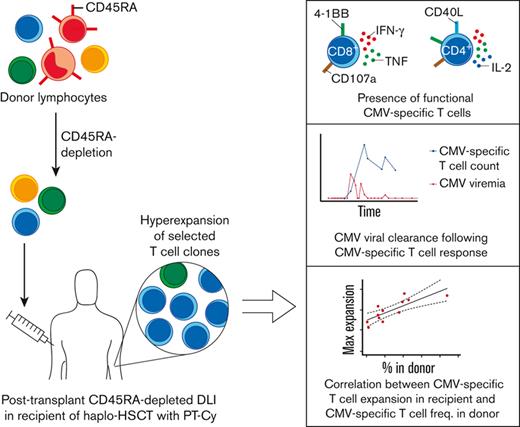
Key Points
Infusions of donor memory T-cells after haplo-HSCT lead to engraftment, persistence, and prominent expansion of selected T-cell clones.
Infused patients harbor highly functional CMV-specific T-cells and their expansion correlates with their frequency in the donor.
Abstract
Haploidentical hematopoietic stem cell transplantation (haplo-HSCT) with post-transplant cyclophosphamide is a curative treatment for many hematological malignancies, yet a majority of patients still suffers from recurrent infections. Post-transplant infusion of memory T-cells could potentially enhance immunological protection without increasing the risk of eliciting acute graft-versus-host disease, which is mainly induced by naïve T-cells. Here, we performed longitudinal analysis of the lymphocyte compartment in 19 patients who underwent haplo-HSCT previously enrolled in a phase II prospective clinical trial (www.clinicaltrials.gov as #NCT04687982), in which they received post-transplant CD45RA-depleted donor lymphocyte infusions (DLI). T-cell receptor sequencing analysis showed that, surprisingly, CD45RA-depleted DLI do not increase T-cell clonal diversity, but lead to prominent expansion of a selected number of infused memory T-cell clones, suggesting recruitment of these cells in the immune response. Pathogen-specific memory T-cells, including cytomegalovirus (CMV)-specific cells, were engrafted and were able to persist for at least 1 month. Deep immunophenotyping revealed strong polyfunctional effector CMV-specific T-cell responses in the majority of patients, with their expansion correlating with the frequency of CMV-specific cells in the donor. These findings provide a rationale behind the suggested improved protection against viral infections in patients receiving CD45RA-depleted DLI.
Introduction
Allogeneic hematological stem cell transplantation (HSCT) has curative potential for multiple hematological malignancies. The development of HSCT from HLA-haploidentical related donors has greatly increased the accessibility of allogeneic HSCT to patients, and use of post-transplant cyclophosphamide (PT-Cy) even allows for the transfer of T-replete grafts in this setting, which enhances immune reconstitution.1,2 PT-Cy decreases the risk of acute graft-versus-host disease (aGvHD) by preferentially targeting highly proliferating cells, thereby either depleting3 or functionally inhibiting4 alloreactive T-cells transferred with the graft. However, the procedure may deplete alloreactive memory T-cells that are otherwise pathogen-specific.5 The loss of antigen-specific memory T-cell precursors might explain why a majority of patients still experiences post-transplant infections, in particular reactivation of latent viruses such as cytomegalovirus (CMV), which are a major cause of nonrelapse morbidity and mortality.6
Studies in mice have shown that aGvHD is mainly caused by alloreactive naïve T-cells.7-10 On the other hand, memory T-cells harbor a poised epigenetic state that, among other factors, allows faster and superior protection against pathogens in comparison with naïve T-cells.11,12 On the basis of these differences, we hypothesize that add-back of donor memory T-cells can enhance immune reconstitution and immunological protection without eliciting aGvHD.
We conducted a phase II clinical trial, in which patients treated with haploidentical-HSCT (haplo-HSCT) with PT-Cy for hematological malignancies received post-transplant infusions of memory T-cells.13On the basis of the principle that naïve T-cells express the CD45RA isoform of the CD45 gene, whereas memory T-cells preferentially express the CD45RO isoform, the donor lymphocyte infusions (DLI) consisted of CD45RA-depleted lymphocytes. The treatment was deemed safe, with only 1 out of 19 treated patients developing grade 2 aGvHD, which was successfully treated by steroids. Comparison with a historical cohort of patients treated with haplo-HSCT with PT-Cy suggested a lower incidence of infections in the patients who received CD45RA-depleted DLI.13
Here, we performed a prospective immunological study on the patients enrolled in the trial to better understand the impact of CD45RA-depleted DLI on immune reconstitution and responses. DLI administration led to a prominent expansion of selected T-cell clones, suggesting recruitment of these cells in the immune response. Pathogen-specific memory T-cells were able to engraft and persist for at least 1 month, potentially explaining the tendency toward a lower incidence of viral infections observed in patients treated with the CD45RA-depleted DLI.
