Key Points
This analysis used US and European stem cell transplant registries and provides a simple and easily applicable predictive system.
Abstract
To develop a prognostic model for patients undergoing allogeneic hematopoietic cell transplantation (allo-HCT) for myelofibrosis (MF), we examined the data of 623 patients undergoing allo-HCT between 2000 and 2016 in the United States (the Center for International Blood and Marrow Transplant Research [CIBMTR] cohort). A Cox multivariable model was used to identify factors prognostic of mortality. A weighted score using these factors was assigned to patients who received transplantation in Europe (the European Bone Marrow Transplant [EBMT] cohort; n = 623). Patient age >50 years (hazard ratio [HR], 1.39; 95% confidence interval [CI], 0.98-1.96), and HLA-matched unrelated donor (HR, 1.29; 95% CI, 0.98-1.7) were associated with an increased hazard of death and were assigned 1 point. Hemoglobin levels <100 g/L at time of transplantation (HR, 1.63; 95% CI, 1.2-2.19) and a mismatched unrelated donor (HR, 1.78; 95% CI, 1.25-2.52) were assigned 2 points. The 3-year overall survival (OS) in patients with a low (1-2 points), intermediate (3-4 points), and high score (5 points) were 69% (95% CI, 61-76), 51% (95% CI, 46-56.4), and 34% (95% CI, 21-49), respectively (P < .001). Increasing score was predictive of increased transplant-related mortality (TRM; P = .0017) but not of relapse (P = .12). The derived score was predictive of OS (P < .001) and TRM (P = .002) but not of relapse (P = .17) in the EBMT cohort as well. The proposed system was prognostic of survival in 2 large cohorts, CIBMTR and EBMT, and can easily be applied by clinicians consulting patients with MF about the transplantation outcomes.
Introduction
Myelofibrosis (MF) is a myeloproliferative neoplasm characterized by clonal myeloid proliferation, extramedullary hematopoiesis, peripheral cytopenias, bone marrow fibrosis, splenomegaly, and a heterogenous symptom burden.1,2 The discovery of the JAK2V617F driver mutation and subsequent introduction of JAK inhibitors into the therapeutic arena has had a significant impact on clinical care of MF.3-7 Despite this, allogeneic hematopoietic cell transplantation (allo-HCT) remains the only curative therapy to date.8,9
The dynamic international prognostic scoring system (DIPSS)10 and DIPSS-plus11 are commonly used for disease risk stratification, and the data regarding the mutations landscape12,13 add further prognostic information. However, these tools were reported mostly in cohorts without transplantations, yet, the transplantation outcomes are dependent not only on disease characteristics, and both patient- and transplantation-related factors affect outcomes. The MPD-101 study reported superior outcomes after a reduced intensity conditioning in patients who received a transplantation from a matched related donor compared with those who received a transplantation from an unrelated donor,14 whereas a prospective study by the European group of blood and marrow transplantation reported that being of younger age (<55 years) and having a matched donor were associated with improved outcomes.15 In addition, transplantation-related mortality (TRM) in patients with MF undergoing allo-HCT is noted to be higher than that in patients with other myeloid malignancies, reportedly as high as 35% at 5 years after transplantation compared with 20% in patients with acute myelogenous leukemia.16 These observations highlight some unique characteristics of the disease, such as an increased inflammatory milieu and extramedullary hematopoiesis as well as the interaction between the host and donor among patients with MF, emphasizing the need for a prognostic score among patients with MF undergoing allo-HCT.
In this study, we used data from the Center for International Blood and Marrow Transplant Research (CIBMTR) registry to identify disease-, patient-, and transplantation-specific variables that are associated with the outcome in patients undergoing allo-HCT for MF; this cohort served as the training set. The primary objective of this study was to develop a simple clinical risk stratification tool prognostic of survival after transplantation. We then sought to determine whether this tool was also prognostic of relapse, TRM, and disease-free survival (DFS). The model was validated in an external cohort of patients reported in the European Bone Marrow Transplant (EBMT) registry.
Patients and methods
Data source
The study was performed through a collaboration between the CIBMTR Chronic Leukemia Working Committee and the EBMT Chronic Malignancies Working Party. The CIBMTR is a nonprofit research collaboration of the National Marrow Donor Program/Be The Match and the Medical College of Wisconsin. More than 330 medical centers worldwide submit to the CIBMTR clinical data about HCT and other cellular therapies. Participating centers are required to report all transplantations consecutively. The CIBMTR ensures data quality through computerized checks for discrepancies, physicians’ review of submitted data, and on-site audits of participating centers. The CIBMTR complies with federal regulations that protect human research participants. The institutional review boards of the Medical College of Wisconsin and the National Marrow Donor Program approved this study. EBMT is a voluntary organization comprising >600 transplantation centers, mainly from Europe. Accreditation as a member-center requires submission of a minimal essential data form from all consecutive patients to a central registry. Since 1996, accredited EBMT centers are subject to on-site audits. Since January 2003, all transplantation centers have been required to obtain written informed consent in accordance with the Declaration of Helsinki 1975 before data registration.
