Key Points
Picomolar levels of circulating FXIa do not initiate clotting but facilitate thrombi-size growth by enhancing TG inside the clot.
Because of its thrombogenic role, FXIa may serve as a target for novel anticoagulant therapies.
Abstract
Inhibitors of coagulation factor XIa (FXIa) are currently being investigated as potential anticoagulant therapies. We hypothesize that circulating FXIa could be a potential target for these therapies. Using previous analyses of FXIa impurities in immune globulin products involved in thrombotic adverse events, we estimated that picomolar levels of FXIa can be thrombogenic. In an in vitro clot-growth assay, 0.1-3 pM of FXIa did not, by itself, activate clotting but increased the size of growing clots. Spatio-temporal reconstruction of thrombin activity inside the clot revealed that FXIa’s effect was limited to the clot-plasma interface, in which FXIa produced a taller than standard wave of thrombin. Factor-depleted plasma and a panel of selective anti-FXIa antibodies showed that exogenous FXIa effects are (1) blocked by anti-FXIa antibodies, (2) independent of FXI activation inside the clot, and (3) larger than the contribution of in situ FXIa. In a thrombin generation (TG) assay, picomolar FXIa did not initiate TG but rather promoted TG triggered by tissue factor or thrombin, suggesting that the effect of FXIa on the thrombin wave is mediated by the elevation of thrombin-triggered TG. In circulating bovine blood, low doses of human FXIa did not initiate clotting but increased the size of stenosis-triggered thrombi. FXIa injection in mice enhanced TG in plasma for at least 6 hours ex vivo, confirming the persistence of circulating FXIa. Our findings suggest that picomolar levels of circulating FXIa may not be able to initiate thrombosis but can facilitate thrombus growth through the facilitation of TG inside the clot.
Introduction
Although existing antithrombotic therapies are effective, they may also carry the risk of bleeding complications. Coagulation factor XI (FXI) is an attractive target for novel antithrombotic therapies that may improve the risk-benefit profile by reducing the risk of bleeding. Zymogen FXI likely plays a limited role in hemostasis as patients with FXI deficiency have mild bleeding symptoms. In contrast, the significant role of FXI in thrombosis is suggested because of (1) a correlation between elevated plasma FXI levels and a history of thrombotic events,1,2 (2) antithrombotic effects of severe FXI deficiency,3 and (3) results of studies assessing the pharmacological inhibition of FXI4 and activated FXI (FXIa)5 in humans and animals. Another indicator is the presence of circulating FXIa in patients with prothrombotic conditions in cardiovascular6-11 and inflammatory diseases12,13 and with trauma,14,15 whereas it is absent in healthy individuals. Interestingly, a longitudinal study of patients with advanced stable coronary artery disease16 demonstrated that early detection of circulating FXIa was a reliable predictor of thrombosis that occurred years later.
Independent evidence of thrombogenic potential for elevated FXI and FXIa was obtained from investigations of thrombotic adverse events (TAEs) caused by 2 plasma-derived products, FXI and immune globulin (IG) concentrates. Treatment of FXI deficiency with early versions of FXI concentrates was effective but was complicated by frequent TAEs.15,17 These TAEs were believed to be caused by the coadministration of FXI with its impurity, FXIa, a theory that was consistent with reduced markers of in vivo thrombin generation (TG), namely thrombin-antithrombin complex formation, in patients who were administered with these, and mitigation of TAE risks in newer FXI products that were manufactured to contain less FXIa. More recently, batches of plasma-derived IG concentrates,18-20 which were recalled from markets for causing TAEs, were found to contain procoagulant activity mediated by FXI and FXIa impurities,21-24 and, again, the changes to the manufacturing processes that reduced FXI and FXIa levels in IG products have also lowered the TAE risks.17
The history of TAEs triggered by impurities, although unfortunate, can offer valuable insights into the mechanisms by which FXI and FXIa can cause thrombosis and improve our understanding of the targets of future anticoagulants. The clinical evidence pertaining to TAEs is limited by the spontaneous nature of adverse event reporting and the complexity of thrombotic event development. The spike in TAEs associated with intravenous IGs was initially related to 1 product, but several others were later reported to have an association between procoagulant activity in vitro and TAEs.21,25 Overall, of the patients who received TAE-implicated product batches, only some ended up developing adverse events.26 Most, but not all, patients had underlying conditions that increased thrombotic risks. The biochemical characterization of the implicated batches revealed increased TG potential mediated by FXIa and possibly other coagulation factor impurities, eg, kallikrein, FXI, and FXII.26
In this study, we present the results of in vitro and animal investigations designed to estimate FXIa levels that may have been administered to patients compared with those of in situ FXIa produced during the blood coagulation reaction. Our findings suggest that the presence of picomolar concentrations of FXIa in blood may be sufficient to promote thrombotic events, which aligns with the available data on TAEs associated with FXI and IG concentrates and suggests that circulating FXIa, rather than or in addition to FXI, can serve as a target for future anticoagulant therapies.
