Key Points
Among 67 children with relapsed/refractory HL treated with ASCT and brentuximab vedotin consolidation, the 3-year PFS was 85%.
Brentuximab vedotin consolidation in pediatric patients with relapsed/refractory HL is associated with a favorable safety profile.
Abstract
Outcomes for children and adolescents with relapsed and refractory Hodgkin lymphoma (HL) are poor, with ∼50% of patients experiencing a subsequent relapse. The anti-CD30 antibody–drug conjugate brentuximab vedotin improved progression-free survival (PFS) when used as consolidation after autologous stem cell transplantation (ASCT) in adults with high-risk relapsed/refractory HL. Data on brentuximab vedotin as consolidative therapy after ASCT in pediatric patients with HL are extremely limited, with data of only 11 patients reported in the literature. We performed a retrospective analysis of 67 pediatric patients who received brentuximab vedotin as consolidation therapy after ASCT for the treatment of relapsed/refractory HL to describe the experience of this regimen in the pediatric population. This is the largest cohort reported to date. We found that brentuximab vedotin was well tolerated with a safety profile similar to that of adult patients. With a median follow-up of 37 months, the 3-year PFS was 85%. These data suggest a potential role for the use of brentuximab vedotin as consolidation therapy after ASCT for children with relapsed/refractory HL.
Introduction
Although the prognosis for children with Hodgkin lymphoma (HL) is excellent, up to 25% of patients experience relapse and require salvage therapy.1-3 In the largest retrospective series, event-free survival (EFS) for patients with relapsed or refractory disease after high-dose therapy with autologous stem cell transplantation (ASCT) is only 42% to 57%, thus there is an urgent clinical need for improved therapies.1,4-9 One approach to reducing the risk of posttransplantation recurrence is through the use of post-ASCT consolidative therapy. Brentuximab vedotin is an anti-CD30 antibody conjugated to the microtubule-disrupting agent monomethyl auristatin E that has demonstrated efficacy in the salvage and frontline settings in adult and pediatric patients with HL.10-13 The use of brentuximab vedotin as consolidation therapy after ASCT in adults with relapsed/refractory HL and risk factors for posttransplantation progression were evaluated in the AETHERA study. In this randomized, placebo-controlled trial, adult patients with risk factors for posttransplantation progression treated with brentuximab vedotin for post-ASCT consolidation had improved progression-free survival (PFS) compared with those treated with placebo (hazard ratio [HR], 0.57; 95% confidence interval [CI], 0.40-0.81; P = .0013), which persisted in a 5-year follow-up (5-year PFS, 59%; 95% CI, 51-66 vs 41%; 95% CI, 33-49, respectively).14,15
Brentuximab vedotin is well tolerated in children with relapsed and refractory CD30+ malignancies, such as anaplastic large cell lymphoma and HL.16 As monotherapy, the most common treatment-related adverse events were pyrexia and nausea, and the most common grade 3 or higher adverse events were neutropenia, increased gamma-glutamyl transpeptidase, and pyrexia.16 In addition, it has been well tolerated and effective in combination with traditional cytotoxic agents both as initial and retrieval therapy.13,17-19 Data on the use of brentuximab vedotin as consolidation therapy in pediatric patients after ASCT are extremely limited, with only 2 single-institution reports on a total of 11 patients available in the literature.20,21 This retrospective multicenter study describes the safety, tolerability, and efficacy of brentuximab vedotin consolidation after ASCT in pediatric patients with relapsed/refractory HL.