Patients
Patients with MF aged ≥40 years who underwent allo-HCT from a related HLA-matched donor (as previously described17), unrelated HLA-matched donor (MURD), or unrelated HLA-mismatched donor (MMURD) reported to the CIBMTR from 2000 to 2016 were included. Patients undergoing syngeneic umbilical cord blood– (n = 18) or mismatched related-donor transplantation (haplo-identical) (n = 73) and patients with missing donor data (n = 2) were excluded from this analysis, in addition to 15 patients whose graft-versus-host disease (GVHD) prophylaxis was by ex vivo T-cell depletion (n = 5) or CD34+-selection procedure (n = 10). An additional 35 patients were removed because of missing date of diagnosis, unknown GVHD prophylaxis, or missing complete 100-day follow-up data. A total of 623 patients met all inclusion criteria and composed the CIBMTR cohort and, hence, were included in the multivariate analysis. The same inclusion criteria were applied for the EBMT cohort and resulted in 2672 candidates, of whom 623 patients had complete data for the required ultimately scoring system components and were included in the multivariate analysis.
Statistical analysis
A total of 623 patients in the CIBMTR cohort were included to develop a Cox regression model, and 623 patients in the EBMT cohort were included for external validation. The scoring model and prognostic variables were constructed and selected based on the CIBMTR data, and the scoring system was then validated on the EBMT data. Ruxolitinib, a JAK1/JAK2 inhibitor, which was the first drug to gain approval by both the US Food and Drug Administration and the European Medicines Agency for treatment of MF,18 was introduced into clinical practice in 2012. We, therefore, tested whether the introduction of ruxolitinib significantly affected the potential findings from this study; 3 cohorts with respect to commercial availability of ruxolitinib were tested: patients who received transplantation before 2012, patients undergoing allo-HCT after 2012 who were treated with ruxolitinib, and patients who received transplantation after 2012 and were not treated with ruxolitinib. Because this variable and interactions with this variable were not significant, the full cohort was used for subsequent analysis. A Cox proportional hazards model with stepwise selection procedure was used to select significant covariates for overall survival (OS), at a significance level of 0.1. A raw score (0, 1, or 2) for each risk factor was assigned based on the magnitude of log hazard ratios (HRs) in the Cox model. Next, scores were calculated for each patient, and a univariable Cox model with those scores were fitted. Finally, the risk score (low, intermediate, or high) was determined based on the HRs of the univariable Cox model with the scores. The new scoring system was evaluated with the validation data set (EBMT). In the CIBMTR cohort, The new scoring system was compared with DIPSS. After construction of the risk score for the main outcome, OS, the analysis was performed for the secondary outcomes including DFS, relapse, and TRM. Probabilities of OS and DFS for each risk group were calculated using the Kaplan Meier estimator, with the variance estimated using Greenwood formula. Probabilities of TRM and relapse were generated using cumulative incidence estimates to accommodate the competing risk event. SAS version 9.4 (SAS Institute, Cary, NC) and R version 4.0 (R Foundation for Statistical Computing, Vienna, Austria) were used for the analysis.
The development of a prognostic scoring system
Variables related to patients, donors, disease, treatment before transplantation, and transplantation itself were included in the analysis (supplemental Table 1). We then constructed a Cox proportional hazards model using the CIBMTR training set that included the following nonmodifiable variables: age, hemoglobin, white blood cell count, platelet count, constitutional symptoms, circulating blasts at diagnosis and at the time of allo-HCT, sex, Karnofsky performance status before allo-HCT, time from the date of diagnosis to allo-HCT, cytogenetics abnormalities, JAK 2 mutation (present or absent), spleen status at allo-HCT, and donor type. A multivariable model identified 3 independent predictors of survival: age, hemoglobin at time of allo-HCT, and donor type.
Results
Patients
Patient data for the CIMBTR and EBMT cohorts are listed in Table 1 and supplemental Table 1. The 2 cohorts were noted with differences in patient-, disease- and transplantation-related variables. The percentage of patients for whom follow-up data were reported was 98%, 94%, 91%, and 89% at 1, 2, 3, and 4 years, respectively, in the CIBMTR cohorts. Although the cohorts included patients with primary MF and secondary MF, the majority in both cohorts were patients with primary MF: 87% in the CIBMTR and 80% in the EBMT cohorts.