Methods
Reagents
FXIa and an inhibitory antihuman FXI monoclonal antibody, αFXI-2 (AHXI-5061),27 were purchased from Haematologic Technologies, Inc, Essex Junction, VT. FXIa activity was calibrated in international units (IU) against the National Institute for Biological Standards and Control Reference Reagent for FXIa (11/236). The potency of 1 pM was 1.7 mIU FXIa/mL. Anti-FXI antibodies O1A6 and ab14E11 were prepared as described.28-30 IG products were purchased from the National Institutes of Health Pharmacy in Bethesda, MD. All other reagents were purchased from reputable vendors (see supplemental Materials).
TG-Fibrin generation assay
Citrated plasma (75% vol/vol) was mixed with Corn Trypsin Inhibitor (CTI, 200 μg/mL), fluorogenic substrate Z-Gly-Gly-Arg-7-Amino-4-methylcoumarin (Z-Gly-Gly-Arg-AMC, 800 μM), and phospholipid vesicles (4 μM). A solution of CaCl2, with or without tissue factor (TF) or β-thrombin, was used to trigger TG. FXIa was added to the plasma immediately after recalcification and immediately before the start of recording. Fluorescence (460 nm) and clot absorbance (490 nm) were recorded at 37°C using a microplate reader (Biotek Synergy H4) and analyzed as described.31 TG was calibrated using an internal thrombin-α2 macroglobulin calibrator from Stago tested in parallel with the test sample. A calibrated automated thrombinography algorithm was used to apply internal calibration and account for the inner-filter effect and substrate consumption.
To evaluate mouse plasma, a slightly modified assay was performed with and without the αFXI-2 antibody (see supplemental Materials).
Clot growth (custom variant of thrombodynamics assay)
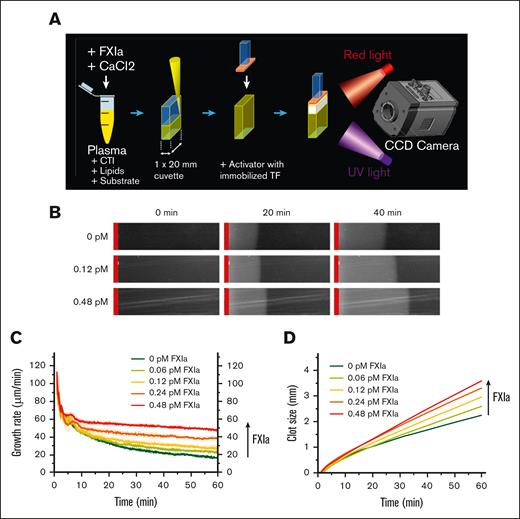
Clot growth was studied in stagnant human plasma using a custom-made video- microscopy system32 (Figure 1A) equipped with a low light Andor camera (Oxford Instruments plc, Abingdon, UK). The concentrations of human plasma, CTI, fluorogenic substrates, and lipids were the same as those used in the TG assay. Anti-FXI antibodies αFXI-2, O1A6, and ab14E11 were preincubated with plasma for 30 minutes, followed by plasma recalcification. FXIa was then mixed in, and the resulting mixture was transferred to a clot-growth chamber. The initiation of clotting (and the start of the experiment) was performed by sliding a TF-coated33 plastic insert into the clot-growth chamber. The TF density at the contact of the TF-covered insert with plasma was modeled after cultured smooth muscle cells.34 Images of visible light (625 nm) scattered by the clot34,35 and fluorescence (365 nm excitation and 441 nm emission) were captured every 10.8 seconds. The clot size growth phase was quantified using the growth rate, which was calculated as the slope of the linear regression for clot size data between 10 and 40 minutes following the onset of clotting. The spatial distribution of thrombin activity inside the clot was calculated using an AMC calibrator (ie, the brightness of each pixel was compared with the brightness of the calibration picture with a known amount of AMC) as described32 (see supplemental Materials).
Thrombogenicity of TAE-implicated batches may be caused by an increase in the spatial clot-growth rate. (A) Clot growth was studied using a video-microscopy-based instrument, as shown in the schematic diagram. Recalcified plasma was placed in a microchamber and brought into contact with a TF-coated activator. (B) Images of growing clots in healthy plasma supplemented with the indicated concentrations of FXIa at 0, 20, and 40 minutes. The TF-coated activator is indicated by the red vertical line. Shown representative of 2--4 repeats. (C) Clot-growth rate over time periods at the indicated FXIa concentration. Shown representative of 2--4 repeats. (D) Clot size over time periods at the indicated FXIa concentration. The vertical arrow indicates increasing concentrations of FXIa. Shown representative of 2--4 repeats.
