TO THE EDITOR:
Immune checkpoint inhibitors (ICI) may complicate response assessment via a flare reaction or pseudoprogression (PP). To avoid the mistake of considering a new agent ineffective when a PP was erroneously diagnosed, it was proposed to add the category called indeterminate response (IR) to the Lugano classification,1 in the lymphoma response to immunomodulatory therapy criteria (LYRIC)2 for metabolic and morphologic evaluation assessed using F-fluorodeoxyglucose positron emission tomography and computed tomography (FDG PET/CT). This follows several potential patterns: (1) ≥50% increase in the overall tumor burden (sum of the product of the perpendicular diameters of up to 6 target measurable nodes and extranodal sites) occurred in the first 12 weeks of therapy and without clinical deterioration (IR1); (2) new lesions or ≥50% increase in the existing lesion(s) without a ≥50% increase in the overall tumor burden at any time during treatment (IR2); or (3) increased FDG uptake of 1 or more lesions without any increase in size or number of lesions (IR3). These 3 patterns are defined as progressive metabolic disease (PMD) per the Lugano criteria. PP was defined as an IR that became a partial or complete metabolic response (PMR or CMR), in comparison with that at baseline, at the next evaluation. The risk of the IR category, reciprocally, is to continue to expose patients to an ineffective drug. Therefore, the aim of this study is to prospectively assess the LYRIC criteria in patients with relapsed/refractory diffuse large B-cell lymphomas (DLBCLs) and follicular lymphomas (FLs) treated with atezolizumab (anti–PD-L1), venetoclax (BCL-2 inhibitor), and obinutuzumab (anti-CD20) in the GATA trial3,4 and to measure the possibility and incidence of PP.
The GATA study is an LYSA-sponsored multicenter phase 2 trial (NCT03276468) evaluating the combination of atezolizumab, obinutuzumab, and venetoclax in patients with biopsy-confirmed R/R DLBCL and FL who failed with least 1 line of therapy (rituximab and anthracycline-containing regimen). The local imaging practitioners used the Lugano classification. In the Lugano classification, patients were classified based on 4 possible response categories: CMR, PMR, no metabolic response (NMR), or PMD. They were also classified in the LYRIC classification based on 3 categories (IR1, IR2, and IR3) when a PMD was observed, per the Lugano criteria. Clinical investigators were warned of the possibility of PP, and a coordinating investigator's approval was required before treatment discontinuation. A centralized imaging review of FDG PET/CT was performed in 1 batch at the end of the study using the GaelO platform https://www.gaelo.fr. Three time points were reviewed: baseline, cycle 4 (C4; 12 weeks), and cycle 8 (C8; 24 weeks), based on the Lugano and LYRIC criteria. This was a preplanned analysis, and the GATA trial is now closed.
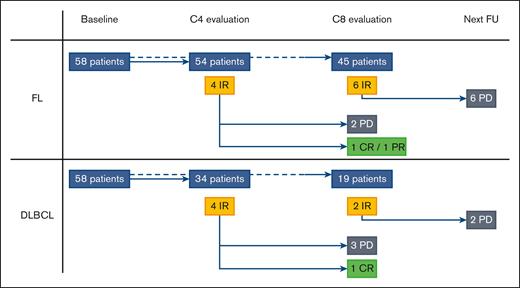
Our study involved 116 patients from the GATA trial, including 58 with FL (median age, 62.5 years) and 58 with DLBCL (median age, 70 years). With a median follow-up of 9 months, the overall metabolic response rate at C8 was 23.6% among patients with DLBCL. With a median follow-up of 14.5 months, the overall metabolic response rate at C8 was 53.6% among patients with FL. Eighty-eight patients (54 with FL and 34 with DLBCL) were evaluated at C4, and 64 at C8 (45 with FL and 19 with DLBCL), all of which were centrally reviewed. Per the Lugano criteria, 84 of 88 reviewed responses (95.5%) were concordant with the local response at C4, and 60 of 64 reviewed responses (93.8%) at C8. The differences included NMR vs PMD (3/8 discordances), CMR vs PMR (2/8), PMR vs PMD (2/8), and PMR vs NMR (1/8). Seven discrepancies were observed for FL and 1 for DLBCL. Per the LYRIC criteria (Figure 1; supplemental Data), 8 out of 88 patients (9.0%; 4/54 with FL and 4/34 with DLBCL) were classified as IR at C4 via a centralized review. During follow-up, 3 cases turned out to be PP (2/4 with FL and 1/4 with DLBCL), and 5 cases showed progressive diseases (PDs). The 3 PP were IR2 or IR3 at C4, and we did not observe any PP as IR1. At C8, 8 out of 64 patients (12.5%; 6 with FL and 2 with DLBCL) were classified as having IR via a centralized review. During follow-up, all of these cases turned out to have PD within 1 or 3 cycles of therapy (Figure 1; supplemental Data). Figure 2 shows a representative example of each, with an IR2 becoming a PP (Figure 2A) and an IR3 proving to be a PD (Figure 2B). Institutional review board approval was obtained during the prospective GATA trial (NCT03276468). This study was conducted in accordance with the Declaration of Helsinki.