Baseline characteristics for patients undergoing allo-HCT for MF from 2000 to 2016, who were included in the CIBMTR and EBMT cohorts
| Variable . | CIBMTR . | EBMT . | P value . |
|---|---|---|---|
| No. of patients | 623 | 623 | |
| Median follow-up of survivors (range), mo | 42 (3-193) | 83 (3-219) | |
| Patient related | |||
| Age at diagnosis, median (range), y | 54 (40-75) | 52 (40-74) | <.01† |
| Age at HCT, median (range), y | 58 (40-76) | 57 (41-74) | <.01† |
| Sex | .12‡ | ||
| Male | 395 (63%) | 421 (68%) | |
| Karnofsky performance status score before HCT | <.01‡ | ||
| 90-100 | 373 (60%) | 313 (50%) | |
| HCT-CI | <.01‡ | ||
| 0 | 113 (18%) | 201 (32%) | |
| 1 | 62 (10%) | 53 (9%) | |
| 2 | 67 (11%) | 41 (7%) | |
| 3+ | 174 (28%) | 84 (13%) | |
| Disease related | |||
| Disease at diagnosis | <.01‡ | ||
| MF | 542 (87%) | 499 (80%) | |
| Polycythemia vera | 32 (5%) | 52 (8%) | |
| Essential thrombocythemia | 49 (8%) | 52 (8%) | |
| Polycythemia vera/essential thrombocythemia | 20 (3%) | ||
| Blast in peripheral blood of >1% at diagnosis | 89 (14%) | 104 (17%) | .09‡ |
| Hemoglobin level <100 g/L at diagnosis | 216 (35%) | 214 (34%) | <.01‡ |
| WBC count >25 × 109/L at diagnosis | 59 (9%) | 49 (8%) | <.01‡ |
| Platelet count at diagnosis, 50 × 109/L–100 × 109/L | 80 (13%) | 87 (14%) | <.01‡ |
| Constitutional symptoms at diagnosis | 183 (29%) | 176 (28%) | <.01‡ |
| Blast in peripheral blood >1% before HCT | 188 (30%) | 200 (32%) | <.01‡ |
| Hemoglobin level <100 g/L before HCT | 442 (71%) | 411 (66%) | .06‡ |
| WBC count >25 × 109/L before HCT | 82 (13%) | 95 (15%) | <.01‡ |
| Platelet count 50 × 109/L–100 × 109/L before HCT | 133 (21%) | 107 (17%) | <.01‡ |
| Constitutional symptoms before HCT | 104 (17%) | 181 (29%) | <.01‡ |
| DIPSS before HCT | |||
| Low | 76 (12%) | ||
| Intermediate-1 | 283 (45%) | ||
| Intermediate-2 | 236 (38%) | ||
| High | 11 (2%) | ||
| Cytogenetics | |||
| Favorable (normal) | 251 (40%) | ||
| Favorable (other) | 113 (18%) | ||
| Unfavorable | 113 (18%) | ||
| Not tested | 34 (5%) | ||
| JAK2 mutation | <.01‡ | ||
| Yes | 202 (32%) | 213 (34%) | |
| Spleen status | <.01‡ | ||
| Normal | 132 (21%) | 81 (13%) | |
| Splenomegaly | 451 (72%) | 304 (49%) | |
| Splenectomy | 23 (4%) | 90 (14%) | |
| Treatment-related, nontransplantation | |||
| Prior therapy | <.01‡ | ||
| Yes | 468 (75%) | 340 (55%) | |
| Missing | 4 (1%) | 55 (9%) | |
| Number of lines of pretreatments | <.01‡ | ||
| 0 | 151 (24%) | 228 (37%) | |
| 1 | 255 (41%) | 126 (20%) | |
| 2 | 108 (17%) | 18 (3%) | |
| ≥3 | 101 (16%) | 16 (3%) | |
| Received Jakafi as prior therapy | <.01‡ | ||
| Yes | 175 (28%) | 85 (14%) | |
| Treatment-related, transplantation | |||
| Time from diagnosis to HCT (mo) | 18 (2-294) | 26 (2-268) | <.01† |
| Donor type | <.01‡ | ||
| HLA-identical sibling | 221 (35%) | 469 (75%) | |
| Well-matched unrelated | 322 (52%) | 107 (17%) | |
| Partially matched unrelated | 80 (13%) | 47 (8%) | |
| Sex match of donor and recipient | .04‡ | ||
| M-M | 257 (41%) | 253 (41%) | |
| M-F | 134 (22%) | 168 (27%) | |
| F-M | 136 (22%) | 107 (17%) | |
| F-F | 93 (15%) | 95 (15%) | |
| Graft source | .58‡ | ||
| Peripheral blood | 554 (89%) | 560 (90%) | |
| Use of TBI | .59‡ | ||
| No | 526 (84%) | 530 (85%) | |
| Conditioning regimen intensity | <.01‡ | ||
| MAC | 285 (46%) | 181 (29%) | |
| RIC | 292 (47%) | 440 (71%) | |
| NMA | 37 (6%) | ||
| GVHD prophylaxis | <.01‡ | ||
| Post-CY + other(s) | 9 (1%) | 14 (2%) | |
| TAC + MMF ± other(s) (except post-CY) | 88 (14%) | 3 (0) | |
| TAC + MTX ± other(s) (except MMF, post-CY) | 281 (45%) | 6 (1%) | |
| TAC + other(s) (except MMF, MTX, post-CY) | 32 (5%) | 7 (1%) | |
| TAC alone | 11 (2%) | 3 (0) | |
| CSA + MMF ± other(s) (except post-CY) | 63 (10%) | 193 (31%) | |
| CSA + MTX ± other(s) (except MMF, post-CY) | 111 (18%) | 258 (41%) | |
| CSA + other(s) (except MMF, MTX, post-CY) | 6 (1%) | 15 (2%) | |
| CSA alone | 12 (2%) | 82 (13%) | |
| Other(s) | 8 (1%) | 15 (2%) | |
| Median follow-up of survivors (range), mo | 42 (3-193) | 83 (3-219) |
| Variable . | CIBMTR . | EBMT . | P value . |
|---|---|---|---|
| No. of patients | 623 | 623 | |
