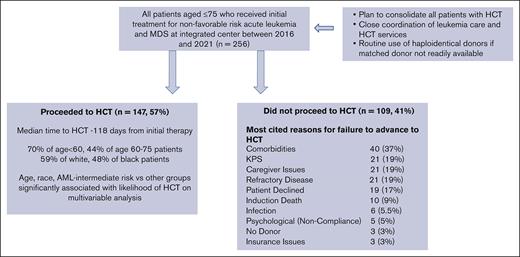
Key Points
Close integration of leukemia and HCT services and the use of haploidentical donors result in greater access to HCT than previously reported.
Racial disparity is reduced by this approach, but caregiver requirements continue to limit HCT access for Black patients.
Abstract
Few patients with nonfavorable risk (NFR) acute leukemia and myeloid dysplasia syndrome (AL/MDS) undergo allogeneic transplantation (HCT). We assessed whether this could be improved by integrating HCT/leukemia care and the use of haploidentical donors. Of 256 consecutive patients aged <75 years who received initial therapy at our center for NFR AL/MDS from 2016 to 2021, 147 (57%) underwent planned HCT (70% for patients aged <60 years). In the logistic regression analysis, age (OR 1.50 per 10-year increment; P < .001) and race (Black vs White [OR 2.05; P = .023]) were significant factors for failure to receive HCT. Reasons for no HCT included comorbidities (37%), poor KPS, lack of caregiver support, refractory malignancy (19% each), and patient refusal (17%). Lack of donor or insurance were rarely cited (3% each). In older patients (≥60 years), comorbidities (49 vs 15%; P < .001) and KPS (25% vs 10%; P = .06) were more common, and lack of caregivers was less common (13% vs 30%; P = .031). In Black vs White patients, lack of caregivers (37% vs 11%; P = .002) was more frequent. The median time from initial treatment to HCT was 118 days and was similar for Black and White patients. Landmark analysis showed that HCT within 6 months of the initial treatment produced better survival. Multivariable analysis showed that HCT resulted in a significant survival benefit (HR 0.60; P = .020). With the above approach, most of the currently treated patients aged <75 years can access planned HCT. Black patients remain at greater risk of not receiving HCT.
Introduction
Hematopoietic cell transplantation (HCT) remains the most effective consolidation therapy for patients with acute leukemia (AL) and myeloid dysplasia syndrome (MDS) with a nonfavorable risk (NFR) profile for disease relapse. However, prior studies have demonstrated that only a minority of patients who may benefit from HCT eventually receive it.1-5 Racial minorities have been demonstrated to face greater barriers to HCT than other patients.6-8 Some historical barriers may be ameliorated by recent developments. For example, the lack of available HLA-matched donors may be overcome by the recent use of wider donor sources, such as HLA-haploidentical related donors (HIDs) and cord blood units. Similarly, newer therapeutic regimens may result in a greater number of patients achieving complete remission. However, other potential obstacles, such as recipient comorbidities, inadequate health insurance coverage, and insufficient caregiver support, continue to be limiting factors for many patients. Delays in HLA-typing and referral to an HCT program can represent significant obstacles even within academic centers1 and may be more problematic if the patients receive induction therapy in an institution without an HCT program or where leukemia care is not closely coordinated with HCT services. These issues may need to be addressed if more patients are to undergo successful transplantation.
At our center, nontransplant care of patients with AL/MDS and HCT is performed by the same physicians within a closely integrated program, avoiding the need for a specific referral to a transplant physician. Furthermore, HLA-typing of patients and potential donors and a preliminary unrelated donor search are routinely performed at the time of initial treatment to avoid delays in having them proceed to undergo HCT. Our center was also among the earliest to routinely use HIDs to achieve timely HCT in patients who do not have immediate access to HLA-matched donors.9,10 Patients with AL and MDS treated at our center were prospectively enrolled in our institutional leukemia database. For patients with NFR who should proceed to undergo HCT, all significant reasons for failure to proceed with transplantation are documented in real time.
In this study, we analyzed all consecutive patients who received initial therapy for nonfavorable AL and MDS at our center, between 2016 and 2021, to assess the rate and speed at which such patients proceeded to receive HCT. We chose this cohort because HID was routinely used in patients without a matched sibling donor and an unfavorable, preliminary unrelated donor search. Furthermore, this period included modern therapeutic strategies to facilitate disease remission, such as hypomethylating agents± venetoclax, CPX-351 for acute myeloid leukemia (AML)/MDS, blinatumomab and inotuzumab for B-lineage acute lymphoblastic leukemia (ALL), and second- and third-generation tyrosine kinase inhibitors for Ph+ ALL and nelarabine. We hypothesized that in this cohort of patients treated using current regimens, within an integrated AL/HCT program, a higher proportion of patients (including older patients aged 60-75 years) would proceed to receive HCT than that previously reported. Furthermore, because self-identified Black patients constitute a relatively large proportion of patients treated at our center, we aimed to assess whether racial disparity in the use of HCT remains a significant problem in this setting and whether documented barriers to HCT differ based on self-identified race.
