Key Points
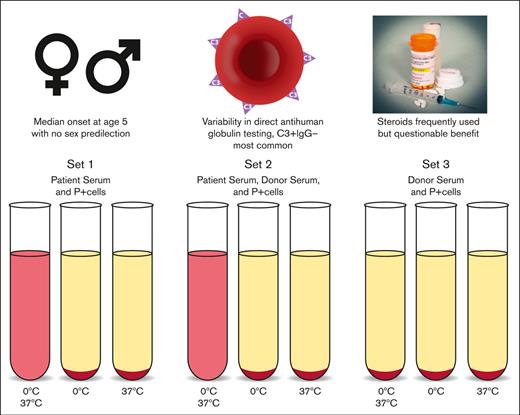
Among 230 reported cases, contemporary PCH has a median onset age of 5 years with no sex predilection.
PCH is associated with significant variability in DAT results, necessitating high clinical suspicion and DL testing.
Abstract
Paroxysmal cold hemoglobinuria (PCH) is a rare autoimmune hemolytic anemia often overlooked as a potential etiology of hemolysis and is challenging to diagnose because of the complicated testing methods required. We performed a systematic review of all reported cases to better assess the clinical, immunohematologic, and therapeutic characteristics of PCH. We systematically analyzed PubMed, Medline, and EMBASE to identify all cases of PCH confirmed by Donath-Landsteiner (DL) testing. Three authors independently screened articles for inclusion, and systematically extracted epidemiologic, clinical, laboratory, treatment, and outcomes data. Discrepancies were adjudicated by a fourth author. We identified 230 cases, with median presentation hemoglobin of 6.5 g/dL and nadir of 5.5 g/dL. The most common direct antiglobulin test (DAT) result was the presence of complement and absence of immunoglobulin G (IgG) bound to red blood cells, although other findings were observed in one-third of cases. DL antibody class and specificity were reported for 71 patients, of which 83.1% were IgG anti-P. The use of corticosteroids is common, although we found no significant difference in the length of hospitalization for patients with and without steroid therapy. Recent reports have highlighted the use of complement inhibitors. Among patients with follow-up, 99% (213 of 216) were alive at the time of reporting. To our knowledge, this represents the largest compilation of PCH cases to date. We discovered that contemporary PCH most commonly occurs in children with a preceding viral infection, corticosteroid use is frequent (but potentially ineffective), and DAT results are more disparate than traditionally reported.
Introduction
Autoimmune hemolytic anemia (AIHA) is an uncommon disorder affecting between ∼1 and 3 per 100 000 patients per year and is characterized by the development of autoantibodies directed against erythrocyte antigens.1,2 Originally described by Dressler in 1854,3 paroxysmal cold hemoglobinuria (PCH) is among the rarest forms of the autoimmune hemolytic anemias, with an estimated annual incidence of 0.04 cases per 100 000 persons.4-7
Historically, PCH was often described as a chronic condition in patients afflicted with tertiary or congenital syphilis who presented with “paroxysms” of hemoglobinuria after exposure to cold temperatures and subsequent rewarming.7 However, the advent and widespread use of effective antitreponemal therapy is believed to have significantly altered the epidemiology and clinical course of this condition.6
Today, the medical consensus derived from anecdotal reports and small case series is that PCH typically presents acutely with intravascular hemolysis and hemoglobinuria after an infectious insult, predominantly in the pediatric population.6 Although resolution of hemolysis is thought to occur within a relatively short timeframe, the anemia may be abrupt and severe, frequently necessitating blood transfusion to prevent hemodynamic collapse. In addition to blood transfusion, various interventions including corticosteroids and other immunosuppressive agents, as well as patient warming have been attempted to ameliorate the hemolysis, however, controversy remains as to whether medical therapy is effective in these patients.
