TO THE EDITOR:
Hematologic malignancies are heterogeneous and have unpredictable illness trajectories. Intensive therapies, such as hematopoietic stem cell transplant (HSCT) and chimeric antigen receptor (CAR) T-cell therapies, have the potential to cure albeit with risks of adverse events and mortality.1 Because of their uncertain prognosis and potential for high symptom burden, patients with hematologic malignancies may benefit from longitudinal outpatient palliative care (PC) comanagement.2,3
PC provides specialized and holistic medical care to people with serious illness4 that can help patients with symptoms,5,6 mood,7 quality of life,7 decision-making, social needs, and end-of-life concerns.2,4,8 PC can lower health care utilization and costs for patients with advanced cancers,9,10 including hematologic malignancies.8 However, patients with hematologic malignancies are less likely to be referred to PC than patients with solid tumor malignancies.11,12 Furthermore, hematologic malignancies are not included in most PC oncology trials.4 There are 2 randomized controlled trials to date, focused on patients with acute myeloid leukemia who are hospitalized for intensive therapies.7,13 There is even less data about outpatient PC integration for patients with hematologic malignancies.14
To address this gap, we report on the early experience of an outpatient palliative service embedded within a malignant hematology clinic. We describe patients with hematologic malignancies who were referred to PC. We examine associations between PC and inpatient health care utilization and costs.
We conducted a single-center, retrospective study at a tertiary medical center to evaluate the following outcomes in patients with hematologic malignancies who received embedded, outpatient PC: (1) referral patterns, (2) inpatient or emergency department (ED) health care utilization, and (3) costs of ED stays or hospitalizations. The institutional review board has reviewed this study, and the research meets requirements for protection of human subjects. The study was conducted in accordance with the Declaration of Helsinki.
An oncology-trained, PC-certified nurse practitioner (NP) staffed an outpatient PC service for 1 full-day per week starting in 2016, with assistance from a PC chaplain and nurses as needed. Oncology clinicians referred patients who had a serious illness and need for specialty symptom management, decision-making support, or family support. After the initial visit, the NP determined the frequency of follow-up visits.
We identified all patients with a hematologic malignancy who initiated outpatient PC between 1 March 2016 and 30 May 2020. Using the electronic medical record, we determined patients’ demographics, clinical characteristics, reason(s) for referral to PC, and number and timing of PC visits. From the health system finance database, we obtained data about inpatient health care utilization and direct costs for the prespecified subset of patients who were followed by PC for ≥6 months. We excluded hospitalizations for HSCT as PC was not expected to impact HSCT.
We calculated frequencies for demographic and clinical characteristics. We used Wilcoxon signed-rank test to compare the number of hospitalizations and ED visits, and their corresponding costs, in the 6 months before initiation of PC to the 6 months after. To examine the impact that age, sex, referral reason, cancer type, and history of HSCT has on hospitalization and ED encounters and costs, we performed bivariate and multivariate linear regression analysis on the log-transformed ratio of our outcomes. We used an alpha of 0.05 to determine statistical significance. We used Stata software (StataCorp LLC, College Station, TX) for our analyses.
A total of 120 patients with hematologic malignancies were referred to PC during the study period (Table 1). The most common cancers were myeloma (41.7%), non-indolent lymphoma (17.5%), and acute myeloid leukemia (15.0%). The most common reasons for referral to PC were symptom management (pain 60%, mood 12.5%, and fatigue 7.5%) and goals of care conversations before HSCT (10%). The median number of PC visits for our cohort was 4 (range, 1-42).
