Key Point
Erythroferrone exacerbates iron overload and ineffective erythropoiesis in a mouse model of β-thalassemia intermedia.
Abstract
β-thalassemia is characterized by chronic hepcidin suppression and iron overload, even in patients who have not undergone transfusion. The HbbTh3/+ (Th3/+) mouse model of nontransfusion–dependent β-thalassemia (NTDBT) partially recapitulates the human phenotype but lacks chronic hepcidin suppression, progressive iron accumulation into adulthood, or the interindividual variation of the rate of iron loading observed in patients. Erythroferrone (ERFE) is an erythroid regulator that suppresses hepcidin during increased erythropoiesis. ERFE concentrations in the sera of patients with NTDBT correlate negatively with hepcidin levels but vary over a broad range, possibly explaining the variability of iron overload in patients. To analyze the effect of high ERFE concentrations on hepcidin and iron overload in NTDBT, we crossed Th3/+ mice with erythroid ERFE–overexpressing transgenic mice. Th3/ERFE-transgenic mice suffered high perinatal mortality, but embryos at E18.5 showed similar viability, appearance, and anemia effects as Th3/+ mice. Compared with Th3/+ littermates, adult Th3/ERFE mice had similarly severe anemia but manifested greater suppression of serum hepcidin and increased iron accumulation in the liver, kidney, and spleen. The Th3/ERFE mice had much higher concentrations of serum ERFE than either parental strain, a finding attributable to both a higher number of erythroblasts and higher production of ERFE by each erythroblast.Th3/+ and Th3/ERFE mice had similar red blood cell count and shortened erythrocyte lifespan, but Th3/ERFE mice had an increased number of erythroid precursors in their larger spleens, indicative of aggravated ineffective extramedullary erythropoiesis. Thus, high ERFE concentrations increase the severity of nontransfusional iron overload and ineffective erythropoiesis in thalassemic mice but do not substantially affect anemia or hemolysis.
Introduction
β-thalassemia intermedia (also called nontransfusion–dependent β-thalassemia [NTDBT]) is a disease characterized by decreased synthesis of β-globin or decreased β-globin stability.1 The resulting deficit in β-globin leads to decreased hemoglobin (Hb) synthesis, accumulation of α-globin and its toxic degradation products, increased apoptosis of erythroblasts, ineffective erythropoiesis, decreased survival of red cells, and anemia. Tissue hypoxia from decreased oxygen delivery leads to elevated erythropoietin (EPO) levels, stimulating the erythropoietic tissues to expand. A consequence of increased but ineffective erythropoiesis is the suppression of hepcidin, the peptide hormone that serves as a master systemic iron regulator produced in the liver, resulting in increased dietary iron absorption.2-5 Over time, iron accumulates in the liver, pancreas, and other organs leading to severe clinical complications in adult patients even when regular erythrocyte transfusions are not used in the management of the disease.1
The Hbbth3/+ mouse (abbreviated here as Th3/+ or in figures as Th3), a model of thalassemia intermedia, recapitulates the following aspects of the phenotype observed in humans: low Hb, altered erythrocyte morphology, decreased erythrocyte survival with increased reticulocyte count, extramedullary erythropoiesis, splenomegaly, and iron overload in the liver and spleen.6,7 Although human patients with thalassemia intermedia demonstrate variably decreased hepcidin production even as adults, Th3/+ mice only have reduced hepcidin expression during the growth phase, and the hepcidin levels normalize to that of a wild-type (WT)–mouse by adulthood, although they are still inappropriately low for the degree of iron overload.2-5,8,9 Although useful for studying the other aspects of thalassemia intermedia, this model does not replicate the variability in the rate of iron accumulation characteristic of human patients with NTDBT10 and the range of phenotype severities.
Erythroferrone (ERFE) is an erythroid suppressor of hepcidin produced by EPO-stimulated erythroblasts.11 The hormone ERFE is produced by erythroid cells in response to hemorrhage, hypoxia, or other erythropoietic stimuli, and suppresses the production of hepcidin and thereby increases iron availability for erythropoiesis.11,12 Recent evidence indicates that ERFE suppresses hepcidin expression by binding to select members of the bone morphogenetic protein (BMP) family13-15 and inhibiting the BMP signaling that stimulates hepcidin production.16 In human patients with NTDBT, serum ERFE levels are variable over a 100-fold range, often greatly increased compared with that in healthy controls and anticorrelate with serum hepcidin concentrations.17 To the extent that ERFE levels can be compared between mice and humans, Th3/+ mice manifest relatively modestly and uniformly increased serum ERFE concentrations.18 Nevertheless, ablation of ERFE in Th3/+ mice normalized the expression of hepcidin messenger RNA (mRNA) at ages 3 and 6 weeks and significantly reduced the liver and spleen iron content at ages 6 and 12 weeks, supporting the concept that ERFE is a key hepcidin suppressor and mediator of iron overload in β-thalassemia.18
To elucidate the role of increased ERFE as a modifier of iron overload and disease phenotype in NTDBT, we crossed Th3/+ mice with mice that overexpressed ERFE in the erythroid cells,16 studied the effects of ERFE overexpression on iron homeostasis in Th3/+ mice at E18.5 and young adult mice that were aged 16 weeks, and analyzed erythropoiesis in middle-aged mice (37-45 weeks).19
Methods
Transgenic mice
All experiments involving mice were conducted with the approval of the University of California, Los Angeles Animal Research Committee. ERFE-overexpressing transgenic mice were generated, and ERFE expression levels were confirmed as previously described, with 1 line having high expression of ERFE (E(H)) and a second line having a moderate expression of ERFE (E(M)).16 Th3/+ mice (Hbbth3/+, JAX #003253) were provided by Stefano Rivella (University of Pennsylvania). Th3/+ mice were crossed with E(H) mice to generate offspring with both the Th3 deletion and high ERFE levels (T-E(H)) as well as Th3/+, E(H), and WT littermates (supplemental Figure 1). Th3/+ mice were crossed with E(M) mice to generate offspring with both the Th3 deletion and moderately high ERFE levels (T-E(M)) as well as Th3/+, E(M), and WT littermates. The breeding scheme is depicted in supplemental Figure 1. At embryonic day 18.5 (E18.5), at 16 weeks, and from 37 to 45 weeks, mice were euthanized using isoflurane inhalation, and blood and tissues of individual embryos or mice were harvested for separate analysis.
