Key Points
In young low-risk patients with DLBCL (aaIPI 0-1), CNS relapse remains a rare event regardless of frontline therapy and prophylaxis.
In young high-risk patients with DLBCL (aaIPI 2-3), intensified therapy including agents crossing the BBB leads to low rates of CNS relapse.
Abstract
Most patients with diffuse large B-cell lymphoma (DLBCL) can be cured with immunochemotherapy such as R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone). Patients with progression or relapse in the central nervous system (CNS) face dismal outcomes. The impact of more aggressive regimens used in frontline therapy has not been systematically investigated in this context. To this end, we analyzed a large cohort of 2203 younger patients with DLBCL treated on 10 German (German Lymphoma Alliance [GLA]/The German High Grade Non-Hodgkin's Lymphoma Study Group [DSHNHL]) and French (The Lymphoma Study Association [LYSA]) prospective phase 2 and 3 trials after first-line therapy with R-CHOP, R-CHOEP (R-CHOP + etoposide), dose-escalated R-CHOEP followed by repetitive stem cell transplantation (R-MegaCHOEP), or R-ACVBP (rituximab, doxorubicin, cyclophosphamide, vindesine, bleomycine, and prednisone) followed by consolidation including multiple drugs crossing the blood-brain barrier (BBB). Patients with DLBCL with an age-adjusted International Prognostic Index (aaIPI) of 0 to 1 showed very low cumulative incidence rates of CNS relapse regardless of first-line therapy and CNS prophylaxis (3-year cumulative incidences 0%-1%). Younger high-risk patients with aaIPI of 2 to 3 had 3-year cumulative incidence rates of 1.6% and 4% after R-ACVBP plus consolidation or R-(Mega)CHO(E)P, respectively (hazard ratio 2.4; 95% confidence interval: 0.8-7.4; P = .118). Thus, for younger high-risk patients, frontline regimens incorporating agents crossing the BBB may reduce often fatal CNS relapse.
Introduction
Relapse in the central nervous system (CNS) is an important cause of treatment failure in patients with diffuse large B-cell lymphoma (DLBCL).1 Patients experiencing CNS relapse continue to show dismal outcomes, emphasizing the unmet need to better understand and prevent CNS relapse. We recently reported that in younger patients with high-risk aggressive B-cell lymphoma (age-adjusted International Prognostic Index [aaIPI] of 2 or 3) failing conventional or high-dose (HD) chemotherapy, up to one-third of all progressions and relapses occurred in the CNS, highlighting that to improve results of modern DLBCL therapy, better prognostication of CNS relapse risk remains of paramount importance.2 The now widely used CNS International Prognostic Index (CNS-IPI), based on simple clinical parameters (age >60 years, lactate dehydrogenase [LDH] greater than normal, Eastern Cooperative Oncology [ECOG] performance status [PS] >1, advanced stage, extranodal involvement >1, and involvement of kidney and/or adrenal glands) and developed in patients treated with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone), defines 3 risk groups (low-, intermediate-, and high-risk) featuring CNS relapse rates between 0.6% and 10.2% at 2 years.1 In addition to these clinical risk factors, molecular subtyping of the lymphoma may help to improve the identification of patients with DLBCL at high risk for relapse in the CNS.3,4 Beyond all models, consequent imaging of the brain and flow cytometry of the cerebrospinal fluid (CSF) in any high-risk patient remain important diagnostic tools. More sensitive technologies, including the search for cell-free tumor DNA in the CSF, may foster the early detection of CNS involvement.5
Although prophylactic intrathecal (IT) injections of methotrexate (MTX) (± cytarabine and prednisolone) are of limited if any effect to prevent CNS relapse,6-8 systemic administration of HD MTX and other cytotoxic drugs (eg, cytarabine, ifosfamide, and etoposide) crossing the blood-brain barrier (BBB) seemed more promising.7,9 However, randomized studies comparing prophylactic strategies have not been reported, and more recent analyses shed doubt on whether IV HD MTX is more effective than IT MTX or has any preventive effect at all.10-12
Whether first-line therapy other than standard R-CHOP influences the frequency of CNS relapse has not been thoroughly addressed. In the prerituximab era, Bernstein et al were unable to demonstrate that aggressive multiagent chemotherapy (methotrexate, bleomycin, doxorubicin, cyclophosphamide, vincristine, and dexamethasone [m-BACOD], prednisone, methotrexate, doxorubicin, cyclophosphamide, etoposide, cytarabine, bleomycin, vincristine, methotrexate [ProMACE-CytaBOM], methotrexate, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin [MACOP-B]) significantly reduced the frequency of CNS relapse as compared with patients treated with CHOP.13 In 2003, the French study group reported that patients treated with the ACVBP (doxorubicin, cyclophosphamide, vindesine, bleomycine, and prednisone) regimen experienced significantly fewer CNS relapses than patients treated with CHOP,14 whereas Boehme et al for the German high-grade lymphoma study group showed that the addition of etoposide to CHOP (CHOEP) significantly reduced the number of CNS relapses.15 With the advent of rituximab, we and others showed that the addition of rituximab to CHOP reduced the risk of CNS relapse, albeit moderately.6,16 Whether more aggressive systemic chemotherapy in combination with rituximab (R-CHOEP, R-ACVBP) compared with standard R-CHOP reduces the risk of CNS relapse has not been investigated. R-CHOEP, combining drugs identical to dose-adusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (DA-EPOCH-R), albeit given at different doses and routes of administration continues to be used in Germany and Scandinavian countries,2,17 whereas R-ACVBP combining an induction phase of R-ACVBP with a consolidation phase comprising HD MTX, rituximab, etoposide, ifosfamide, and cytarabine, is used in the French-speaking world.18,19 Here, we report on the risk of CNS relapse in large cohorts of younger patients from prospective phase 2 and 3 trials treated with R-CHOP, R-CHOEP, or R-ACVBP and compare the incidence and quality of CNS relapses.