Thrombogenicity of TAE-implicated batches may be caused by an increase in the spatial clot-growth rate. (A) Clot growth was studied using a video-microscopy-based instrument, as shown in the schematic diagram. Recalcified plasma was placed in a microchamber and brought into contact with a TF-coated activator. (B) Images of growing clots in healthy plasma supplemented with the indicated concentrations of FXIa at 0, 20, and 40 minutes. The TF-coated activator is indicated by the red vertical line. Shown representative of 2--4 repeats. (C) Clot-growth rate over time periods at the indicated FXIa concentration. Shown representative of 2--4 repeats. (D) Clot size over time periods at the indicated FXIa concentration. The vertical arrow indicates increasing concentrations of FXIa. Shown representative of 2--4 repeats.
Closed-loop bovine blood circulation model
Citrated venous bovine whole blood supplemented with heparin (0.3 U/mL) and recalcified with CaCl2 (0.6 mmol/L), with or without human FXIa, was placed in a closed polyvinyl chloride tubing circuit (internal diameter 6.4 mm and length 78 cm) with a stenotic section to facilitate blood clot deposit. The blood was circulated with the help of a roller pump at 100 mL/min for 30 minutes at room temperature. The thrombi were photographed and weighed under wet and dry conditions. Platelet counts were measured using a Hemavet 950 FS analyzer before and after the experiment.
FXIa administration in mice
All animal procedures were performed in accordance with protocols approved by the US Food and Drug Administration Center for Biologics Evaluation and Research Animal Care and Use Committee. CD1 mice (Charles River Laboratories, Frederick, MD) received human FXIa or saline via a lateral tail vein injection. Citrated blood was collected from the submandibular vein, and platelet free plasma was separated and frozen at −80°C.
Results
Effect of added FXIa on spatial clot growth and TG in healthy plasma
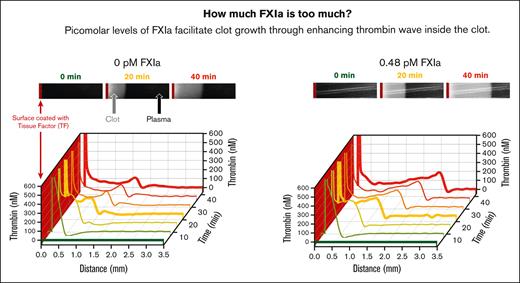
Estimates of procoagulant activity in TAE-implicated IG products suggest thrombogenicity of circulating FXIa at subpicomolar to picomolar levels (see supplemental Materials). Previous studies indicated that the FXI pathway becomes important under conditions of limited TF exposure, for example, either when very low TF levels are mixed with whole blood or plasma, or when plasma comes into contact with a TF-coated surface (Figure 1A) to trigger the growth of fibrin clots.32,34,36 In TF-surface triggered experiments, subpicomolar concentrations of FXIa increased the rate of the clot size growth phase in a dose-dependent manner (Figure 1B-D) but had no effect on the clot initiation time (∼2 minutes, data not shown) and the first 5 minutes of clot growth (Figure 1C,D). Note that in the control condition without added FXIa, the clot size growth rate slowed down by 70% within an hour (ie, from 60 μm/min at 5 minutes to 16 μm/min at 60 minutes); in the presence of FXIa, the rate of deceleration was slower (in a dose-dependent manner) (Figure 1C).
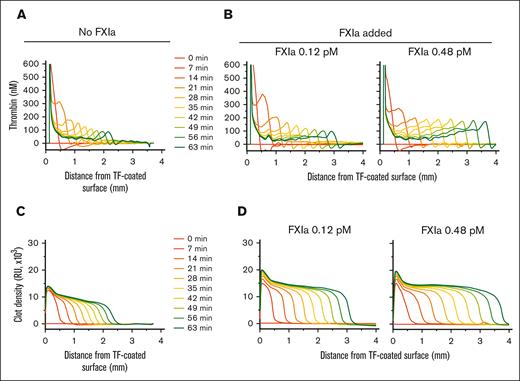
Analysis of thrombin activity distribution inside the clots showed increased TG at the clot-plasma interface in the presence of FXIa. Spatio-temporal patterns of thrombin activity appeared as waves at the outer edge of the fibrin clot, which were higher in the presence of FXIa than in its absence (compare panels A and B of Figure 2). The height of the TG wave decreased over time without the presence of FXIa (Figure 2A), whereas the addition of FXIa stabilized the height and travel rate of the TG wave, both of which increased in an FXIa dose-dependent manner (Figure 2B). In addition, the light scattered by the clot, indicative of clot density (measured in relative units), was brighter in experiments with subpicomolar concentrations of FXIa than without FXIa (compare panels C and D of Figure 2). Previously, the amount of light scattering under these conditions was proportional to fibrinogen concentration,34 suggesting denser clots in the presence of FXIa.