Methods
We performed a retrospective analysis of pediatric patients who received brentuximab vedotin as consolidative therapy after ASCT for the treatment of relapsed/refractory HL. Patients were aged ≤21 years at the time of ASCT. Patient data were collected from 15 institutions within the United States and Canada. Patients who initiated treatment with brentuximab vedotin as consolidation therapy from January 2014 until January 2021 were included in the study. Data collected included disease presentation, presentation at relapse, salvage therapy, treatment response, ASCT, and posttransplantation consolidation with brentuximab vedotin. Data on imaging prior to transplantation were collected, including Deauville scores from FDG-positron emission tomography (PET) when available. Investigators also reported whether the PET was interpreted as PET-positive or PET-negative to allow for the determination of PET status among sites that did not report for Deauville scores. For sites that did report Deauville scores, a negative PET was considered Deauville ranging from 1 to 3. Data collected on brentuximab vedotin treatment included the timing of drug initiation, duration of treatment, reasons for early termination (defined as discontinuation of treatment after fewer than 16 cycles), and toxicities. Data included all grades of neuropathy and all other grade 3 or 4 toxicities. In addition, we collected data on risk factors for posttransplantation recurrence as defined in the AETHERA trial, including relapse <12 months from completion of frontline therapy, refractory to frontline therapy, the best response of partial remission or stable disease before salvage treatment, the presence of B symptoms or extranodal involvement at relapse, and 2 or more systemic treatments before ASCT.14,15
Overall Survival was defined as the time from transplantation to death from any cause. Patients alive were censored at their last follow-up. PFS was defined as the time from transplantation to relapse, progression, or death. Patients alive without relapse or progression were censored at their last follow-up. Survival rates were estimated using a Kaplan-Meier estimator and given with 95% CIs. The association between risk factors and PFS was assessed via the estimation of HR and 95% CI, using the Cox proportional hazards model. The research protocol was approved by local site institutional review boards. Study data were collected and managed using REDCap (Research Electronic Data Capture) tools hosted at Memorial Sloan Kettering Cancer Center.22,23
Results
Patient characteristics
Seventy-two patients were identified from 15 academic centers. Five patients were excluded from the analysis: 2 for receiving treatment with brentuximab vedotin in combination with a checkpoint inhibitor during post-ASCT consolidation, 1 for undergoing ASCT after age 21, and 2 for an initial diagnosis of non-HL. The median age at initial diagnosis was 15 years (range, 4-20 years). The stages at initial diagnosis were stage II (n = 27; 40%), stage III (n = 18; 27%), and stage IV (n = 22; 33%). Thirty-six patients (54%) had bulky disease, and 29 (43%) patients had B symptoms. The most commonly used frontline regimen was doxorubicin, bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide (ABVE-PC), which was used for 37 patients (55%; Table 1). Other regimens included doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD) (n = 12; 18%); vincristine, etoposide, prednisone, doxorubicin, –cyclophosphamide, vincristine, prednisone, dacarbazine (OEPA-COPDAC) (n = 8; 12%); mechlorethamine, etoposide, adriamycin, bleomycin, vincristine, vinblastine, prednisone (Stanford V) (n = 3; 4%); and bleomycin, etoposide, cyclophosphamide, vincristine, prednisone, procarbazine (BEACOPP) (n = 2; 3%). One patient received AVD and nivolumab. Two patients received brentuximab vedotin in their upfront therapy, both in combination with AVE-PC chemotherapy. Twenty-nine patients (43%) received frontline radiation therapy (RT).
Upfront treatment regimens
| Upfront regimen . | % receiving RT . | |
|---|---|---|
| ABVE-PC | 37 (55%) | 17 (46%) |
| ABVD | 12 (18%) | 1 (8%) |
| OEPA-COPDAC | 8 (12%) | 4 (50%) |
| Stanford V | 3 (4%) | 7 (70%) |
| BEACOPP | 2 (3%) | |
| BV-AVE-PC | 2 (3%) | |
| Nivolumab-AVD | 1 (1%) | |
| Other | 2 (3%) | |
| Upfront regimen . | % receiving RT . | |
|---|---|---|
| ABVE-PC | 37 (55%) | 17 (46%) |
| ABVD | 12 (18%) | 1 (8%) |
| OEPA-COPDAC | 8 (12%) | 4 (50%) |
| Stanford V | 3 (4%) | 7 (70%) |
| BEACOPP | 2 (3%) | |
| BV-AVE-PC | 2 (3%) | |
| Nivolumab-AVD | 1 (1%) | |
| Other | 2 (3%) | |
ABVE-PC, doxorubicin, bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide; ABVD, doxorubicin, bleomycin, vinblastine, dacarbazine; AVD, doxorubicin, vinblastine, dacarbazine; BEACOPP, bleomycin, etoposide, cyclophosphamide, vincristine, prednisone, procarbazine; BV-AVE-PC, brentuximab vedotin, doxorubicin, vincristine, etoposide, prednisone, cyclophophamide; OEPA-COPDAC, vincristine, etoposide, prednisone, doxorubicin, cyclophosphamide, vincristine, prednisone, dacarbazine; Stanford V, mechlorethamine, etoposide, adriamycin, bleomycin, vincristine, vinblastine, prednisone.