Methods
Patients
The results presented here are of the immunological analysis of samples from patients enrolled in a phase II single-center prospective study (www.clinicaltrials.gov as #NCT04687982).13 Inclusion criteria for the study were age ≥18 years and HSCT with PT-Cy for hematological disease, with myeloablative condition regimens, reduced intensity conditioning regimens, or nonmyeloablative condition regimens. Exclusion criteria were active grade 2 to 4 aGvHD, uncontrolled infection, severe cytopenia, and progressive disease at the time of enrollment. In total, 19 patients received CD45RA-depleted DLI, prepared from donor apheresis using CliniMACS and the CD45RA-depletion product line (Miltenyi Biotec). Patient characteristics are summarized in supplemental Table 1. Patients received up to 3 CD45RA-depleted DLI, each 4 to 6 weeks apart and with dose escalation. The first DLI consisted of fresh cells and a dose of 5×105 CD3/kg bodyweight, the second and third doses used thawed, cryopreserved aliquots with a dose of 1×106 and 5×106 CD3/kg bodyweight, respectively. Patients were monitored for CMV reactivation using polymerase chain reaction, twice a week from day +15 to day +100 and then weekly until day +180. Twelve patients (63%) experienced CMV reactivation before receiving the first DLI. Patients who received DLI were compared to control patients treated with haplo-HSCT with PT-Cy analyzed in previous studies,14,15 including only patients who would have hypothetically matched the inclusion criteria for the clinical study. Blood samples from 10 additional patients treated with haplo-HSCT with PT-Cy at our institute were used as controls for T-cell receptor sequencing (TCR-seq) analysis. The trial was approved by the local independent Ethics committee (approval no. ONC-2016-002) and the clinical work was approved by the internal review board of the Istituto di Ricovero e Cura a Carattere Scientifico Humanitas Research Hospital (protocols 8/2/2013 and 1397) and conducted in accordance with the Declaration of Helsinki. Signed written informed consent was obtained from all patients and donors.
TCR-seq
RNA was extracted from 3×105 cells per sample using the Qiagen RNeasy Micro kit. For DLI samples not containing a sufficient number of cells, peripheral blood mononuclear cells from the donor were manually depleted of CD45RA+ cells using CD45RA MicroBeads (Milteny Biotec). Two DLI samples were excluded because of insufficient RNA integrity as determined by Tape Station. Of the remaining 48 samples, libraries were constructed using the SMARTer Human TCR αβ Profiling Kit v2 (Takara Bio USA), from which the CDR3 region of the TCR β chain was sequenced in a single run on the Illumina NextSeq. The frequency of TCR β chain clonotypes was determined using MiXCR (version 3.0.14).16,17 Libraries contained 2334 to 44 388 functional CDR3β clonotypes. Exploratory analysis and diversity estimation of the TCR β repertoire were performed with the Immunarch package (version 0.6.6)18 for R, whereas repertoire overlap analysis was performed using VDJtools version 1.2.119. The clonotype scatter plot was produced using a custom script based on the Matplotlib package (version 3.4.3). Annotation of clonotypes was performed with the Immunarch package, using the immune receptor databases VDJDB, McPAS-TCR, and PIRD TBAdb.
Cell culture
Cryopreserved peripheral blood mononuclear cells were either thawed and stained directly, or rested for 6 hours at 37°C 5% CO2 and then stimulated for 16 hours at 37°C 5% CO2, in presence of GolgiPlug and GolgiStop (both BD Biosciences), 1 μg/mL soluble anti-CD28 (BD Biosciences), and either a pool of 138 peptides (15mers with 11 amino acids overlap) derived from CMV-pp65 protein (1 μg/mL; JPT Technologies) or peptide solvent (dimethyl sulfoxide) for unstimulated samples. Culture medium consisted of RPMI-1640 (Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (Sigma-Aldrich), 1% Ultraglutamine, and 1% penicillin-streptomycin (both Lonza).
Flow cytometry
Detailed flow cytometry procedures have been previously described.20 In brief, a fraction of the freshly thawed peripheral blood mononuclear cells were labeled with Zombie Aqua Fixable Viability dye and a flow cytometry panel designed to analyze natural killer (NK)-cell, γδ T-cell, and innate lymphoid cell (ILC) phenotypes (supplemental Table 2). The cells cultured overnight were labeled with Zombie Aqua Fixable Viability dye and a flow cytometry panel designed to analyze T-cell phenotypes (supplemental Table 3). Fixation and staining for intracellular markers were performed with the FoxP3 transcription factor buffer kit (eBioscience). Cells were acquired on a BD FACSymphony A5.
Manual flow cytometric analysis
Flow cytometric compensation and gating were performed using FlowJo version 9. The gating strategy is shown in supplemental Figure 1A-B. Frequencies of CMV-specific T-cells were determined by the sum of interferon gamma (IFN-γ)-, tumor necrosis factor (TNF)- and interleukin 2 (IL-2)-expressing cells. Boolean gating was used to define expression of combinations of cytokines. Background correction was applied using the unstimulated samples, and negative values were set to 0. Effector function analysis was performed with Simplified Presentation of Incredibly Complex Evaluations (SPICE; version 5).21
Unsupervised high-dimensional analysis
Flow cytometric compensation and gating was performed using FlowJo version 9. After removing aggregates and CD14+ cells and gating for live CD3+ lymphocytes, a maximum of 1000 CD8+ events and CD4+ events from unstimulated samples were exported per bulk T-cell profiling. Within the CD8+ and CD4+ gates, Boolean gating was applied for 4-1BB, IFN-γ, TNF and IL-2. A maximum of 1000 activation marker-positive cells from peptide-stimulated and unstimulated samples were exported for CMV-specific T-cell analysis. For NK-cell profiling, aggregates, CD14+, CD3+, and lineage+ cells were removed. NK-cells were identified based on expression of CD56 and/or CD16, and a maximum of 2000 events were exported per sample. Exported events were biexponentially transformed in FlowJo version 10 and clustered with PhenoGraph (version 1.5.3) as previously described,22 using a pipeline in Python available at http://github.com/luglilab/Cytophenograph. The following K number of nearest neighbors were used: 50, 100, 50, 50, and 30 for bulk CD8+ T-cell, bulk CD4+ T-cell, NK-cell, CMV-specific CD8+ T-cell, and CMV-specific CD4+ T-cell analyses, respectively. A fixed seed of 123456 was used for all analyses. Clusters representing <1% of the total events were excluded from downstream analysis. Balloon plots and heatmaps were generated using the ggplot2 package for R. For calculating background-corrected CMV-specific T-cell cluster frequencies, cluster frequencies were first expressed as frequencies among total CD8+ or CD4+ T-cells. Background correction was then applied by subtracting the frequency of a given cluster found in an unstimulated sample from the frequency of the same cluster found in the same sample stimulated with CMV peptides, and negative values were set to 0.