Measurement of iron-related and hematologic parameters
Liver, kidney, spleen, and heart nonheme iron as well as serum iron were measured by using a colorimetric assay per the manufacturer’s protocol (Sekisui Diagnostics). Before sampling, tissues were pulverized in liquid nitrogen to reduce variation resulting from regional differences in tissue iron deposition. Complete blood counts were measured by using a HemaVet blood analyzer (Drew Scientific). The distribution of nonheme iron in deparaffinized formalin-fixed paraffin-embedded sections of the kidney, liver, and spleen was visualized by using Perls’ Prussian blue stain or diaminobenzidine-enhanced Perls’ stain. Slides were counterstained with Nuclear Fast Red solution (Vector) and visualized with a Nikon Eclipse E600 microscope and SPOT Imaging Software.
RNA isolation and measurement of gene expression
Total RNA isolation was performed using TRIzol (Thermo Fisher Scientific). Complementary DNA was synthesized using the iScript complementary DNA Synthesis Kit (Bio-Rad) following the manufacturer’s protocol. Relative mRNA expression for genes of interest was determined via quantitative reverse transcription polymerase chain reaction using SsoAdvanced Universal SYBR Green Supermix (Bio-Rad) and measured on a CFX-96 reverse transcription polymerase chain reaction detection system (Bio-Rad).
Quantification of serum proteins and metabolites
Serum hepcidin concentrations were determined using enzyme-linked immunosorbent assay as previously detailed.11 Serum Erfe concentrations were determined using the Mouse ERFE enzyme–linked immunosorbent assay Kit (Intrinsic LifeSciences), serum EPO using the Quantikine Mouse EPO Kit (R&D Systems), serum urea nitrogen using the QuantiChrom Urea Assay Kit (BioAssay Systems), serum creatinine using the Mouse Creatinine Kit (Crystal Chem), and serum lactate dehydrogenase using the Lactate Dehydrogenase Activity Kit (Sigma-Aldrich).
Flow cytometric analysis of erythropoiesis, reticulocyte count, and erythrocyte survival
For analysis of erythropoietic cell populations,20 single-cell suspensions of the bone marrow (BM) were obtained by flushing mouse femurs with phosphate-buffered saline (PBS)/0.5% bovine serum albumin (BSA)/2mM EDTA and passing the suspension through a 27 1⁄2 G needle 3 to 4 times. Single-cell suspensions of the spleen were obtained by mashing mouse spleens through a 40 μm cell strainer. The cell strainer was rinsed with 3 mL cold PBS/0.5%BSA buffer. The splenic cells were centrifuged at 300 g for 5 minutes, then resuspended in PBS/0.5% BSA buffer.
Approximately 2 × 106 single cells were resuspended at a concentration of 106/80 μL in PBS/0.5% BSA, then incubated with anti-mouse CD16/CD32 for 15 minutes at 4°C. Cells were subsequently stained with APC(allophycocyanin)-conjugated–rat anti-mouse CD44, PE(phycoerythrin)-conjugated–rat anti-mouse CD45, and FITC(fluorescein isothiocyanate)-conjugated–rat anti-mouse TER119 for 30 minutes at 4°C. Cells were washed twice with PBS/0.5% BSA, then stained with the viability marker 7-AAD (Invitrogen) at 4°C for 10 minutes in the dark before proceeding immediately to flow analysis.
For reticulocyte counts, mouse blood was obtained by cheek bleed, and 2.5 μL of blood was diluted into 500 μL Hanks balanced salt solution (HBSS) containing 1% BSA (HBSS/BSA). Samples were incubated with 1 μL PE-conjugated–rat anti-mouse TER119 antibody in the dark at room temperature for 1 hour. Cells were washed twice with fresh HBSS/BSA, then stained with 2 μL thiazole orange solution (TO-1; Sigma, stock solution 2.5 mg/mL in PBS with 0.1% sodium azide) in the dark at room temperature for 30 minutes. Cells were washed twice with HBSS/BSA, then resuspended in 500 μL PBS containing 1% BSA. The percentage fraction of reticulocytes in blood was determined via fluorescence-activated cell sorting analysis.
For measurements of the erythrocyte survival, the mice were given 700 μg of EZ-LinkSulfo-NHS-LC-Biotin (Thermo Fisher Scientific) in PBS using retro-orbital injection 4 times during ∼36 to 48 hours. Weekly cheek bleeds (25-50 μL) were performed to quantify the fraction of biotinylated erythrocytes. 2.5 μL of whole blood was resuspended in HBSS/BSA, then incubated with 4 μL of APC-conjugated streptavidin (Thermo Fisher Scientific) and 1 μL of PE-conjugated–rat anti-mouse TER-119 at room temperature for 1 hour. The samples were then washed, resuspended in HBSS/BSA, then analyzed via flow cytometry to determine the percentage of biotinylated (APC-streptavidin-conjugated) Ter119+ erythrocytes.
All the antibodies used for flow cytometry were purchased from BD Biosciences. Flow cytometry analysis was performed using the Attune NxT Flow Cytometer (Thermo Fisher) at the UCLA Flow Cytometry Core, and data were analyzed with the FlowJo software package (version 10.8.1). Unstained cells were used as a negative control. The gating strategy is shown in supplemental Figure 2.
Statistical analysis
The primary outcomes were the differences between Th3/+ and T-E(M) groups, with additional very limited secondary comparisons reported in Figure 2. Statistical analysis was performed by using the statistical package included in Prism version 9 (GraphPad). P < .05 was considered statistically significant. Means of Th3/+ and T-E(M) groups, defined based on the genotype, were compared using two-tailed unpaired t tests. The analysis of observed vs expected ratios when analyzing transgene inheritance was performed using binomial testing and an expected transgene incidence of 50%. Fisher exact test was used to analyze differences in the phenotypic incidence between the groups. Correlations between 2 parameters were analyzed using Pearson correlation.