Patient characteristics at relapse for the 67 patients are presented in Table 2. The median age at the time of relapsed/refractory disease was 17 years (range, 8-21 years). Twenty-four patients (36%) were diagnosed with treatment-refractory disease and 43 (64%) with relapsed disease. In relapsed and refractory patients, the median time from the end of upfront treatment to relapsed/refractory disease was 5.9 months (range, 0.0-52.0 months), with 27 of 43 patients (64%) experiencing a relapse <12 months from the completion of their initial therapy. At the time of relapsed/refractory disease, 13 patients (19%) had B symptoms, and 23 (35%) had extranodal disease. Relapse characteristics broken down by initial therapy regimen are provided in supplemental Table 1.
Baseline patient characteristics
| Characteristic . | n = 67 . |
|---|---|
| Age at diagnosis (y); median (range) | 15 (4-20) |
| Age at relapse/refractory disease (y); median (range) | 17 (8-21) |
| Sex | |
| Female | 34 (51%) |
| Male | 33 (49%) |
| Relapse or refractory | |
| Relapsed disease | 43 (64%) |
| Refractory disease | 24 (36%) |
| Timing of initial relapse | |
| Relapse <12 months | 27 (64%) |
| Relapse ≥12 months | 15 (36%) |
| Unknown | 1 |
| Stage at relapse | |
| I | 2 (3%) |
| II | 31 (47%) |
| III | 7 (11%) |
| IV | 26 (39%) |
| Unknown | 1 |
| B symptoms | 13 (19%) |
| Bulk disease | 20 (30%) |
| Extranodal disease | 23 (35%) |
| Salvage regimens before ASCT | |
| 1 | 50 (75%) |
| 2 | 12 (18%) |
| 3 | 5 (7%) |
| Treatment with brentuximab before ASCT | |
| At any time | 46 (69%) |
| Immediately before ASCT | 41 (61%) |
| PET response before ASCT | |
| Negative | 45 (69%) |
| Positive | 20 (31%) |
| Unknown | 2 |
| BMT conditioning | |
| BEAM | 40 (60%) |
| CBV | 10 (15%) |
| Other∗ | 17 (25%) |
| RT given in salvage | 30 (45%) |
| Characteristic . | n = 67 . |
|---|---|
| Age at diagnosis (y); median (range) | 15 (4-20) |
| Age at relapse/refractory disease (y); median (range) | 17 (8-21) |
| Sex | |
| Female | 34 (51%) |
| Male | 33 (49%) |
| Relapse or refractory | |
| Relapsed disease | 43 (64%) |
| Refractory disease | 24 (36%) |
| Timing of initial relapse | |
| Relapse <12 months | 27 (64%) |
| Relapse ≥12 months | 15 (36%) |
| Unknown | 1 |
| Stage at relapse | |
| I | 2 (3%) |
| II | 31 (47%) |
| III | 7 (11%) |
| IV | 26 (39%) |
| Unknown | 1 |
| B symptoms | 13 (19%) |
| Bulk disease | 20 (30%) |
| Extranodal disease | 23 (35%) |
| Salvage regimens before ASCT | |
| 1 | 50 (75%) |
| 2 | 12 (18%) |
| 3 | 5 (7%) |
| Treatment with brentuximab before ASCT | |
| At any time | 46 (69%) |
| Immediately before ASCT | 41 (61%) |
| PET response before ASCT | |
| Negative | 45 (69%) |
| Positive | 20 (31%) |
| Unknown | 2 |
| BMT conditioning | |
| BEAM | 40 (60%) |
| CBV | 10 (15%) |
| Other∗ | 17 (25%) |
| RT given in salvage | 30 (45%) |
BEAM, carmustine, etoposide, cytarabine, melphalan; CBV, carmustine, cyclophosphamide, etoposide.