Patients and methods
Consecutive patients aged <75 years who received initial treatment for NFR, AML, ALL, and MDS at our center between January 2016 and December 2021 were included in this analysis. Data were extracted retrospectively from our institutional AL and HCT databases, entered in real time. The study was approved by the institutional review board and was performed in accordance with the Declaration of Helsinki. HCT was the targeted and planned consolidation therapy for all such patients at our institution during this period, and HID were routinely used for HCT when an optimally HLA-matched donor was unavailable. Specifically, patients who did not have a matched sibling donor (MRD) and whose preliminary unrelated donor search was unfavorable or those with anticipated short-lived remissions underwent HCT using an HID as soon as feasible, if an HID was available and significant donor-specific HLA antibodies were not present.
Planning for HCT was started at the time of commencement of initial therapy. Patients and family donors were HLA-typed at the starting point, and a preliminary unrelated donor search was performed as soon as the results were available. A dedicated PhD-level psychologist and social worker were employed in the leukemia/HCT program. Formal assessments by the program’s dedicated PhD psychologist and licensed social worker regarding caregiver availability for HCT and other psychosocial conditions relevant to HCT were initiated while receiving initial treatment to facilitate early assistance with psychosocial issues that may limit access to HCT. A dedicated leukemia coordinator was assigned to each patient to facilitate logistics of care and donor search and was responsible for integrating care with the program’s transplant coordinators to enable the patients progression to undergo HCT as rapidly as feasible. The Northside Hospital provided a generous financial assistance program for patients from the State of Georgia who were indigent or had significant gaps in their insurance coverage, which might have affected their ability to proceed to undergo HCT. This program was need-based and allowed several patients without insurance coverage to proceed to undergo allogeneic HCT. Patients residing more than 40 miles from the transplant center typically received free accommodation for 6 months after HCT or longer if post-HCT complications required frequent visits.
All patients were required to have <5% bone marrow blasts based on morphology and flow cytometry results, without evidence of circulating blasts in the peripheral blood, to proceed to undergo HCT. Hematopoietic recovery from remission induction therapy and complete molecular/cytogenetic remission were not required if these criteria were met. During the period that the study population was treated, 12 patients with NFR AML in complete remission (CR) received autologous HCT followed by pembrolizumab maintenance for 6 months after transplantation in a clinical trial (NCT02771197). These patients were excluded from the analysis.
NFR was defined as follows: AML-intermediate and unfavorable genetic risk per the European Leukemia Net 2017 classification or core binding factor (CBF) AML with a c-KIT mutation; ALL-poor risk genetic risk group per the National Comprehensive Cancer Network (NCCN) guidelines version 1.2002 for patients aged <40 years and >40 years; and MDS with excess blasts, and all patients with chronic myelo-monocytic leukemia (CMML) receiving antileukemic therapy. Data regarding patient characteristics, treatment received, progression to HCT, reasons for failure to proceed to HCT, survival, and relapse were prospectively entered into our leukemia database, from which they were extracted for this analysis. For all patients who failed to proceed to undergo HCT, the electronic medical record was reviewed by the principal investigator of the study to confirm/validate the reasons documented in the database.
Statistical methods
Two-sample comparisons were evaluated using the Wilcoxon rank sum test for continuous variables and the score test for dichotomous variables as well as Fisher exact test for categorical variables consisting more than 2 levels. We performed landmark analysis to assess whether transplantation treatment was protective against a mortality event. Based on the 6-month landmark, we constructed 2 groups, including those with and without a transplantations within the landmark. The Kaplan-Meier method was used to estimate survival probabilities, and the log-rank test was used to compare survival outcomes between the 2 groups.
Multivariable analyses included logistic regression analysis for transplant receipt and Cox proportional hazards regression analysis for survival end points. We considered the following variables in the logistic regression analysis: age at diagnosis, sex, race (White, Black, or other), ethnicity, diagnosis and risk (ALL, AML–intermediate risk, AML–poor risk, or MDS), distance from the transplantation center, and the year of treatment (2016-2018 or 2019-2021). Our aim in the Cox analysis was to evaluate the effects of transplantation on the survival end points. Therefore, we retained the time of transplantation receipt as a time-dependent covariate in Cox models for the overall survival and disease-free survival (DFS). We further considered the same set of variables for the logistic regression analysis. We tested whether age had a linear effect on all outcome variables. In the logistic regression model for the time of transplantation receipt, linearity for age was held approximately, and continuous age was used in this regression model. Linearity for age was violated using the Cox model. For Cox analysis, we categorized age into 3 equal-size groups (≤50, 51-64, and 65-75), and these categorized ages were used. For each regression model, we implemented a forward stepwise selection algorithm. A variable was selected if P < .05. For the variables in the Cox models, we tested the proportionality by including and testing time-dependent covariates. The proportionality was maintained for all variables in the final Cox model.
We reported 2 sided P values. P < .05 was considered as statistically significant. All statistical analyses were performed using SAS software (version 9.4, SAS Institute, Cary, NC).