Results
Overexpression of ERFE in Th3/+ mice results in impaired survival
Thalassemic mice with increased ERFE expression were generated by crossing 2 different mouse lines overexpressing ERFE [high expression, E(H) or moderate expression, E(M)] with Th3/+ mice, thus generating WT, Th3/+, E(H) or E(M), and T-E(H) or T-E(M) mice (supplemental Figure 1). Given that Th3/+ and ERFE-transgenic mice are both heterozygous, we expected to generate equal ratios of all 4 groups of mice. To our surprise, T-E(H) pups, from Th3/+ and E(H) crosses, were produced at a severely diminished rate (supplemental Figure 3A), with only one T-E(H) mouse surviving to adulthood out of 13 litters. When assessed at embryonic day E18.5, pup ratios were close to the expected Mendelian ratios of 25% per genotype, suggesting impaired postnatal or perinatal survival rather than impaired embryonic viability. A similar but less extreme pattern of impaired survival was observed in T-E(M) pups produced by breeding Th3/+ mice with E(M) mice, with 8.4% of T-E(M) pups surviving up to weaning age compared with the expected 25% (Figure 1A). Although most Th3/+ x E(M) pregnancies resulted from crosses between E(M) females and Th3/+ males, impaired T-E(M) pup survival was observed with either maternal genotype, suggesting that it is due to the ERFE expression of the pup alone rather than any interaction between maternal and pup’s ERFE expressions.21
Impaired postnatal survival of T-E(M) mice. Th3/+ (Th3) mice were bred with ERFE-overexpressing Line-M (E[M]) mice. (A) Expected and observed percentages of generated offspring with specified genotypes at either 3 weeks after birth or in utero at embryonic day E18.5. Total numbers are in parentheses next to percentages. (B-H) Pups were harvested at E18.5, and blood and fetal livers were collected for analysis. Blood: (B) RBC counts, (C) Hb level, (D) hematocrit, (E) MCV. Fetal liver: (F) Erfe mRNA, (G) Hamp mRNA, and (H) nonheme iron concentrations. Statistics: (A) significant differences from expected proportions were assessed using χ2 test (∗∗∗∗P < .0001). (B-H) P values were assessed using two-tailed unpaired t test (∗∗∗∗P < .0001) between the Th3 and T- E(M) groups only.
Impaired postnatal survival of T-E(M) mice. Th3/+ (Th3) mice were bred with ERFE-overexpressing Line-M (E[M]) mice. (A) Expected and observed percentages of generated offspring with specified genotypes at either 3 weeks after birth or in utero at embryonic day E18.5. Total numbers are in parentheses next to percentages. (B-H) Pups were harvested at E18.5, and blood and fetal livers were collected for analysis. Blood: (B) RBC counts, (C) Hb level, (D) hematocrit, (E) MCV. Fetal liver: (F) Erfe mRNA, (G) Hamp mRNA, and (H) nonheme iron concentrations. Statistics: (A) significant differences from expected proportions were assessed using χ2 test (∗∗∗∗P < .0001). (B-H) P values were assessed using two-tailed unpaired t test (∗∗∗∗P < .0001) between the Th3 and T- E(M) groups only.
At E18.5, T-E(H) or T-E(M) mice had no worse anemia than Th3/+ mice (Figure 1B-E; supplemental Figure 3B-E), indicating that the increased ERFE levels in double mutants (Figure 1F; supplemental Figure 3F) impaired survival in a manner not attributable to anemia. In fact, T-E(H) mice at E18.5 had somewhat higher Hb, mean corpuscular hemoglobin (MCH), and mean corpuscular volume (MCV) compared with their Th3/+ littermates (supplemental Figure 3C-E). This increase could be attributed to an increased iron mobilization in the fetal liver for erythropoiesis, because T-E(H) mice had lower hepcidin (as assessed using fetal liver Hamp mRNA) and liver nonheme iron levels relative to Th3/+ mice (supplemental Figure 3G-H). The improvement in Hb was not observed in T-E(M) mice, likely because of their moderate expression of ERFE, lesser suppression of fetal hepcidin than in T-E(H) mice (although still significantly lower than that in Th3/+ fetuses; Figure 1G), and no significant difference in fetal liver iron content between Th3/+ and T-E(M) mice at E18.5 (Figure 1H).
Erfe overexpression does not substantially alter erythrocyte parameters in adult thalassemic mice
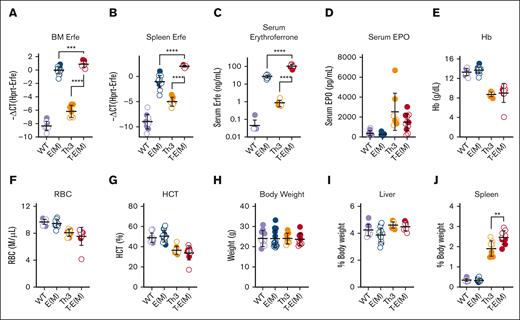
In the surviving adult T-E(M) mice, we detected an expected increase in ERFE mRNA levels in the BM and spleen (Figure 2A-B), resulting in significantly higher serum ERFE levels without any significant change in serum EPO levels in the T-E(M) mice compared with both Th3/+ and E(M) mice (Figure 2C-D). We did not detect a substantial effect of ERFE overexpression on the complete blood counts at age 16 weeks compared with that in the Th3/+ littermates (Figure 2E-G; supplemental Figure 4A-D). A small increase in mean corpuscular hemoglobin in T-E(M) compared with that in Th3/+ littermates (supplemental Figure 4A) is consistent with the expected effect of an iron overload in T-E(M). Body weights were also not significantly different across all 4 groups (WT, E(M), Th3/+, and T-E(M)) at 16 weeks (Figure 2H), and there were also no differences in liver, kidney, heart, and body weight percentages (Figure 2I; supplemental Figure 5A-B). T-E(M) mice did, however, have significantly larger spleens compared with Th3/+ mice (Figure 2J), suggesting the worsening of ineffective erythropoiesis, which is explored further.
ERFE overexpression does not alter blood erythrocyte parameters in adult thalassemic mice. Mice generated from Th3 × E(M) breedings were analyzed at 16 weeks. Measurements in males are depicted in closed circles and females in open circles. (A-C) Erythroferrone levels were assessed by (A) BM Erfe mRNA, (B) spleen Erfe mRNA levels, (C) serum ERFE. (D) Serum EPO. (E-J) Erythrocyte parameters: (E) Hb levels, (F) RBC count, and (G) hematocrit. (H-J) Body and organ weights: (H) total body weight, (I,J) liver and spleen weights as a percentage of total body weight. P values were assessed by two-tailed unpaired t test (∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001) between the Th3 and T-E(M) groups only and (A-C) also between E(M) and T-E(M).