Patient characteristics
| . | N = 120 (range), (%) . | . |
|---|---|---|
| Median age at enrollment (y) | 59 (24-89) | |
| Female | 58 (48.0) | |
| Race | ||
| Non-Hispanic White | 77 (64.0) | |
| Hispanic | 14 (11.7) | |
| Asian | 11 (9.2) | |
| Black | 8 (6.7) | |
| Cancer type | ||
| Myeloma | 50 (41.7) | |
| Non-indolent lymphoma | 21 (17.5) | |
| Acute myeloid leukemia | 18 (15.0) | |
| Myeloproliferative neoplasm | 14 (11.7) | |
| Reasons for referral | ||
| Pain | 72 (60.0) | |
| Mood | 15 (12.5) | |
| Fatigue | 9 (7.5) | |
| Goals of care conversations before HSCT | 12 (10.0) |
| . | N = 120 (range), (%) . | . |
|---|---|---|
| Median age at enrollment (y) | 59 (24-89) | |
| Female | 58 (48.0) | |
| Race | ||
| Non-Hispanic White | 77 (64.0) | |
| Hispanic | 14 (11.7) | |
| Asian | 11 (9.2) | |
| Black | 8 (6.7) | |
| Cancer type | ||
| Myeloma | 50 (41.7) | |
| Non-indolent lymphoma | 21 (17.5) | |
| Acute myeloid leukemia | 18 (15.0) | |
| Myeloproliferative neoplasm | 14 (11.7) | |
| Reasons for referral | ||
| Pain | 72 (60.0) | |
| Mood | 15 (12.5) | |
| Fatigue | 9 (7.5) | |
| Goals of care conversations before HSCT | 12 (10.0) |
| Reasons for hospitalization . | Before PC . | After PC . |
|---|---|---|
| Chemotherapy/radiotherapy | 21 | 10 |
| Pain | 7 | 4 |
| New cancer diagnosis | 3 | 0 |
| Relapsed disease | 3 | 0 |
| Orthopedic procedure | 2 | 1 |
| Hernia | 2 | 0 |
| Biopsy | 2 | 0 |
| Viral upper respiratory infection | 2 | 2 |
| Treatment toxicity | 1 | 0 |
| Gastric anastomotic leak | 1 | 0 |
| Pacemaker placement | 1 | 0 |
| Myocardial infarction | 1 | 0 |
| Dysphagia | 1 | 0 |
| Fever | 1 | 5 |
| Bowel ischemia | 1 | 0 |
| Nausea/vomiting | 1 | 1 |
| Gastrointestinal bleeding | 1 | 1 |
| Failure to thrive | 1 | 0 |
| Altered mental status | 1 | 0 |
| Bacterial infection | 0 | 3 |
| Disease progression | 0 | 2 |
| Subdural hematoma | 0 | 1 |
| Adrenal insufficiency | 0 | 1 |
| Acute kidney injury | 0 | 1 |
| Reasons for hospitalization . | Before PC . | After PC . |
|---|---|---|
| Chemotherapy/radiotherapy | 21 | 10 |
| Pain | 7 | 4 |
| New cancer diagnosis | 3 | 0 |
| Relapsed disease | 3 | 0 |
| Orthopedic procedure | 2 | 1 |
| Hernia | 2 | 0 |
| Biopsy | 2 | 0 |
| Viral upper respiratory infection | 2 | 2 |
| Treatment toxicity | 1 | 0 |
| Gastric anastomotic leak | 1 | 0 |
| Pacemaker placement | 1 | 0 |
| Myocardial infarction | 1 | 0 |
| Dysphagia | 1 | 0 |
| Fever | 1 | 5 |
| Bowel ischemia | 1 | 0 |
| Nausea/vomiting | 1 | 1 |
| Gastrointestinal bleeding | 1 | 1 |
| Failure to thrive | 1 | 0 |
| Altered mental status | 1 | 0 |
| Bacterial infection | 0 | 3 |
| Disease progression | 0 | 2 |
| Subdural hematoma | 0 | 1 |
| Adrenal insufficiency | 0 | 1 |
| Acute kidney injury | 0 | 1 |
Thirty-seven (30.8%) patients died by the date of chart review. Of the decedents, 17 (45.9%) died at home with hospice services, 3 (8.1%) died in a residential hospice setting, 3 (8.1%) died in the hospital with comfort care, and the remaining 14 (37.8%) died during a hospitalization without a preceding transition to comfort care.