Defined by the presence of a biphasic antibody, the confirmatory test for PCH was developed >100 years ago by Julius Donath and Karl Landsteiner for whom the pathologic antibody bears their name.4-8 Although the mechanism of hemolysis was not completely elucidated by Donath and Landsteiner, because the existence of complement was newly discovered and unexplored at that time, they did implicate complement in the pathogenesis.6 It was eventually discovered that at cold temperatures, this antibody adsorbs and fixes the early components of complement onto the surface of red blood cells (RBCs).7-10 As warming occurs, the antibody dissociates and activation of the full complement cascade results in intravascular lysis and release of free hemoglobin, leading to the classic hemoglobinuria.7-10
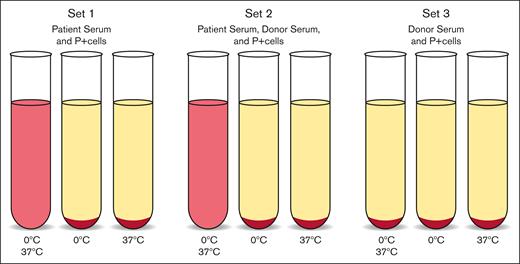
Despite being the disease for which the first described immunohematology test was utilized for diagnosis, PCH remains a challenging clinical and laboratory diagnosis owing to its rarity and complicated testing methods. Recent cases of PCH have highlighted the diagnostic delay associated with accurate laboratory assessment, because patients may be misdiagnosed with cold antibody-mediated AIHA (cAIHA) based on a direct antiglobulin test (DAT) positive only for complement.11 Although the DAT may represent a useful screening test, detection of the pathognomonic Donath-Landsteiner (DL) antibody is challenging, as it requires specific specimen preparation, incubation temperatures, and extensive technologist time (Figure 1).12,13 Furthermore, because this assay is rarely performed, testing is often only available at specialized centers and reference laboratories.
DL test.12,13 This test is diagnostic of PCH and can be performed with patient serum or whole blood although serum is preferred. Testing requires receipt of sample at 37°C to prevent binding of antibodies to patient cells. Three sets of 3 tubes are prepared as follows: (1) patient serum, (2) patient serum and normal serum, and (3) normal serum only. Normal serum is added to patient serum to supplement complement factors, and normal serum alone should act as a negative control. P-antigen–positive reagent cells are added to each tube. The first tube of each set is incubated in ice at ∼0°C and then brought to 37°C, the second tube of each set is incubated in ice only, and the third tube is incubated at 37°C only. Hemolysis in the first tube of sets 1 and 2 is a positive test.
DL test.12,13 This test is diagnostic of PCH and can be performed with patient serum or whole blood although serum is preferred. Testing requires receipt of sample at 37°C to prevent binding of antibodies to patient cells. Three sets of 3 tubes are prepared as follows: (1) patient serum, (2) patient serum and normal serum, and (3) normal serum only. Normal serum is added to patient serum to supplement complement factors, and normal serum alone should act as a negative control. P-antigen–positive reagent cells are added to each tube. The first tube of each set is incubated in ice at ∼0°C and then brought to 37°C, the second tube of each set is incubated in ice only, and the third tube is incubated at 37°C only. Hemolysis in the first tube of sets 1 and 2 is a positive test.
Given that much of the data regarding PCH have been derived from individual case reports and small series, we sought to further characterize the epidemiology, clinical and immunohematologic features, and therapeutic interventions via analysis of all reported PCH cases to date. Furthermore, because this disease has transitioned from a chronic disease affecting patients with syphilis to a predominantly acute condition in patients with other inciting stimuli, we have included a temporal analysis to identify and analyze the clinical and epidemiologic features of contemporary PCH.
Methods
This study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and was designed to collect and review the clinical demographics, laboratory findings, immunohematologic features, therapies, and outcomes of all cases of confirmed PCH reported in the literature.
We systematically analyzed PubMed, Medline, and EMBASE (supplemental Figure 1) from inception to November 23, 2022 for all articles describing PCH using keywords and search terms (supplemental Table 1).
Citations and abstracts were screened by 3 authors for relevance to the subject by using the following search terminology: Donath-Landsteiner, PCH, DL hemolytic anemia, and biphasic antibody. Publications were assessed against formal inclusion/exclusion criteria by 3 authors working independently. The criteria for inclusion were: (1) description of at least 1 human case of PCH defined as a positive DL test; (2) all patient ages and sexes; (3) case reports, case series, conference abstracts, and retrospective studies; and (4) articles in English. Exclusion criteria were: (1) duplicate articles; (2) reports describing theoretical cases of PCH or not describing a case of PCH; (3) reports with negative or inconclusive DL testing; (4) reports not documenting DL testing; (5) cases of PCH in animals; (6) articles without patient information; and (7) articles not in English.