Results
Patient characteristics
The characteristics of 256 patients who received initial therapy for NFR AL and MDS, 147 patients who underwent HCT, and 109 patients who failed to proceed to undergo HCT are shown in Table 1. For all patients, the median age was 58 years (48% were 60 years or older) and 26% self-identified as Black. AML was the most frequent diagnosis (67%). For both AML and ALL, poor-risk disease was more common (70% and 81%, respectively) than intermediate-risk or standard disease. The median number of days from diagnosis to the start of initial treatment was 5 days (range, 1-155 days). For patients who received an HCT, additional details regarding the HCT, including regimen intensity and donor source, are provided in the supplemental Table.
Characteristics of patients treated
| . | All patients (N = 256) . | Transplant (N = 147) . | No transplant (N = 109) . | P value . |
|---|---|---|---|---|
| Age at treatment, y, median (range) | 58 (18, 75) | 54 (18, 75) | 62 (21, 75) | < .001 |
| D from diagnosis to initial treatment, median (range) | 5 (1, 155) | 5 (1, 155) | 6 (1, 28) | .23 |
| D from initial treatment to transplant, median (range) | 118 (58, 621) | |||
| Distance from hospital, median (range) | 29.5 (2.4, 882) | 27.4 (3.4, 882) | 33.8 (2.4, 174) | .12 |
| Male sex | 131 (51%) | 72 (49%) | 59 (54%) | .45 |
| Race | .027 | |||
| White | 174 (68%) | 102 (70%) | 72 (66%) | |
| Black | 67 (26%) | 32 (22%) | 35 (32%) | |
| Other | 14 (6%) | 12 (8%) | 2 (2%) | |
| Ethnicity | .22 | |||
| Hispanic | 26 (10%) | 18 (12%) | 8 (7%) | |
| Non-Hispanic | 230 (90%) | 129 (88%) | 101 (93%) | |
| Diagnosis | .28 | |||
| AML | 171 (67%) | 93 (63%) | 78 (26%) | |
| ALL | 73 (28%) | 45 (31%) | 28 (71%) | |
| MDS | 12 (5%) | 9 (6%) | 3 (3%) | |
| Risk categories in patients with AML | .044 | |||
| Intermediate risk | 51 (30%) | 34 (37%) | 17 (22%) | |
| Poor risk | 120 (70%) | 59 (63%) | 61 (78%) | |
| Risk categories in patients with ALL | .13 | |||
| Standard risk | 14 (19%) | 6 (13%) | 8 (29%) | |
| Poor risk | 59 (81%) | 39 (87%) | 20 (71%) | |
| Y of initial treatment | .61 | |||
| 2016-2018 | 124 (48%) | 69 (47%) | 55 (50%) | |
| 2019-2021 | 132 (52%) | 78 (53%) | 54 (50%) | |
| Number of survivors | 139 | 101 | 38 | |
| Survivor follow-up from initial treatment (mo), median (range) | 35.0 (5.9, 76.8) | 36.3 (5.9, 76.8) | 28.3 (5.9, 73.6) | .45 |
| . | All patients (N = 256) . | Transplant (N = 147) . | No transplant (N = 109) . | P value . |
|---|---|---|---|---|
| Age at treatment, y, median (range) | 58 (18, 75) | 54 (18, 75) | 62 (21, 75) | < .001 |
| D from diagnosis to initial treatment, median (range) | 5 (1, 155) | 5 (1, 155) | 6 (1, 28) | .23 |
| D from initial treatment to transplant, median (range) | 118 (58, 621) | |||
| Distance from hospital, median (range) | 29.5 (2.4, 882) | 27.4 (3.4, 882) | 33.8 (2.4, 174) | .12 |
| Male sex | 131 (51%) | 72 (49%) | 59 (54%) | .45 |
| Race | .027 | |||
| White | 174 (68%) | 102 (70%) | 72 (66%) | |
| Black | 67 (26%) | 32 (22%) | 35 (32%) | |
| Other | 14 (6%) | 12 (8%) | 2 (2%) | |
| Ethnicity | .22 | |||
| Hispanic | 26 (10%) | 18 (12%) | 8 (7%) | |
| Non-Hispanic | 230 (90%) | 129 (88%) | 101 (93%) | |
| Diagnosis | .28 | |||
| AML | 171 (67%) | 93 (63%) | 78 (26%) | |
| ALL | 73 (28%) | 45 (31%) | 28 (71%) | |
| MDS | 12 (5%) | 9 (6%) | 3 (3%) | |
| Risk categories in patients with AML | .044 | |||
| Intermediate risk | 51 (30%) | 34 (37%) | 17 (22%) | |
| Poor risk | 120 (70%) | 59 (63%) | 61 (78%) | |
| Risk categories in patients with ALL | .13 | |||
| Standard risk | 14 (19%) | 6 (13%) | 8 (29%) | |
| Poor risk | 59 (81%) | 39 (87%) | 20 (71%) | |
| Y of initial treatment | .61 | |||
| 2016-2018 | 124 (48%) | 69 (47%) | 55 (50%) | |
| 2019-2021 | 132 (52%) | 78 (53%) | 54 (50%) | |
| Number of survivors | 139 | 101 | 38 | |
| Survivor follow-up from initial treatment (mo), median (range) | 35.0 (5.9, 76.8) | 36.3 (5.9, 76.8) | 28.3 (5.9, 73.6) | .45 |
Progression to HCT
One hundred forty-seven of the 256 patients (57%) underwent HCT within a median of 118 days from initial therapy (Table 1). Forty-seven percent were received transplantation between 2016 and 2018, whereas the remaining 53% received transplantation between 2019 and 2022. Patients received a graft from an HID in 44%, an MRD in 32%, and an MUD in 24%. Upon performing univariate analysis, the proportion of patients who underwent HCT was higher in younger patients (age, <60 years) than in older patients (age, 60-75 years; 70% vs 44%; P < .001) but not significantly different in self-identified Black vs White patients (48% vs 59%; P = .13), female vs male patients (60% vs 55%; P = .42), and in patients with AML vs ALL (54% vs 62%; P = .30). For patients with AML, a higher proportion of patients with intermediate-risk disease underwent HCT than those with poor-risk disease (67% vs 49%; P = .036).