Methods
Patients and treatment
This study represents a joint analysis of the French Lymphoma Study Alliance (LYSA) and the German Lymphoma Alliance (GLA), formerly the Deutsche Studiengruppe Hochmaligne Non-Hodgkin Lymphome (DSHNHL). All patients in this analysis were treated on prospective clinical trials LNH03-1B,20 LNH03-2B,18 LNH03-3B,21 LNH07-3B,22 FLYER,23 MInT,24 UNFOLDER,25 MegaCHOEP phase 226 and phase 3,27 and DENSE-R-MegaCHOEP28 launched by LYSA or GLA/DSHNHL.
Patients who are HIV-negative with newly diagnosed DLBCL, aged between 18 and 60 years, covering all risk groups of the aaIPI were included in this analysis. Patients with CNS involvement at diagnosis as well as patients with a history of transformed lymphoma were excluded. CNS involvement was diagnosed if patients presented with typical clinical symptoms and/or imaging suggested brain lesions compatible with CNS lymphoma and/or lymphoma cells were detected in the CSF. In German trials, CNS staging at initial diagnosis was not mandatory, but it was performed in case of any clinical signs and symptoms. In French trials, initial diagnostics included CSF cytology.
The respective study designs, including the modalities used to prevent CNS relapse, are summarized in supplemental Table 1. In brief, first-line therapy comprised 4 to 8 courses of standard CHOP or 6 to 8 cycles of CHOEP given at 2- or 3-week intervals in combination with rituximab. In the R-MegaCHOEP trial, patients randomized to HD therapy received 4 courses of dose-escalated CHOEP (MegaCHOEP), necessitating the infusion of autologous hematopoietic stem cells in combination with rituximab.27 Patients on DSHNHL studies received CNS prophylaxis with IT MTX, including CSF analysis only if bone marrow (BM), testes, or lymph nodes in the upper neck or head were involved.
The R-ACVBP regimen comprised 3 or 4 courses of R-ACVBP followed by consolidation, depending on the risk profile of patients. Patients with IPI 0 or 1 received consolidation with 2 cycles of HD MTX (3 g/m2), 4 cycles of rituximab (375 mg/m2), etoposide (300 mg/m2), and ifosfamide (1500 mg/m2), followed by 2 cycles of cytarabine (100 mg/m2) subcutaneously for 4 days.18,20 Patients with aaIPI of 2 to 3 received 2 cycles of HD MTX followed by BEAM (carmustine, etoposide, cytarabine, and melphalan) and autologous stem cell transplantation (ASCT) for consolidation.21,22 Patients treated with R-ACVBP were to receive 4 prophylactic IT injections of 15 mg of MTX during courses 1 to 4 of systemic chemotherapy, including CSF analysis.
In all LYSA and DSHNHL/GLA trials included, patients received regular follow-up, and the site of relapse was reliably captured in the case report forms, including a precise description of potential CNS manifestation. Imaging of the CNS and CSF analysis during follow-up were not regularly performed but were initiated in case of any new neurological symptoms. Baseline patient characteristics, treatment, and outcome details were retrieved from the LYSA data files at the Lymphoma Academic Research Organisation and the DSHNHL data center at the Institute for Medical Informatics, Statistics, and Epidemiology, University of Leipzig, Leipzig, Germany. All patients gave informed consent. This analysis was done according to the Declaration of Helsinki and guidelines of Good Clinical Practice.
Statistical analysis
Progression-free survival (PFS), defined as time from study inclusion (randomization/registration) to progression, relapse, or death from any cause; and overall survival (OS), defined as time from inclusion to death from any cause, were estimated according to Kaplan-Meier, and differences between groups were compared by log-rank test. Kaplan-Meier estimates at 3 years, with 95% confidence intervals (CIs), were calculated. Multivariable analyses were done using Cox proportional hazard models adjusted for the factors of the aaIPI (LDH greater than normal, ECOG PS >1, stage III/IV), extralymphatic involvement, sex, B symptoms, BM involvement, and bulky disease >10 cm.