Epidemiologic and clinical data related to patient age, sex, pregnancy status, medical comorbidities, infectious disease history, vaccination history, and presenting signs and symptoms were analyzed. Laboratory data included presenting hemoglobin and hematocrit, hemoglobin and hematocrit nadir, highest lactate dehydrogenase level, indirect antiglobulin test and antibody specificity results, DAT results with polyspecific and monospecific reagents, elution studies, cold agglutinin titer and thermal amplitude testing, and DL testing including immunoglobulin class and antigen specificity. Therapy information included RBC transfusion (yes/no) and number of RBC units, medical interventions (corticosteroids, complement inhibitors, other therapeutic agents), and the use of plasma exchange.
Outcome data (alive or deceased at the time of report, time to hospital discharge and/or resolution of hemolysis, and whether PCH was considered acute or chronic/relapsing) were obtained from all reported cases.
We assessed the quality of reported data, including the possibility of bias via the Joanna Briggs Institute Critical Appraisal Checklist for Case Reports (supplemental Table 2). Assessment for adverse events (#7) and takeaway lessons (#8) were not performed as these qualities were not considered applicable in this review.
As part of a meta-analysis, we performed statistical analysis using GraphPad PRISM version 9.2.0 (GraphPad Software, LLC, San Diego, CA) to calculate the median age of patients at diagnosis, the median hemoglobin value at both presentation and nadir, the median number of RBC units transfused, and the median length of hospitalization. We also specifically compared patients who received corticosteroid therapy with those that did not by assessing the average age, average presenting hemoglobin, average hemoglobin nadir, and average hospital length of stay for these 2 groups.
Results
Data quality
The quality of the data was assessed using an 8-question scale (the Joanna Briggs Institute Critical Appraisal Checklist for Case Reports), although, 2 of the questions were not considered applicable to this review. The strongest evidence included patient demographics, clinical history, and diagnostic testing (supplemental Figure 2). The highest risk of bias involved patients’ condition/signs/symptoms at the time of presentation and patients’ postintervention condition, with more than one-third of reports lacking this information (supplemental Figure 2).
Epidemiology
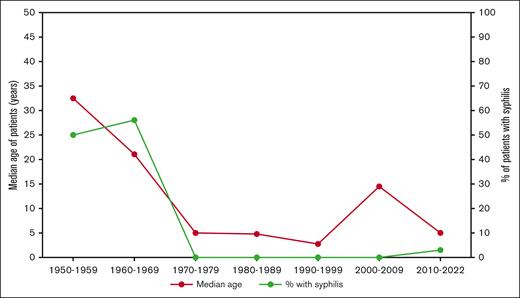
We included 125 individual articles describing a total of 230 patients with PCH confirmed by a positive DL test (supplemental Table 3). The patient population comprised 124 males and 106 females (1.17:1 male-to-female ratio). Patient age ranged from 8 months to 91 years, with a median age of 5 years (interquartile range [IQR], 2.6-51 years). Over the entire study time period, ∼51.7% (119/230) of cases occurred in patients aged ≤5 years, whereas a second smaller peak of cases was observed in patients aged between 50 and 80 years (21.7%, 50/230) (Figure 2). However, as the incidence of chronic syphilis decreased in the latter half of the twentieth century,14 there was a distinct change in both the age at which patients were diagnosed with PCH and the proportion of patients with PCH secondary to syphilis (Figure 3). Between 1950 and 1959, the median age of patients with PCH was 32.5 years and no cases were diagnosed in patients aged <19 years. Conversely, between 2010 and 2022, the median age of patients was 5 years, with 57% of cases occurring in patients aged <10 years.
Number of patients diagnosed with PCH by age and median hemoglobin nadir for each age group.
Number of patients diagnosed with PCH by age and median hemoglobin nadir for each age group.
Median age of PCH diagnosis and percentage of patients with paroxysmal cold hemoglobinuria secondary to syphilis.
Median age of PCH diagnosis and percentage of patients with paroxysmal cold hemoglobinuria secondary to syphilis.