For multivariable assessment of factors predicting failure to proceed to undergo HCT, a logistic regression analysis was performed. Factors assessed were age at initial treatment, sex, race (White, Black, and Asian), ethnicity (Hispanic and non-Hispanic), diagnosis (AML, ALL, and MDS), disease risk (poor risk and other), distance from transplant center, and the year of treatment (2016-2018 and 2019-2021). Using a forward, stepwise selection algorithm, a variable was selected if the P < .05. Age was categorized to test whether the effect was linear and the linearity was stable. Because of the interaction between diagnosis and disease risk, these variables were combined into 5 categories: AML–intermediate risk, AML–poor risk, ALL-standard risk, ALL-poor risk, and MDS. The following variables were significantly associated with a failure to proceed to undergo HCT (Table 2): Age (odds ratio [OR], 1.50 per 10-year increment; P < .001), race (Black vs White [OR 2.05; P = .023]), diagnosis and risk group (AML–poor risk vs AML–intermediate risk [OR 2.23; P = .029]), and ALL-standard risk for AML-intermediate-risk (OR 3.26; P = .07).
Logistic regression analysis of factors associated with failure to proceed to undergo HCT
| Variable . | Effect . | OR . | 95% CI . | P value . |
|---|---|---|---|---|
| Age | Per 10-y increment | 1.50 | 1.21--1.86 | < .001 |
| Race | Black vs White | 2.05 | 1.11--3.79 | .023 |
| Diagnosis and disease risk | AML–poor risk vs AML–intermediate risk | 2.23 | 1.09--4.58 | .029 |
| ALL-standard risk vs AML–intermediate risk | 3.26 | 0.92--11.55 | .07 | |
| ALL-poor risk vs AML–intermediate risk | 1.55 | 0.66--3.64 | .31 | |
| MDS- vs AML–intermediate risk | 0.65 | 0.15--2.87 | .57 |
| Variable . | Effect . | OR . | 95% CI . | P value . |
|---|---|---|---|---|
| Age | Per 10-y increment | 1.50 | 1.21--1.86 | < .001 |
| Race | Black vs White | 2.05 | 1.11--3.79 | .023 |
| Diagnosis and disease risk | AML–poor risk vs AML–intermediate risk | 2.23 | 1.09--4.58 | .029 |
| ALL-standard risk vs AML–intermediate risk | 3.26 | 0.92--11.55 | .07 | |
| ALL-poor risk vs AML–intermediate risk | 1.55 | 0.66--3.64 | .31 | |
| MDS- vs AML–intermediate risk | 0.65 | 0.15--2.87 | .57 |
Reasons for failure to proceed to HCT
For the 109 patients who failed to proceed to undergo HCT, the reasons documented in the database and confirmed via a review of the electronic medical record are shown in Table 3. Significant comorbidities were the most cited reasons (37%). Inadequate KPS, lack of caregivers, and refractory disease were cited by 19% of patients. Approximately 17% of patients declined a transplantation while meeting all other criteria. Importantly, the lack of suitable donors and inadequate insurance coverage were cited as the reasons for only 3% each.
Reasons cited for failure to proceed to HCT
| Reasons cited for no HCT . | (Total number of patients = 109) . |
|---|---|
| Comorbidities | 40 (37%) |
| KPS | 21 (19%) |
| Caregiver issues | 21 (19%) |
| Refractory disease | 21 (19%) |
| Patient declined | 19 (17%) |
| Induction death | 10 (9%) |
| Infection | 6 (5.5%) |
| Psychological (noncompliance) | 5 (5%) |
| No donor | 3 (3%) |
| Insurance issues | 3 (3%) |
| Reasons cited for no HCT . | (Total number of patients = 109) . |
|---|---|
| Comorbidities | 40 (37%) |
| KPS | 21 (19%) |
| Caregiver issues | 21 (19%) |
| Refractory disease | 21 (19%) |
| Patient declined | 19 (17%) |
| Induction death | 10 (9%) |
| Infection | 6 (5.5%) |
| Psychological (noncompliance) | 5 (5%) |
| No donor | 3 (3%) |
| Insurance issues | 3 (3%) |
We compared the reasons for the failure to proceed to HCT in the different subgroups of our treated patients. For patients aged from 60 to 75 years, comorbidities (49% vs 15%; P < .001) and KPS (25% vs 10%; P = .06) were more commonly cited than in patients aged <60 years, whereas the lack of caregivers (13% vs 30%; P = .031) was less common (Table 4). There were no significant differences between female and male patients with respect to the reasons cited for failure to proceed to HCT. However, death during induction therapy (17% vs 6%; P = .05) and the lack of caregiver support for HCT (37% vs 11%; P = .002) were more commonly cited for Black than for White patients (Table 5). Refractory disease was more common in patients with AML than in patients with ALL who failed to proceed to undergo HCT (26% vs 4%; P = .012) (Table 6).