Statistical analysis
GraphPad Prism software (version 7) and the R stats package were used for statistical analyses. Statistical significance was tested with Wilcoxon, Mann-Whitney or Kruskal-Wallis test. P values <.05 were considered significant.
Results
Limited contribution of CD45RA-depleted DLI on lymphocyte counts after haplo-HSCT
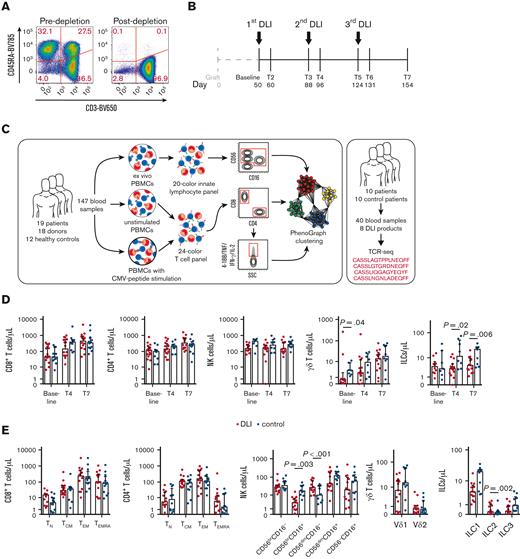
Nineteen recipients of haplo-HSCT with PT-Cy were enrolled in a phase II clinical study around day +50 after transplantation, in which they received DLI with the aim of enhancing protection against opportunistic pathogens. The DLI product was depleted of CD45RA+ cells, thus consisting almost exclusively of memory T-cells (Figure 1A). Patients received up to 3 DLI with increasing cell numbers, each about 1 month apart. Blood samples were collected just before DLI administration, 1 week after DLI administration, and 1 month after the third DLI (Figure 1B). Blood samples of the patients, their donors, and of healthy controls harboring CMV-specific T-cells were analyzed through high-dimensional flow cytometry and bulk TCR-seq, to reveal the dynamics and immunological phenotypes of NK-cells and total and CMV-specific T-cells (Figure 1C).
Immune reconstitution after haplo-HSCT with CD45RA-depleted DLI. (A) CD45RA expression by lymphocytes in the DLI product before and after depletion of CD45RA+ cells. A representative DLI product is shown. (B) Schematic overview of DLI administration and blood sample collection. Median days post-transplant are indicated. (C) Experimental approach. Blood samples from patients who received DLI, their donors, and unrelated healthy controls were stained with a flow cytometry panel allowing the detection of innate lymphocytes. The remaining cells of each sample were cultured overnight in presence or absence of a CMV-peptide library, and stained with a T-cell–focused flow cytometry panel. Unstimulated samples were used for bulk T-cell analysis, whereas CMV-specific cells were identified through Boolean gating for activation markers in response to CMV-peptide stimulation. NK cells, bulk, and CMV-specific T-cell phenotypes were analyzed in more detail with unsupervised clustering. In addition, TCR sequencing of blood and DLI samples from patients who received DLI and control patients was performed to assess the impact of the DLI at the clonal level. (D) Median lymphocyte counts with interquartile range in patients who underwent haplo-HSCT and received or did not receive CD45RA-depleted DLI (n = 7-17/group). Statistical significance was determined with Mann-Whitney test. (E) Median lymphocyte counts with interquartile range at T7 in patients who underwent haplo-HSCT and received or did not receive CD45RA-depleted DLI (n = 10-14/group). Statistical significance was determined with Mann-Whitney test. ILC1, group 1 innate lymphoid cell; ILC2, group 2 innate lymphoid cell; ILC3, group 3 innate lymphoid cell; TCM, central memory T cell; TEM, effector memory T cell; TEMRA, effector memory T cell re-expressing CD45RA; TN, naïve T cell.
Immune reconstitution after haplo-HSCT with CD45RA-depleted DLI. (A) CD45RA expression by lymphocytes in the DLI product before and after depletion of CD45RA+ cells. A representative DLI product is shown. (B) Schematic overview of DLI administration and blood sample collection. Median days post-transplant are indicated. (C) Experimental approach. Blood samples from patients who received DLI, their donors, and unrelated healthy controls were stained with a flow cytometry panel allowing the detection of innate lymphocytes. The remaining cells of each sample were cultured overnight in presence or absence of a CMV-peptide library, and stained with a T-cell–focused flow cytometry panel. Unstimulated samples were used for bulk T-cell analysis, whereas CMV-specific cells were identified through Boolean gating for activation markers in response to CMV-peptide stimulation. NK cells, bulk, and CMV-specific T-cell phenotypes were analyzed in more detail with unsupervised clustering. In addition, TCR sequencing of blood and DLI samples from patients who received DLI and control patients was performed to assess the impact of the DLI at the clonal level. (D) Median lymphocyte counts with interquartile range in patients who underwent haplo-HSCT and received or did not receive CD45RA-depleted DLI (n = 7-17/group). Statistical significance was determined with Mann-Whitney test. (E) Median lymphocyte counts with interquartile range at T7 in patients who underwent haplo-HSCT and received or did not receive CD45RA-depleted DLI (n = 10-14/group). Statistical significance was determined with Mann-Whitney test. ILC1, group 1 innate lymphoid cell; ILC2, group 2 innate lymphoid cell; ILC3, group 3 innate lymphoid cell; TCM, central memory T cell; TEM, effector memory T cell; TEMRA, effector memory T cell re-expressing CD45RA; TN, naïve T cell.