Clinical findings and laboratory results
Among 128 patients in whom presenting signs/symptoms were reported, hemoglobinuria/dark urine was most common (68.8% of patients), whereas more than a quarter of patients either endorsed or had objective evidence of jaundice and/or scleral icterus (27.3% of patients) and fever (25.8% of patients) (Table 1).
Presenting signs and symptoms of patients with PCH
| Sign/symptom . | Number (%) of patients∗ . |
|---|---|
| Dark urine/hemoglobinuria | 88 (68.8) |
| Jaundice/scleral icterus | 35 (27.3) |
| Fever | 33 (25.8) |
| Weakness/fatigue/malaise | 27 (21.3) |
| Pallor | 22 (17.3) |
| Nausea/vomiting | 17 (13.4) |
| Abdominal pain | 17 (13.4) |
| Chills/rigors | 10 (7.9) |
| Headache | 3 (2.4) |
| Confusion/disorientation | 3 (2.4) |
| Anorexia | 3 (2.4) |
| Back/flank pain | 3 (2.4) |
| Shortness of breath | 3 (2.4) |
| Myalgia/arthralgia | 2 (1.6) |
| Rash | 2 (1.6) |
| Sign/symptom . | Number (%) of patients∗ . |
|---|---|
| Dark urine/hemoglobinuria | 88 (68.8) |
| Jaundice/scleral icterus | 35 (27.3) |
| Fever | 33 (25.8) |
| Weakness/fatigue/malaise | 27 (21.3) |
| Pallor | 22 (17.3) |
| Nausea/vomiting | 17 (13.4) |
| Abdominal pain | 17 (13.4) |
| Chills/rigors | 10 (7.9) |
| Headache | 3 (2.4) |
| Confusion/disorientation | 3 (2.4) |
| Anorexia | 3 (2.4) |
| Back/flank pain | 3 (2.4) |
| Shortness of breath | 3 (2.4) |
| Myalgia/arthralgia | 2 (1.6) |
| Rash | 2 (1.6) |
Among 128 patients in whom presenting signs/symptoms were reported; >1 symptom may have been reported in a single patient.
The hemoglobin value at presentation was available for 149 individuals, with a median of 6.5 g/dL (IQR, 4.8-9.0 g/dL), ranging from 2.3 to 14.7 g/dL. The hemoglobin nadir was reported in 197 patients, with a median nadir of 5.5 g/dL (IQR, 4.4-7.2 g/dL), ranging from 2.0 to 16.1 g/dL. The upper hemoglobin nadir range was higher than the upper hemoglobin range at presentation because of the inclusion of more cases reporting a hemoglobin nadir than a presenting hemoglobin. Furthermore, reports of patients with the highest hemoglobin nadir values did not include presenting hemoglobin values (eg, the presenting hemoglobin was not reported for the patient with a hemoglobin nadir of 16.1 g/dL, which presumably would have been >16.1 g/dL).
The lowest median hemoglobin nadir was observed in the 10-to-19-year-old age group (4.8 g/dL; range, 2.3-10.6 g/dL), followed by the 0-to-9- year-old (5.3 g/dL; range, 2.0-12.9 g/dL) and 20-to-29- year-old (5.4 g/dL; range, 2.7-16.1 g/dL) age groups.
Multiple antecedent microbial agents and infectious processes were identified before the onset of PCH, most frequently upper respiratory tract infections without a specifically identified causative organism (88 patients), gastroenteritis (15 patients), syphilis (12 patients, 75% of whom were diagnosed with PCH before 1963), and Mycoplasma pneumoniae (8 patients). No syphilis-associated PCH cases (median age: 47 years) were identified in the pediatric population, although 2, 19-year-old males and 1, 22-year-old male were reported to have PCH secondary to syphilis. Numerous other infectious diseases were implicated in single case reports, including chicken pox, measles, malaria, and leishmaniasis (Table 2).