Frequency of progression to undergo HCT and reasons documented for failure to proceed to undergo HCT among the subgroup of patients treated based on age
| . | Age < 60 (N = 133) . | Age 60-75 (N = 123) . | P value . |
|---|---|---|---|
| Number of patients proceeding to undergo HCT | 93 (70%) | 54 (44%) | < .001 |
| Reasons for failure to proceed to undergo HCT | (N = 40) | (N = 69) | |
| Comorbidities | 6 (15%) | 34 (49%) | < .001 |
| KPS | 4 (10%) | 17 (25%) | .06 |
| Caregiver issues | 12 (30%) | 9 (13%) | .031 |
| Refractory disease | 8 (20%) | 13 (19%) | .88 |
| Patient declined | 8 (20%) | 11 (16%) | .59 |
| Induction death | 5 (5%) | 5 (7%) | .36 |
| . | Age < 60 (N = 133) . | Age 60-75 (N = 123) . | P value . |
|---|---|---|---|
| Number of patients proceeding to undergo HCT | 93 (70%) | 54 (44%) | < .001 |
| Reasons for failure to proceed to undergo HCT | (N = 40) | (N = 69) | |
| Comorbidities | 6 (15%) | 34 (49%) | < .001 |
| KPS | 4 (10%) | 17 (25%) | .06 |
| Caregiver issues | 12 (30%) | 9 (13%) | .031 |
| Refractory disease | 8 (20%) | 13 (19%) | .88 |
| Patient declined | 8 (20%) | 11 (16%) | .59 |
| Induction death | 5 (5%) | 5 (7%) | .36 |
Frequency of progression to undergo HCT and reasons documented for failure to proceed to undergo HCT among the subgroup of patients treated based on race
| . | Black (N = 67) . | White (N = 174) . | P value . |
|---|---|---|---|
| Number of patients proceeding to undergo HCT | 32 (48%) | 102 (59%) | .13 |
| Reasons for failure to proceed to HCT | (N = 35) | (N = 72) | |
| Comorbidities | 11 (31%) | 28 (39%) | .45 |
| KPS | 6 (17%) | 14 (19%) | .77 |
| Caregiver issues | 13 (37%) | 8 (11%) | .002 |
| Refractory disease | 6 (17%) | 15 (21%) | .65 |
| Patient declined | 5 (14%) | 13 (18%) | .62 |
| Induction death | 6 (17%) | 4 (6%) | .05 |
| . | Black (N = 67) . | White (N = 174) . | P value . |
|---|---|---|---|
| Number of patients proceeding to undergo HCT | 32 (48%) | 102 (59%) | .13 |
| Reasons for failure to proceed to HCT | (N = 35) | (N = 72) | |
| Comorbidities | 11 (31%) | 28 (39%) | .45 |
| KPS | 6 (17%) | 14 (19%) | .77 |
| Caregiver issues | 13 (37%) | 8 (11%) | .002 |
| Refractory disease | 6 (17%) | 15 (21%) | .65 |
| Patient declined | 5 (14%) | 13 (18%) | .62 |
| Induction death | 6 (17%) | 4 (6%) | .05 |
Frequency of progression to undergo HCT and reasons documented for failure to proceed to undergo HCT among the subgroup of patients treated based on AML vs ALL
| . | AML (N = 171) . | ALL (N = 73) . | P value . |
|---|---|---|---|
| Number of patients proceeding to undergo HCT | 93 (54%) | 45 (62%) | .30 |
| Reasons for failure to proceed to HCT | (N = 78) | (N = 28) | |
| Comorbidities | 27 (35%) | 13 (46%) | .27 |
| KPS | 17 (22%) | 4 (14%) | .39 |
| Caregiver issues | 14 (18%) | 6 (21%) | .69 |
| Refractory disease | 20 (26%) | 1 (4%) | .012 |
| Patient declined | 13 (17%) | 5 (18%) | .89 |
| Induction death | 6 (8%) | 3 (11%) | .62 |
| . | AML (N = 171) . | ALL (N = 73) . | P value . |
|---|---|---|---|
| Number of patients proceeding to undergo HCT | 93 (54%) | 45 (62%) | .30 |
| Reasons for failure to proceed to HCT | (N = 78) | (N = 28) | |
| Comorbidities | 27 (35%) | 13 (46%) | .27 |
| KPS | 17 (22%) | 4 (14%) | .39 |
| Caregiver issues | 14 (18%) | 6 (21%) | .69 |
| Refractory disease | 20 (26%) | 1 (4%) | .012 |
| Patient declined | 13 (17%) | 5 (18%) | .89 |
| Induction death | 6 (8%) | 3 (11%) | .62 |
Time to HCT
The time from treatment to HCT for the patients who underwent transplantation and the subgroups analyzed are shown in (Table 7). The majority of patients (117 of 147; 80%) received an HCT within 6 months of initial therapy. Two patients who eventually received an HCT experienced disease relapse from CR1 while waiting for an HCT and received a transplantation in the second remission after reinduction. All others received transplantation during the first remission. The median time to HCT was longer in patients with ALL (median, 136 days) and shorter in patients with MDS (median, 92 days) than in those with AML (median, 111 days; P < .001 and .022 vs AML, respectively). For patients with ALL, this was likely due to our center’s policy of administering 4 cycles of the Hyper-CVAD (cyclophosphamide, vincristine, adriamycin, dexamethasone) regimen (through cycle 2B) before HCT. Patients receiving a matched sibling donor HCT (median, 113 days) had a shorter time to transplantation than those receiving MUD HCTs(median, 140 days; P = .018), but not those receiving HID transplantations (median, 113 days). Race, ethnicity, age, and sex did not significantly affect the time to HCT.