From day +50 (baseline) to day +150 (T7), counts of CD8+, CD4+, and γδ T cells gradually increased, whereas counts of NK cells and ILCs remained stable (supplemental Figure 2). The number of CD8+ T cells significantly increased 1 week after administration of the third DLI. Considering that the number of infused T cells was low compared with the total T-cell pool and no immediate increase in CD4+ T-cell counts was observed after DLI administration, the increase in CD8+ T-cell counts suggests that infused CD8+ T cells had expanded.
We then compared cell counts of major lymphocyte subsets of the patients who received DLI with that of control patients who underwent haplo-HSCT analyzed in a previous study.14,15 Counts of CD8+ T cells, CD4+ T cells, and NK cells were similar between the 2 groups, but rare ILC numbers were slightly decreased in the patients who received DLI compared with the controls (Figure 1D). Manual gating of flow data revealed that besides minor alterations in rare NK cell and ILC subsets, CD8+ and CD4+ naïve and memory subsets were remarkably similar (Figure 1E).
High-dimensional profiling reveals the T- and NK-cell dynamics after DLI infusion
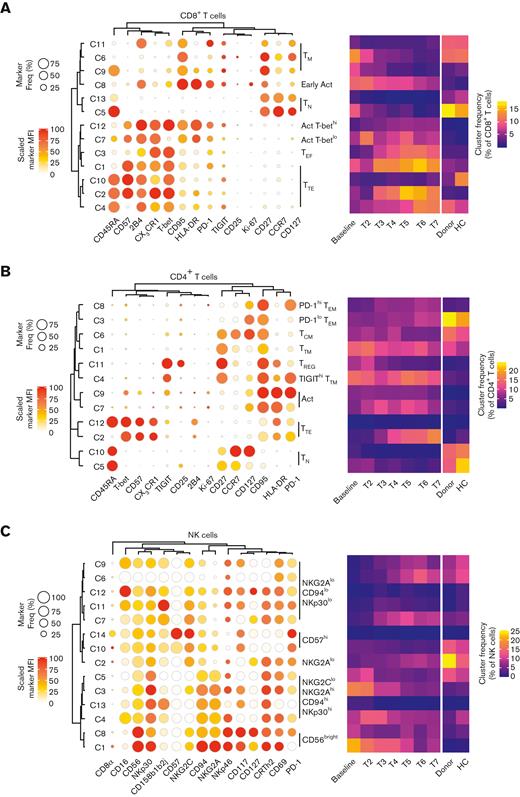
We analyzed T- and NK-cell phenotypes of patients who received DLI in great detail through uniform manifold approximation and projection (supplemental Figure 3) and unsupervised clustering using the PhenoGraph algorithm (Figure 2A-C and supplemental Figure 4),22 thereby taking advantage of all markers included in the high-dimensional flow panels. Patients who underwent transplant showed a strong defect in naïve T cells (CD8 clusters 5 and 13 and CD4 clusters 5 and 10), an increase in frequency of regulatory T cells (CD4 cluster 11) and HLA-DRhiCD95hi activated phenotypes (CD8 clusters 7, 8, and 12 and CD4 clusters 7 and 9) that waned over time, and an accumulation of T-bethiCX3CR1hi effector (CD8 cluster 3) and terminal effector clusters expressing CD45RA and/or CD57 (CD8 clusters 1, 2, and 4 and CD4 cluster 2), corroborating previous analyses.14 Interestingly, programmed death receptor 1 expression was increased on CD4+ effector memory cells (cluster 8), whereas the patients also uniquely harbored a programmed death receptor 1hiTIGIThiCD27hiCD127loCCR7lo CD4+ transitional memory subset (cluster 4). Within the NK-cell compartment, the frequency of cluster 1 of CD56bright cells and cluster 3 of CD56dim cells, both featuring high levels of NKG2A, CD94, and NKp30, was greatly increased at baseline but diminished during follow-up. Instead, CD56dim/loNKG2AloCD94loNKp30lo cells (clusters 6, 7, and 9) increased over time, as previously reported.15 Taken together, CD45RA-depleted DLI appear to have only a limited impact on lymphocyte reconstitution at the phenotypic, bulk level.
T- and NK-cell dynamic changes after haplo-HSCT with CD45RA-depleted DLI. (A-C) Total CD8+ T cells (A), CD4+ T cells (B) and NK cells (C) were clustered by PhenoGraph. Cluster identities are revealed by balloon plots, whereas the heatmaps show median cluster dynamics in patients who received DLI (n = 19) at different time points, their donors (n = 18), and unrelated CMV+ healthy controls (n = 12). Samples containing <50 total CD8+ T cells, CD4+ T cells, or NK cells were excluded from temporal analysis. Act, activated; HC, healthy control; TCM, central memory T cell; TEF, effector T cell; TEM, effector memory T cell; TEMRA, effector memory T cell re-expressing CD45RA; TM, memory T cell; TN, naïve T cell; TREG, regulatory T cell; TTE, terminal effector T cell; TTM, transitional memory T cell.
T- and NK-cell dynamic changes after haplo-HSCT with CD45RA-depleted DLI. (A-C) Total CD8+ T cells (A), CD4+ T cells (B) and NK cells (C) were clustered by PhenoGraph. Cluster identities are revealed by balloon plots, whereas the heatmaps show median cluster dynamics in patients who received DLI (n = 19) at different time points, their donors (n = 18), and unrelated CMV+ healthy controls (n = 12). Samples containing <50 total CD8+ T cells, CD4+ T cells, or NK cells were excluded from temporal analysis. Act, activated; HC, healthy control; TCM, central memory T cell; TEF, effector T cell; TEM, effector memory T cell; TEMRA, effector memory T cell re-expressing CD45RA; TM, memory T cell; TN, naïve T cell; TREG, regulatory T cell; TTE, terminal effector T cell; TTM, transitional memory T cell.