Metabolic evaluation per the LYRIC criteria of the GATA trial. In accordance with the LYRIC criteria, 8 patients (4 with FL and 4 with DLBCL; yellow rectangles) were classified as C4. During follow-up, 3 cases turned out to be PP (2 with FL and 1 with DLBCL; green rectangles) and 5 cases of real PDs (black rectangles). At C8 evaluation, 8 patients (6 with FL and 2 with DLBCL; yellow rectangles) were classified as IR. During the follow-up, all of these cases turned out to be real PDs (black rectangles) within 1 or 3 cycles of therapy. CR, complete response; PR, partial response; FU, follow-up.
Metabolic evaluation per the LYRIC criteria of the GATA trial. In accordance with the LYRIC criteria, 8 patients (4 with FL and 4 with DLBCL; yellow rectangles) were classified as C4. During follow-up, 3 cases turned out to be PP (2 with FL and 1 with DLBCL; green rectangles) and 5 cases of real PDs (black rectangles). At C8 evaluation, 8 patients (6 with FL and 2 with DLBCL; yellow rectangles) were classified as IR. During the follow-up, all of these cases turned out to be real PDs (black rectangles) within 1 or 3 cycles of therapy. CR, complete response; PR, partial response; FU, follow-up.
Illustration of the 2 IR and their evolution. (A) At baseline, 1 case harboring metabolic lymph nodes in the right supraclavicular, axillary, and inguinal areas at baseline (blue and red arrows). At C4, a new left inguinal lesion appeared, with an increase in the existing right supraclavicular lesions (blue arrows) while metabolic regression was observed (red arrows), showing an IR2 perthe LYRIC criteria. At C8, no metabolic activity was observed in the supraclavicular, axillary, or inguinal areas, indicating a complete metabolic response while retrospectively setting the PP at C4. (B) At baseline, 1 case harbored metabolic nodes in the left iliac and inguinal areas at baseline (blue and red arrows). At C4, 2 lesions showed increased metabolic uptake in the left iliac and inguinal areas, without morphological modification (blue arrows), whereas metabolic regression was observed in the other lesions (red arrow), showing an IR3 per the LYRIC criteria. At C8, there was an increase of metabolic lesions in the para aortic, left iliac, and left inguinal areas pointing to a “true” progression.
Illustration of the 2 IR and their evolution. (A) At baseline, 1 case harboring metabolic lymph nodes in the right supraclavicular, axillary, and inguinal areas at baseline (blue and red arrows). At C4, a new left inguinal lesion appeared, with an increase in the existing right supraclavicular lesions (blue arrows) while metabolic regression was observed (red arrows), showing an IR2 perthe LYRIC criteria. At C8, no metabolic activity was observed in the supraclavicular, axillary, or inguinal areas, indicating a complete metabolic response while retrospectively setting the PP at C4. (B) At baseline, 1 case harbored metabolic nodes in the left iliac and inguinal areas at baseline (blue and red arrows). At C4, 2 lesions showed increased metabolic uptake in the left iliac and inguinal areas, without morphological modification (blue arrows), whereas metabolic regression was observed in the other lesions (red arrow), showing an IR3 per the LYRIC criteria. At C8, there was an increase of metabolic lesions in the para aortic, left iliac, and left inguinal areas pointing to a “true” progression.