Time to HCT from initial therapy
| . | D-to-transplant . | P value . | |
|---|---|---|---|
| N . | Median (range) . | ||
| Whole cohort | 147 | 118 (58, 621) | |
| Age at treatment | |||
| <60 | 93 | 114 (58, 453) | − |
| 60-75 | 54 | 130.5 (65, 621) | .17 |
| Sex | |||
| Female | 75 | 117 (58, 621) | − |
| Male | 72 | 119.5 (65, 549) | .89 |
| Race | |||
| White | 102 | 121.5 (58, 621) | − |
| Black | 32 | 112 (75, 281) | .80 |
| Asian | 11 | 165 (59, 358) | .67 |
| Ethnicity | |||
| Non-Hispanic | 129 | 118 (58, 621) | − |
| Hispanic | 18 | 118 (90, 453) | .86 |
| Diagnosis | |||
| AML | 93 | 111 (59, 621) | − |
| ALL | 45 | 136 (91, 549) | < .001 |
| MDS | 9 | 92 (58, 425) | .022 |
| Risk in AML patients | |||
| Intermediate | 34 | 113 (59, 358) | − |
| Poor | 59 | 111 (86, 621) | .76 |
| Donor type | |||
| MRD | 47 | 113 (58, 425) | − |
| MUD | 35 | 140 (83, 297) | .018 |
| HID | 65 | 113 (72, 621) | .74 |
| Pre-BMT status | |||
| CR1 | 122 | 118.7 (59, 621) | − |
| Other | 25 | 117 (58, 453) | .89 |
| . | D-to-transplant . | P value . | |
|---|---|---|---|
| N . | Median (range) . | ||
| Whole cohort | 147 | 118 (58, 621) | |
| Age at treatment | |||
| <60 | 93 | 114 (58, 453) | − |
| 60-75 | 54 | 130.5 (65, 621) | .17 |
| Sex | |||
| Female | 75 | 117 (58, 621) | − |
| Male | 72 | 119.5 (65, 549) | .89 |
| Race | |||
| White | 102 | 121.5 (58, 621) | − |
| Black | 32 | 112 (75, 281) | .80 |
| Asian | 11 | 165 (59, 358) | .67 |
| Ethnicity | |||
| Non-Hispanic | 129 | 118 (58, 621) | − |
| Hispanic | 18 | 118 (90, 453) | .86 |
| Diagnosis | |||
| AML | 93 | 111 (59, 621) | − |
| ALL | 45 | 136 (91, 549) | < .001 |
| MDS | 9 | 92 (58, 425) | .022 |
| Risk in AML patients | |||
| Intermediate | 34 | 113 (59, 358) | − |
| Poor | 59 | 111 (86, 621) | .76 |
| Donor type | |||
| MRD | 47 | 113 (58, 425) | − |
| MUD | 35 | 140 (83, 297) | .018 |
| HID | 65 | 113 (72, 621) | .74 |
| Pre-BMT status | |||
| CR1 | 122 | 118.7 (59, 621) | − |
| Other | 25 | 117 (58, 453) | .89 |
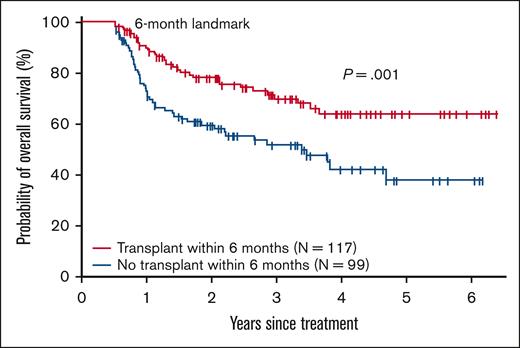
Effect of HCT on survival
The effect of HCT on survival was assessed using a landmark analysis. The results are shown in Figure 1. Patients who underwent HCT within 6 months of initial treatment had a significantly superior survival compared with patients who did not proceed to undergo HCT within 6 months 3-year survival 70% vs 52% (P = .004 using log-rank test). We also assessed factors associated with survival following initial therapy using Cox multivariable analysis (Table 8). The transplantation was retained in the Cox models and coded as a time-dependent covariate. Other variables considered in Cox analysis included age at diagnosis (≤50, 51-64, and 65-75), sex, race (White, Black, and Asian), ethnicity (non-Hispanic and Hispanic), diagnosis (ALL, AML, and MDS), disease risk (poor risk and other), and the year of treatment (2016-2018 and 2019-2021). A forward, stepwise selection algorithm was implemented. The proportionality was tested using time-dependent covariate. Proportionality was held for all variables included in the Cox models. HCT vs no HCT was associated with a significantly improved survival (hazard ratio [HR], 0.60; P = .02) other significant covariates for survival were age 51 to 64 vs < 50 (HR 1.96; P = .009) age >65 vs <50 (HR 2.22; P = .002) and the year of initial therapy from 2019 to 2021 vs from 2016 to 2018 (HR 0.66; P = .033)
Landmark analysis of overall survival by receipt of HCT within 6 months of the initial treatment.
