blue symbols) or B12 control (red symbols). Each symbol represents 1 patient sample. Asterisks indicate statistical significance using Wilcoxon matched-pair signed rank test for comparison of different treatments applied to individual patient samples and Mann-Whitney test for comparison of different patient groups. ∗P < .05; ∗∗P < .001; ∗∗∗P< .001.
Epcoritamab induced both CD4 and CD8 T-cell expansion in vitro. On day 7, the median increase in CD4+ T cells with epcoritamab compared with the B12 control condition was 13-fold for the treatment-naive group (P = .016), sixfold for ibrutinib and acalabrutinib-treated group (P = .019 and P = .001; respectively), and sevenfold for BTKi progressive disease group (P = .016). The median increase of CD8+ T-cell counts with epcoritamab was 15-fold for the treatment-naive patients (P = .016), sevenfold and fourfold for patients treated with ibrutinib and acalabrutinib,respectively, (P = .009 and P = .003, respectively), and fourfold for patients with progressing disease while on BTKi (P = .016; Figure 3D).
Epcoritamab induces Th1 polarization and memory T-cell differentiation
Th1, Th2, and Th17 T-helper subsets were identified via flow cytometry and staining for CCR6 and CXCR3 (supplemental Figure 5A). Ex vivo, Th1:Th2 ratios were biased toward Th2 in all patient samples (supplemental Figure 5B). In vitro, epcoritamab promoted Th1 polarization as early as on day 3 (supplemental Figure 5C).34 After 7 days, the median Th1:Th2 ratio in B12 and epcoritamab-treated PBMCs increased from 0.2 to 0.6 (P = .03) for treatment-naive, from 0.4 to 3 (P = .002) for ibrutinib-treated, from 0.7 to 2 (P = .005) for acalabrutinib-treated, and from 0.4 to 2 (P = .16) for the progressive disease cohorts (Figure 4A). We also found a significant increase in the concentration of Th1 cytokines in cell culture supernatants on day 7 (Figure 4B). For example, the median concentration of interferon-gamma was <50 pg/mL in B12 control-treated samples but was >2000 pg/mL in epcoritamab-treated samples (P ≤ .03). Likewise, concentrations of tumor necrosis factor α (P ≤ .02) and granulocyte-macrophage colony-stimulating factor (P = .008) significantly increased in epcoritamab-treated samples compared with that in controls. Although we detected increases in the concentration of the Th2 cytokines, such as IL-5, upon epcoritamab treatment, their concentrations remained very low (≤ 300 ρg/mL).
Epcoritamab shifts T-cell differentiation toward Th1 polarization and enhances its differentiation into EM T cells. (A) Th1 and Th2 polarization of CD4+ T cells was assessed based on CCR6 and CXCR3 expression. Th1:Th2 ratio (log2 transformed) was calculated using the percentage of Th1 and Th2 subsets within CD4+ T cells in PBMCs from patients who were TN (n = 7), IBR (n = 10), ACA (n = 11), and RES (n = 7) after 7 days of culture with epcoritamab (blue symbols) or B12 control (red symbols). Each symbol represents 1 patient sample. (B) Th1 (interferon-gamma, tumor necrosis factor α, and granulocyte-macrophage colony-stimulating factor) and Th2 (interleukin-6 [IL-6] and IL-5) cytokine levels measured via Luminex cytokine assays in cell supernatants harvested after 7 days of exposure to epcoritamab (EPCO) or B12 control (B12) for samples from patients who were TN (n = 8), IBR (n = 8), and ACA (n = 8). (C) T-cell differentiation in CD4+ or CD8+ subsets was assessed via flow cytometry, separating naive T cells, CM T cells, EM T cells, and effector T cells, based on CCR7 and CD45RO expression for samples from patients who were TN (n = 7), IBR (n = 10), ACA (n = 11), and RES (n = 7) after 7 days of culture with EPCO or B12. Pie charts represent the median proportion of each subset. Asterisks indicate statistically significant expansion of CD4 and CD8 T-effector memory or T-central memory in cultures treated with EPCO compared with B12 as determined using Wilcoxon matched-pair signed rank test. ∗P< .05; ∗∗P< .01.