Infections reported in patients associated with development of PCH
| Infection/microbial agent . | Patients (#)∗ . |
|---|---|
| Upper respiratory tract infection | 88 |
| Gastroenteritis | 15 |
| Syphilis | 12 |
| Mycoplasma pneumoniae | 8 |
| Haemophilus influenza | 6 |
| Respiratory syncytial virus | 6 |
| Viral exanthem | 4 |
| Viral illness | 4 |
| Adenovirus | 3 |
| Chicken pox (Varicella zoster) | 3 |
| Influenza A | 3 |
| Measles | 2 |
| Parovirus B19 | 2 |
| Bronchitis | 1 |
| Chest infection | 1 |
| Cytomegalovirus | 1 |
| Epstein-Bar virus | 1 |
| Echovirus | 1 |
| Escherichia coli urinary tract infection | 1 |
| Glandular fever | 1 |
| Klebsiella pneumoniae | 1 |
| Leishmania infantum | 1 |
| Malaria | 1 |
| Mononucleosis | 1 |
| Mumps | 1 |
| Sepsis | 1 |
| Staphylococcus aureus meningitis | 1 |
| Streptococcus pneumoniae | 1 |
| Streptococcus pyogenes | 1 |
| Urinary tract infection | 1 |
| Infection/microbial agent . | Patients (#)∗ . |
|---|---|
| Upper respiratory tract infection | 88 |
| Gastroenteritis | 15 |
| Syphilis | 12 |
| Mycoplasma pneumoniae | 8 |
| Haemophilus influenza | 6 |
| Respiratory syncytial virus | 6 |
| Viral exanthem | 4 |
| Viral illness | 4 |
| Adenovirus | 3 |
| Chicken pox (Varicella zoster) | 3 |
| Influenza A | 3 |
| Measles | 2 |
| Parovirus B19 | 2 |
| Bronchitis | 1 |
| Chest infection | 1 |
| Cytomegalovirus | 1 |
| Epstein-Bar virus | 1 |
| Echovirus | 1 |
| Escherichia coli urinary tract infection | 1 |
| Glandular fever | 1 |
| Klebsiella pneumoniae | 1 |
| Leishmania infantum | 1 |
| Malaria | 1 |
| Mononucleosis | 1 |
| Mumps | 1 |
| Sepsis | 1 |
| Staphylococcus aureus meningitis | 1 |
| Streptococcus pneumoniae | 1 |
| Streptococcus pyogenes | 1 |
| Urinary tract infection | 1 |
>1 infection was reported in some patients.
In addition to infectious etiologies, we identified 21 patients with antecedent hematologic malignancies, including chronic lymphocytic leukemia (CLL, n = 5), monoclonal gammopathy of undetermined significance/multiple myeloma (n = 5), non-Hodgkin lymphoma (type not specified, n = 4), myelodysplastic syndrome (MDS, n = 4), primary myelofibrosis (n = 1), diffuse large B-cell lymphoma (n = 1), and T-cell acute lymphoblastic leukemia (n = 1).
DAT results were reported for 97.0% (223 of 230) of patients (Table 3). The most common finding among patients with reported results was a positive DAT using a polyspecific reagent, with C3 but not immunoglobulin G (IgG) bound to RBCs using monospecific reagents (66.4%, 148 of 223), consistent with previously reported findings. Among patients with reported DAT results, 9.0% (20 of 223) had a positive DAT and 6.3% (14 of 223) had a negative DAT, but monospecific reagents to assess for individual IgG and C3 components were either not available or not used. Notably, 3 patients had a positive DAT with IgG but not C3 detected, 14 patients had negative results using only a polyspecific reagent, and 10 had negative results with both polyspecific and monospecific reagents.
Direct antiglobulin test results for patients with PCH
| Polyspecific reagent . | Monospecific reagents . | Number of patients (%) . | |
|---|---|---|---|
| IgG . | C3 . | ||
| + | – | + | 148 (64.3) |
| + | NR | NR | 20 (8.7) |
| + | + | + | 18 (7.8) |
| – | NR | NR | 14 (6.1) |
| – | – | – | 10 (4.3) |
| NR | NR | NR | 7 (3.0) |
| NR | – | + | 6 (2.6) |
| + | + | – | 3 (1.3) |
| – | – | + | 2 (0.9) |
| NR | NR | + | 1 (0.4) |
| + | NR | + | 1 (0.4) |
| Polyspecific reagent . | Monospecific reagents . | Number of patients (%) . | |
|---|---|---|---|
| IgG . | C3 . | ||
| + | – | + | 148 (64.3) |
| + | NR | NR | 20 (8.7) |
| + | + | + | 18 (7.8) |
| – | NR | NR | 14 (6.1) |
| – | – | – | 10 (4.3) |
| NR | NR | NR | 7 (3.0) |
| NR | – | + | 6 (2.6) |
| + | + | – | 3 (1.3) |
| – | – | + | 2 (0.9) |
| NR | NR | + | 1 (0.4) |
| + | NR | + | 1 (0.4) |
NR, not reported.