Any CNS involvement, with or without progression or relapse at other localizations, is defined a CNS event. CNS events occurred as first progression/relapse (CNS relapse) or as later event after involvement of other regions. In case of combined relapse (involvement of CNS and non-CNS manifestations), all lymphoma manifestations were detected at the same time point. The cumulative incidence curves of CNS relapse were calculated with “CNS relapse” as event and PFS events without CNS involvement as competing events. All other patients were censored with PFS time. We only included CNS events as part of the first progression/relapse in the analysis of cumulative incidences because we aim particularly to uncover the impact of frontline therapy on the occurrence of CNS relapse. Three-year cumulative incidence rates are presented. Analyzing the time to CNS relapse for patients with aaIPI of 2 to 3, cause-specific hazard models were used, considering competing risk events adjusted for ECOG >1, >1 extralymphatic involvement, sex, B symptoms, BM involvement, bulky disease >10 cm, and additionally for involvement of kidney and/or adrenal gland. Analyses were performed for aaIPI groups (0, 1, and 2-3) separately to investigate the treatment effects (R-ACVBP vs R-CHOEP) in comparable trial populations. A sensitivity analysis was done for aaIPI of 2 to 3, excluding patients from LNH07-3B because this study followed a positron emission tomography (PET)–guided approach to selecting therapy. All analyses were intent-to-treat.
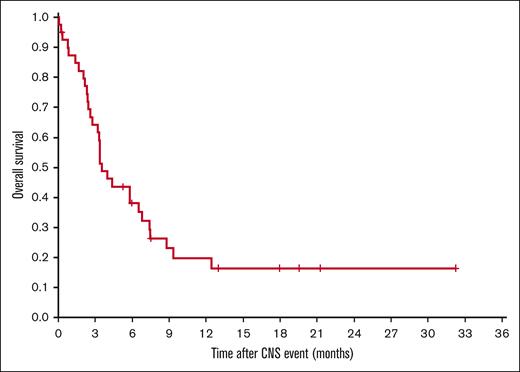
OS after a CNS event, defined as time from the CNS event to death from any cause, was estimated according to Kaplan-Meier, and 1-year rates with 95% CI are presented. For comparison of patient characteristics, χ2 and, if necessary, Fisher exact test were used. For comparison of ages, Mann-Whitney U test was used. The 2-sided significance level was set at P < .050. No adjustments were made for multiple comparisons. Statistical analyses were performed with IBM SPSS Statistics 26 and 28 software (SPSS, Chicago, IL) and R (version 3.6.0, package ‘cuminc’).
Results
Survival analysis
A total of 2203 patients with DLBCL between 18 and 60 years old treated in German and French clinical phase 2 or 3 trials were included in this analysis. Study designs are summarized in supplemental Table 1. Overall, 455 French patients were treated with R-ACVBP including consolidation, 1304 patients from France and Germany received R-CHOP at 2- or 3-week intervals, and 444 patients from Germany received R-CHOEP (n = 305) or R-MegaCHOEP (n = 139) (Table 1). Because PFS and OS did not show significant differences, patients treated with R-CHOEP or R-MegaCHOEP were analyzed together.
Overview of included patients according to aaIPI and first-line therapy
| Number of patients . | R-ACVBP (n = 455) . | R-CHOP (n = 1304) . | R-CHOEP (n = 444) . |
|---|---|---|---|
| aaIPI of 0 without bulk (n = 652) | |||
| LNH03-1B | 76 | ||
| FLYER | — | 399 | |
| MInT | — | 58 | 55 |
| UNFOLDER | — | 64 | — |
| aaIPI of 1 (n = 924) | |||
| LNH03-2B | 134 | 134 | — |
| MInT | — | 105 | 85 |
| UNFOLDER | — | 466 | — |
| aaIPI of 2-3 (n = 627) | |||
| LNH03-3B | 164 | — | — |
| LNH07-3B∗ | 81 | 78 | |
| MegaCHOEP phase 2 | — | — | 47 |
| MegaCHOEP phase 3 | — | — | 189 |
| DENSE-R-MegaCHOEP | — | — | 68 |
| Number of patients . | R-ACVBP (n = 455) . | R-CHOP (n = 1304) . | R-CHOEP (n = 444) . |
|---|---|---|---|
| aaIPI of 0 without bulk (n = 652) | |||
| LNH03-1B | 76 | ||
| FLYER | — | 399 | |
| MInT | — | 58 | 55 |
| UNFOLDER | — | 64 | — |
| aaIPI of 1 (n = 924) | |||
| LNH03-2B | 134 | 134 | — |
| MInT | — | 105 | 85 |
| UNFOLDER | — | 466 | — |
| aaIPI of 2-3 (n = 627) | |||
| LNH03-3B | 164 | — | — |
| LNH07-3B∗ | 81 | 78 | |
| MegaCHOEP phase 2 | — | — | 47 |
| MegaCHOEP phase 3 | — | — | 189 |
| DENSE-R-MegaCHOEP | — | — | 68 |
PET-guided trial.