Typical profiles of thrombin activity (FIIa) and clot density. (A-B) Thrombin concentration inside of the clot at the indicated time points (A) without added FXIa or (B) with 0.12 or 0.48 pM of FXIa. (C-D) Clot density profiles at the indicated time points (C) without added FXIa or (D) with 0.12 or 0.48 pM of FXIa. Profiles are shown for the experiments presented in Figure 1 (representative of 2--4 repeats).
Typical profiles of thrombin activity (FIIa) and clot density. (A-B) Thrombin concentration inside of the clot at the indicated time points (A) without added FXIa or (B) with 0.12 or 0.48 pM of FXIa. (C-D) Clot density profiles at the indicated time points (C) without added FXIa or (D) with 0.12 or 0.48 pM of FXIa. Profiles are shown for the experiments presented in Figure 1 (representative of 2--4 repeats).
Relative contributions of FXI activation and added FXIa
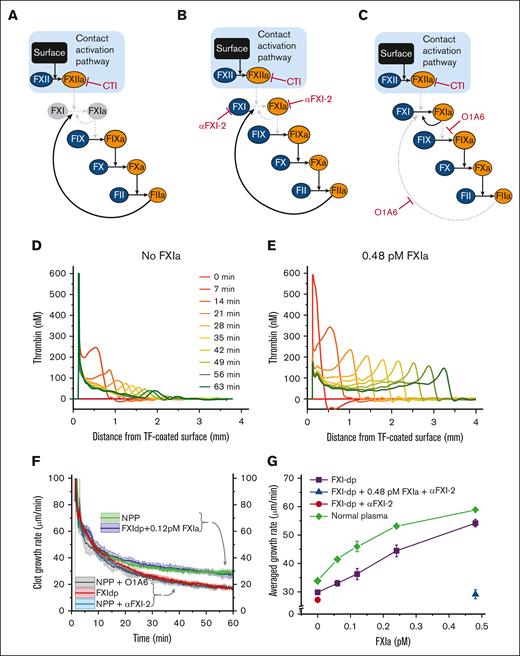
To understand whether the effect of picomolar concentrations of FXIa depends upon the activation of plasma FXI, we performed experiments in FXI-deficient plasma supplemented with CTI (added to minimize artifactual contact activation of FXI by FXIIa; Figure 3A). Under these conditions, the addition of 0.48 pM FXIa enhanced thrombin wave height (Figure 3D-E) and propagation rate, almost doubling it (Figure 3G; violet line), as compared with conditions with no FXIa present. In another experiment, healthy plasma was treated with CTI and anti-FXI antibodies αFXI-2 (which inhibits FXI and FXIa; Figure 3B) or O1A6 (which inhibits FXI activation via thrombin; Figure 3C). Both anti-FXI antibodies (Figure 3F; light blue and gray curves) reduced the usual clot-growth rate (34.5 ± 0.5 μm/min; green curve) to the level observed in FXI-deficient plasma (29.9 ± 0.5 μm/min; red curve; see Figure 3F). Interestingly, the growth rate in healthy plasma was similar to that of FXI-deficient plasma supplemented with only 0.12 pM of FXIa (Figure 3F; blue and green curves), indicating that FXIa concentrations >0.12 pM can outperform the in situ FXI activation.
Regulation of clot growth by endogenous FXI zymogen. Diagrams illustrate the 3 experimental conditions to modulate the interaction of FXI with FIX and thrombin. In all experiments, FXI activation by FXIIa is blocked by CTI. Solid black arrows illustrate active pathways, and dashed gray arrows indicate inhibited pathways. Zymogens and enzymes are represented by blue and orange ellipses, respectively. (A) Thrombin-mediated activation of FXI is blocked in the absence of FXI (ie, in FXI-deficient plasma). (B) Antibody αFXI-2 inhibits FXI and FXIa. (C) Antibody O1A6 inhibits FXI activation by thrombin and FIX activation by FXIa. (D) Condition A: TG profiles inside the growing clot in FXI-deficient plasma without FXIa. Shown representative of 2 repeats. (E) Condition A + FXIa: TG profiles inside a growing clot in FXI-deficient plasma supplemented with 0.48 pM of FXIa. Shown representative of 2 repeats. (F) Clot-growth rate in healthy normal pooled plasma (NPP; green curve), FXI-deficient plasma with (blue curve) or without (red curve) 0.12 pM FXIa, and in healthy plasma treated with αFXI-2 (light blue curve) or O1A6 (gray curve). The standard deviation of the averaged clot-growth rate is indicated by the colored shades around the curves. Note that the addition of 0.12 pM FXIa to FXI-deficient plasma normalized the clot-growth rate. Shown representative of 2--4 repeats. (G) The average rate of clot growth as a function of FXIa activity. FXIa increased the average clot-growth rate in healthy (green) and FXI-deficient (purple) plasma in a dose-dependent manner. The antibody for FXI and FXIa, αFXI-2, neutralized the effect of FXIa in FXI-deficient plasma (blue triangle) but did not affect clot growth in untreated FXI-deficient plasma (red circle), confirming the specificity of αFXI-2 to FXIa. Shown representative of 1--4 repeats.