Epcoritamab shifts T-cell differentiation toward Th1 polarization and enhances its differentiation into EM T cells. (A) Th1 and Th2 polarization of CD4+ T cells was assessed based on CCR6 and CXCR3 expression. Th1:Th2 ratio (log2 transformed) was calculated using the percentage of Th1 and Th2 subsets within CD4+ T cells in PBMCs from patients who were TN (n = 7), IBR (n = 10), ACA (n = 11), and RES (n = 7) after 7 days of culture with epcoritamab (blue symbols) or B12 control (red symbols). Each symbol represents 1 patient sample. (B) Th1 (interferon-gamma, tumor necrosis factor α, and granulocyte-macrophage colony-stimulating factor) and Th2 (interleukin-6 [IL-6] and IL-5) cytokine levels measured via Luminex cytokine assays in cell supernatants harvested after 7 days of exposure to epcoritamab (EPCO) or B12 control (B12) for samples from patients who were TN (n = 8), IBR (n = 8), and ACA (n = 8). (C) T-cell differentiation in CD4+ or CD8+ subsets was assessed via flow cytometry, separating naive T cells, CM T cells, EM T cells, and effector T cells, based on CCR7 and CD45RO expression for samples from patients who were TN (n = 7), IBR (n = 10), ACA (n = 11), and RES (n = 7) after 7 days of culture with EPCO or B12. Pie charts represent the median proportion of each subset. Asterisks indicate statistically significant expansion of CD4 and CD8 T-effector memory or T-central memory in cultures treated with EPCO compared with B12 as determined using Wilcoxon matched-pair signed rank test. ∗P< .05; ∗∗P< .01.
Using CCR7 and CD45RO expression, measured via flow cytometry, we identified T-cell subsets as naive, central memory (CM), effector memory (EM), and effector T cells (supplemental Figure 5D). Ex vivo, no significant difference in the T-cell compartment composition was observed between the patient groups (supplemental Figure 5E). After 7 days, the median frequency of T-EM among CD4+ T cells was higher in epcoritamab-treated cultures than in controls: 39% vs 17% (P = .006) in patients who were ibrutinib-treated, 37% vs 25% (P = .04) in those who were acalabrutinib-treated, and 60% vs 18% (P = .016) in those who were treatment-naive, respectively (Figure 4C). Likewise, among CD8+ T cells the median frequencies of T-EM in epcoritamab-treated cultures vs in controls were 57% vs 42% (P = .03) for ibrutinib-treated, 55% vs 35% (P = .04) for acalabrutinib-treated, and 58% vs 32% (P = .07) in treatment-naive patients. Numerically, the frequency of CM T cells increased in all epcoritamab-treated cultures compared with that in controls, but the change reached statistical significance only for CD8 T cells from patients who were treatment-naive and those treated with ibrutinib (Figure 4C). Conversely, the frequency of naive T cells significantly decreased in all cultures. In samples from patients who had progressing disease while on BTKi, the proportion of CM and EM was very high in all conditions, and no statistically significant shifts were observed (P > .1).
In summary, the addition of epcoritamab to CLL PBMCs in vitro promoted Th1 polarization and differentiation in EM and CM T cells, with a concurrent reduction in the frequency of naive T cells.
Epcoritamab mediates the CLL-directed cytotoxicity by autologous T cells in vivo
Next, we tested epcoritamab in a PDX mouse model.35 PBMCs from 7 patients were injected into 35 mice, with 5 mice per patient. Three patients were treatment-naive and 4 patients had progressing disease while on BTKi therapy (supplemental Table 3). We excluded 4 mice showing low engraftment on day 2. The 31 mice with acceptable human cell engraftment were stratified based on the engraftment level (supplemental Figure 6) to receive epcoritamab (n = 17) or B12 control (n = 14) on days 3 and 10 (Figure 5A). CLL cell burden in the blood and spleens was quantified via flow cytometry (Figure 5B).