ERFE overexpression does not alter blood erythrocyte parameters in adult thalassemic mice. Mice generated from Th3 × E(M) breedings were analyzed at 16 weeks. Measurements in males are depicted in closed circles and females in open circles. (A-C) Erythroferrone levels were assessed by (A) BM Erfe mRNA, (B) spleen Erfe mRNA levels, (C) serum ERFE. (D) Serum EPO. (E-J) Erythrocyte parameters: (E) Hb levels, (F) RBC count, and (G) hematocrit. (H-J) Body and organ weights: (H) total body weight, (I,J) liver and spleen weights as a percentage of total body weight. P values were assessed by two-tailed unpaired t test (∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001) between the Th3 and T-E(M) groups only and (A-C) also between E(M) and T-E(M).
ERFE overexpression exacerbated iron overload in thalassemic mice
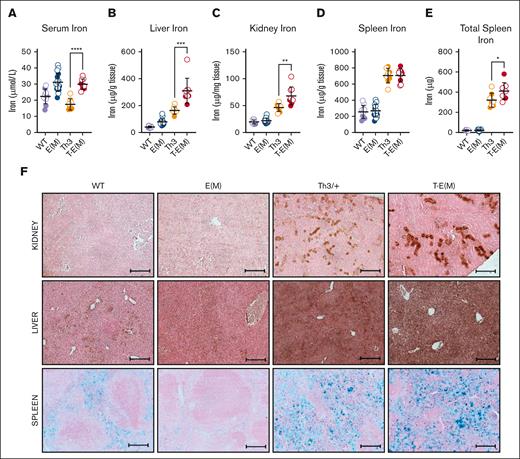
Moderate ERFE overexpression by itself had little effect on iron loading but the overexpression of ERFE in the context of thalassemia worsened the iron-loading phenotype significantly. At the age of 16 weeks, T-E(M) mice had higher serum iron levels than Th3/+ mice (Figure 3A) as well as significantly higher liver and kidney nonheme iron concentrations than both the E(M) and Th3/+ littermates (Figure 3B-C). Heart nonheme iron concentrations were unaffected across all groups (supplemental Figure 5C). Although there was no difference in spleen nonheme iron concentrations between T-E(M) and Th3/+ mice (Figure 3D), T-E(M) mice had significantly higher total spleen iron because of the increased spleen size (Figure 3E). Diaminobenzidine-enhanced Perls’ staining for ferric iron deposits in tissue sections from the male (Figure 3F) and female (supplemental Figure 6) mice revealed a notable increase in tubular iron accumulation in kidneys from the T-E(M) and Th3/+ mice compared with that from WT and E(M) mice. Kidney function appeared worse in Th3/+ and T-E(M) mice compared with that in WT and E(M) mice, as reflected by their serum blood urea nitrogen and creatinine levels (supplemental Figure 7A-B). Although kidney iron was significantly higher in T-E(M) than in Th3/+ mice (Figure 3C), there was no difference in serum blood urea nitrogen and creatinine levels between Th3/+ and T-E(M) mice (supplemental Figure 7A-B). Perls’ stain of liver sections (Figure 3F; supplemental Figure 6) revealed iron deposition in hepatocytes of T-E(M) mice, less prominent but still detectable staining can be observed in Th3/+ mice, and smaller amounts of iron were detected in hepatocytes of WT and E(M) mice. Splenic iron staining (Figure 3F; supplemental Figure 6) was increased in both Th3/+ and T-E(M) mice, presumably from the dominant effect of thalassemic hemolysis but E(M) mice showed relative iron depletion compared with that in WT mice because of loss of iron from splenic macrophages caused by ERFE-mediated hepcidin suppression.
Although T-E(M) mice and E(M) mice had similarly elevated serum iron levels (Figure 3A) at an age of 16 weeks, the pronounced iron overload in the tissues of T-E(M) mice suggests that earlier in life, they experienced rapid iron accumulation with even higher iron concentrations, as was described in Th3/+ mice.8 In addition, the significantly increased spleen iron in T-E(M) and Th3/+ mice compared with E(M) mice (Figure 3E) is likely because of the combination of increased extramedullary hematopoiesis in the spleen and the increased red blood cell (RBC) recycling burden in the spleen caused by hemolysis of thalassemic erythrocytes. However, we did not, detect elevated lactate dehydrogenase in Th3 or T-E(M) mice (supplemental Figure 4E).
ERFE overexpression exacerbates iron overload in thalassemic mice. Mice generated from Th3 × E(M) breedings were analyzed at 16 weeks of age. Males are depicted as closed circles and females as open circles. (A-E) Nonheme iron concentrations were assessed in (A) serum, (B) liver, (C) kidney, and (D) spleen: (E) total splenic nonheme iron, (F) iron visualized in formalin-fixed paraffin-embedded sections sections of the kidney (top), liver (middle) and spleen (bottom) from male 16-week-old mice using diaminobenzidine-enhanced Perls’ stain (kidney and liver) and Perls’ stain (spleen). Original magnification ×10 (F). Scale bars represent 200 μm (F). (A-F) P values were assessed by two-tailed unpaired t test (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001) between the Th3 and T-E(M) groups only.
ERFE overexpression exacerbates iron overload in thalassemic mice. Mice generated from Th3 × E(M) breedings were analyzed at 16 weeks of age. Males are depicted as closed circles and females as open circles. (A-E) Nonheme iron concentrations were assessed in (A) serum, (B) liver, (C) kidney, and (D) spleen: (E) total splenic nonheme iron, (F) iron visualized in formalin-fixed paraffin-embedded sections sections of the kidney (top), liver (middle) and spleen (bottom) from male 16-week-old mice using diaminobenzidine-enhanced Perls’ stain (kidney and liver) and Perls’ stain (spleen). Original magnification ×10 (F). Scale bars represent 200 μm (F). (A-F) P values were assessed by two-tailed unpaired t test (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001) between the Th3 and T-E(M) groups only.