Regulation of clot growth by endogenous FXI zymogen. Diagrams illustrate the 3 experimental conditions to modulate the interaction of FXI with FIX and thrombin. In all experiments, FXI activation by FXIIa is blocked by CTI. Solid black arrows illustrate active pathways, and dashed gray arrows indicate inhibited pathways. Zymogens and enzymes are represented by blue and orange ellipses, respectively. (A) Thrombin-mediated activation of FXI is blocked in the absence of FXI (ie, in FXI-deficient plasma). (B) Antibody αFXI-2 inhibits FXI and FXIa. (C) Antibody O1A6 inhibits FXI activation by thrombin and FIX activation by FXIa. (D) Condition A: TG profiles inside the growing clot in FXI-deficient plasma without FXIa. Shown representative of 2 repeats. (E) Condition A + FXIa: TG profiles inside a growing clot in FXI-deficient plasma supplemented with 0.48 pM of FXIa. Shown representative of 2 repeats. (F) Clot-growth rate in healthy normal pooled plasma (NPP; green curve), FXI-deficient plasma with (blue curve) or without (red curve) 0.12 pM FXIa, and in healthy plasma treated with αFXI-2 (light blue curve) or O1A6 (gray curve). The standard deviation of the averaged clot-growth rate is indicated by the colored shades around the curves. Note that the addition of 0.12 pM FXIa to FXI-deficient plasma normalized the clot-growth rate. Shown representative of 2--4 repeats. (G) The average rate of clot growth as a function of FXIa activity. FXIa increased the average clot-growth rate in healthy (green) and FXI-deficient (purple) plasma in a dose-dependent manner. The antibody for FXI and FXIa, αFXI-2, neutralized the effect of FXIa in FXI-deficient plasma (blue triangle) but did not affect clot growth in untreated FXI-deficient plasma (red circle), confirming the specificity of αFXI-2 to FXIa. Shown representative of 1--4 repeats.
Amplification of TG by subpicomolar levels of FXIa
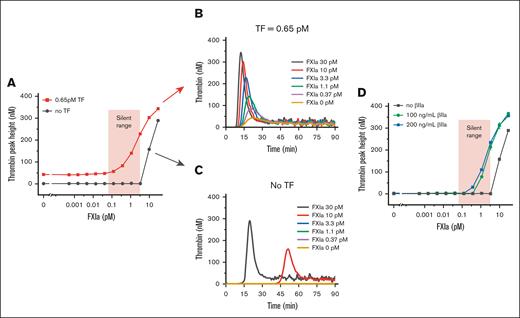
Although subpicomolar FXIa was sufficient to promote TF-activated clot growth, FXIa did not result in homogeneous plasma clotting in the experimental chamber, suggesting that low doses of FXIa require TF as a cotrigger. To test the effect of FXIa at the site of TF exposure more accurately, we performed a microplate-based TG assay in healthy plasma, with or without added lipidated TF. At concentrations from 0.065 to 10 pM, FXIa dose-dependently facilitated TG triggered by TF (Figure 4A, B) but did not induce TG in the absence of TF (Figure 4A,C), that is, subpicomolar concentrations of FXIa relied on TF to produce its effect.
Low FXIa concentrations do not trigger TG but promote TG in the presence of added procoagulant stimuli. (A) Effect of increasing FXIa concentrations on thrombin peak height in recalcified, but not activated, plasma (black) and TF-activated plasma (red). The red shaded area indicates the range of silent FXIa concentrations that do not initiate TG but enhance TG when TF is introduced. Shown representative of 2 repeats. (B) TG curves in TF-triggered (0.65 pM TF, 20 mM CaCl2) NPP (from panel A). Shown representative of 2 repeats. (C) TG curves of the recalcified NPP without TF (from panel A). Shown representative of 2 repeats. (D) Effect of increasing FXIa concentrations on thrombin peak height in recalcified plasma supplemented with the indicated β-thrombin concentrations. Red shaded area indicates the range of silent FXIa from the panel A. Shown representative of 2 repeats.