Other BMT conditioning regimens included gemcitabine, busulfan, melphalan, and vorinostat (8 patients); busulfan, gemcitabine, and melphalan (4 patients); etoposide and melphalan (3 patients); and cyclophosphamide and etoposide (2 patients).
Before high-dose chemotherapy and ASCT, patients had received a median of 1 prior line of salvage therapy (range, 1-3). The most common salvage regimen was brentuximab vedotin/bendamustine (n = 26; 39%; Table 3). Forty-six patients (69%) received brentuximab vedotin at any point during the pre-ASCT salvage treatment, with 41 patients (61%) receiving it in the line of therapy immediately before ASCT (Table 2). RT was used before ASCT in 2 patients (3%) and as part of transplantation consolidation in 28 patients (42%). Based on treating physician interpretation, 45 (69%) patients achieved a PET-negative response before transplantation, whereas 20 patients (31%) proceeded to undergo transplantation with a PET-positive response. PET response was unknown in 2 patients. Among the 60 patients with available Deauville scores, 45 (75%) had a Deauville score of 1 to 3 just before ASCT, and 15 (25%) had reported Deauville scores of 4 and 5. A summary of risk factors for relapse after ASCT, as defined by the AETHERA study, is presented in Table 4. Sixty-three of the 67 patients (94%) had at least 1 risk factor.
Salvage regimens before ASCT
| Salvage regimen . | |
|---|---|
| Brentuximab/bendamustine | 26 (39%) |
| Brentuximab/gemcitabine | 15 (22%) |
| Gemcitabine/vinorelbine | 10 (15%) |
| Ifosfamide/vinorelbine | 9 (13%) |
| Brentuximab | 8 (12%) |
| Ifosfamide/carboplatin/etoposide | 6 (9%) |
| Ifosfamide/gemcitabine/vinorelbine | 4 (6%) |
| Brentuximab/nivolumab | 4 (6%) |
| Gemcitabine/vinblastine/liposomal doxorubicin | 1 (1%) |
| Bendamustine/gemcitabine/vinorelbine | 1 (1%) |
| Other | 6 (9%) |
| Salvage regimen . | |
|---|---|
| Brentuximab/bendamustine | 26 (39%) |
| Brentuximab/gemcitabine | 15 (22%) |
| Gemcitabine/vinorelbine | 10 (15%) |
| Ifosfamide/vinorelbine | 9 (13%) |
| Brentuximab | 8 (12%) |
| Ifosfamide/carboplatin/etoposide | 6 (9%) |
| Ifosfamide/gemcitabine/vinorelbine | 4 (6%) |
| Brentuximab/nivolumab | 4 (6%) |
| Gemcitabine/vinblastine/liposomal doxorubicin | 1 (1%) |
| Bendamustine/gemcitabine/vinorelbine | 1 (1%) |
| Other | 6 (9%) |
Risk factors for relapse at the time of ASCT
| Risk factor . | |
|---|---|
| Relapse <12 months after frontline therapy∗ | 27 (41%) |
| Refractory to frontline therapy | 24 (36%) |
| Presence of extranodal involvement at relapse | 23 (34%) |
| Partial response/stable disease pre-ASCT | 22 (33%) |
| ≥2 systemic treatments pre-ASCT | 17 (25%) |
| Presence of B symptoms at relapse | 13 (19%) |
| Number of risk factors | |
| 0 | 4 (6%) |
| ≥1 | 63 (94%) |
| ≥2 | 44 (66%) |
| ≥3 | 14 (21%) |
| Risk factor . | |
|---|---|
| Relapse <12 months after frontline therapy∗ | 27 (41%) |
| Refractory to frontline therapy | 24 (36%) |
| Presence of extranodal involvement at relapse | 23 (34%) |
| Partial response/stable disease pre-ASCT | 22 (33%) |
| ≥2 systemic treatments pre-ASCT | 17 (25%) |
| Presence of B symptoms at relapse | 13 (19%) |
| Number of risk factors | |
| 0 | 4 (6%) |
| ≥1 | 63 (94%) |
| ≥2 | 44 (66%) |
| ≥3 | 14 (21%) |
One patient’s time to relapse was unknown; this patient has 3 other risk factors, and therefore, these data would not change their risk classification.