DLI-derived pathogen-specific memory T-cells engraft and are recruited in the immune response
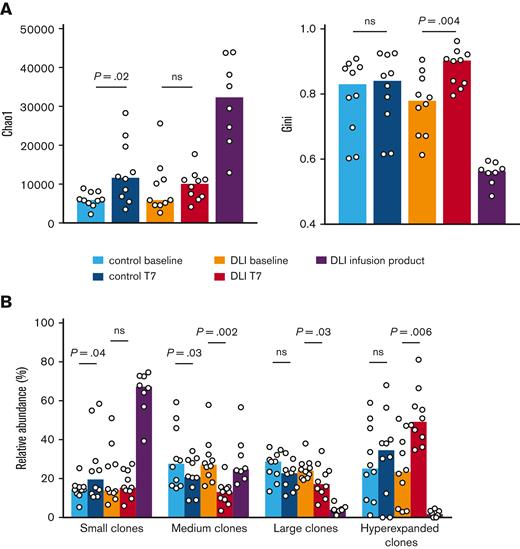
Memory T cells detected in the patients could be derived from either the DLI or the initial graft. To gain more insight in the specific contribution of DLI-derived clonotypes on immune reconstitution, we performed TCR-seq on blood samples of 10 patients who underwent haplo-HSCT and received CD45RA-depleted DLI and 10 control patients who underwent haplo-HSCT but did not receive DLI. We analyzed the clonal diversity by computing both the Chao1 estimator, a measure of TCR richness, and the Gini coefficient, a measure of the inequality among clonotype size within the repertoire. Even though the DLI lacked naïve T cells, the clonal diversity of the patient samples was even lower, indicated by a low Chao1 estimator and high Gini coefficient (Figure 3A) and consistent with a previous report on the very clonal nature of the T-cell pool in patients who underwent HSCT.23 Nevertheless, TCR richness significantly increased for control patients from day +50 to day +150, whereas that for the patients who received the DLI did not. Instead, the inequality among clonotypes increased during follow-up of the patients who received DLI, suggesting expansion of certain clones. The increased clonal diversity at T7 for control patients was attributed to a modest though significant increase in small clones, at the expense of medium clones (Figure 3B). For patients receiving DLI, no change was observed for small clones. Instead, there was a strong increase in hyperexpanded clones (constituting >1% of all TCR β sequences24), at the expense of medium and large clones. This suggests that CD45RA-depleted DLI do not increase clonal diversity of the T-cell pool, but may nonetheless significantly contribute to T-cell reconstitution through recruitment of infused clones in the immune response. Reactivation of CMV may have a strong impact on the clonality of the T-cell pool through the process of memory inflation of CMV-specific T cells.25 Yet, the difference in hyperexpanded clones between the 2 patient groups could not be solely attributed to a difference in the incidence of CMV reactivation (supplemental Figure 5A), suggesting that this is a DLI-dependent phenomenon.
CD45RA-depleted DLI induce hyperexpansion of selected T-cell clones. TCR-seq was performed on samples of patients who received DLI collected at baseline and T7 (n = 10 patients), the DLI (n = 8), and samples of control patients (n = 10 patients) collected around day +50 and day +150. (A) Left graph shows the Chao1 diversity index and right graph shows the Gini diversity index. Medians are indicated. Statistical significance was determined by Wilcoxon test. (B) Clonotypes were categorized into small, medium, large, and hyperexpanded clones which constituted <0.01%, 0.01%–0.1%, 0.1%–1%, and >1% of the repertoire, respectively. Medians are indicated. Statistical significance was determined by Wilcoxon test.
CD45RA-depleted DLI induce hyperexpansion of selected T-cell clones. TCR-seq was performed on samples of patients who received DLI collected at baseline and T7 (n = 10 patients), the DLI (n = 8), and samples of control patients (n = 10 patients) collected around day +50 and day +150. (A) Left graph shows the Chao1 diversity index and right graph shows the Gini diversity index. Medians are indicated. Statistical significance was determined by Wilcoxon test. (B) Clonotypes were categorized into small, medium, large, and hyperexpanded clones which constituted <0.01%, 0.01%–0.1%, 0.1%–1%, and >1% of the repertoire, respectively. Medians are indicated. Statistical significance was determined by Wilcoxon test.
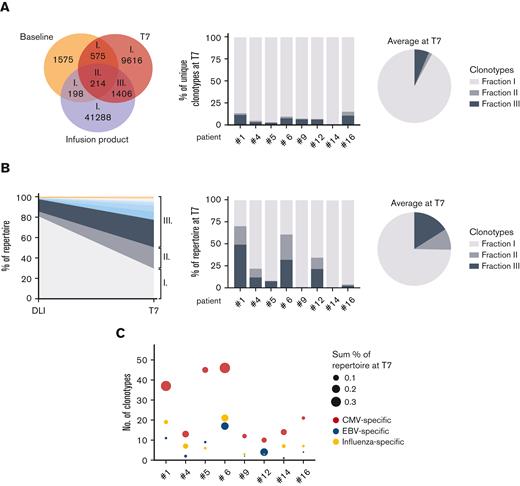
To further validate this finding, we explored the clonal overlap among baseline, T7, and DLI samples of 8 patients who received DLI. Clonotypes overlapping between T7 and DLI, but not between T7 and baseline, were present in all 8 patients (Figure 4A; supplemental Figure 5B). These DLI-derived clonotypes constituted up to 11.9% (mean 6.5%) of all unique clonotypes at T7. Upon looking at their relative abundance, the contribution of DLI-derived clonotypes was even more pronounced (mean 16.0%; Figure 4B; supplemental Figure 5C). In patient #1, DLI-derived clonotypes made up 49.5% of the entire repertoire in terms of abundance 1 month after the final infusion. The 10 most abundant clones in this fraction contributed to 22.7% of the entire repertoire, underlining the expanded nature of these clones. Because CMV can strongly affect T-cell reconstitution after haplo-HSCT with PT-Cy,14 we asked whether CMV-specific T cells could be detected within the DLI-derived clones. Indeed, public clonotypes associated with CMV-specificity were detected in all patients, as were Epstein-Barr virus– and influenza-specific clonotypes (Figure 4C). These DLI-derived pathogen-specific cells could have been administered with any of the 3 DLI and so their ability to persist is at least 1 month.