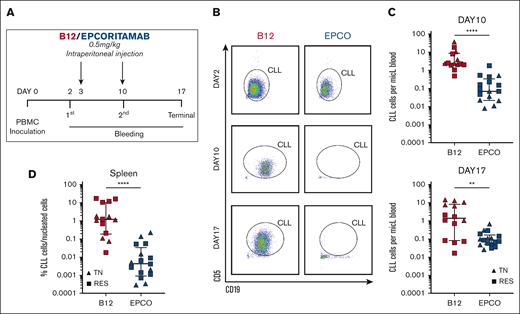
Epcoritamab eliminates CLL cells in PDXs. (A) PBMCs from patients who were TN (n = 3, triangles) and RES (n = 4, squares) were injected into NSG mice on experimental day 0. Cell engraftment was confirmed on day 2 via flow cytometry. Mice were then treated once weekly with either epcoritamab or B12 control (0.5 mg/kg). Four or 5 mice were included for each patient and divided between the 2 treatment groups (n = 7 patients; n = 31 mice). (B) Representative flow cytometry dot plots of peripheral blood from mice treated with either epcoritamab (EPCO) or B12 control (B12) on day 2 (top, before treatment), day 10 (middle, after 1 injection), and day 17 (bottom, after 2 injections). (C) Comparison of the mean CLL cell count in the peripheral blood between EPCO-treated (blue) and B12-treated (red) groups on experimental days 10 and 17. (D) Percentage of CLL cells among nucleated cells in the spleen on experimental day 17 between the EPCO-treated (blue) and B12-treated (red) groups. The median and interquartile ranges are indicated. Asterisks indicate statistical significance using Mann-Whitney test. ∗∗P< .01; ∗∗∗∗P< .0001.
Epcoritamab eliminates CLL cells in PDXs. (A) PBMCs from patients who were TN (n = 3, triangles) and RES (n = 4, squares) were injected into NSG mice on experimental day 0. Cell engraftment was confirmed on day 2 via flow cytometry. Mice were then treated once weekly with either epcoritamab or B12 control (0.5 mg/kg). Four or 5 mice were included for each patient and divided between the 2 treatment groups (n = 7 patients; n = 31 mice). (B) Representative flow cytometry dot plots of peripheral blood from mice treated with either epcoritamab (EPCO) or B12 control (B12) on day 2 (top, before treatment), day 10 (middle, after 1 injection), and day 17 (bottom, after 2 injections). (C) Comparison of the mean CLL cell count in the peripheral blood between EPCO-treated (blue) and B12-treated (red) groups on experimental days 10 and 17. (D) Percentage of CLL cells among nucleated cells in the spleen on experimental day 17 between the EPCO-treated (blue) and B12-treated (red) groups. The median and interquartile ranges are indicated. Asterisks indicate statistical significance using Mann-Whitney test. ∗∗P< .01; ∗∗∗∗P< .0001.
Compared with mice that received B12 control, the median leukemic cell burden in mice treated with epcoritamab was reduced by 71% after 1 injection (day 10; P < .0001) and by 94% after the second injection (day 17; P = .003; Figure 5C). In the spleen, tumor infiltration was reduced by >99% in epcoritamab-treated mice (P < .0001; Figure 5D). There was no apparent difference in the efficacy of epcoritamab against CLL cells obtained from treatment-naive patients or patients who had progressing disease while continuously recceiving BTKi treatment. We attempted to engraft samples from patients in remission on BTKi but were unable to achieve acceptable engraftment for in vivo studies.
Epcoritamab effectively induced CLL-directed cytotoxicity in vivo, which, in our PDX model, was dependent on autologous T cells. We observed a high degree of activity against circulating as well as splenic CLL cells in samples from treatment-naive patients and patients who had progressing disease while receiving BTKi therapy.