The median time from ASCT to the start of brentuximab vedotin consolidation was 57.5 days (range, 30-209 days). The median number of brentuximab vedotin cycles completed after ASCT was 12. Twenty-four patients (36%) completed all 16 cycles of consolidation, including 1 patient who received more than 16 cycles. Reasons for completing fewer than 16 cycles of brentuximab vedotin consolidation included an adverse event (n = 27), patient/family decision (n = 4), relapse (n = 4), and shorter duration preplanned by the medical team (n = 5). Treatment was still ongoing for 3 patients.
The most frequent treatment-related adverse event was neuropathy, reported in 28 of 67 patients (42%), with peripheral sensory neuropathy (PSN) reported in 9 of 67 patients (13%), peripheral motor neuropathy (PMN) reported in 4 of 67 patients (6%), and combined PSN and PMN reported in 15 of 67 patients (22%; Table 5). The incidence of PSN and PMN based on grade was PSN grade 1: 10%, grade 2: 16%, grade 3: 9%; and grade unknown: 1%; PMN grade 1: 1%, grade 2: 18%, and grade 3: 9%. The most common grade 3 or 4 treatment-related adverse events were cytopenias (neutropenia in 20 of 67 patients [30%], thrombocytopenia in 8 of 67 [12%], and anemia in 6 of 67 [9%]). Of note, grade 3 or 4 pulmonary toxicity/pneumonitis was reported in 6 of 67 patients (9%); this included 2 patients with pneumonitis, 2 patients with pulmonary nodules of unclear etiology, 1 patient with pulmonary infiltrates, and 1 patient with restrictive and obstructive pulmonary disease. Twenty-seven (40%) patients discontinued treatment because of an adverse event. The reasons cited for discontinuation of brentuximab vedotin were peripheral neuropathy in 19 of 27 patients (70%), cytopenia in 3 of 27 patients (11%), pneumonitis in 3 of 27 patients (11%), and an infusion reaction in 1 of 27 patients (4%). In 1 individual, the reason for discontinuation was multifactorial, including pneumonitis, cytopenias, and infections. The median number of cycles of brentuximab vedotin administered to patients who discontinued treatment because of an adverse event was 10 (range, 1.0-15.0).