ERFE overexpression further suppresses hepcidin levels in adult thalassemic mice
In T-E(M) mice, the reduction of liver hepcidin mRNA (Hamp) caused by ERFE overexpression was not significant compared with Th3/+ littermates (Figure 4A), but serum hepcidin concentrations were significantly lower in T-E(M) mice (Figure 4B). Because hepcidin levels are affected by iron-level status, we examined whether hepcidin production is inappropriately low by indexing liver Hamp mRNA and serum hepcidin levels to liver nonheme iron concentration. Both ratios were significantly lower in T-E(M) mice than in Th3/+ mice (Figure 4C,D), indicating that increased ERFE further worsens thalassemic iron loading by inhibiting the hepcidin response to iron overload. Interestingly, despite the increase in ERFE and inappropriately low hepcidin in T-E(M) mice compared with that in Th3/+ mice at 16 weeks and E18.5, we did not observe any significant differences in hepatic mRNAs encoding the biomarkers of hepatic BMP signaling Id1, Smad7 or Atoh8, nor in 16-week-old mice in mRNAs encoding the ligands Bmp2 or Bmp6 (supplemental Figure 8).
ERFE overexpression further suppresses hepcidin levels in adult thalassemic mice. Mice generated from Th3 × E(M) breedings were analyzed at 16 weeks. Data for males are depicted in closed circles and for females in open circles. Liver hepcidin Hamp mRNA (A) and serum hepcidin protein concentration (B) are shown and also expressed as a ratio to liver nonheme iron concentration (LIC) to determine the appropriateness of hepcidin levels relative to iron status (C,D). P values were assessed by two-tailed unpaired t test (∗∗P < .01; ∗∗∗∗P < .0001) between the Th3 and T-E(M) groups only.
ERFE overexpression further suppresses hepcidin levels in adult thalassemic mice. Mice generated from Th3 × E(M) breedings were analyzed at 16 weeks. Data for males are depicted in closed circles and for females in open circles. Liver hepcidin Hamp mRNA (A) and serum hepcidin protein concentration (B) are shown and also expressed as a ratio to liver nonheme iron concentration (LIC) to determine the appropriateness of hepcidin levels relative to iron status (C,D). P values were assessed by two-tailed unpaired t test (∗∗P < .01; ∗∗∗∗P < .0001) between the Th3 and T-E(M) groups only.
High serum Erfe in T-E(M) mice is a result of the combination of an expanded population of thalassemic erythroblasts and high expression of Erfe in individual erythroblasts
T-E(M) and Th3/+ mice had similarly increased expression in the spleen and BM of glycophorin A mRNA (Gypa), a specific marker of erythroid cells as well as the transferrin receptor 1 mRNA (Figure 5A-B, D-E), consistent with the erythroblast expansion characteristic of ineffective erythropoiesis. We observed that the increased Erfe mRNA in BMs and spleens of mice expressing transgenic Erfe strongly correlated with the expansion of the erythroblast population as measured by Gypa mRNA (Figure 5C,F), suggesting that high serum ERFE concentrations in T-E(M) mice result from a combination of an expanded erythroblast population (the thalassemia effect) and high expression of Erfe in each erythroblast (the transgenic expression effect).
Increased density of erythroid cells in erythropoietic organs of Th3 and T-E(M) mice contributes to enhanced ERFE production. Mice generated from Th3 × E(M) breeding were analyzed at 16 weeks. Males are depicted as closed circles and females as open circles. Erythroid precursor cell density was estimated in (A-C) BM and (D-F) spleen (extramedullary) by measuring (A,D) Gypa mRNA and (B,E) Tfrc mRNA. (C,F) Correlation between Gypa mRNA and Erfe mRNA in (C) BM and (D) spleen of E(M) and T-E(M) mice. (C,F) P values and R2 were determined using Pearson correlation analysis.
Increased density of erythroid cells in erythropoietic organs of Th3 and T-E(M) mice contributes to enhanced ERFE production. Mice generated from Th3 × E(M) breeding were analyzed at 16 weeks. Males are depicted as closed circles and females as open circles. Erythroid precursor cell density was estimated in (A-C) BM and (D-F) spleen (extramedullary) by measuring (A,D) Gypa mRNA and (B,E) Tfrc mRNA. (C,F) Correlation between Gypa mRNA and Erfe mRNA in (C) BM and (D) spleen of E(M) and T-E(M) mice. (C,F) P values and R2 were determined using Pearson correlation analysis.
High ERFE concentrations increase extramedullary erythropoiesis in Th3/+ mice but do not substantially alter the erythroid maturation pattern or peripheral erythroid parameters
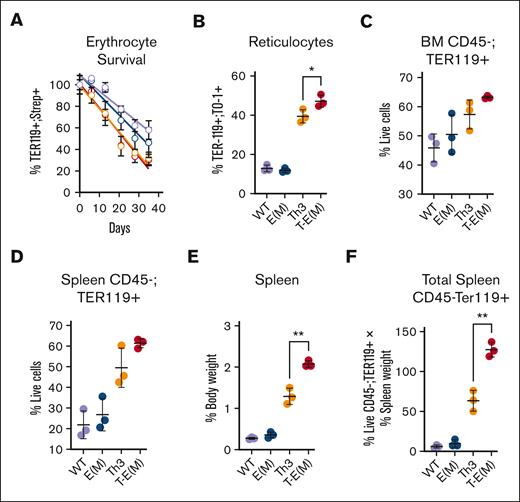
Although we did not observe any significant differences between T-E(M) mice and Th3/+ mice in their shortened erythrocyte survival (Figure 6A), a small increase in circulating reticulocytes was detected in T-E(M) mice compared with that in Th3/+ mice (Figure 6B). ERFE overexpression had minor or no effects on the number, Hb content, or size of circulating Th3/+ erythrocytes (supplemental Figure 9) and did not modify the abnormal maturation patterns of thalassemic cells in the marrow or the spleen further (supplemental Figure 10). However, after accounting for the much larger spleen size of T-E(M) mice, there was a substantial increase in the total number of erythroid cells in the spleen with a smaller but similar trend in the BM (Figure 6C-F). In view of the similar maturation patterns of erythroid precursors, the increased number of total erythroid cells in the spleen of T-E(M) vs Th3/+ mice implies an increase in erythropoiesis not matched by a decrease in erythrocyte survival or increase in the number of circulating mature erythrocytes, overall signifying exacerbation of ineffective erythropoiesis.