| Median follow-up of survivors (range), mo | 42 (3-193) | 83 (3-219) | |
| Patient related | |||
| Age at diagnosis, median (range), y | 54 (40-75) | 52 (40-74) | <.01† |
| Age at HCT, median (range), y | 58 (40-76) | 57 (41-74) | <.01† |
| Sex | .12‡ | ||
| Male | 395 (63%) | 421 (68%) | |
| Karnofsky performance status score before HCT | <.01‡ | ||
| 90-100 | 373 (60%) | 313 (50%) | |
| HCT-CI | <.01‡ | ||
| 0 | 113 (18%) | 201 (32%) | |
| 1 | 62 (10%) | 53 (9%) | |
| 2 | 67 (11%) | 41 (7%) | |
| 3+ | 174 (28%) | 84 (13%) | |
| Disease related | |||
| Disease at diagnosis | <.01‡ | ||
| MF | 542 (87%) | 499 (80%) | |
| Polycythemia vera | 32 (5%) | 52 (8%) | |
| Essential thrombocythemia | 49 (8%) | 52 (8%) | |
| Polycythemia vera/essential thrombocythemia | 20 (3%) | ||
| Blast in peripheral blood of >1% at diagnosis | 89 (14%) | 104 (17%) | .09‡ |
| Hemoglobin level <100 g/L at diagnosis | 216 (35%) | 214 (34%) | <.01‡ |
| WBC count >25 × 109/L at diagnosis | 59 (9%) | 49 (8%) | <.01‡ |
| Platelet count at diagnosis, 50 × 109/L–100 × 109/L | 80 (13%) | 87 (14%) | <.01‡ |
| Constitutional symptoms at diagnosis | 183 (29%) | 176 (28%) | <.01‡ |
| Blast in peripheral blood >1% before HCT | 188 (30%) | 200 (32%) | <.01‡ |
| Hemoglobin level <100 g/L before HCT | 442 (71%) | 411 (66%) | .06‡ |
| WBC count >25 × 109/L before HCT | 82 (13%) | 95 (15%) | <.01‡ |
| Platelet count 50 × 109/L–100 × 109/L before HCT | 133 (21%) | 107 (17%) | <.01‡ |
| Constitutional symptoms before HCT | 104 (17%) | 181 (29%) | <.01‡ |
| DIPSS before HCT | |||
| Low | 76 (12%) | ||
| Intermediate-1 | 283 (45%) | ||
| Intermediate-2 | 236 (38%) | ||
| High | 11 (2%) | ||
| Cytogenetics | |||
| Favorable (normal) | 251 (40%) | ||
| Favorable (other) | 113 (18%) | ||
| Unfavorable | 113 (18%) | ||
| Not tested | 34 (5%) | ||
| JAK2 mutation | <.01‡ | ||
| Yes | 202 (32%) | 213 (34%) | |
| Spleen status | <.01‡ | ||
| Normal | 132 (21%) | 81 (13%) | |
| Splenomegaly | 451 (72%) | 304 (49%) | |
| Splenectomy | 23 (4%) | 90 (14%) | |
| Treatment-related, nontransplantation | |||
| Prior therapy | <.01‡ | ||
| Yes | 468 (75%) | 340 (55%) | |
| Missing | 4 (1%) | 55 (9%) | |
| Number of lines of pretreatments | <.01‡ | ||
| 0 | 151 (24%) | 228 (37%) | |
| 1 | 255 (41%) | 126 (20%) | |
| 2 | 108 (17%) | 18 (3%) | |
| ≥3 | 101 (16%) | 16 (3%) | |
| Received Jakafi as prior therapy | <.01‡ | ||
| Yes | 175 (28%) | 85 (14%) | |
| Treatment-related, transplantation | |||
| Time from diagnosis to HCT (mo) | 18 (2-294) | 26 (2-268) | <.01† |
| Donor type | <.01‡ | ||
| HLA-identical sibling | 221 (35%) | 469 (75%) | |
| Well-matched unrelated | 322 (52%) | 107 (17%) | |
| Partially matched unrelated | 80 (13%) | 47 (8%) | |
| Sex match of donor and recipient | .04‡ | ||
| M-M | 257 (41%) | 253 (41%) | |
| M-F | 134 (22%) | 168 (27%) | |
| F-M | 136 (22%) | 107 (17%) | |
| F-F | 93 (15%) | 95 (15%) | |
| Graft source | .58‡ | ||
| Peripheral blood | 554 (89%) | 560 (90%) | |
| Use of TBI | .59‡ | ||
| No | 526 (84%) | 530 (85%) | |
| Conditioning regimen intensity | <.01‡ | ||
| MAC | 285 (46%) | 181 (29%) | |
| RIC | 292 (47%) | 440 (71%) | |
| NMA | 37 (6%) | ||
| GVHD prophylaxis | <.01‡ | ||
| Post-CY + other(s) | 9 (1%) | 14 (2%) | |
| TAC + MMF ± other(s) (except post-CY) | 88 (14%) | 3 (0) | |
| TAC + MTX ± other(s) (except MMF, post-CY) | 281 (45%) | 6 (1%) | |
| TAC + other(s) (except MMF, MTX, post-CY) | 32 (5%) | 7 (1%) | |
| TAC alone | 11 (2%) | 3 (0) | |
| CSA + MMF ± other(s) (except post-CY) | 63 (10%) | 193 (31%) | |
| CSA + MTX ± other(s) (except MMF, post-CY) | 111 (18%) | 258 (41%) | |
| CSA + other(s) (except MMF, MTX, post-CY) | 6 (1%) | 15 (2%) | |
| CSA alone | 12 (2%) | 82 (13%) | |
| Other(s) | 8 (1%) | 15 (2%) | |
| Median follow-up of survivors (range), mo | 42 (3-193) | 83 (3-219) |
Nonmodifiable variables are indicated in italic.
CSA, cyclosporine; Cy, cyclophosphamide; HCT-CI, HCT-specific comorbidity index; MAC, myeloablative conditioning; MMF, mycophenolate mofetil; MTX, methotrexate; NMA, nonmyeloablative; RIC, reduced intensity conditioning; TAC, tacrolimus; TBI, total body irradiation; WBC, white blood cell.