Treatment-related adverse events
| Adverse event . | |
|---|---|
| Neuropathy | 28 (42%) |
| PSN | 9 (13%) |
| PMN | 4 (6%) |
| Both PMN and PSN | 15 (22%) |
| Neutropenia | 20 (30%) |
| Thrombocytopenia | 8 (12%) |
| Anemia | 6 (9%) |
| Pulmonary toxicity | 6 (9%) |
| Transaminitis | 5 (7%) |
| Infusion reaction | 4 (6%) |
| Nausea/vomiting | 3 (4%) |
| Cardiotoxicity | 2 (3%) |
| Secondary malignancy | 1 (2%) |
| Adverse event . | |
|---|---|
| Neuropathy | 28 (42%) |
| PSN | 9 (13%) |
| PMN | 4 (6%) |
| Both PMN and PSN | 15 (22%) |
| Neutropenia | 20 (30%) |
| Thrombocytopenia | 8 (12%) |
| Anemia | 6 (9%) |
| Pulmonary toxicity | 6 (9%) |
| Transaminitis | 5 (7%) |
| Infusion reaction | 4 (6%) |
| Nausea/vomiting | 3 (4%) |
| Cardiotoxicity | 2 (3%) |
| Secondary malignancy | 1 (2%) |
Outcomes
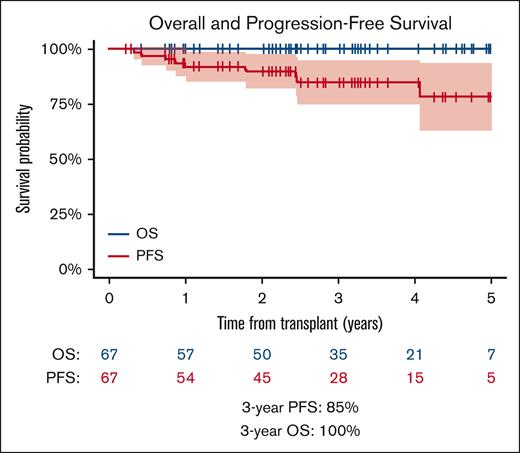
With a median follow-up of 37 months (range, 3-75 months), the 3-year PFS was 85% (95% CI, 75-95) (Figure 1A). The 3-year overall survival was 100%, and no deaths were observed. At the end of follow-up, 58 patients (87%) were alive with no evidence of disease, whereas 9 (13%) patients were alive with disease. There was 1 secondary malignancy reported: a patient who had a second relapse of HL 24 months after ASCT and subsequently developed acute myeloid leukemia 1 year later. This patient was alive without either disease.
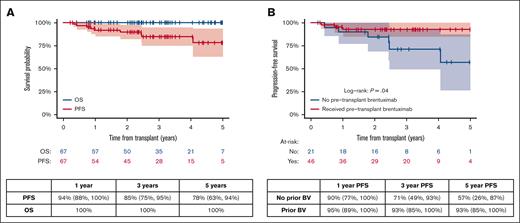
Overall survival and PFS. (A) Overall survival and PFS in all patients (B) PFS based on pretransplantation–brentuximab vedotin exposure; PFS includes 95% CI.
Overall survival and PFS. (A) Overall survival and PFS in all patients (B) PFS based on pretransplantation–brentuximab vedotin exposure; PFS includes 95% CI.
A univariable analysis of potential risk factors for progression did not detect any factors associated with PFS in this cohort, including relapse <12 months from the end of upfront therapy (HR, 0.40; 95% CI, 0.08-1.91), primary refractory disease (HR, 2.21; 95% CI, 0.59-8.24), response to the last salvage therapy partial response/stable disease (HR, 0.90; 95% CI, 0.23-3.62), extranodal disease at relapse (HR, 1.49; 95% CI, 0.40-5.55), B symptoms at relapse (HR, 3.41; 95% CI, 0.91-12.7), and 2 or more salvage therapies before ASCT (HR, 1.29; 95% CI, 0.32-5.18). Furthermore, when patients were grouped based on the number of risk factors, there was no significant difference in PFS (supplemental Figure 1). The 3-year PFS for patients with 0 or 1 risk factor was 90% (95% CI, 78-100); for patients with ≥2 risk factors, it was 83% (95% CI: 70-96]; and for patients with ≥3 risk factors, it was 76% (95% CI, 52-100; P = .6 and P = .5 for 0-1 vs ≥2 and 0-2 vs ≥3 risk factors, respectively).
Twenty of the 67 patients (30%) proceeded to undergo transplantation with a positive FDG-PET, as defined by the treating physician. Relapse occurred in 4 of 20 patients who had a positive FDG-PET just before ASCT and in 4 of 45 patients who had a negative FDG-PET just before ASCT. At 3 years, the PFS was 76% for patients who were FDG-PET–positive before ASCT (95% CI, 53-98) and 92% for patients who were FDG-PET–negative before ASCT (95% CI, 83-100; P = .2).