Low FXIa concentrations do not trigger TG but promote TG in the presence of added procoagulant stimuli. (A) Effect of increasing FXIa concentrations on thrombin peak height in recalcified, but not activated, plasma (black) and TF-activated plasma (red). The red shaded area indicates the range of silent FXIa concentrations that do not initiate TG but enhance TG when TF is introduced. Shown representative of 2 repeats. (B) TG curves in TF-triggered (0.65 pM TF, 20 mM CaCl2) NPP (from panel A). Shown representative of 2 repeats. (C) TG curves of the recalcified NPP without TF (from panel A). Shown representative of 2 repeats. (D) Effect of increasing FXIa concentrations on thrombin peak height in recalcified plasma supplemented with the indicated β-thrombin concentrations. Red shaded area indicates the range of silent FXIa from the panel A. Shown representative of 2 repeats.
Furthermore, we suggested that the effect of picomolar concentrations of FXIa on the height and movement rate of spatio-temporal thrombin waves in microchamber experiments could be explained based on the synergy between FXIa and thrombin, of which the latter could act like TF, revealing the FXIa's potential. To test this hypothesis in the TG experiments, the TF trigger was replaced with β-thrombin (βIIa) because βIIa lacks the ability to clot fibrinogen (and thus can be mixed with plasma) but can activate FV, FVIII, and FXI. As expected, picomolar concentrations of FXIa produced TG, but only in the presence of βIIa (Figure 4D).
FXIa increases the size of thrombi in circulating blood
To study the effect of FXIa on thrombus size growth in circulating whole blood, we used a model of catheter thrombogenicity, which is a closed loop with a stenosis thrombus formed in heparinized bovine blood. The concentration of human FXIa in this experiment was ∼100-fold higher than that used in clot-growth experiments to account for the 10-fold lower sensitivity of bovine plasma to human FXIa observed in a preliminary in vitro bridging study. Likewise, the experimental heparin concentration was chosen in titration studies to prevent blood coagulation in stagnant blood but allow for thrombus formation in circulating blood with added FXIa. The selected FXIa dose did not induce clotting in heparinized bovine plasma in the absence of TF and thrombin triggers. As expected, when thrombi were induced by stenosis, their sizes and weights increased with the FXIa dose (Figure 5A,B). FXIa also reduced the platelet count at the end of the experiment, indicating their accumulation in growing thrombi (Figure 5B; supplemental Table 1).
FXIa promotes clot formation in circulating whole bovine blood and TG after intravenous injection in mice. (A) Images of blood clots formed in circulating whole bovine blood spiked with (from left to right) 0, 10, 50, 100, 200, and 500 pM human FXIa. Shown representative of 2 repeats. (B) The weight of thrombi (from panel A) increased in an FXIa concentration-dependent manner, whereas the concentration of platelets in the remaining liquid blood (after 30 min of circulation) decreased (raw data are provided in supplemental Table 1). Shown representative of 2 repeats. (C) Schematic depiction of the approach used to characterize the effect of FXIa on TG by calculating ΔTPH between ex vivo TG experiments with and without the anti-FXI antibody (αFXI-2). (D) TG assay-based pharmacokinetics of intravenously administered human FXIa in mice. Blood from ∼2 to 5 mice was collected at the indicated time points and tested using the TG assay, as indicated in panel C. Mean ± S.D is shown. TPH, thrombin peak height; ΔTPH, change in TPH; i.v., intravenously.
FXIa promotes clot formation in circulating whole bovine blood and TG after intravenous injection in mice. (A) Images of blood clots formed in circulating whole bovine blood spiked with (from left to right) 0, 10, 50, 100, 200, and 500 pM human FXIa. Shown representative of 2 repeats. (B) The weight of thrombi (from panel A) increased in an FXIa concentration-dependent manner, whereas the concentration of platelets in the remaining liquid blood (after 30 min of circulation) decreased (raw data are provided in supplemental Table 1). Shown representative of 2 repeats. (C) Schematic depiction of the approach used to characterize the effect of FXIa on TG by calculating ΔTPH between ex vivo TG experiments with and without the anti-FXI antibody (αFXI-2). (D) TG assay-based pharmacokinetics of intravenously administered human FXIa in mice. Blood from ∼2 to 5 mice was collected at the indicated time points and tested using the TG assay, as indicated in panel C. Mean ± S.D is shown. TPH, thrombin peak height; ΔTPH, change in TPH; i.v., intravenously.