∗Missing data are presented in the supplemental Tables.
Kruskal Wallis test.
Pearson χ2 test.
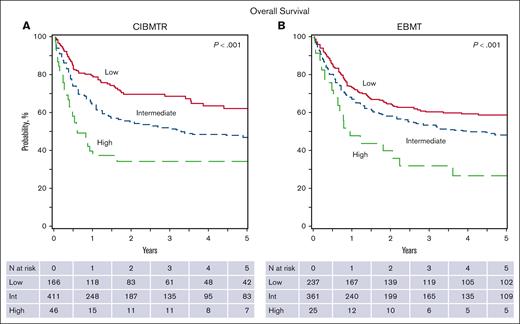
The OS rates at 1,3, and 5 years were 65.7% (95% confidence interval [CI], 61.9-69.4), 54.6% (95% CI, 50.4-58.7), and 49.9% (95% CI, 45.5-54.3), respectively (Figure 1). DFS at 1, 3, and 5 years were 39.7% (95% CI, 35.7-43.7), 31.1% (95% CI, 27.3-34.9), and 26.5% (95% CI, 22.7-30.5), respectively. Rates of TRM at 1, 3, and 5 years were 20.6% (95% CI, 17.4-23.9), 24.7% (95% CI, 21.3-28.3), and 27.1% (95% CI, 23.5-31), respectively. The relapse rates at 1, 3, and 5 years were 39.7% (95% CI, 35.8-43.6), 44.2% (95% CI, 40.2-48.3), and 46.3% (95% CI, 42.2-50.5), respectively, in the CIMBTR cohort.
Prognostic scoring system
Table 2 summarizes the variables relevant to OS identified in the multivariable analysis of data of the patients in the CIBMTR cohort. Based on an HR ≥1.5, a weighted score of 2 was assigned to a hemoglobin level <100 g/L at the time of allo-HCT and having a MMURD, whereas other factors were assigned a score of 0 or 1, based on an HR ≤1 and from 1 to 1.5. The overall score ranged from 0 to 5, with increasing scores indicating greater risk (supplemental Table 2). Based on HR of the univariate Cox model with raw scores, we created a 3-category system: low, score from 1 to 2 (HR, 1-1.5); intermediate, score from 3 to 4 (HR, 1.5-2.5); and high, score from 5 (HR, >2.5).
Multivariable analysis
| OS (CIBMTR) . | . | 95% CI . | 95% CI . | . | Overall . | . | . |
|---|---|---|---|---|---|---|---|
| Age (y) at HCT . | HR . | Lower limit . | Upper limit . | P value . | P value . | n . | . |
| ≥50 | 1 | .0694 | 86 | 0 | |||
| >50 | 1.382 | 0.975 | 1.96 | .0694 | 537 | 1 | |
| Hemoglobin before HCT | |||||||
| ≥100 g/L | 1 | .0011 | 148 | 0 | |||
| <100 g/L | 1.631 | 1.217 | 2.185 | .0011 | 475 | 2 | |
| Donor type | |||||||
| HLA-identical siblings | 1 | .0048 | 221 | 0 | |||
| Well-matched URD | 1.284 | 0.997 | 1.654 | .0527 | 322 | 1 | |
| Partially matched URD | 1.776 | 1.251 | 2.521 | .0013 | 80 | 2 | |
| Contrast | |||||||
| Well-matched URD vs partially matched URD | 0.7232 | 0.5197 | 1.0065 | .0546 |
| OS (CIBMTR) . | . | 95% CI . | 95% CI . | . | Overall . | . | . |
|---|---|---|---|---|---|---|---|
| Age (y) at HCT . | HR . | Lower limit . | Upper limit . | P value . | P value . | n . | . |
| ≥50 | 1 | .0694 | 86 | 0 | |||
| >50 | 1.382 | 0.975 | 1.96 | .0694 | 537 | 1 | |
| Hemoglobin before HCT | |||||||
| ≥100 g/L | 1 | .0011 | 148 | 0 | |||
| <100 g/L | 1.631 | 1.217 | 2.185 | .0011 | 475 | 2 | |
| Donor type | |||||||
| HLA-identical siblings | 1 | .0048 | 221 | 0 | |||
| Well-matched URD | 1.284 | 0.997 | 1.654 | .0527 | 322 | 1 | |
| Partially matched URD | 1.776 | 1.251 | 2.521 | .0013 | 80 | 2 | |
| Contrast | |||||||
| Well-matched URD vs partially matched URD | 0.7232 | 0.5197 | 1.0065 | .0546 |
The HR for death (using the low-risk group as reference) was 1.64 (95% CI, 1.23-2.18) for the intermediate-risk group, and 2.65 (95% CI, 1.70-4.14) for the high-risk group (overall P = .0002; Table 3). This 3-category system was predictive for DFS with a HR for death of 1.44 (95% CI, 1.14-1.81) for the intermediate-risk group, and 1.83 (95% CI, 1.24-2.71) for the high-risk group (overall P = .0015; supplemental Table 3) and for TRM as well; the HR for death was 1.63 (95% CI, 1.10-2.44) for the intermediate-risk group, and 3.09 (95% CI, 1.75-5.48) for the high-risk group (overall P = .0017; supplemental Table 3). This prognostic system was not predictive of relapse.