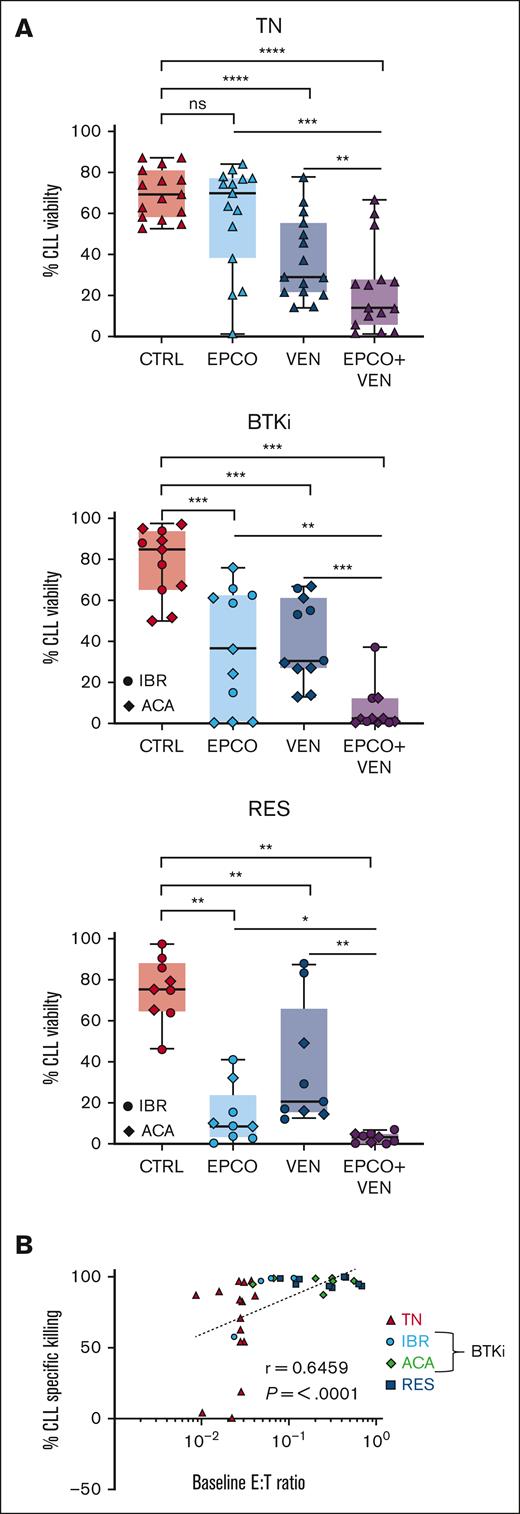
The combination of epcoritamab with venetoclax is more cytotoxic than either agent alone
The Bcl-2 inhibitor venetoclax, combined with anti-CD20 antibodies, is effective in treating patients with progressing disease while on BTKi.18-21 Here, we tested the addition of venetoclax to epcoritamab in vitro. We titrated the dose of venetoclax to 5nM to achieve half-maximal killing of CLL cells after 48 hours of culture (data not shown). PBMCs were incubated for up to 7 days with B12/dimethyl sulfoxide control vs epcoritamab, venetoclax, or both agents combined. After 7 days of venetoclax, the median CLL cell viability was 29% (range, 22%-55%) in treatment-naive patients (P < .0001, vs control), 31% (range, 27%-61%) in patients treated with BTKi (P = .001), and 21% (range, 15%-66%) in patients with progressing disease while receiving a BTKi (P = .004). In PBMCs treated with the combination of epcoritamab and venetoclax, the CLL cell viability decreased significantly compared with that in PBMCs treated with either agent alone (Figure 6A): it decreased to 14% (range, 6%-28%) for treatment-naive patients (P ≤ .006), to 2% (range, 0.6%-12%) for patients treated with BTKi (P ≤ .005), and to 3% (range, 0.6%-5%) for patients with progressing disease while receiving a BTKi (P ≤ .01). As seen with single-agent epcoritamab, the E:T ratio and cytotoxicity activity were highly correlated for the combination of epcoritamab with venetoclax (r = 0.6; P < .0001; Figure 6B). The addition of venetoclax did not differentially reshape the T-cell compartment compared with single-agent epcoritamab (supplemental Figure 7A-B).To account for interpatient variations in T-cell frequency, we enriched CLL cells to > 95% by depleting autologous T cells and adding healthy donor PBMCs as the T-cell source at a fixed 1:3 E:T ratio. Within 48 hours, CLL cell viability had significantly decreased in the presence of epcoritamab combined with venetoclax vs single agents in all 3 patient groups (P ≤ .05; supplemental Figure 7C-D). Taken together, using autologous or allogeneic T cells as effectors, the combination of epcoritamab and venetoclax was more active than either agent alone.