Landmark analysis of overall survival by receipt of HCT within 6 months of the initial treatment.
Multivariable analysis of survival following initial therapy
| Variable . | Effect . | HR . | 95% CI . | P value . |
|---|---|---|---|---|
| Transplantation | Yes vs no | 0.60 | 0.40 – 0.92 | .020 |
| Age | 51-64 vs ≤50 | 1.96 | 1.18 – 3.24 | .009 |
| ≥65 vs ≤50 | 2.22 | 1.33 – 3.69 | .002 | |
| Y of treatment | 2019-2021 vs 2016-2018 | 0.66 | 0.44 – 0.97 | .033 |
| Variable . | Effect . | HR . | 95% CI . | P value . |
|---|---|---|---|---|
| Transplantation | Yes vs no | 0.60 | 0.40 – 0.92 | .020 |
| Age | 51-64 vs ≤50 | 1.96 | 1.18 – 3.24 | .009 |
| ≥65 vs ≤50 | 2.22 | 1.33 – 3.69 | .002 | |
| Y of treatment | 2019-2021 vs 2016-2018 | 0.66 | 0.44 – 0.97 | .033 |
Discussion
In this study, we analyzed 256 consecutive patients aged 75 years or younger who received initial treatment for NFR AL/MDS at our center between 2016 and 2021 to determine the proportion of patients who underwent planned HCT and the barriers cited by patients who failed to proceed to undergo HCT. We demonstrated that 57% of all patients (70% of all treated patients aged <60 years) underwent HCT within a median of 118 days since the start of the initial therapy. Among the older patients (age, 60-75 years), 44% underwent HCT. Importantly, the analysis was not restricted to patients in CR but included all patients who received initial therapy for AL/MDS, thus addressing both success in inducing remission and the efficacy of transition to HCT. The rate of HCT observed in this study was higher than that historically reported. For example, in a population based Swedish study of all patients aged diagnosed between 1997 and 2006, 35% of patients with AML and 34% of patients with ALL aged <55 years underwent HCT.11 In another population-based study from the UK, only 5% of all patients with AML diagnosed between 1997 and 2007 (7%-13% of age groups under 60 years) underwent HCT.12 Similar results were reported for a more recently treated cohort (2000-2014) from Denmark, where only 196 of 1391(14%) treated patients with NFR AML aged from 15 to 70 years eventually underwent HCT.4The rate of HCT has been particularly low for patients older than 50 –or 55 years, even when those patients were treated in a tertiary referral academic center. For example, Estey at al reported that in patients aged >50 years with high-risk AML/MDS treated at the MD Anderson Cancer Center between 2001 and 2003, only 53 (20%) of 259 treated patients were referred for HCT, and only 14 (5.4%) eventually underwent HCT despite being enrolled in a formal protocol to evaluate them for HCT.1
The patients in our study were treated with a combined AL/HCT program. The resulting integration of HCT services and donor search/patient suitability assessment for HCT into the initial treatment of these patients may have contributed to the higher rate of HCT observed in our patients compared with that reported historically from other centers. Consistent with this hypothesis, Pagel et al13 found that a coordinated attempt to identify donors rapidly and prospectively, encouraging HCT for patients at high risk within a Southwestern Oncology Group (SWOG)-led intergroup protocol (S1203), increased the proportion that underwent HCT. Specifically, 65% of patients with AML at high risk aged <60 years in CR1 underwent HCT compared with a historical rate of 40%. Although encouraging, the rate of HCT reported by Pagel et al represents a smaller percentage of patients actually treated (70 of 159; 44%) than that observedin our study (70% for patients aged <60 years). One reason for this difference may be that our patients represent a more recently treated cohort (2016-2021) vs that reported by Pagel (2012-2015). However, a more important factor underlying the higher rate of HCT observed in our study was likely the use of HID in patients without access to an MRD or an adequately matched unrelated donor. Such donors were not used in the study by Pagel et al and most historical reports but represented 44% of donors used for our patients. The lack of a suitable donor was cited as an obstacle in only 3% of the patients in our study. The use of routine and early HID significantly expanded donor access, particularly among patients who were in the minority groups, which constituted a significant proportion of our treated population. Our findings provide evidence that the use of HID has significantly affected timely donor access and has enabled a larger proportion of patients to advance to undergo HCT.