The Lugano criteria using the Deauville visual scale are currently widely used interpretation criteria for the response assessment of lymphoma.5 However, this concept of PP is not reflected in this classification. Thus, LYRIC criteria have been issued to reflect PP in patients with lymphoma treated with immunotherapy using the IR category. The building of the LYRIC criteria was mostly based on data from Hodgkin lymphoma (HL) due to the activity of ICI in this setting. Compared with the Lugano classification, the LYRIC criteria appear to be more flexible because IR allows treatment to continue until the next evaluation, which determines whether the patient was in PP or PD. To our knowledge, this is the first prospective study that evaluates per the LYRIC criteria as a secondary end point of a clinical trial offering a checkpoint inhibitor.
In our analysis, 8 cases were classified as IR per LYRIC criteria at the C4 evaluation. Per the Lugano criteria, these cases were necessarily classified as PMD. Five cases were subsequently confirmed as PD at the next evaluation (2 FL and 3 DLBCL cases). In other words, in our study, the concept of IR has contributed to the unnecessary exposure of 5.7% of the patients to ineffective therapy. Reciprocally, the same concept has contributed to avoiding the mistake of considering a new agent ineffective in 3.4% of patients, because we observed 3 IRs at C4 that became a complete response or partial response at C8 (2 with FL and 1 with DLBCL). The rate of patients with at least 1 pseudoprogressive lesion upon early FDG PET/CT evaluation was equal to or lower than that reported in other retrospective studies.6-8 Notably, no delayed PPs were observed. Therefore, according to our data, if LYRIC is used in this context, it should only be used for early evaluation (after 4 cycles or 16 weeks in our study). Moreover, an alternative would be to use the Lugano criteria, and in case of PMD without clinical deterioration, to continue the treatment with the plan for an early re-evaluation (after 2 cycles/8 weeks). This proposal is not supported by any data but seems reasonable given the additional complexity brought about by LYRIC and the scarcity of PPs in this context.
The applicability of our results is limited by the scope of our study, that is, the therapeutic field of non-HL (NHL), as well as the small number of patients. Furthermore, this must be interpreted in the context of the relatively low response rates in this study, and more broadly, with ICI in NHL. More precisely, the evaluation of the PP percentage might have been influenced by the progression of 28 patients before any interim assessment. Similarly, potential bias was introduced by the required coordinating investigators’ approval to discontinue treatment. Future studies will seek to extend these results to larger cohorts of NHL, and to patients with HL. Moreover, LYRIC are used in all clinical trials evaluating immune therapies and are time consuming. Therefore, we believe that a similar analysis should be performed in the context of chimeric antigen receptor T cells and bispecific antibodies.
Acknowledgments: The authors thank the patients and their families, Lymphoma Study Association, and Lymphoma Academic Research Organization.
Contributions: Y.A.T. and C.H. contributed to the overall design and performed the research; Y.A.T., C.H., and R.O.C. analyzed the data and performed the statistical analyses; Y.A.T., C.H., C. Baillet, E.B., E.N.-V., J.M.S.D.c., C. Bailley, S.K., S.G., E.G., R.G., N.M., L.Y., S.L.G., H.T., R.H., F.M., and G.C. provided clinical care and collected data; and all authors critically reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Charles Herbaux, Hematology, CHU Montpellier, Montpellier, France; e-mail: c-herbaux@chu-montpellier.fr.
References
Author notes
Data are available on request from the corresponding author, Charles Herbaux (c-herbaux@chu-montpellier.fr).
The full-text version of this article contains a data supplement.