To determine the impact of brentuximab vedotin therapy before ASCT on the outcome, we performed a subanalysis comparing the PFS among patients exposed to brentuximab vedotin before ASCT (n = 46) with that among patients who were brentuximab vedotin–naive (n = 21). Among the 46 patients with previous brentuximab vedotin exposure, 2 received brentuximab vedotin in both upfront and salvage therapy. The remaining received brentuximab vedotin as part of salvage therapy only. Three-year PFS in brentuximab vedotin–exposed vs brentuximab vedotin–naive patients was 93% (95% CI, 85-100) vs 71% (95% CI, 49-53; P = .04), respectively (Figure 1B).
Discussion
Here, we report the largest series, to our knowledge, of pediatric patients treated with brentuximab vedotin consolidation after ASCT for relapsed/refractory HL. Brentuximab vedotin was well tolerated in our cohort, with rates of adverse events similar to those in prior reports about such pediatric and adult patients.13,18,19,21 Two small studies have reported a total of 11 pediatric patients treated with this approach.20,21 In a retrospective review at St. Jude Children’s Research Hospital, 4 of 5 patients with relapsed/refractory HL received all 16 doses of brentuximab vedotin consolidation. With a follow-up of 29 to 76 months, all patients who were in CR at the time of ASCT were alive and remained in CR at the time of review.20 Adverse events included PMN, nausea, rash, dyspepsia, and fatigue. In a separate retrospective study describing 6 pediatric patients, 2 discontinued treatment early secondary to grade 3 motor and sensory neuropathies.21 Other toxicities reported included grade 3 neutropenia, fatigue, and bone pain. PFS after ASCT for the 6 patients was 12, 18, 22, 24, 30, and 30 months.21 In the AETHERA study, the rates of all grades of PSN, PMN, and grade 3 or 4 neutropenia were 56%, 23%, and 29%, respectively, with 33% discontinuing consolidation early because of treatment-related adverse events. Our cohort was similar, with 37% and 28% of patients experiencing PSN and PMN of any grade, respectively, 30% of patients experiencing grade 3 or 4 neutropenia, and 40% discontinuing therapy early because of an adverse event.
In our cohort, 6 of 67 patients (9%) experienced grade 3 or 4 pulmonary toxicity in comparison with 8 of 167 patients (5%) in the AETHERA trial.14 The rate of pulmonary toxicity in our cohort could be related to brentuximab, conditioning with carmustine, etoposide, cytarabine, and melphalan (BEAM) before ASCT, RT, or a combination of these therapies. Of note, among the 6 patients who experienced grade 3 or 4 pulmonary toxicity, 4 had received RT to the mediastinum, and 3 received BEAM conditioning.
Prior retrospective studies in pediatric patients with relapsed/refractory HL have identified similar risk factors used in the AETHERA as predictors of posttransplantation progression.8,24 In our study, 63 of 67 patients had at least 1 risk factor for post-ASCT recurrence, and 44 of 67 patients had 2 or more risk factors. Despite this, with a median follow-up of 37 months, the 3-year PFS was 85% (95% CI, 75-95). Overall, these outcomes are excellent in comparison with those of other large cohorts from earlier treatment eras, in which the EFS after ASCT ranged from 42% to 57%.24,25 Other smaller studies have also reported excellent outcomes, particularly among patients treated in the last 20 years, with an EFS after ASCT ranging from 85% to 87%.8,9 The explanation for exceptional outcomes in our group is likely multifactorial, including (1) an inherent selection bias in that our cohort included only patients who completed ASCT and started brentuximab consolidation, (2) improved salvage therapies, (3) increased use of FDG-PET to determine remission before ASCT and a significant portion of patients in our cohort (69%) entering ASCT with a negative FDG-PET, and (4) a potential benefit from the use of brentuximab vedotin consolidation.