DLI-derived memory T cells are engrafted and recruited in the immune response. (A) Venn diagram shows the clonal overlap in number of unique clonotypes of patient #1. The bar graph shows the clonal overlap at T7 with the DLI separately for patients who received DLI as frequency of the total number of unique clonotypes at T7, whereas their average is shown in the pie chart. (B) Left graph shows the relative abundance of clonotypes overlapping between the DLI and T7 of patient #1. Roman numerals indicate the clonotype fractions shown in (A). The top 10 most abundant clones of fraction III are highlighted in color. The bar graph shows the abundance of overlapping clonotypes at T7 separately for patients who received DLI, whereas their average is shown in the pie chart. (C) DLI-derived engrafted clonotypes (fraction III) of each patient were analyzed for the presence of public TCR sequences known to harbor specificity for CMV-, Epstein-Barr virus- or influenza-derived epitopes.
DLI-derived memory T cells are engrafted and recruited in the immune response. (A) Venn diagram shows the clonal overlap in number of unique clonotypes of patient #1. The bar graph shows the clonal overlap at T7 with the DLI separately for patients who received DLI as frequency of the total number of unique clonotypes at T7, whereas their average is shown in the pie chart. (B) Left graph shows the relative abundance of clonotypes overlapping between the DLI and T7 of patient #1. Roman numerals indicate the clonotype fractions shown in (A). The top 10 most abundant clones of fraction III are highlighted in color. The bar graph shows the abundance of overlapping clonotypes at T7 separately for patients who received DLI, whereas their average is shown in the pie chart. (C) DLI-derived engrafted clonotypes (fraction III) of each patient were analyzed for the presence of public TCR sequences known to harbor specificity for CMV-, Epstein-Barr virus- or influenza-derived epitopes.
Magnitude of the CMV-specific T-cell response in patients who received DLI correlates with the abundance of CMV-specific T cells in the donor
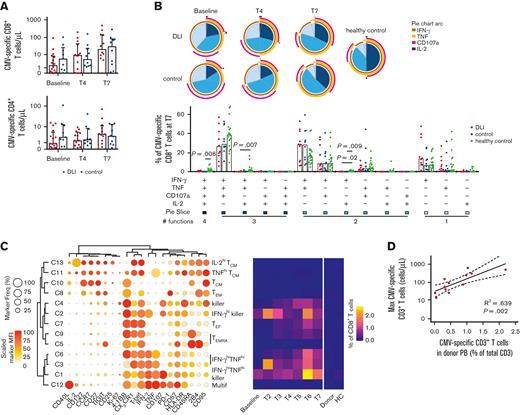
CMV is one of the major pathogens causing infections early after HSCT, even when using T-replete grafts of a CMV+ donor,6 and despite the transfer of CMV-specific T cells with haplo-HSCT and PT-Cy.5 DLI of enriched CMV-specific T cells from a donor have been reported to help resolve CMV infection.26 Because CMV-specific memory T cells were transferred with the DLI, engrafted, and persisted, we analyzed their number and phenotype in an in vitro CMV-pp65 peptide library stimulation assay. Comparing patients who received DLI to control patients revealed no significant difference in absolute numbers of CMV-specific T cells during follow-up, but a large interpatient variability was apparent (Figure 5A). We next explored the quality of the CMV-specific T-cell response by examining specific combinations of effector functions. Early after transplant, practically all CMV-specific CD8+ T cells expressed IFN-γ (Figure 5B; supplemental Figure 6A). During follow-up, the contribution of CD8+ T cells producing solely IFN-γ decreased, and that of cells with 3 effector functions (CD107a, IFN-γ, and TNF) increased, as previously reported.14 IL-2 production was rare, and by T7 even significantly decreased in both patient cohorts compared to healthy controls. In contrast, IL-2 production was relatively common in the CMV-specific CD4+ T-cell compartment, with the 2 patient groups again showing a very similar spectrum of effector functions (supplemental Figure 6B, 7A).
Magnitude of recipient CMV-specific T-cell responses correlates with the abundance of CMV-specific T cells in the donor. (A) Counts of CMV-specific T cells in CMV-reactivating patients who underwent haplo-HSCT and received or did not receive CD45RA-depleted DLI (n = 9-16/group). Medians with interquartile range are shown. Statistical significance was determined with Mann-Whitney test. (B) Effector functions of CMV-specific CD8+ T cells in patients who underwent haplo-HSCT and received or did not receive CD45RA-depleted DLI, and in CMV+ healthy controls (n = 6-20/group in pie charts, n = 10-20/group in bar graphs). Samples containing <35 CMV-specific CD8+ T cells were excluded from analysis. Medians are shown. Statistical significance was determined by Kruskal-Wallis and posthoc Dunn’s test with Bonferroni correction. (C) CMV-specific CD8+ T cell PhenoGraph cluster identities are revealed by balloon plot, whereas the heatmap shows median cluster dynamics in CMV-reactivating patients who received DLI (n = 17), their donors (n = 16), and CMV+ healthy controls (n = 12). Samples containing <50 total CD8+ T cells were excluded from temporal analysis. (D) Linear regression with 95% confidence interval bands on the maximum measured abundance of CMV-specific T cells in the patients who received DLI vs the frequency of CMV-specific T cells in the peripheral blood of the donor. Only patients with a clear CMV-specific T cell response are included in the analysis (n = 12; one patient was excluded because of missing donor sample). Count values were log-transformed. HC, healthy control; Multif, multifunctional; TCM, central memory T cell; TEF, effector T cell; TEM, effector memory T cell; TEMRA, effector memory T cell re-expressing CD45RA.