The combination of epcoritamab with venetoclax induced a higher degree of CLL cytotoxicity in vitro than either agent alone. PBMCs from patients who were TN (n = 15), patients treated with BTKi (BTKi, n = 11; IBR, 4; and ACA, 7), and patients who were RES (n = 9) were cultured with either B12/dimethyl sulfoxide control (CTRL), epcoritamab (EPCO), 5nM venetoclax (VEN), or the combination of epcoritamab with venetoclax (EPCO + VEN). (A) CLL cell viability after 7 days of culture. (B) Spearman correlation of baseline E:T ratios and percentage of CLL cell–specific lysis in samples from all 3 groups cultured with EPCO + VEN for 7 days. Asterisks indicate statistical significance using Wilcoxon matched-pair signed rank test for the comparison of different treatments applied to individual patient samples. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
The combination of epcoritamab with venetoclax induced a higher degree of CLL cytotoxicity in vitro than either agent alone. PBMCs from patients who were TN (n = 15), patients treated with BTKi (BTKi, n = 11; IBR, 4; and ACA, 7), and patients who were RES (n = 9) were cultured with either B12/dimethyl sulfoxide control (CTRL), epcoritamab (EPCO), 5nM venetoclax (VEN), or the combination of epcoritamab with venetoclax (EPCO + VEN). (A) CLL cell viability after 7 days of culture. (B) Spearman correlation of baseline E:T ratios and percentage of CLL cell–specific lysis in samples from all 3 groups cultured with EPCO + VEN for 7 days. Asterisks indicate statistical significance using Wilcoxon matched-pair signed rank test for the comparison of different treatments applied to individual patient samples. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Discussion
CLL cells are sensitive to T-cell attack, as demonstrated with donor lymphocyte infusion after allogeneic stem cell transplantation43 and successes with chimeric antigen receptor (CAR) T-cell therapy.44,45 bsAbs offer an off-the-shelf approach that depends on the cytotoxic effector functions of autologous T cells. The cellular immune dysfunction characteristic of CLL hampers CAR T-cell efficacy46-48 and may also hamper the activity of bsAbs. Here, we tested epcoritamab across a clinical spectrum of CLL disease course by including samples from patients who were treatment-naive, those treated with BTKi, and those with progressing disease while on BTKi therapy. In vitro, epcoritamab-induced CLL cytotoxicity was readily demonstrable in PBMCs from treatment-naive patients and was enhanced in samples from patients with ongoing BTKi therapy, including patients with progressive disease. Epcoritamab expanded CD4+ and CD8+ T cells, induced memory T-cell differentiation, and promoted Th1 polarization. These attributes are associated with long-term protective responses to cancer immunotherapy,49,50 including in patients with aggressive lymphoma treated with CD19-targeting CAR T cells.51
Low CD20 expression is characteristic of CLL, as are low E:T ratios, given the high number of circulating tumor cells. We found that epcoritamab-mediated cytotoxicity did not correlate with CD20 expression, consistent with observations on lymph node biopsy specimen from patients with B-cell lymphomas.38 Efficacy of epcoritamab in PDXs of lymphoma has also been reported in the presence of rituximab,37 and although rituximab interfered with epcoritamab binding to CD20-expressing cells in vitro, tumor-cell cytotoxicity was minimally affected by clinically relevant concentrations of rituximab.52 Taken together, epcoritamab-mediated cytotoxicity can be achieved despite low antigen density in tumor cells. This contrasts with reported associations of low CD20 expression with reduced benefits of anti-CD20 antibodies in CLL.53,54 Although anti-CD20 antibodies improve response rates and survival in combinations with chemotherapy,55,56 BTKis can interfere with Fc receptor–mediated effector functions,57-59 and the benefit of combining anti-CD20 mAbs with BTKis remains ill-defined.60-62 Therefore, anti-CD20 bsAbs that direct cytotoxic T cells against tumor cells and are equally effective against cells with low target antigen density represent an attractive new combination partner to BTKi therapy.
In contrast to our data from earlier studies with the CD19/CD3 bsAb,34,35 epcoritamab-mediated cytotoxicity correlated with E:T ratios. Samples from patients receiving BTKi therapy, including those whose disease progressed, had higher E:T ratios compared with samples from treatment-naive patients, partly explaining the superior activity of the bsAb in patients receiving BTKis. In addition, BTKis, by transcriptionally downregulating immunosuppressive molecules in CLL cells, create an environment favorable to cellular immunotherapy.34 Consistently, we found that epcoritamab induced a higher degree of T-cell activation, proliferation, and expression of cytotoxic effectors in T cells from patients receiving BTKis than in treatment-naive patients, irrespective of which BTKi the patients received.