ERFE overexpression augments ineffective extramedullary erythropoiesis in older adult thalassemic mice. (A-B) Mice generated from Th3 × E(M) breeding were injected retroorbitally with NHS-Sulfo-Biotin 4 times in 48 hours, and then ∼50 μL weekly cheek bleeds were performed to obtain peripheral blood for analysis. Erythroid cells were analyzed via flow cytometry using PE-conjugated–anti-TER119 to identify erythroid lineage, APC-conjugated streptavidin to detect biotinylation, and TO-1 to detect RNA-containing reticulocytes. (A) Percentage of biotinylated erythrocytes over the course of 5 weeks (color scheme same as that in panels B-F). (B) Percent reticulocytes at terminal bleed defined as TER119+; TO-1+ cells relative to TER119+ cells. (C-F) Erythroid cells in BMs and spleens were harvested from mice at ∼37 to 45 weeks. Dead cells, and CD45+ cells, were excluded by 7-AAD and CD45 staining, and live erythroid cells from BM (C) or spleen (D) were counted via flow cytometry as live TER119+ cells. (E) Spleen weight as the percentage of body weight (F) Total splenic erythroid cells indices adjusted for body size were estimated by multiplying the values in panel D with the relative spleen size in panel E. (B-E) P values were assessed using two-tailed unpaired t test (∗ P < .05; ∗∗ P < .005) between the Th3 and T-E(M) groups only. N = 3 for each group.
ERFE overexpression augments ineffective extramedullary erythropoiesis in older adult thalassemic mice. (A-B) Mice generated from Th3 × E(M) breeding were injected retroorbitally with NHS-Sulfo-Biotin 4 times in 48 hours, and then ∼50 μL weekly cheek bleeds were performed to obtain peripheral blood for analysis. Erythroid cells were analyzed via flow cytometry using PE-conjugated–anti-TER119 to identify erythroid lineage, APC-conjugated streptavidin to detect biotinylation, and TO-1 to detect RNA-containing reticulocytes. (A) Percentage of biotinylated erythrocytes over the course of 5 weeks (color scheme same as that in panels B-F). (B) Percent reticulocytes at terminal bleed defined as TER119+; TO-1+ cells relative to TER119+ cells. (C-F) Erythroid cells in BMs and spleens were harvested from mice at ∼37 to 45 weeks. Dead cells, and CD45+ cells, were excluded by 7-AAD and CD45 staining, and live erythroid cells from BM (C) or spleen (D) were counted via flow cytometry as live TER119+ cells. (E) Spleen weight as the percentage of body weight (F) Total splenic erythroid cells indices adjusted for body size were estimated by multiplying the values in panel D with the relative spleen size in panel E. (B-E) P values were assessed using two-tailed unpaired t test (∗ P < .05; ∗∗ P < .005) between the Th3 and T-E(M) groups only. N = 3 for each group.
Discussion
The severity of NTDBT manifestations is highly variable from patient to patient,22 but most patients develop systemic iron overload as adults, even if they never underwent transfusion. Iron overload predominantly affects the liver.10,23 The severity of the iron overload varies greatly even between unchelated patients of similar age and genotype.10 A number of genetic modifiers of disease severity have been identified, but these efforts mainly focused on the severity of anemia22 and not iron overload.
One potential cause of difference between different patients in the severity of iron overload is the variable production of erythroid regulators of iron homeostasis.24 Studies in human subjects and mice have documented that augmented erythropoietic activity, whether in response to blood loss, EPO, or ineffective erythropoiesis, generates 1 or more signals that suppress the production of the iron-regulatory hormone hepcidin.3,4,7,8,12,25,26 ERFE, a glycoprotein erythrokine produced by EPO-stimulated erythroblasts, is a key erythroid regulator whose physiological and pathological role as an erythroid hepcidin suppressor is supported by both laboratory mouse models11,16,18,27 and studies in patients with iron-loading anemias.17,28,29 In particular, knocking out the Erfe gene in the Th3/+ mouse model fully restored hepcidin mRNA expression at ages 3 and 6 weeks; significantly reduced liver and spleen iron content at 6 and 12 weeks of age; and slightly ameliorated erythropoiesis, as measured per the reduced spleen index, red cell distribution width, and MCV, but did not improve anemia.18 In another study of Th3/+ mice, treatment with monoclonal antibodies against ERFE started at the age of 4 weeks decreased splenomegaly, serum and liver iron concentrations, and reticulocyte counts with an increase in Hb concentrations.14
Single timepoint serum ERFE measurements in patients with NTDBT documented dramatic interpatient variability in serum ERFE concentrations that were anticorrelated with their serum hepcidin concentrations.17 By contrast, the Th3/+ mouse model of NTDBT shows a uniform and relatively mild increase in ERFE16,18 accompanied by low serum hepcidin during the period of rapid growth and erythroid expansion (ages 3-8 weeks).8,18 This study aimed to understand the potential impact of high ERFE concentrations on the adult phenotype of NTDBT.
We transgenically augmented erythroid ERFE in the Th3/+ mouse model. We had previously generated 3 erythroid-specific ERFE-transgenic mouse lines, E(L), E(M), and E(H), with a low, medium, and high level of ERFE overexpression, respectively.16 Serum ERFE concentrations in E(L)-line are similar to those of Th3/+ mice,16 so we crossed the E(M)- and E(H)-lines with the Th3/+ mice (all strains are heterozygous) and characterized the progeny, which included WT, Th3/+, E, and T-E mice. Unexpectedly, T-E(H) mice had severely impaired perinatal survival compared with the parental lines, with only 1 mouse surviving to adulthood from 13 litters. Prenatally, at E18.5, the T-E(H) embryos appeared similar to Th3/+ embryos, constituted close to the predicted 25% Mendelian ratio, and had slightly higher Hb and slightly lower fetal liver iron concentration than Th3/+ embryos (supplemental Figure 3; Table 1). Even the T-E(M) mice suffered ∼50% perinatal mortality compared with either Th3/+ mice or E(M) mice, despite prenatal RBC, Hb, hematocrit, MCV, and fetal liver iron concentrations that were indistinguishable from Th3/+ embryos. Seventeen mice survived to adulthood and were analyzed for this report (Figure 1). We previously noted a somewhat increased perinatal mortality and neurological problems in the E(H) transgenic mice,16 so we surmise that the expected increase in ERFE concentrations in T-E(H) mice compared with E(H) mice further exacerbated this problem. However, unlike mice, humans are protected by fetal Hb from any perinatal adverse effects of their NTDBT. For this reason, we did not further investigate the potential nonhematological causes of perinatal mortality in T-E mice.