FXIa pharmacokinetics in mice
FXIa pharmacokinetics remain unknown; however, slow FXIa clearance may explain both the existence of circulating FXIa in patients with thrombosis 6-8,37 and TAEs after picomolar doses of FXIa. Indeed, in our microchamber experiments (Figures 1 and 2), the procoagulant effect of FXIa was apparently stable for at least 40 minutes, as observed in the sustained FXIa dose-dependent parameters of growing clots, that is, stable growth rates with congruent thrombin waves of a consistent height. To estimate FXIa clearance in vivo, human FXIa or buffer was injected into healthy CD1 mice. Then plasma was collected and tested in a TG assay (chromogenic and enzyme-linked immunosorbent assays were not sensitive enough to detect FXIa activity) in the presence of either an aFXI-2 antibody or a buffer to obtain a change in thrombin peak height (TPH), as schematically shown in Figure 5C. Thus, we used the aFXI-2 antibody to characterize the effect of FXIa on TG by calculating the change in TPH. The ex vivo TG assay results showed that FXIa-dependent (ie, αFXI-2 blocked) activity was present in mouse plasma for at least 4 hours after injection (Figure 5D).
Discussion
In this study, we showed that picomolar quantities of exogenous FXIa (1 pM equivalent to 1.7 mIU/mL) may not be able to initiate coagulation in vitro, in circulating blood or living mice. However, when coagulation is triggered by TF or thrombin, the thrombogenic potential of these FXIa doses is revealed through increased TG, accelerated growth of fibrin clots and thrombi in circulating blood, and formation of a spatio-temporal wave of thrombin inside the growing clots. Our results are in agreement with data presented by Grundmann et al,24 who also observed the procoagulant activity of subpicomolar FXIa concentrations with a modified TG method in diluted plasma supplemented with excess lipids.
Our study was inspired by earlier investigations on unexpected TAEs in patients receiving IG products, which established a connection between FXIa administration (in the form of impurities in the administered pharmaceutical product) and thrombosis in humans. Although it was not possible to measure the amount of FXIa that is definitively responsible for TAEs, our analysis of TAE-associated IG lots pointed to picomolar levels of FXIa. In this study, we demonstrated that subpicomolar levels (∼ 1 mIU/mL) of FXIa can promote clot growth. The thrombogenicity of these small traces of FXIa is likely driven by (1) the relative persistence of FXIa activity, as observed in in vitro and mouse experiments and (2) FXIa’s ability to facilitate the initial TG (as triggered by either TF or thrombin existing inside the clot) and expand it into a sustained wave of thrombin that moves within the clot, ultimately potentiating clot growth.
The thrombin wave observed in our clot-growth experimental system32 was previously suggested to depend on self-sustained activation of FXI by thrombin because clot growth was reduced in FXI-deficient plasma. In another clot-growth study, a relatively high FXIa level of 150 pM increased clot growth and resulted in spontaneous clotting.38 Here, we used a panel of specific antibodies to clarify that FXI activation, although real, is a relatively minor regulator of in vitro clot growth compared with traces of exogenous FXIa. We show that 0.48 pM FXIa, or ∼0.0016% of plasma FXI level, can double the clot-growth rate, whereas activation of FXI by thrombin contributes to a mere 20% increase in the clot-growth rate. Importantly, we demonstrated that this effect of exogenous FXIa on clot growth is limited by increasing the height of thrombin waves that reside inside the growing clot.
In our experiments, 0.12 pM FXIa was sufficient to induce the same clot growth in FXI-deficient plasma, as was observed in healthy plasma, suggesting that the effect of 0.12 pM FXIa is equivalent to that of FXI activated inside the growing clot in healthy plasma. A similarly low estimate was made in a previous investigation of FXIa-contaminated concentrates of FXI by von dem Borne et al.36 In that study, FXI-deficient plasma supplemented with femtomolar concentrations of exogenous FXIa produced standard clotting times, and the addition of 0.125 pM FXIa affected clot lysis in the same manner as FXI concentrate.
Our results indicate that circulating FXIa may be a potent procoagulant with a crucial thrombogenic role in patients who are at risk. Indeed, the procoagulant effects of exogenous FXIa can be blocked with inhibitory anti-FXI antibodies, suggesting that circulating FXIa could serve as a potential target for novel anti-FXIa therapies. Animal models and early phase clinical trials in humans with novel anticoagulants targeting FXIa demonstrated an antithrombotic effect and a potentially reduced bleeding risk. The expectation of lower bleeding risks compared with those of the currently available anti-FIIa and anti-FXa direct oral anticoagulant therapies is based on the relatively mild bleeding phenotype of FXI deficiency. This is in contrast to the spontaneous bleeding tendency in FVIII and FIX deficiencies, which are the 2 coagulation proteins needed for the generation of FXa and thrombin.