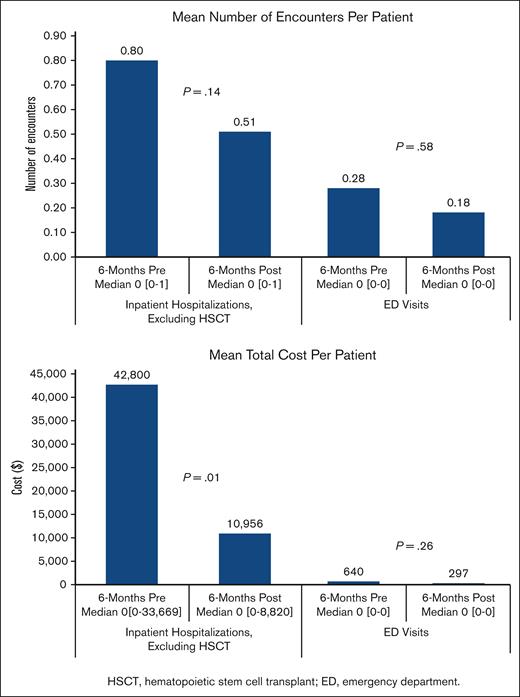
In the subset of 65 patients who followed PC for ≥6 months, mean hospitalization direct costs (excluding hospitalizations for HSCT) were lower during the 6 months after PC enrollment compared with the previous 6 months ($42 800 vs $10 956; P = .01; Figure 1). With a sample size of 65, 80% power, and 2-sided alpha of 0.05, our effect size was small to medium (ie, 0.353). We did not find significant differences in the number of hospitalizations (P = .14), number of ED visits (P = .58), or ED costs in the 6 months before vs the 6 months after PC enrollment (P = .26).
Health care utilization before and after PC. Comparison of the total costs and encounters for patients (n = 65) in the 6 months before and after PC enrollment. Mean difference and median (interquartile range) were calculated using Wilcoxon signed-rank test.
Health care utilization before and after PC. Comparison of the total costs and encounters for patients (n = 65) in the 6 months before and after PC enrollment. Mean difference and median (interquartile range) were calculated using Wilcoxon signed-rank test.
We conducted bivariate and multivariate linear regression analyses using clinically meaningful patient factors that may impact health care utilization (Table 2). In bivariate analyses, PC referral reason was associated with hospitalizations (P = .02) and hospitalization costs (P = .04) (Table 2). Enrollment age, sex, HSCT, and referral reason and cancer type (groups listed in Table 1) were not associated with health care utilization in multivariate analyses (Table 2).
Bivariate and multivariate linear regression analyses of determinants on total hospitalization costs
| . | Total hospitalization costs (excluding HSCT) in the 6 months before vs 6 months after . | |||||
|---|---|---|---|---|---|---|
| Bivariate . | Multivariate . | |||||
| Beta coeff . | 95% CI . | P value . | Beta coeff . | 95% CI . | P value . | |
| Enrollment age (y) | –0.004 | –0.049 to 0.041 | 0.846 | 0.011 | –0.052 to 0.075 | 0.705 |
| Sex | –0.250 | –2.136 to 1.636 | 0.782 | 0.281 | –1.963 to 2.526 | 0.789 |
| Referral reason | 0.371 | 0.016 to 0.725 | 0.041 | 0.436 | –0.020 to 0.892 | 0.059 |
| Cancer type | 0.128 | –0.616 to 0.873 | 0.720 | 0.280 | –0.639 to 1.200 | 0.519 |
| HSCT | 0.200 | –1.687 to 2.088 | 0.825 | –0.300 | –2.531 to 1.931 | 0.775 |
| . | Total hospitalization costs (excluding HSCT) in the 6 months before vs 6 months after . | |||||
|---|---|---|---|---|---|---|
| Bivariate . | Multivariate . | |||||
| Beta coeff . | 95% CI . | P value . | Beta coeff . | 95% CI . | P value . | |
| Enrollment age (y) | –0.004 | –0.049 to 0.041 | 0.846 | 0.011 | –0.052 to 0.075 | 0.705 |
| Sex | –0.250 | –2.136 to 1.636 | 0.782 | 0.281 | –1.963 to 2.526 | 0.789 |
| Referral reason | 0.371 | 0.016 to 0.725 | 0.041 | 0.436 | –0.020 to 0.892 | 0.059 |
| Cancer type | 0.128 | –0.616 to 0.873 | 0.720 | 0.280 | –0.639 to 1.200 | 0.519 |
| HSCT | 0.200 | –1.687 to 2.088 | 0.825 | –0.300 | –2.531 to 1.931 | 0.775 |
HSCT, hematopoietic stem cell transplant; CI, confidence interval; coeff, coefficient.