Primer sequences used for quantitative reverse transcription polymerase chain reaction
| . | F:5′---3′ . | R:5′---3′ . |
|---|---|---|
| mHprt | CTGGTTAAGCAGTACAGCCCC | CGAGAGGTCCTTTTCACCAGC |
| mHamp | CCTATCTCCATCAACAGATG | AACAGATACCACACTGGGAA |
| mErfe | ATGGGGCTGGAGAACAGC | TGGCATTGTCCAAGAAGACA |
| mId1 | ACCCTGAACGGCGAGATCA | TCGTCGGCTGGAACACATG |
| mBmp2 | GATCTGTACCGCAGGCACTC | CCGTTTTCCCACTCATCTCT |
| mBmp6 | ATGGCAGGACTGGATCATTGC | CCATCACAGTAGTTGGCAGCG |
| mGypa | ATGGCAGGGATTATCGGAAC | CACCCTCAGGAGATTGGATG |
| mTfrc | TCATGAGGGAAATCAATGATC | GCCCCAGAAGATATGTCGGAA |
| mSmad7 | GCAGGCTGTCCAGATGCTGT | GATCCCCAGGCTCCAGAAGA |
| mAtoh8 | CACCATCAGCGCAGCCTTC | CCATAGGAGTAGCACGGCACC |
| . | F:5′---3′ . | R:5′---3′ . |
|---|---|---|
| mHprt | CTGGTTAAGCAGTACAGCCCC | CGAGAGGTCCTTTTCACCAGC |
| mHamp | CCTATCTCCATCAACAGATG | AACAGATACCACACTGGGAA |
| mErfe | ATGGGGCTGGAGAACAGC | TGGCATTGTCCAAGAAGACA |
| mId1 | ACCCTGAACGGCGAGATCA | TCGTCGGCTGGAACACATG |
| mBmp2 | GATCTGTACCGCAGGCACTC | CCGTTTTCCCACTCATCTCT |
| mBmp6 | ATGGCAGGACTGGATCATTGC | CCATCACAGTAGTTGGCAGCG |
| mGypa | ATGGCAGGGATTATCGGAAC | CACCCTCAGGAGATTGGATG |
| mTfrc | TCATGAGGGAAATCAATGATC | GCCCCAGAAGATATGTCGGAA |
| mSmad7 | GCAGGCTGTCCAGATGCTGT | GATCCCCAGGCTCCAGAAGA |
| mAtoh8 | CACCATCAGCGCAGCCTTC | CCATAGGAGTAGCACGGCACC |
In surviving adult mice, our examination of the modulatory effect of transgenic erythroid–specific ERFE in the Th3/+ mouse model of thalassemia intermedia indicated that augmented ERFE affected iron homeostasis much more on the Th3/+ background than on WT background (Figure 3B,C), causing more severe iron overload in T-E(M) than in Th3/+ mice or E(M) mice (Figure 3A-C). We attribute this synergistic effect to the increased number of transgenic ERFE–expressing erythroblasts in T-E(M) mice compared with E(M) mice, causing a greater than additive increase in serum ERFE levels (serum ERFE, means ± standard deviation: Th3 = 0.87 ± 0.35 ng/mL, E[M] = 27.4 ± 5.0 ng/mL, T-E[M] = 104.7 ± 23.7 ng/mL; Figure 2C).
Serum hepcidin concentrations in adults were significantly lower in T-E(M) than in Th3/+ mice (Figure 4), resulting in higher serum iron, higher liver iron concentration, higher kidney iron concentration, and higher total spleen iron (Figure 3A,B,C) but not higher heart iron concentration (Figure 3D). The lack of cardiac iron overload is also noted in human patients with NTDBT.10 This increase in serum iron and iron stores in T-E(M) compared with those in Th3/+ mice had no detectable effect on the RBC, Hb, hematocrit, or MCV, again, consistent with human NTDBT in which decreasing iron overload through chelation did not affect the frequency of urgent transfusions30 and has not been reported to alter erythroid parameters.
Kidney tubular iron overload in T-E(M) and Th3/+ mice (Figure 3F) was accompanied by similar mild renal impairment in both (supplemental Figure 7). Although renal impairment in patients with NTDBT has been noted,31 to our knowledge, this is the first observation of this pathology in a rodent model of NTDBT. Among the possible etiologies, the adverse effects of tubular iron overload and renal injury from chronic anemia leading to glomerular hyperfiltration32 may be most applicable to our model.
We provide additional evidence that increased ERFE concentrations lead to iron overload in Th3/+ mice by suppressing hepcidin expression in the liver relative to the hepatic iron load and decreasing hepcidin concentrations in blood (Figures 1 and 4). We searched for the expected suppression of BMP signaling in T-E(M) compared with that in Th3/+ in adult mice but found only suggestive trends (supplemental Figure 8). One of the possible reasons may be that hepatic iron loading and high serum iron (Figure 3) counter the effect of ERFE by driving up BMP signaling. Indeed, in the fetal liver (Figure 1G) where hepatic iron overload does not have enough time to develop and oppose Erfe-mediated effects on hepcidin expression, the suppression of Hamp mRNA expression in T-E(M) mice was very robust (Figure 1G). However, Id1, Smad7, and Atoh8 levels had still not decreased (supplemental Figure 3F-E). As noted by others,33 biomarkers of BMP signaling respond poorly to acute erythroid stimuli, a discrepancy that raises the possibility that other (possibly non-BMP) pathways contribute to hepcidin suppression in response to erythroid stimulation33 or ERFE.