Magnitude of recipient CMV-specific T-cell responses correlates with the abundance of CMV-specific T cells in the donor. (A) Counts of CMV-specific T cells in CMV-reactivating patients who underwent haplo-HSCT and received or did not receive CD45RA-depleted DLI (n = 9-16/group). Medians with interquartile range are shown. Statistical significance was determined with Mann-Whitney test. (B) Effector functions of CMV-specific CD8+ T cells in patients who underwent haplo-HSCT and received or did not receive CD45RA-depleted DLI, and in CMV+ healthy controls (n = 6-20/group in pie charts, n = 10-20/group in bar graphs). Samples containing <35 CMV-specific CD8+ T cells were excluded from analysis. Medians are shown. Statistical significance was determined by Kruskal-Wallis and posthoc Dunn’s test with Bonferroni correction. (C) CMV-specific CD8+ T cell PhenoGraph cluster identities are revealed by balloon plot, whereas the heatmap shows median cluster dynamics in CMV-reactivating patients who received DLI (n = 17), their donors (n = 16), and CMV+ healthy controls (n = 12). Samples containing <50 total CD8+ T cells were excluded from temporal analysis. (D) Linear regression with 95% confidence interval bands on the maximum measured abundance of CMV-specific T cells in the patients who received DLI vs the frequency of CMV-specific T cells in the peripheral blood of the donor. Only patients with a clear CMV-specific T cell response are included in the analysis (n = 12; one patient was excluded because of missing donor sample). Count values were log-transformed. HC, healthy control; Multif, multifunctional; TCM, central memory T cell; TEF, effector T cell; TEM, effector memory T cell; TEMRA, effector memory T cell re-expressing CD45RA.
PhenoGraph clustering analysis revealed that in the patients who received DLI, CMV-specific CD8+ T cells predominantly display an effector phenotype (ie, most abundant clusters 1-4, 6, 7, and 9) featuring high expression of T-bet, CX3CR1, and CD57, and low expression of CCR7, CD27, and CD127 (Figure 5C; supplemental Figure 8). 4-1BB and IFN-γ were the most commonly expressed activation markers. Likewise, the most prevalent CMV-specific CD4+ T-cell clusters (2, 3, 7, and 9) displayed an effector phenotype expressing T-bet, CX3CR1, and CD57, although several memory phenotypes were also visible, though at low frequencies (supplemental Figure 7B and8). Of interest was the existence of multifunctional cluster 12 which expressed all 3 cytokines IFN-γ, TNF, and IL-2, together with CD40L and 4-1BB, a phenotype that was previously associated with CMV viremia control after haplo-HSCT with PT-Cy.14 Overall, the observed phenotypes and effector functions are very similar to those of patients who underwent haplo-HSCT but did not receive post-transplant DLI,14 suggesting that antigen-specific cells infused with the DLI are not qualitatively different from those initially infused with the graft.
Overlaying the total frequency of CMV-specific T-cell clusters on CMV viremia revealed that 13 out of 19 patients controlled CMV viremia after the development of a strong CMV-specific T-cell response (supplemental Figure 9). Nevertheless, 3 patients (#4, #7, and #8) did not develop a CMV-specific T-cell response, despite the presence of multiple CMV viremia blips. Notably, patient #4 received the graft and DLI from a CMV+ donor, suggesting that not all patients might benefit from adoptive transfer of donor-derived CMV-specific T cells in this setting. Finally, patients #2 and #14 neither developed CMV-specific T cells nor CMV viremia, indicating that the virus did not reactivate in these patients. For those patients who were able to mount a strong CMV-specific T-cell response, the maximum expansion of CMV-specific cells positively correlated with the frequency of CMV-specific T cells in the peripheral blood of the donor (Figure 5D), suggesting that besides the quality, the quantity of transferred pathogen-specific memory T cells is also of importance.
Discussion
T-replete haplo-HSCT with PT-Cy results in low rates of GvHD, nonrelapse mortality, and favorable immune reconstitution,27 nonetheless patients still often suffer from opportunistic pathogens, in particular CMV reactivation.6 In a phase II clinical trial, we investigated whether post-transplant infusions of memory T cells could enhance immune protection without increasing the risk of aGvHD. Cumulative incidence of viral infection from day +43 onwards was lower in the DLI cohort compared with a cohort of patients who underwent haplo-HSCT without post-transplant DLI (32% vs 53%, respectively).13 Here, we report the in-depth immunological analysis and show that infused pathogen-specific memory T cells persist and clonally expand, potentially explaining the low incidence of viral infections in patients receiving CD45RA-depleted DLI.