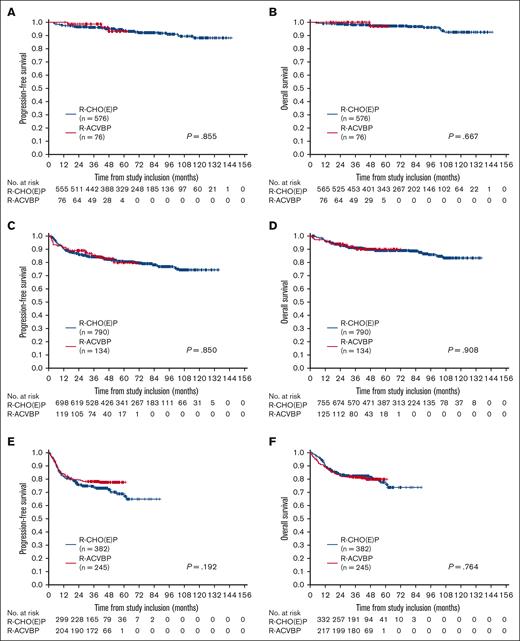
First, we compared PFS and OS according to aaIPI risk groups and first-line therapy (Figure 1). For patients with aaIPI of 0 and no bulk, 3-year PFS rates were 99% (95% CI, 96%-100%) and 96% (95% CI, 94%-98%) after R-ACVBP (n = 76) and R-CHO(E)P (n = 576), respectively, without any significant differences between treatment groups. The 3-year OS rates were 100% and 98% (95% CI, 97%-99%), respectively. (Figure 1A-B). For patients with aaIPI of 1, 3-year PFS and OS rates were estimated at 85% (95% CI, 79%-92%)/84% (95% CI, 82%-87%) and 91% (95% CI, 86%-96%)/91% (95% CI, 89%-93%) after R-ACVBP (n = 134) and R-CHO(E)P (n = 790), respectively (Figure 1C-D). Patients with aaIPI of 2 to 3 showed 3-year PFS rates of 78% (95% CI, 73%-83%) and 74% (95% CI, 69%-78%) and 3-year OS rates of 81% (95% CI, 76%-86%) and 82% (95% CI, 78%-86%) after first-line therapy with R-ACVBP (n = 245) or R-(Mega)CHO(E)P (n = 382), respectively (Figure 1E-F). In multivariable analyses adjusted for extralymphatic involvement, sex, B symptoms (aaIPI = 0); elevated LDH, stage III/IV, extralymphatic involvement, sex, B symptoms, BM involvement, and bulky disease (aaIPI = 1); or ECOG >1, extralymphatic involvement, sex, B symptoms, BM involvement, and bulky disease (aaIPI = 2-3); there was no significant difference in PFS and OS according to treatment arms. A separate analysis of the 3 treatment strategies (R-ACVBP, R-CHOP, and R-CHOEP) showed comparable results (supplemental Figure 1; supplemental Table 2).
Survival analysis. PFS (A,C,E) and OS (B,D,F) for patients with aaIPI of 0 without bulk (A-B), aaIPI of 1 (C-D), and aaIPI of 2 to 3 (E-F) according to first-line therapy (R-ACVBP vs R-CHO(E)P). Log-rank P values are presented.
Survival analysis. PFS (A,C,E) and OS (B,D,F) for patients with aaIPI of 0 without bulk (A-B), aaIPI of 1 (C-D), and aaIPI of 2 to 3 (E-F) according to first-line therapy (R-ACVBP vs R-CHO(E)P). Log-rank P values are presented.
Baseline characteristics of patients with CNS event
Overall, 40 of 2203 young patients with DLBCL (1.8%) experienced progression or relapse in the CNS. Thirty-three CNS events (82%) represented the first progression or relapse for affected patients. For 7 patients, CNS events occurred after prior progression or relapse outside the CNS. As expected, 27 of these 33 CNS relapses occurred within the first 12 months after study inclusion. Interestingly, the 6 remaining CNS relapses (18%) occurred >40 months after study inclusion. Overall, 16 patients had CNS events during first-line treatment. For 39 of 40 patients with CNS events, the precise sites of CNS involvement were reported at initial diagnosis: 15 CNS relapses occurred in brain parenchyma, 16 CNS events affected the meninges (in imaging or CSF), in 2 cases there was a solid intraspinal lymphoma manifestation, and in 6 cases combined CNS involvement was detected. Overall, 11 patients showed an isolated CNS relapse after prior complete remission of all peripheral manifestations, and 14 patients showed isolated progress in the CNS during first-line treatment. In 10 patients, a combined CNS relapse with concomitant lymphoma manifestations outside the CNS occurred, and 5 patients showed progressive disease within and outside the CNS. This analysis shows that for a remarkable fraction of patients (15/40, 37.5%), CNS relapse does not only affect the isolated CNS compartment but is frequently associated with a lack of systemic control of the disease.
Comparing patients with (n = 40) and without a CNS event (n = 2163) revealed several baseline characteristics significantly associated with a CNS event (Table 2). Overall, patients with CNS event showed a higher aaIPI at initial diagnosis (P < .001). Each factor of the aaIPI: elevated LDH (49% vs 80%, P < .001), poor ECOG PS (8% vs 25%, P = .002), and advanced stage III/IV disease (46% vs 70%, P = .003) conveyed a significantly higher risk for CNS events compared with patients lacking the respective characteristics. Other significant factors were bulky disease >10 cm (21% vs 35%, P = .029), B symptoms (29% vs 49%, P = .009), and BM involvement (6% vs 18%, P = .012). Involvement of the kidney and/or adrenal gland occurred in 4% of patients without a CNS event compared with 10% of patients with a CNS event (P = .057). Patients with CNS event showed a higher CNS-IPI at initial diagnosis (P < .001). In the entire study cohort, only 1 of 640 patients with CNS-IPI of 0 experienced a late CNS event (0.2%; 95% CI, 0.004-0.9) >4 years after study inclusion. In 1399 patients with CNS-IPI of 0 or 1, 16 CNS events occurred (1.1%; 95% CI, 0.7-1.9). A total of 665 patients of intermediate risk with CNS-IPI of 2 to 3 showed 17 CNS events (2.6%; 95% CI, 1.5-4.1). Finally, 139 patients showed a high CNS-IPI of 4-5 with 7 patients (5%; 95% CI, 2-10) experiencing a CNS event. Twenty patients displayed a CNS-IPI of 5, with 4 patients (20%; 95% CI, 6-44) showing a CNS event.