In addition to being the largest study on the use of brentuximab vedotin consolidation in pediatric patients, to our knowledge, our series of 67 pediatric patients is also the largest report of outcomes for pediatric patients with relapsed/refractory HL in the brentuximab era. Patients included in this study received initial therapy that is reflective of commonly used chemotherapy protocols of the study period, but this has rapidly evolved in recent years. Although 46 of 67 patients received brentuximab vedotin as a component of their salvage regimen, only 2 of 67 patients received brentuximab vedotin in their initial therapy. Recent trials incorporating brentuximab vedotin into upfront regimens for both pediatric and adult patients have resulted in significant improvements compared with traditional chemotherapy, and, in the near future, brentuximab vedotin may be considered standard of care for the upfront treatment of many pediatric patients with high-risk HL.13,18,26 The impact that upfront use of brentuximab vedotin will have on the efficacy of its inclusion in salvage and post-ASCT consolidation treatment remains unknown.
Limitations of this study include its retrospective nature, the lack of uniform treatment before ASCT, the variability in off-therapy image surveillance across centers, and the variation in time to initiation of brentuximab vedotin consolidation.14 In addition, this retrospective study did not include central radiology review. The interpretation of FDG-PET, including the Deauville score or PET-positive vs PET-negative status, was determined by the local radiologist and oncologist. Thus, there is likely variability in the definition of CR before transplantation; however, this is reflective of the real-world experience.
In contrast to the AETHERA study, which excluded patients with prior brentuximab vedotin exposure, in this study, 69% of patients received brentuximab vedotin before ASCT, which is reflective of the common use of brentuximab vedotin–containing salvage regimens during the time these data were collected.16,17,19,27 In our cohort, patients with prior brentuximab exposure had an improved 3-year PFS compared with those who were brentuximab–naive. Caution should be used when interpreting these data; however, it is possible that the selection of patients for brentuximab maintenance is enriched for patients who had a response to brentuximab vedotin before ASCT.
In summary, in the largest report of brentuximab vedotin consolidation therapy after ASCT in children with relapsed/refractory HL, we demonstrated this regimen is well tolerated, with the toxicity comparable with that observed in adults. The combination of modern salvage chemotherapy followed by ASCT and posttransplantation brentuximab vedotin consolidation resulted in an excellent PFS in a population with historically poor outcomes. Prospective studies evaluating or incorporating post-ASCT consolidation with brentuximab vedotin or other agents for pediatric patients are warranted.
Acknowledgment
This work was supported by the National Institutes of Health/National Cancer Institute Cancer Center Support grant P30 CA008748.
Authorship
Contribution: C.J.F., P.D.H.-M., and L.G.-R. substantially contributed to conception and design of the study; A.M., C.J.F., L.G.-R., and J.R. analyzed and interpreted the data; A.K.F., J.K., K.J.L., L.J.M., R.E.N., M.P., F.W., A.P.Y., P.D.H.-M., and L.G.-R. provided patient data; C.J.F., L.G.-R., and J.R. wrote the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: K.J.L. reports consulting or advisory roles at Jazz Pharmaceuticals and research funding from Abbott Diagnostics. L.G.-R. reports consulting roles at Janssen and Merck. C.J.F., J.R., I.B., B.C., H.D., R.J.D., and J.E.F. received clinical trial support from Seattle Genetics. The remaining authors declare no competing financial interests.
Correspondence: Jaclyn Rosenzweig, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: rosenzwj@mskcc.org; Lisa Giulino-Roth, Weill Cornell Medical College, 525 E 68th St, Payson 695, New York, NY 10065; e-mail: lgr2002@med.cornell.edu; and Christopher J. Forlenza, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: forlenzc@mskcc.org.
References
Author notes
∗C.J.F. and J.R. contributed equally to this study.
Data are available on request from the corresponding author, Jaclyn Rosenzweig (rosenzwj@mskcc.org).
The full-text version of this article contains a data supplement.