OS by prognostic score in CIMBTR and EBMT cohorts
| CIBMTR . | ||||||
|---|---|---|---|---|---|---|
| . | . | 95% CI . | 95% CI . | . | Overall . | . |
| Rank . | HR . | Lower limit . | Upper limit . | P value . | P value . | n . |
| Low | 1 | Reference | .0002 | 166 | ||
| Intermediate | 1.636 | 1.226 | 2.183 | .0008 | 411 | |
| High | 2.649 | 1.695 | 4.138 | < .0001 | 46 | |
| Contrast | ||||||
| Intermediate vs high | 0.6177 | 0.4186 | 0.9114 | .0152 | ||
| CIBMTR . | ||||||
|---|---|---|---|---|---|---|
| . | . | 95% CI . | 95% CI . | . | Overall . | . |
| Rank . | HR . | Lower limit . | Upper limit . | P value . | P value . | n . |
| Low | 1 | Reference | .0002 | 166 | ||
| Intermediate | 1.636 | 1.226 | 2.183 | .0008 | 411 | |
| High | 2.649 | 1.695 | 4.138 | < .0001 | 46 | |
| Contrast | ||||||
| Intermediate vs high | 0.6177 | 0.4186 | 0.9114 | .0152 | ||
| EBMT . | ||||||
|---|---|---|---|---|---|---|
| . | . | 95% CI . | 95% CI . | . | Overall . | . |
| Rank . | HR . | Lower limit . | Upper limit . | P value . | P value . | n . |
| Low | 1 | Reference | .0011 | 237 | ||
| Intermediate | 1.336 | 1.054 | 1.694 | .0167 | 361 | |
| High | 2.353 | 1.442 | 3.839 | .0006 | 25 | |
| Contrast | ||||||
| Intermediate vs high | 0.5678 | 0.3544 | 0.9097 | .0186 | ||
| EBMT . | ||||||
|---|---|---|---|---|---|---|
| . | . | 95% CI . | 95% CI . | . | Overall . | . |
| Rank . | HR . | Lower limit . | Upper limit . | P value . | P value . | n . |
| Low | 1 | Reference | .0011 | 237 | ||
| Intermediate | 1.336 | 1.054 | 1.694 | .0167 | 361 | |
| High | 2.353 | 1.442 | 3.839 | .0006 | 25 | |
| Contrast | ||||||
| Intermediate vs high | 0.5678 | 0.3544 | 0.9097 | .0186 | ||
Considering that spleen size and splenectomy are a matter of debate regarding transplantation outcomes in patients with MF, we examined size of a healthy spleen vs one with splenomegaly vs with splenectomy. Patients who underwent a splenectomy had worse OS, but there was no difference in survival when comparing patients with splenomegaly vs those with normal spleen size. Yet, a very small proportion of patients in this analysis had a splenectomy (4% in the CIBMTR cohort and 14% of the EBMT cohort), which limits the ability to draw conclusions on the role of splenectomy in outcomes in these cohorts.
Application of CIBMTR scoring system to the EBMT cohort for validation
The prognostic score was applied to an external EBMT cohort for validation. Analyzed data within this data set comprised data from 623 patients for whom complete data were available. When compared with the CIBMTR cohort, the 1-, 3-, and 5-year OS were 68.6% (95% CI, 64.9-72.2), 55.0% (95% CI, 51.0-58.9), and 51.2% (95% CI, 47.1-55.2) in the EBMT cohort, respectively (Figure 1). In the EBMT cohort, the 3-year incidence of TRM was 27.9% (95% CI, 24.4-31.6), and relapse was 24.3% (95% CI, 20.9-27.8). The 3-category system was prognostic of OS (P = .0011), DFS (P = .0007), and TRM (P = .0021), and, again, was not predictive of relapse (P = .1673; supplemental Table 4). Because of a significantly longer follow-up time in the EBMT cohort (83 vs 42 months in the CIBMTR cohort), a sensitivity analysis was performed on the EBMT cohort, censoring the data at 42 months (supplemental Table 5), showing similar results.
Comparison of proposed scoring system with DIPSS score
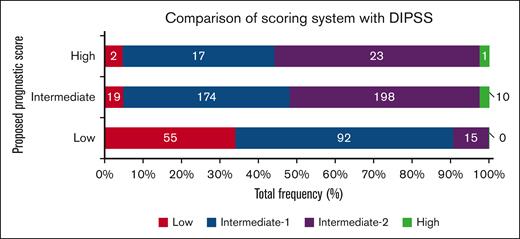
To study how the proposed new scoring system compares with the DIPSS prognostic tool, we generated a cross table with the proposed score (rows) and the DIPSS classification (Figure 2). This included a total of 606 patients from the CIBMTR cohort for whom a DIPSS score was available. This comparison showed agreement on patients classified as being at low and high risk based on the DIPSS score; however, patients characterized as being at intermediate-1 and intermediate-2 risk were distributed among all risk categories in the new proposed model. The performance of the new scoring system is favorable compared with the DIPSS as indicated by the differences seen in the HRs and the c-statistics when the follow-up was censored at 36 months after transplantation. (supplemental Table 6).
Overall survival in the CIBMTR and EBMT cohorts by the prognostic scoring system classification. (A) CIBMTR; (B) EBMT.
Overall survival in the CIBMTR and EBMT cohorts by the prognostic scoring system classification. (A) CIBMTR; (B) EBMT.