The increase in spleen size in T-E(M) compared with that in the Th3/+ group suggested that ERFE exacerbates hemolysis and/or ineffective erythropoiesis in T-E(M) mice, because more precursors were needed to maintain comparable Hb levels. To assess the peripheral hemolysis and ineffective medullary and extramedullary erythropoiesis at chronic stages, we performed additional analyses (Figure 6; supplemental Figures 9 and 10) on the surviving 37- to 45-week-old mice. In T-E(M) compared with in Th3/+ mice, we found no change in erythrocyte count or erythrocyte lifespan measured by the loss of biotinylated erythrocytes from circulation. There were only minor increases in the blood Hb levels, MCV and erythropoietic output, as estimated via reticulocytosis. The abnormal erythroblast maturation pattern was similar in T-E(M) and Th3/+ mice. However, we documented robustly increased numbers of erythroid cells in the spleen of T-E(M) compared with those in the spleen of Th3/+ mice, when splenic mass was taken into account. Because the maturation patterns in T-E(M) and Th3/+ cells were similar, the larger number of total erythroid cells in the spleen signifies a proportional increase in the number of cells at each erythroblast stage. On aggregate, these findings indicate that in thalassemic mice high ERFE promotes ineffective erythropoiesis, predominantly in the enlarged spleen.
We conclude that increased ERFE concentrations in the blood of T-E(M) compared with that in the blood of Th3/+ mice, by suppressing hepcidin and increasing plasma iron concentrations, enhance ineffective extramedullary erythropoiesis, iron overload, and splenomegaly. This is the opposite phenotype to the Th3/+ mice with transgenic or monoclonal antibody–mediated ERFE ablation in which spleen size and iron overload were consistently reduced.14,18
The remarkable increase in expression and blood concentrations of ERFE in the T-E(M) mice, compared with that in its parental genotypes (Figure 2), is dependent on both Erfe expression per erythroid precursor and the increased mass of erythroid precursors (Figure 5). The effect of erythroid ERFE augmentation in the mouse NTDBT model suggests how genetic variations in ERFE expression in erythroblasts could be amplified by the greatly expanded erythroblast populations characteristic of ineffective erythropoiesis and could contribute to human variations in plasma hepcidin concentrations and systemic iron overload observed in patients with NTDBT. Indeed, in another human anemia with ineffective erythropoiesis, congenital dyserythropoietic anemia type 2A (CDAIIA), the ERFE A260S polymorphic variant was greatly enriched in a subset of patients with severe disease (worse anemia and more iron overload). ERFE A260S is predicted to cause the stabilization of ERFE mRNA leading to its increased expression. The ERFE A260S variant increased serum ERFE concentrations in patients with CDAIIA by much more than it did in healthy controls, leading to worse iron overload in ERFE-A260S-carrying patients with CDAII as judged by serum ferritin concentrations.29
More generally, our model also illustrates how ineffective erythropoiesis amplifies the effect of EPO-stimulated secretion of ERFE (and potentially other erythroid suppressors of hepcidin). The combined effect of massive erythroblast expansion in anemias with ineffective erythropoiesis and EPO-stimulated secretion of ERFE by each erythroblast11 may account for the greater propensity of these patients to iron overload34 as compared with patients with anemias caused by peripheral hemolysis but with effective erythropoiesis. The same mechanism may also apply to other putative erythroid hepcidin suppressors.
Our study provides further preclinical support for targeting increased ERFE in patients with NTDBT and other anemias of ineffective erythropoiesis. Therapeutic monoclonal antibodies against ERFE are being developed14,35 and may offer an additional means for examining the role of ERFE in human iron-loading anemias with ineffective erythropoiesis and for preventing iron overload in these conditions.
Acknowledgments
The authors acknowledge the support from the UCLA Flow Cytometry Core in obtaining and analyzing data.
The work was supported by National Institutes of Health grants (1R01DK126680) (T.G.), with supplement and R01HD096863 (J.O. and E.N., respectively).
Authorship
Contributions: J.O. and V.Z. designed and performed experiments, analyzed the data, and wrote the manuscript; and E.N. and T.G. conceived the project, designed experiments, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: T.G. and E.N. are shareholders in Intrinsic LifeSciences and Silarus Therapeutics and have received consulting fees from Disc Medicine, FibroGen, AstraZeneca, Ionis Pharmaceuticals, and Rallybio. T.G. has also received consulting fees from Alnylam Pharmaceuticals, Akebia Therapeutics, Global Blood Therapeutics, Gossamer Bio, Pharmacosmos, Sierra Oncology, and Silence Therapeutics. E.N. received consulting fees from GSK, Novo Nordisk, Protagonist, and Shield Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Tomas Ganz, CHS 47-200J, Department of Medicine, David Geffen School of Medicine, UCLA, 10833 Le Conte Ave, Los Angeles, CA 90095-1690; e-mail: tganz@mednet.ucla.edu.
References
Author notes
∗J.O. and V.Z. are joint first authors.
Data are available on request from the corresponding author, Tomas Ganz (tganz@mednet.ucla.edu).
The full-text version of this article contains a data supplement.


![Impaired postnatal survival of T-E(M) mice. Th3/+ (Th3) mice were bred with ERFE-overexpressing Line-M (E[M]) mice. (A) Expected and observed percentages of generated offspring with specified genotypes at either 3 weeks after birth or in utero at embryonic day E18.5. Total numbers are in parentheses next to percentages. (B-H) Pups were harvested at E18.5, and blood and fetal livers were collected for analysis. Blood: (B) RBC counts, (C) Hb level, (D) hematocrit, (E) MCV. Fetal liver: (F) Erfe mRNA, (G) Hamp mRNA, and (H) nonheme iron concentrations. Statistics: (A) significant differences from expected proportions were assessed using χ2 test (∗∗∗∗P < .0001). (B-H) P values were assessed using two-tailed unpaired t test (∗∗∗∗P < .0001) between the Th3 and T- E(M) groups only.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/7/14/10.1182_bloodadvances.2022009307/2/m_blooda_adv-2022-009307-gr1.jpeg?Expires=1769184642&Signature=oVkn72iFM--qk-HhH3tQ9dmDiywZ9vlzWsfTDcRzcAKlIEJJ2mizZMQA1qugeY2uOkS8yGypcRJzHZNGgo9Ws07Lz0zlSOuUtu2icASOArHL7NB-GuWmq2gbMzD1c5u3D2ugXJO9WM42dLYHrhkI60Hfb2ppI2RbKJClQpOCrDD6vonvAJr9aiGvRHFcmqNuvgGbIT3uSFpxba7Cm9F01OJgebupcxC52t4ShkYT5De8BROBLh30Y5Va23fWIjMA9qMSKw7ZQFrr5482jEWL3TN~4SCokGvKzw3~4uetQbL9WR-NTZwaRJYJ7UpbI14nkQ1zNewTT9czohfT0zjMNg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)