In the setting of limited PC involvement for hematologic malignancies in the United States, we report on the early experience of an embedded, outpatient PC service in a large academic medical center. Patients with a wide range of diagnoses were referred, mostly for symptom management and advance care planning. Nearly half of decedents following PC ultimately used hospice services. In the subset of patients who followed PC for at least 6 months, inpatient costs (excluding hospitalizations for HSCT) were significantly lower after PC. Taken together, our findings suggest the value of longitudinal outpatient PC for hematologic malignancies.
Our findings are consistent with prior literature that show that early, outpatient PC is associated with lower end-of-life health care utilization for patients with cancer9,15 and that specialty PC increases the likelihood of patients with hematologic malignancies dying at home or in a residential hospice rather than a hospital.16 In our study, the total hospitalization costs were lower despite no significant difference in the number of hospitalizations; this was likely owing to fewer admissions for chemotherapy (Table 1). We also may have been underpowered to detect significant changes in number of encounters. Larger and longer-term studies are warranted to determine whether and how outpatient PC impacts other health care utilization parameters.
Our study has important limitations and should be seen as hypothesis generating. First, our study reports on a specific cohort of 120 patients, who were seen in one embedded PC program at a single academic medical center. The patients who were referred to and seen by PC were a minority in the malignant hematology and HSCT clinics. This introduces potential bias as our cohort is potentially different from patients who were not referred to PC or chose not to attend. We were unable to compare the health care utilization of our cohort to a control group, nor was our sample size sufficient for propensity score matching. Instead, we used a pre-post methodology to assess associations between PC and health care utilization. However, costs could differ based on timing of chemotherapy or notable symptoms. To provide further context, we list the reasons for hospitalizations in the pre- and post-periods.
In conclusion, longitudinal outpatient PC for people with hematologic malignancies is feasible and has benefits upstream from the end-of-life period.17 Large, multisite, and controlled trials are needed to confirm our findings and determine which aspects of PC are most impactful.
Acknowledgments: The authors thank Li Zhang, biostatistician, for her guidance on the statistical analysis.
The National Institute on Aging (NIA) T32 AG000212 (M.T.), American Society of Hematology (ASH) RTAF (K.L.S.). This content is the responsibility of the authors and does not necessarily represent the views of the NIA, its board of governors or methodology committee, or ASH.
Contribution: M.T., K. Bischoff, and M.R. developed the study concept, study design, and prepared the manuscript; K. Bischoff, C.A., and G.N.M. provided additional content expertise; M.T., K.L.S., and K. Berry acquired data; M.T. and D.O. conducted statistical analysis; and M.T., K. Bischoff, K.L.S., K. Berry, D.O., B.F., N.S., R.O., C.A., J.V., E.C., N.S.L., G.N.M., and M.R. edited and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mazie Tsang, Division of Hematology/Oncology, Mayo Clinic Arizona, PX CB 03 HEMONC, 5881 East Mayo Blvd, Phoenix, AZ 85054; e-mail: tsang.mazie@mayo.edu.
References
Author notes
∗M.T. and K.B. contributed equally to this work.
Data are available on request from the corresponding author, Mazie Tsang (tsang.mazie@mayo.edu).
Presented at the American Society of Clinical Oncology 2022 Annual Meeting and the 62nd annual meeting of the American Society of Hematology (virtual), 5-8 December 2020.