Categorization of patients based on the proposed scoring system vs the DIPSS. Colored bars represent the DIPSS on the x-axis, within the stratification based on the new scoring system in rows.
Categorization of patients based on the proposed scoring system vs the DIPSS. Colored bars represent the DIPSS on the x-axis, within the stratification based on the new scoring system in rows.
Study limitations
This study included patients treated over a long time period, from 2000 to 2016, a period during which there had been changes and advances in the field of stem cell transplantation, whether related to HLA typing, prevention, and management of GVHD; improvement in detection and treatment of infectious diseases; and the introduction of new conditioning regimens that are not captured in this analysis. Of particular importance are the increased use of haplo-identical donors, which were excluded from this analysis because of very small numbers and will require follow-up studies, and the role of molecular mutations in prognostic scores of transplantations for MF. Furthermore, differences in practices between the United States and Europe as well insurance policies that affects transplantation referrals are beyond the scope of this analysis but need to be acknowledged. Lastly, the CIBMTR cohort did not have any patients with a score of 0, so no conclusions could be drawn from that group of patients.
Discussion
In this large analysis, using CIBMTR and EBMT cohorts, we present a new scoring tool prognostic of outcomes in patients with MF undergoing allo-HCT. Using a multivariate analysis, we identified 3 simple and clinically relevant variables: patient age, pretransplantation hemoglobin level, and donor type, to be prognostic for OS, DFS, and TRM.
The current available prognostic scoring systems such as the DIPSS, the DIPSS-plus, and, more recently, the mutation-enhanced international prognostic score system for transplantation-age patients with primary myelofibrosis (MIPSS-70),19 were validated for patients with primary MF and can discriminate clearly between patients with MF who are at low or intermediate risk or with poor prognosis. Several studies using these prognostic systems in transplantation cohorts have found them to be predictive, as expected, based on disease biology; however, the effects of transplantation-related factors, such as conditioning intensity and type of donors varied in the different studies.20,21 Here, we note that the proposed system correlated well with the low- and high-risk DIPSS groups; however, for patients in the intermediate-1 and intermediate-2 risk groups, the new proposed system reassigned them to a different group in 45% of intermediate-1 risk group cases and in 16% of intermediate-2 risk group cases. This is particularly important in cases for which the DIPSS score is predictive of worse transplantation outcomes; for the same patients, if an HLA-matched related donor is available, the new score would be intermediate and associated with better outcomes than what the DIPSS scoring system would predict. In contrast, for patients with favorable outcomes based on the DIPSS score, if an unrelated donor is the only option, transplantation outcomes are predicted to be worse. This comparison between the DIPSS and the new transplantation-specific scoring system highlights that specific transplantation-related factors and, particularly, the graft source (ie, donor) are important and can overcome high-risk disease features. The findings, however, do not suggest that one scoring system is superior to the other but, rather, that they complement each other.
In this analysis, having a HLA-matched related donor was associated with better outcomes compared with both HLA-MURD and mismatched donors, which has been reported previously in patients with MF undergoing allo-HCT.22-24 At the same time, the use of alternative donors such as haplo-identical donors and mismatched unrelated donors is growing rapidly.25 A recent study by Kunte et al26 reported overall good outcomes among 69 patients with MF who received transplantation using haplo-identical donors; at 3 years, the OS, relapse free survival, and GVHD-free relapse free survival were 72% (95% CI, 59-81), 44% (95% CI, 29-59), and 30% (95% CI, 17-43), respectively. Of note, the majority of patients included within this study (80%) received transplantation after 2016, with data being suggestive of changes in trends in recent years.
The impact of the spleen size, and intervention to reduce the spleen size via splenic radiation or splenectomy, on transplantation outcomes remain a matter of debate. A large analysis from EBMT27 that included 1195 patients with MF who received transplantation between 2000 and 2017 reported that splenectomy was associated with lower rates of TRM but increased risk of relapse, without a significant effect on OS. This analysis highlights that, although pretransplant splenectomy was done more commonly in the early years (in 28.3% before 2009 compared with in 14.1% after 2009), the numbers decreased further after the introduction of ruxolitinib in 2012. A very small proportion of patients in this analysis had a splenectomy, which limits the ability to draw conclusions on the role of splenectomy in outcomes in this cohort. Data suggest that the treatment with ruxolitinib before transplantation improves outcomes, whether by its effects on inflammatory milieu or by effect on spleen size,28 and further studies to address this question in the era of JAK-STAT inhibitors are needed.
More recently, next-generation sequencing has been incorporated into contemporary predictive models for MF and several studies have evaluated its prognostic power in the context of allo-HCT as well. In a study by Gagelmann et al29 incorporating clinical, molecular, and transplant-related factors to create the MF transplantation scoring system, patient age and unrelated donor (matched or mismatched) were predictive for outcomes similar to the findings from our analysis. The MF transplantation scoring system included data on molecular mutations; the presence of mutations in ASXL1 gene and non-CALR/MPL driver mutations genotype were predictive of outcomes, whereas a high number of mutations (>3) and the presence of so-called high-risk mutations were not. Several other retrospective analyses evaluated the role of molecular mutations and outcomes of patients with MF undergoing allo-HCT with conflicting conclusions.30-33 Our proposed system lacks any data on molecular mutations because such data were not available in the earlier years of this study period nor collected systematically until very recently. However, the conflicting findings regarding the prognostic power of molecular mutations in the context of allo-HCT, and the lack of standardization of next-generation sequencing assays with relation to the genes involved in different panels, the levels of detection that are considered pathogenic, validation, and clinical interpretation34 suggest that further and larger studies are needed to determine the prognostic role of mutational analysis in predicting transplant outcomes in patients with MF.