Main characteristics of patients with DLBCL according to the occurrence of CNS event
| . | All patients (n = 2203) . | Patients without CNS event (n = 2163) . | Patients with CNS event (n = 40) . | P value∗ . |
|---|---|---|---|---|
| Male, n (%) | 1292 (59) | 1266 (59) | 26 (65) | .410 |
| Female, n (%) | 911 (41) | 897 (41) | 14 (35) | |
| Age, median (range), y | 47 (18-60) | 47 (18-60) | 51 (19-60) | .370 |
| LDH > upper normal value, n (%) | 1102 (50) | 1070 (49) | 32 (80) | <.001 |
| ECOG >1, n (%) | 193 (9) | 183 (8) | 10 (25) | .002 |
| Stage III/IV, n (%) | 1031 (47) | 1003 (46) | 28 (70) | .003 |
| aaIPI, n (%) | ||||
| 0 | 652 (30) | 651 (30) | 1 (2) | <.001 |
| 1 | 924 (42) | 906 (42) | 18 (45) | |
| 2 | 479 (22) | 468 (22) | 11 (28) | |
| 3 | 148 (7) | 138 (6) | 10 (25) | |
| Extralymphatic involvement, n (%) | 1210 (55) | 1183 (55) | 27 (68) | .107 |
| Extralymphatic involvement >1, n (%) | 509 (23) | 496 (23) | 13 (32) | .155 |
| Bulky disease† (>10 cm), n (%) | 462 (21) | 448 (21) | 14 (35) | .029 |
| B symptoms, n (%)† | 654 (30) | 635 (29) | 19 (49) | .009 |
| BM involvement, n (%) | 142 (6) | 135 (6) | 7 (18) | .012 |
| Kidney involvement at staging, n (%) | 56 (3) | 53 (2) | 3 (8) | .079 |
| Adrenal gland involvement at staging, n (%) | 33 (1) | 31 (1) | 2 (5) | .119 |
| Kidney and/or adrenal gland involvement at staging, n (%) | 81 (4) | 77 (4) | 4 (10) | .057 |
| CNS-IPI, n (%) | ||||
| 0 | 640 (29) | 639 (30) | 1 (2) | <.001 |
| 1 | 759 (34) | 744 (34) | 15 (38) | |
| 2 | 414 (19) | 403 (19) | 11 (28) | |
| 3 | 251 (11) | 245 (11) | 6 (15) | |
| 4 | 119 (5) | 116 (5) | 3 (8) | |
| 5 | 20 (1) | 16 (1) | 4 (10) | |
| CNS-IPI groups, n (%) | .002 | |||
| 0-1, low risk | 1399 (64) | 1383 (64) | 16 (40) | |
| 2-3, intermediate risk | 665 (30) | 648 (30) | 17 (42) | |
| 4-5, high risk | 139 (6) | 132 (6) | 7 (18) | |
| MTX prophylaxis (at least 1 course), n (%) | — | |||
| MTX IT† | 780 (38) | 766 (38) | 14 (40) | |
| HD MTX IV | 344 (16) | 342 (16) | 2 (5) | |
| CNS event, n (%) | — | — | ||
| At first progression/relapse (within the first 3 y) | 33 (1.5) (27) | 33 (82) (27) | ||
| After first progression/relapse (within the first 3 y) | 7 (0.3) (4) | 7 (18) (4) |
| . | All patients (n = 2203) . | Patients without CNS event (n = 2163) . | Patients with CNS event (n = 40) . | P value∗ . |
|---|---|---|---|---|
| Male, n (%) | 1292 (59) | 1266 (59) | 26 (65) | .410 |
| Female, n (%) | 911 (41) | 897 (41) | 14 (35) | |
| Age, median (range), y | 47 (18-60) | 47 (18-60) | 51 (19-60) | .370 |
| LDH > upper normal value, n (%) | 1102 (50) | 1070 (49) | 32 (80) | <.001 |
| ECOG >1, n (%) | 193 (9) | 183 (8) | 10 (25) | .002 |
| Stage III/IV, n (%) | 1031 (47) | 1003 (46) | 28 (70) | .003 |
| aaIPI, n (%) | ||||
| 0 | 652 (30) | 651 (30) | 1 (2) | <.001 |
| 1 | 924 (42) | 906 (42) | 18 (45) | |
| 2 | 479 (22) | 468 (22) | 11 (28) | |
| 3 | 148 (7) | 138 (6) | 10 (25) | |
| Extralymphatic involvement, n (%) | 1210 (55) | 1183 (55) | 27 (68) | .107 |
| Extralymphatic involvement >1, n (%) | 509 (23) | 496 (23) | 13 (32) | .155 |
| Bulky disease† (>10 cm), n (%) | 462 (21) | 448 (21) | 14 (35) | .029 |
| B symptoms, n (%)† | 654 (30) | 635 (29) | 19 (49) | .009 |
| BM involvement, n (%) | 142 (6) | 135 (6) | 7 (18) | .012 |
| Kidney involvement at staging, n (%) | 56 (3) | 53 (2) | 3 (8) | .079 |
| Adrenal gland involvement at staging, n (%) | 33 (1) | 31 (1) | 2 (5) | .119 |
| Kidney and/or adrenal gland involvement at staging, n (%) | 81 (4) | 77 (4) | 4 (10) | .057 |
| CNS-IPI, n (%) | ||||
| 0 | 640 (29) | 639 (30) | 1 (2) | <.001 |