Considering the clonal nature of the T-cell pool in patients who underwent haplo-HSCT, we initially hypothesized that DLI could increase clonal diversity. We did not find evidence for this. Instead, we detected hyperexpansion of a small number of clones, suggesting recruitment of infused clones in the immune response. These results are in line with findings from murine studies demonstrating antigen-driven expansion of T cells after lymphocyte depletion.28 Through such clonal expansion, DLI can have a profound effect on the T-cell repertoire, exemplified by patient #1 who had DLI-derived T cells constituting almost 50% of the entire clonal repertoire 1 month after the third DLI. Besides expansion of directly infused clones, DLI might also affect the dynamics of T cells transplanted with the initial graft.29
We detected public TCRs associated with the response against CMV, Epstein-Barr virus, and influenza in the fraction of engrafted clones that were present in the infusion product, but absent at baseline. These cells were likely derived from the DLI, although we cannot exclude the possibility that they were also present at baseline at very low frequency and thereby not detected. Furthermore, these clonotypes could have been administered with any of the 3 DLI, and so their ability to persist is at least 1 month. The DLI-derived clones that showed the greatest expansion did not feature public TCRs associated with the response against CMV, Epstein-Barr virus, or influenza virus, but this does not rule out the possibility that these cells were in fact responding to such antigens. Indeed, CMV reactivation may have a profound effect on post-transplant T-cell reconstitution.30 A future study should couple antigen-specificity of DLI T cells with clonality to investigate this possibility. An alternative explanation for the expansion of certain infused T-cell clones is that these clones react to alloantigens. Even though adoptive transfer of memory T cells do not strongly induce aGvHD, they are still able to proliferate in response to allogeneic peripheral blood mononuclear cells in vitro.5 In this regard, it would be interesting to follow a larger cohort of patients long-term to determine if CD45RA-depleted DLI lowers the relapse rate because of an improved graft-versus-tumor response.
Despite expansion of infused memory T-cell clones, no major effects were found on the global phenotype of the T-cell compartment, which early after transplant is composed mainly of activated and effector phenotypes, with low frequencies of naïve and early memory cells probably because of the highly inflammatory milieu and delayed thymic output.5,31 It can be expected that even though DLI-derived memory T cells could be initially of an early memory phenotype, the clones that did not expand meant they contributed little to the overall T-cell pool, whereas clones that hyperexpanded likely adopted an activated and effector phenotype, thus resulting in no net change of overall phenotype of the T-cell compartment. No major changes in the effector functions and phenotypes of CMV-specific T cells were observed for patients who were treated with DLI. Still, a protective CMV-specific T-cell response might be a numbers game, because there was a positive correlation between the maximum expansion of CMV-specific cells in the recipient and the frequency of CMV-specific cells in the donor. With this in mind and considering that all infusions even up to the highest number were deemed safe,13 we hypothesize that a single administration with a maximum number of memory T cells should be applied.
The use of CD45RA-depleted T cells has also been studied in other transplantation protocols.32-34 In a randomized trial of TCRαβ-depleted allogeneic HSCT, post-transplant CD45RA-depleted DLI were associated with improved recovery of CMV-specific T cells in CMV-seropositive recipients, though CMV viremia incidence did not differ.34 A study comparing CD45RA-depleted vs CD3-depleted haplo-HSCT found improved T-cell reconstitution and reduced CMV and adenovirus viremia for CD45RA-depleted haplo-HSCT.32 These studies further underline the potential protective effect of adoptive transfer of CD45RA-depleted T cells in the allogeneic transplant setting.
Collectively, our data show that post-transplant CD45RA-depleted DLI facilitate engraftment of pathogen-specific memory T cells in recipients of haplo-HSCT. These cells persist and can greatly expand, and thereby have a substantial impact on the clonal repertoire. In the case of CMV-specific T cells, pathogen-specific memory T cells are highly functional. As such, CD45RA-depleted DLI-derived T cells are expected to contribute to control of opportunistic pathogens without increasing the risk of aGvHD.
Acknowledgments
This work was funded by the the Italian Ministry of Health (Grant Giovani Ricercatori GR-2013-02359185 [E.L. and R.C.]) and the European Research Council (ERC-StG-2014 PERSYST #640511 [E.L.]). E.L. is a CRI Lloyd J. Old STAR (CRI award 3914). The purchase of a FACSymphony A5 was defrayed in part by a grant from the Italian Ministry of Health (agreement 82/2015).
Authorship
Contribution: J.J.P.v.B. and E.L. conceived the study; C.D.P., B.S., I.T., R.C., D.M., R.M., J.M., R.C., A.S., L.C., and S.B. provided clinical samples and clinical information; J.J.P.v.B, F.D.P., E.Z., M.C., and A.S. performed the experiments; C.P., G.B., and J.C. sequenced the TCRs. J.J.P.v.B., S.P., and C.D.V. analyzed the data; J.J.P.v.B. and E.L. wrote the manuscript; L.C., S.B., D.M., and E.L. supervised the study; and all authors contributed to and approved the final manuscript.
Conflict-of-interest disclosure: E.L. received honoraria and in-kind reagents from BD Biosciences Italy, preclinical funding from Bristol Myers Squibb on topics unrelated to the content of this manuscript and royalties for a patent that describes methods for generating and isolating TSCM cells. J.J.P.v.B. is currently employed at Janssen. E.Z. is currently employed at Sanofi. A.S. has received honoraria as advisory board member with Bristol Myers Squibb, Servier, Gilead, Pfizer, Eisai, Bayer and Merck Sharp & Dhome; speaker’s bureau member with Takeda, Roche, AbbVie, Amgen, Celgene, AstraZeneca, Lilly, Sandoz, Novartis, Bristol Myers Squibb, Servier, Gilead, Pfizer, Arqule, and Eisai; and consultancy from Arqule.
Correspondence: Enrico Lugli, Laboratory of Translational Immunology, IRCCS Humanitas Research Hospital, via Manzoni 56, 20089 Rozzano, Milan, Italy; e-mail: enrico.lugli@humanitasresearch.it.
References
Author notes
For original data, contact the corresponding author, Enrico Lugli (enrico.lugli@humanitasresearch.it).
The full-text version of this article contains a data supplement.