In summary, despite significant differences between the 2 cohorts, the proposed model was predictive of outcomes in these 2 large data sets. As highlighted earlier, we acknowledge several limitations, which are inherent to the nature of the study being a retrospective analysis of registry-reported data. Despite this, this analysis resulted in a simple, clinically relevant, and easily applicable score that, in addition to the current available risk scores, may help in the counseling of patients with MF undergoing allo-HCT.
Acknowledgments
The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute, the National Heart, Lung, and Blood Institute, and the National Institute of Allergy and Infectious Diseases; HHSH250201700006C from the Health Resources and Services Administration; and N00014-20-1-2832 and N00014-21-1-2954 from the Office of Naval Research. Support is also provided by Be the Mazak Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, and from the following commercial entities: AbbVie, Actinium Pharmaceuticals Inc, Adaptive Biotechnologies Corporation, ADC Therapeutics, Adienne SA, Allogene, Allovir Inc, Amgen Inc, Anthem, Astellas Pharma US, AstraZeneca, Atara Biotherapeutics, BeiGene, bluebird bio Inc, Bristol Myers Squibb Co, CareDx Inc, CRISPR, CSL Behring, CytoSen Therapeutics Inc, Eurofins Viracor, DBA Eurofins Transplant Diagnostics, Fate Therapeutics, Gamida-Cell Ltd, Gilead, GlaxoSmithKline, HistoGenetics, Incyte Corporation, Iovance, Janssen Research & Development LLC, Janssen/Johnson & Johnson, Jasper Therapeutics, Jazz Pharmaceuticals Inc, Kadmon, Karius, Kiadis Pharma, Kite, Kyowa Kirin, Legend Biotech, Magenta Therapeutics, Mallinckrodt Pharmaceuticals, Medac GmbH, Medexus Pharma, Merck & Co, Millennium, the Takeda Oncology Co, Miltenyi Biotec Inc, MorphoSys, Novartis Pharmaceuticals Corporation, Omeros Corporation, OptumHealth, Orca Biosystems Inc, Ossium Health Inc, Pfizer Inc, Pharmacyclics LLC, Priothera, Sanofi, Sanofi-Aventis US Inc, Sobi Inc, Stemcyte, Takeda Pharmaceuticals, Talaris Therapeutics, Terumo Blood and Cell Technologies, TG Therapeutics, Vertex Pharmaceuticals, and Xenikos BV.
Authorship
Contribution: R.T., K.W.A., N.E.-M., and W.S. conceptualized and designed the study; CIBMTR provided financial support and performed data collection and assembly; R.T., K.W.A., N.E.-M., and W.S. performed data analysis; R.T. and W.S. wrote the manuscript; and all authors interpreted the data and approved the final version of the manuscript.
Conflict-of-interest disclosure: M.B. reports research support to the institute from Novartis. V.G. reports consultancy work for Novartis, Incyte, Bristol Myers Squibb (BMS)-Celgene, Sierra Oncology, MorphoSys, Pfizer, and Takeda, and received a research grant through the institution from Novartis and Incyte. H.A. reports serving on the advisory board and speaker bureau for Incyte and BMS. U.G. reports payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing, or education events. C.B. reports serving as an advisory board member of Kite and Novartis (unrelated to this study). D.R. reports serving as a speaker bureau and advisory boards for Incyte. S.G. reports as speaker for Seattle Genetics and Kite Pharma, and advisory board for Sanofi, BMS, Daiichi Sankyo, Astellas, Janssen, and AstraZeneca. T.J. reports institutional research support from CTI Biopharma, Syneos Health, Incyte, and advisory board participation with BMS, Incyte, and CTI. M.A.K.-D. reports consultancy for Daiichi Sankyo. J.A.Y. reports honorarium for 1-time advisory board meeting with Kite and Omeros, and research funding from Gilead. M.R.G. reports consulting fees from and advisory board involvement at AbbVie, Agios, Amgen, Astellas, Blueprint Medicines, BMS, Cardinal Health, CTI Biopharma, Daiichi Sankyo, Gamida-Cell, Gilead, Incyte, Invitae, Karius, Ono Pharmaceutical, Pfizer, Pharmacosmos, Premier, Sierra Oncology, Stemline, and Trovagene; stock ownership with Medtronic; medical writing for Incyte, Amgen, Jazz, Janssen, and Genentech/Roche; and research support from Incyte, Genetech/Roche, and Janssen. T.N. reports clinical trial research support from Novartis to the institution and clinical trial support (drug only supply) from Karyopharmto the institution. The remaining authors declare no competing financial interests.
Correspondence: Roni Tamari, Department of Medicine, Bone Marrow Transplant Service, Memorial Sloan Kettering Cancer Center Department of Medicine, Weill Cornell Medical College, David H. Koch Center, 530 E 74th St, New York, NY 10021; e-mail: tamarir@mskcc.org.
References
Author notes
CIBMTR supports accessibility of research in accord with the National Institutes of Health data sharing policy and the National Cancer Institute Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases de-identified datasets that comply with all relevant global regulations regarding privacy and confidentiality.
Data are available on request from the corresponding author, Roni Tamari (tamarir@mskcc.org).
The full-text version of this article contains a data supplement.