| 1 | 759 (34) | 744 (34) | 15 (38) | |
| 2 | 414 (19) | 403 (19) | 11 (28) | |
| 3 | 251 (11) | 245 (11) | 6 (15) | |
| 4 | 119 (5) | 116 (5) | 3 (8) | |
| 5 | 20 (1) | 16 (1) | 4 (10) | |
| CNS-IPI groups, n (%) | .002 | |||
| 0-1, low risk | 1399 (64) | 1383 (64) | 16 (40) | |
| 2-3, intermediate risk | 665 (30) | 648 (30) | 17 (42) | |
| 4-5, high risk | 139 (6) | 132 (6) | 7 (18) | |
| MTX prophylaxis (at least 1 course), n (%) | — | |||
| MTX IT† | 780 (38) | 766 (38) | 14 (40) | |
| HD MTX IV | 344 (16) | 342 (16) | 2 (5) | |
| CNS event, n (%) | — | — | ||
| At first progression/relapse (within the first 3 y) | 33 (1.5) (27) | 33 (82) (27) | ||
| After first progression/relapse (within the first 3 y) | 7 (0.3) (4) | 7 (18) (4) |
P value for the comparison of patients with CNS failure vs patients without CNS event.
Some missing values: bulky disease (9/9/0), B symptoms (7/6/1), and MTX IT (143/138/5).
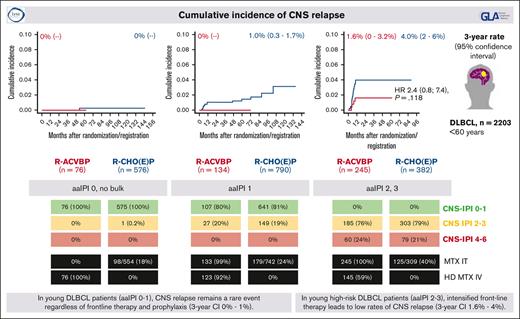
Cumulative incidence of CNS relapse according to aaIPI and first-line therapy
Next, we compared the cumulative incidences of CNS relapse according to aaIPI risk factors and the first-line therapy administered. The different median observation time for OS in patients treated with R-ACVBP or R-CHO(E)P (aaIPI of 0 without bulky disease: 43 vs 68 months; aaIPI of 1: 43 vs 67 months; aaIPI of 2-3: 45 vs 41 months) should be recognized. Among patients with aaIPI of 0 without bulky disease (n = 652), 1 single patient (0.2%) experienced a late CNS relapse (supplemental Table 3). The 3-year cumulative incidences of CNS relapse were 0% in all treatment arms.
Among patients with aaIPI of 1, 134 patients treated with R-ACVBP did not experience any CNS relapse. A total of 705 patients treated with R-CHOP showed 11 CNS relapses (1.6%) and 85 patients treated with R-CHOEP showed 2 CNS relapses (2.4%) (supplemental Table 4). The 3-year cumulative incidences of CNS relapse were 0%, 1.0% (0.3%-1.7%), and 1.2% (0%-3.6%), respectively.
Overall, 245 patients with aaIPI of 2 or 3 received treatment with R-ACVBP, including consolidation. Four CNS relapses were recognized (1.6%), and the 3-year cumulative incidence after R-ACVBP was 1.6% (0%-3.2%) (Figure 2). A total of 382 patients with aaIPI of 2 or 3 were treated with R-CHOP (n = 78), R-CHOEP (n = 165), or R-MegaCHOEP (n = 139). Fifteen CNS relapses (3.9%) were noticed (supplemental Table 5). The corresponding 3-year cumulative incidence of CNS relapse for R-CHO(E)P was 4% (2.0%-6.0%) (Figure 2). Comparing the time to CNS relapse for R-(Mega)CHO(E)P vs R-ACVBP, the multivariable, cause-specific, hazard model adjusted for prognostic factors showed no significant difference R-(Mega)CHO(E)P vs R-ACVBP, hazard ratio 2.4 (95% CI, 0.8-7.4; P = .118). Notably, the patients treated with R-ACVBP or R-CHOP within the LNH07-3B trial received HD MTX and high dose therapy (HDT)/ASCT in case of positive PET scan after 2 cycles of chemotherapy and salvage therapy in case of positive PET scan after 4 cycles of chemotherapy (supplemental Table 1).22 This is important to mention because patients with positive interim PET might represent high-risk patients for CNS relapse, and these patients were treated with an intensified approach in the LNH07-3B trial.22 In 78 patients receiving PET-guided R-CHOP, no CNS event was observed. Two CNS events occurred in the PET-guided R-ACVBP arm (supplemental Figure 2A).
Cumulative incidence of CNS relapse for patients with aaIPI of 2 to 3 according to first-line therapy (R-ACVBP vs R-CHO[E]P). Adjusted hazard ratio (HR) with 95% CI is presented.