Epcoritamab mediates potent in vivo cytotoxicity against CLL cells in the PDX model. Reflecting the situation in patients, the activity of the bsAb in this model depends on the effector functions of autologous T cells. Limitations of the model include a lack of survival end points because the CLL xenografts do not lead to the death of the host.35,42 Furthermore, interpatient variability in engraftment limits interpatient comparisons. Nevertheless, the significant reduction in tumor burden after only 2 injections of epcoritamab clearly demonstrates the potent activity of bsAbs in vivo. Importantly, epcoritamab was also effective in mice xenografted with samples from patients with progressing disease while receiving BTKis, a high-risk group with a poor prognosis.15,63
The BCL-2 inhibitor venetoclax, combined with anti-CD20 antibodies, is a commonly used second-line therapy for patients resistant or intolerant to BTKis. Venetoclax increased the cytotoxicity of epcoritamab against CLL cells in vitro, including in PBMCs from patients with progressing disease while on BTKis. These data support investigations of epcoritamab in combination with venetoclax in patients with progressing disease while on BTKis or as consolidation for patients at an increased risk of failure with single-agent BTKi therapy.64 Venetoclax has been reported to reshape the T-cell compartment by reducing the number of follicular helper and regulatory T cells and improving T-cell metabolic function through the depletion of CLL cells.65,66 However, the effects of venetoclax and its combination with epcoritamab on immune function in patients with CLL need to be further investigated.
In summary, our results assert the potential of epcoritamab as a novel treatment option for patients with CLL and support clinical investigations of epcoritamab in combination with BTKis or venetoclax and as salvage therapy in patients with progressing disease while receiving BTKi therapy.
Acknowledgments
The authors thank patients for participating and donating samples to make this research possible, Susan Soto and Pia Nierman for protocol support, and the NHLBI Flow Cytometry Cores and the NHLBI Animal Facility. This work is supported by the Intramural Research Program of the National Institutes of Health, The National Heart, Lung, and Blood Institute, and Genmab.
Authorship
Contribution: M.M., E.G., C.C., M.W.-B., E.C.W.B., C.S., and A.W. conceptualized the study; M.M., E.G., D.E., J.H., and J.L. performed experiments; M.M., E.G., C.S., and A.W. analyzed data and figures; M.M., C.S., and A.W. wrote the original draft; A.W. and C.S. supervised and acquired funding; and all authors wrote, reviewed, and edited the manuscript.
Conflict-of-interest disclosure: A.W. received research support from Pharmacyclics LLC, an AbbVie Company, Acerta Pharma, a member of the AstraZeneca group, Merck, Nurix, Verastem, and Genmab. C.S. received research funding from Genmab. M.W.-B., I.H.H., E.B., J.C., and C.C. are currently Genmab employees and holders of stock options. E.S.-G., E.B.R., and P.K.E.-B. are currently AbbVie employees and holders of stock options. The remaining authors declare no competing financial interests.
Correspondence: Adrian Wiestner, Hematology Branch, The National Heart, Lung, and Blood Institute, National Institutes of Health, Bldg 10, CRC 3-5140, 10 Center Dr, Bethesda, MD 20892-1202; e-mail: wiestnera@mail.nih.gov.
References
Author notes
∗M.M. and E.G. are joint first authors.
Presented in abstract form at the 63rd annual meeting of the American Society of Hematology, Atlanta, GA, 11 December 2021.
Data are available on request from the corresponding author, Adrian Wiestner (wiestnera@mail.nih.gov).
The full-text version of this article contains a data supplement.