Patients from racial minorities, particularly Black patients, historically have had less access to HCT than their White counterparts.6-8,14 At our center, 26% of patients with NFR AL/MDS treated within the study period self-categorized as Black/African American. Therefore, our study provided an opportunity to determine the extent of any persistent racial disparity in the use of HCT despite the relatively contemporary treatment cohort, extensive use of HID, and the integrated strategy that existed in our program. Multivariable analysis demonstrated that Black patients were significantly less likely to proceed to undergo HCT at our center, despite the given factors (OR of failing to receive HCT = 2.05; P = .023). We also demonstrated that a lack of caregivers and a higher rate of induction death, presumably because of greater socioeconomic deprivation and slower access to initial care, were significant factors underlying this disparity. These factors will need to be addressed if full equity of access to HCT is to be achieved among Black patients. Interestingly, we found that the time from initial therapy to HCT was not prolonged in Black patients who proceeded to undergo HCT when compared with their White counterparts (112 vs 122 days), which is likely attributable to the use of HID and the integration of AL and HCT programs at our center. In a recently published report from Detroit looking at the time to HCT for patients evaluated from 2009 to 2016, Black patients had a significantly longer median time to HCT and a smaller proportion of patients receiving HCT within 6 months of evaluation.15 However, consistent with our observations, there was a significant decrease in this disparity observed in their study between patients first evaluated in 2009 to 2013 vs those first evaluated between 2014 and 2016.
We found that older patients access HCT at a lower rate than younger patients, as has been reported by other observers.14,15 Comorbidities and poor KPS were the major barriers to HCT in this group and may be less amenable to mitigation by the transplant center than other reported obstacles. Conversely, lack of caregiver support was cited as a more frequent problem in patients aged under 60 years. This may be because of the greater spousal work commitments and childcare responsibilities in this age group. Insurance issues were cited as a reason for the failure to advance to HCT in only 3% of our patients. This issue might have been a smaller problem at our center than observed in other settings, because a generous charitable program to facilitate HCT in indigent patients was in effect during this period.
In a landmark analysis comparing patients who received HCT within 6 months of initial therapy with those who did not, and in Cox multivariable analysis, HCT was associated with a survival advantage in our study. A survival benefit of HCT has been previously reported,4,5,11 and our findings confirm the value of consolidative HCT in this population in the current era.
In summary, our findings suggest that in the current era, with the routine use of HIDs and closely integrated leukemia/MDS treatment and HCT services, a much higher proportion of patients proceed to undergo HCT than that historically reported. They also show that the results observed, including an overall survival of 70% for those who underwent transplantation within 6 months of initial treatment and access to HCT for a higher proportion of patients than previously achieved, can be provided outside the setting of a traditional academic medical center.
Separate providers and teams for AL and MDS treatment services and HCT are common, particularly among large academic medical centers. These data suggest that the provision of these services by the same team or at least very closely integrated teams may help improve access to HCT for patients. Although it may not be easy to reorganize the structure of such centers, the provision of navigators that help coordinate such services between departments may mitigate the effects of this separation. Furthermore, jointly funded ancillary staff, such as psychologists and social workers, who recognize and target psychosocial barriers to HCT early in the course of initial treatment may also improve the proportion of patients who successfully proceed to undergo HCT at such centers.
Although these factors might have helped lower the severe racial disparity regarding the access to HCT that has been historically reported, Black patients continue to face obstacles to full equity in this goal. Our data provided insights into the remaining barriers that were most prominent in this group. Specific initiatives that addresses caregiver shortages and slower access to leukemia care in this population may be necessary to address this disparity further.
Refractory malignancy was a significant obstacle to HCT access in our analysis (cited in 19% of cases not proceeding to undergo transplantation). An attempt to proceed with HCT without further attempts at induction therapy was shown to be noninferior to conventional salvage and led to earlier HCT in a recently reported randomized trial from Germany.16 This approach may further improve access to HCT for some patients.
Acknowledgments
The work of Katelin Jackson for managing the Northside Hospital BMT/Leukemia Database is acknowledged. Northside Hospital Cancer Institute is acknowledged for funding the statistical analysis.
Authorship
Contribution: A.B. contributed to the study conception, design, data analysis, and authorship of the manuscript; X.Z. contributed to the statistical analysis, data analysis, and editing of the manuscript; and L.E.M., H.K.H., L.B.-R., S.R.S., and M.S. edited the manuscript and provided the patient data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Asad Bashey, Blood and Marrow Transplant Program at Northside Hospital, 5670 Peachtree Dunwoody Rd, Suite 1000, Atlanta, GA 30342; e-mail: abashey@bmtga.com.
References
Author notes
Deidentified individual data are available on request from the corresponding author, Asad Bashey (abashey@bmtga.com).
The full-text version of this article contains a data supplement.