Cumulative incidence of CNS relapse for patients with aaIPI of 2 to 3 according to first-line therapy (R-ACVBP vs R-CHO[E]P). Adjusted hazard ratio (HR) with 95% CI is presented.
To compare conventional R-CHOEP vs R-ACVBP without the impact of interim PET, we excluded the patients from the LNH07-3B trial. Considering only the 164 patients treated with R-ACVBP within the LNH03-3B trial, the corresponding 3-year cumulative incidence of CNS relapse was 1.2% (0%-2.9%) with a hazard ratio of R-(Mega)CHOEP vs R-ACVBP accounting of 4.1 (95% CI, 0.9-18.2; P = .062) (supplemental Figure 2B).
As expected, patients experiencing CNS events showed very unfavorable outcomes (Figure 3). With a median time of observation of 18 months, 1-year OS after a CNS event was 20% (95% CI, 6-33).
Discussion
Since our initial report in 2009,6 most retrospective analyses failed to show that patients with DLBCL benefit from CNS prophylaxis with IT MTX.3,29,30 More recently, the efficacy of prophylaxis with IV HD MTX has also been questioned.10,12 Taken together, the lack of progress in establishing more effective CNS prophylaxis emphasizes the necessity to continue searching for alternative strategies to reduce the frequency of CNS relapse. Here, we demonstrate that the incidence of CNS relapse in younger, well-documented study patients with aaIPI of 0 or 1 is very low, making further efforts to reduce their number challenging to impossible. Patients falling into these low-risk groups and mostly presenting with a low CNS-IPI can be spared any CNS-directed diagnostic and prophylactic procedures. Attempts to improve the situation would also be hampered by the necessity of treating very large numbers of patients to document any statistically significant improvement.
For younger patients with aaIPI of 2 or 3 and treated with the R-ACVBP regimen, the 3-year cumulative incidence rate of CNS relapse was intriguingly low (1.6%) and still lower than observed with the R-CHO(E)P regimen (4.0%). It should be mentioned that the overall CNS relapse rates observed in this analysis are lower than expected, for example, by applying the CNS-IPI, possibly reflecting the superiority of aggressive regimens over R-CHOP, especially when patients are treated on clinical studies. Taking into account earlier reports on the ACVBP regimen before rituximab was added to chemotherapy,14 the aggregate data available today suggest that (R-)ACVBP can effectively prevent CNS relapse also in younger patients with high-risk DLBCL (aaIPI of 2 or 3), who run the highest risk of CNS relapse of all patients amenable to more aggressive chemotherapy. R-ACVBP in comparison with other aggressive therapies, such as R-CHOEP or DA-EPOCH-R, not only comprises dose-escalated induction chemotherapy but also includes a distinct consolidation part consisting of HD MTX, ifosfamide, etoposide, and cytarabine, or HD MTX, BEAM (including escalated etoposide and cytarabine), and ASCT.18 Moreover, R-ACVBP includes 4 IT injections of MTX administered to all patients regardless of specific regions involved by lymphoma. Although the benefit of IT MTX remains controversial, the combination of aggressive systemic therapy, IT prophylaxis for all patients, and consolidation with multiple drugs crossing the BBB might effectively reduce the incidence of CNS relapse. Which of the unique therapeutic features of R-ACVBP actually makes the difference is impossible to decide; rather, the combination of all consolidation elements may be necessary to obtain optimal results with R-ACVBP. Moreover, it should be underscored that also etoposide has been reported to cross the BBB possibly explaining the low rates of CNS relapse seen in this study after the R-(Mega)CHOEP regimen.15,31 Although our study was a large multicentric analysis of trial patients, 1 limitation remains that nearly all considered young high-risk patients with aaIPI of 2 to 3 were treated with intensified approaches, so that a direct comparison to R-CHOP–treated high-risk patients could not be made. Thus, our hypothesis that the low rate of CNS events is really because of the effect of agents crossing the BBB needs further verification in retrospective and prospective studies.
Unfortunately, most elderly patients with DLBCL and a high risk of CNS relapse may not be able to benefit from being treated with R-ACVBP or other regimens more aggressive than R-CHOP because of toxicities precluding their routine administration to older and unfit patients.18,32 Despite improved OS rates reported for younger patients with an aaIPI of 1, R-ACVBP was associated with significantly increased hematological toxicity, febrile neutropenia (grade ≥3: 38% vs 9%), and mucositis (grade ≥3: 18% vs 0%) in comparison with conventional R-CHOP.18 Therefore, the search for better diagnostic and prognostic markers as well as optimizing systemic therapy, including new drugs with less toxicity, crossing the BBB must continue.33,34 Further research is needed to determine what role interim PET might play in guiding frontline therapy and potentially preventing CNS relapse.22
The prognosis of patients with CNS relapse remains poor. Overall, only 4 smaller phase 2 studies have systematically analyzed therapeutic strategies for patients with secondary CNS relapse.35-38 Best results have been reported for intensive regimens incorporating several drugs penetrating the BBB followed by consolidation with HDT/ASCT.36 In the Marietta trial, patients achieved a 2-year OS of 46%.36 In the cohort of young patients with DLBCL in this study, 1-year OS was only 20%. Thus, it will be essential to further explore the therapeutic potential of novel drugs crossing the BBB, such as ibrutinib, lenalidomide, and checkpoint inhibitors.33,39,40 A GLA phase 2 study investigating R-CHOEP plus the Bruton tyrosine kinase inhibitor ibrutinib in young patients with high-risk DLBCL completed patient accrual and awaits analysis (EudraCT-No. 2017-003256-22). In small case series, promising activity has also been reported for chimeric antigen receptor T-cell therapy.41
To conclude, we demonstrate that the risk of relapse in the CNS for younger patients with low-risk DLBCL (aaIPI of 0 and 1) is very low and further efforts to improve CNS prophylaxis may neither be warranted nor feasible. Younger patients with high-risk (aaIPI of 2 and 3) disease may benefit from aggressive immunochemotherapy, including consolidation with drugs crossing the BBB. Because the toxicities observed with aggressive chemotherapy remain significant and may preclude such treatment in the elderly, the search for more effective and less toxic CNS prophylaxis must continue to improve the overall results of first-line therapy in DLBCL.