![Epcoritamab induces a high degree of CLL cytotoxicity in vitro that is enhanced by prior treatment with BTKis. CLL cell viability was assessed in PBMCs from patients with CLL after culture with either epcoritamab or the B12 nontargeting control antibody (6.6nM): treatment-naive (TN); n = 13, triangles); ibrutinib-treated (IBR; n = 12, circles); acalabrutinib-treated (ACA; n = 14, diamonds) patients; and patients with progressing disease while receiving a BTKi (RES; n = 7). CLL cell viability (A) after 3 and (B) after 7 days in culture with B12 or epcoritamab. (C) Percentage of specific lysis of CLL cells by epcoritamab was calculated as follows: ([%B12-treated CLL viability – %epcoritamab-treated CLL viability] ÷ [%B12-treated CLL viability] × 100). Asterisks indicate statistical significance using Wilcoxon matched-pair signed rank test for comparison of different treatments applied to individual patient samples and Mann-Whitney test for the comparison of different patient groups. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/7/15/10.1182_bloodadvances.2022009517/2/m_blooda_adv-2022-009517-gr1.jpeg?Expires=1765904185&Signature=vPKgWDvc3BSvTVc4sJe39w1C4GVCV~ZLKgQxep~n6XwAyDLTTcwlaVh2VbshoDxh8ovuvn18ksuAuInAs2ZNKSB~JqQfp-emy64iEfNBjJVxevtLqM3knPOhZqgQIJxniTZ1i08UPZInSzHH0zbWvlexBooiy0m8~1TmEdWK45YhCxtvSgCIvIEdQZQHkzWPkxYkFN00vqLTV~iv3xNBU4v1JYugO1mce68RCQ0yaDthcHFQpP1fxtwdPTatSlCNhGoPLUDS-s5ALNs~av-E9ZxQd0aIZRuqxCF-5u7U5ngd5RlHIZjV-QjNH0VrnXJG8O5xp4sUb3gY2HqER~MeBw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Epcoritamab shifts T-cell differentiation toward Th1 polarization and enhances its differentiation into EM T cells. (A) Th1 and Th2 polarization of CD4+ T cells was assessed based on CCR6 and CXCR3 expression. Th1:Th2 ratio (log2 transformed) was calculated using the percentage of Th1 and Th2 subsets within CD4+ T cells in PBMCs from patients who were TN (n = 7), IBR (n = 10), ACA (n = 11), and RES (n = 7) after 7 days of culture with epcoritamab (blue symbols) or B12 control (red symbols). Each symbol represents 1 patient sample. (B) Th1 (interferon-gamma, tumor necrosis factor α, and granulocyte-macrophage colony-stimulating factor) and Th2 (interleukin-6 [IL-6] and IL-5) cytokine levels measured via Luminex cytokine assays in cell supernatants harvested after 7 days of exposure to epcoritamab (EPCO) or B12 control (B12) for samples from patients who were TN (n = 8), IBR (n = 8), and ACA (n = 8). (C) T-cell differentiation in CD4+ or CD8+ subsets was assessed via flow cytometry, separating naive T cells, CM T cells, EM T cells, and effector T cells, based on CCR7 and CD45RO expression for samples from patients who were TN (n = 7), IBR (n = 10), ACA (n = 11), and RES (n = 7) after 7 days of culture with EPCO or B12. Pie charts represent the median proportion of each subset. Asterisks indicate statistically significant expansion of CD4 and CD8 T-effector memory or T-central memory in cultures treated with EPCO compared with B12 as determined using Wilcoxon matched-pair signed rank test. ∗P< .05; ∗∗P< .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/7/15/10.1182_bloodadvances.2022009517/2/m_blooda_adv-2022-009517-gr4.jpeg?Expires=1765904185&Signature=zPgcEbQgmp6h919VcjLwsm2W3MH0r-Cbo-9QfnWcQKCe8XPWu18RTOTbhXDlvbCLykW418zM0GjoHIiXzejt-Evm5-pE6IsxqOII~~8W2xV8ZOimA1z~5bIPV5qDQRLc-YVGTFxZuvDPJB6DoTwGOOx7bmsB3glWg9Qemmd8kQbYpUt5RwIbHKAynqBPeYiADo1dJ2krVAU9M0fB7iQHP379Mb7XFHHQZeusgf-IWXgiTZtr8Nl2g45ild3FVJMNKyBoUghfS775EpXTh~TpIaYIQ4WJxoIcz3AR-2BJt1LFHFWM0uolMRQ3zcEaISeYo-bZV5xhmCAsNgCg3ztm4w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)