The reasons for the strong anticoagulant effect of anti-FXIa therapies may be as complex as the potential involvement of FXI in thrombosis. FXIa inhibitors can block FXI activation mediated by thrombin, FXIIa, and FXIa itself (via autoactivation), which are thought to promote TG inside the thrombus. Another antithrombotic target of anti-FXIa therapies is circulating FXIa, which is associated with past thrombotic events.6-16 Our in vitro studies indicate that circulating FXIa is a thrombogenic risk that supersedes that of FXIa produced inside the clot. Therefore, we suggest that during the clinical development of anti-FXIa therapies, researchers should consider measuring FXIa activity levels in patients before and after therapy administration. It is plausible that a test for circulating FXIa could identify patients who are most likely to benefit from anti-FXIa therapy and determine the optimal dose for them.
The duration of elevated thrombosis risk after inadvertent administration of FXIa is not known. In some patients who received TAE-implicated IGs, clinical thrombosis developed quickly, whereas for others this developed many hours after administration,21,39,40 suggesting that FXIa clearance may take hours. In contrast, Ruhl et al estimated the in vitro half-life of FXIa in healthy human plasma to be only 130 seconds.41 Other authors have observed FXIa activity after 1 to 3 hours of incubation in whole blood or plasma.37,38 Our data provide evidence in support of FXIa activity persistence, as manifested by intensified clot growth 1 hour after the addition of FXIa to plasma and many hours after administration of human FXIa into mice.
An important limitation of our study is the use of platelet-poor plasma. Platelets play a significant role in coagulation by providing a procoagulant surface and releasing procoagulant substances and inhibitors. Furthermore, platelets have a direct impact on FXI/FXIa by secreting FXIa inhibitors while also protecting FXIa from inhibition by binding to the platelet surface. Therefore, the effect of FXIa on clot growth in platelet-rich plasma requires further investigation. Another important limitation of this study is the lack of data from the plasma of patients who received IG concentrates tainted with FXIa. Although experiments with patient plasma would significantly add to the study, the collection of such samples is technically impossible because the IG products tainted with FXIa were withdrawn from the market. We demonstrated that human FXIa can be detected in mouse blood hours after FXIa injection. Although these results are in remarkable agreement with the evidence of circulating FXIa activity in thrombotic patients, interspecies differences in inhibitor concentrations and activities toward human FXIa complicate the generalization of the observed slow clearance of FXIa to human conditions.
Taken together, our results suggest the need to monitor FXIa activity in (1) plasma-derived IG product batches to prevent the distribution of unsafe medicines to patients who are at risk and (2) patients who are at risk to better manage and treat their coagulopathy.
Acknowledgments
The authors thank former FDA student fellows Samuel A. Woodle and Willi Li for their assistance with early experiments as well as Joanne Berger (FDA Library), Daniel Sloper (National Center for Toxicological Research), and Joseph W. Jackson (ORISE Fellow at FDA) for their help in editing the manuscript. This study was supported by funding from the US Food and Drug Administration Office of Women’s Health (M.V.O.). F.I.A. acknowledges support from the Russian Science Foundation (#22-15-00164). This presentation reflects the views of the authors and should not be construed as representing the views or policies of the US Food and Drug Administration.
Authorship
Contribution: L.A.P. and M.V.O. designed the experiments, analyzed the data, and wrote the manuscript; L.A.P., Q.L., and Y.L. designed and performed the experiments; A.M.S. conducted preliminary experiments; D.E.S., E.I.T., and F.I.A. provided product samples, reagents, and analytical instrumentation, respectively; M.V.O. supervised the project and the preparation of the manuscript; and all authors participated in drafting the manuscript.
Conflict-of-interest disclosure: A.M.S. is a recipient of the Oak Ridge Institute for Science and Education Fellowship, distributed via an interagency agreement with the US Department of Energy and the Food and Drug Administration. E.I.T. and Oregon Health & Science University (OHSU) report financial interest in Aronora Inc., a company that may have a commercial interest in the results of this research. This potential conflict of interest has been reviewed and managed by the OHSU conflict-of-interest-in-research committee. The remaining authors declare no competing financial interests.
Correspondence: Mikhail V. Ovanesov, US Food and Drug Administration, 10903 New Hampshire Ave, White Oak Building 52/72, Room 4206, Silver Spring, MD 20993; e-mail: mikhail.ovanesov@fda.hhs.gov.
References
Author notes
Original data and protocols are available on request from the corresponding author, Mikhail V. Ovanesov (mikhail.ovanesov@fda.hhs.gov).
The full-text version of this article contains a data supplement.