Acknowledgments
The authors thank the patients and their families, all investigators, and staff involved in data collection and analyses, and Beate Mann (IMISE Leipzig) for technical assistance.
Authorship
Contribution: C.T., M.Z., B.A., and N.S. conceptualized and designed the study; C.T., L.R., M.Z., and B.A. contributed to manuscript writing; F.F and N.S. prepared the initial manuscript draft; and all authors were responsible for collection, assembly, analysis, and interpretation of data, approved the final manuscript, and are accountable for all aspects of the work.
Conflict-of-interest disclosure: C.T. received honoraria from Roche, Amgen, Janssen, Celgene, and Gilead Science/Kyte Beigene; is in a consulting/advisory role for Roche, Gilead Sciences, Janssen, Celgene, Novartis, BeiGene, and Bristol Myers Squibb (BMS)/Celgene; and received research funding, travel, and accommodations expenses from Roche and Novartis outside the submitted work. C.R. received research grants from AbbVie, Amgen, Novartis, BMS-Celgene, Jazz Pharmaceuticals, Agios, Chugai, MaaT Pharma, Astellas, Roche, Daiichi-Sankyo, and IQVIA, and is an adviser for AbbVie, Janssen, Jazz Pharmaceuticals, Novartis, Celgene, Otsuka, Astellas, Daiichi-Sankyo, Macrogenics, Pfizer, Roche, Servier, and Takeda. V.P. received travel support from AbbVie, Amgen, BMS, Gilead, and Roche. O.S. received research funding from Roche, Takeda, and Gilead, and is on the advisory board of and received honoraria from Celgene, Roche, Takeda, Gilead, BMS, Merck, AbbVie, and Janssen outside the submitted work. F.M. received research funding from Roche, Takeda, and Gilead, and is on the advisory board of and received honoraria from Celgene, Roche, Takeda, Gilead, BMS, Merck, AbbVie, and Janssen outside the submitted work. H.T. is on the advisory board of and received honoraria from Roche. G.L. received research funding from AstraZeneca, Agios, Aquinox, Bayer, Celgene, Gilead, Janssen, Morphosys, Novartis, Roche, and Verastem, and received honoraria from ADC Therapeutics, AbbVie, Amgen, AstraZeneca, Bayer, BMS, Celgene, Constellation, Genmab, Gilead, Incyte, Janssen, Karyopharm, Miltenyi, Morphosys, NanoString, Novartis, and Roche. N.S. received research funding from Janssen and received honoraria from Riemser/Esteve, Takeda, Kite/Gilead, and Novartis outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Norbert Schmitz, Department of Medicine A for Hematology, Oncology, and Pneumology, Münster University Hospital, Domagkstr 3, 48149 Münster, Germany; e-mail: norbert.schmitz@ukmuenster.de.
References
Author notes
∗C.T., B.A., and F.F. contributed equally to this study.
†M.Z. and N.S. contributed equally to this study.
Presented in part at the annual meeting of the American Society of Clinical Oncology, Chicago, IL, 4-8 June 2021.
Data sets of this study will be provided on request to the corresponding author, Norbert Schmitz (norbert.schmitz@ukmuenster.de).
The full-text version of this article contains a data supplement.

![Cumulative incidence of CNS relapse for patients with aaIPI of 2 to 3 according to first-line therapy (R-ACVBP vs R-CHO[E]P). Adjusted hazard ratio (HR) with 95% CI is presented.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/7/15/10.1182_bloodadvances.2022008888/2/m_blooda_adv-2022-008888-gr2.jpeg?Expires=1769165052&Signature=thFzV0pAY5zidf48ZLJr6yqnNl4cc7yC3YtdCPvOgKmZ26i324r750HQWFGxdUX5R4IvCzRuUydsCVmEPnLXlwOL57hSOMQAmy0TA7b-gww3Hl-TABk7GckoLJnoeBe96UVxiMyAfj5iNd48PMPWfKR7xIFvZrelT1JFzF7aW8hk2bJDALO3Ea1WLB0ZdMfxxTnn015jByv2aDhMbJRbwltbhjvbGQUAWWkvZjPA9HsxFnGA-ou6IBXkGQhJZfmkEjELMxTju5GbQISJizpVp2q~1r7xUtyuui6P5yo630tksvZkIlH8P-pSmrd0qxp-V2PQx3pJUPAbGc5k6Gn~Bg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)