To meet our inclusion criteria, all patients had positive DL antibody results. Most cases did not report the antigen specificity of the DL antibody; however, among the 71 patients in whom antibody class and antigen specificity were reported, 59 (83.1%) were IgG class with specificity for the P-antigen. Other antibody isotypes and antigen specificities were rare but included IgG anti-I (n = 2), IgM anti-I (n = 2), anti-PP1Pk (Tja) (n = 2), IgG anti-i (n = 1), IgM anti-P (n = 1), IgA anti-P (n = 1), anti–Pr-like (n = 1), and IgM type with no specificity determined (n = 2).
Therapy and outcomes
Transfusion data were reported in 164 patients, 119 (72.6%) of whom received an RBC transfusion. Among 52 patients for whom the number of units was reported, the median number of units was 2 (IQR, 2-4), and ranged from 1 to 34 units over the hospitalization. Transfusion of P-antigen–negative RBCs was attempted in 2 patients because of severe, transfusion-refractory anemia with variable response. Most endorsed an attempt to keep the patient in a warm environment and ensuring that the transfused blood was warm.
The most common medical treatment for patients with PCH was corticosteroids, with 94 patients receiving at least 1 dose. The total number of doses, frequency, and route of administration were seldom reported. Other common treatments included antimicrobial therapy (n = 26), intravenous immunoglobulin (n = 10), and rituximab (n = 10). Three patients received complement inhibitor therapy (eculizumab). A variety of other medications and treatment modalities were trialed (Table 4). Patients who received additional medications tended to be older (median age of 58.5 years) and have underlying hematologic neoplasms. A total of 72 patients received no medication or intervention excluding transfusion, and 30 patients received no blood transfusion, medication, or other intervention. Notably, 4 patients (all females, median age of 18.5 years) received therapeutic plasma exchange (TPE) with varying results. Three of the 4 patients underwent a total of 3 TPE procedures, whereas the number of procedures was not reported for the fourth patient. The patients required a median of 9 units of RBCs, suggesting a more severe presentation.
Therapies employed in patients with PCH
| Therapy . | Patients (n = 229)∗ . |
|---|---|
| None | 72 |
| Steroids | 57 |
| Antimicrobials | 13 |
| Steroids, antimicrobials | 8 |
| Steroids, IVIG | 5 |
| Steroids, rituximab | 4 |
| Steroids, CHOP | 3 |
| Steroids, azathioprine | 2 |
| Steroids, chlorambucil | 2 |
| Antimicrobials, IVIG | 1 |
| Rituximab | 1 |
| Rituximab, fludarabine, cyclophosphamide, danazol | 1 |
| Steroids, androgen therapy | 1 |
| Steroids, antimicrobials, IVIG | 1 |
| Steroids, azathioprine, rituximab | 1 |
| Steroids, cyclophosphamide, rituximab | 1 |
| Steroids, danazol | 1 |
| Steroids, eculizumab, antimicrobials | 1 |
| Steroids, eculizumab, antimicrobials, TPE | 1 |
| Steroids, eculizumab, cyclophosphamide | 1 |
| Steroids, IVIG, antimicrobials | 1 |
| Steroids, IVIG, rituximab | 1 |
| Steroids, IVIG, TPE | 1 |
| Steroids, rituximab, cyclophosphamide | 1 |
| Steroids, TPE | 1 |
| TPE | 1 |
| Therapy . | Patients (n = 229)∗ . |
|---|---|
| None | 72 |
| Steroids | 57 |
| Antimicrobials | 13 |
| Steroids, antimicrobials | 8 |
| Steroids, IVIG | 5 |
| Steroids, rituximab | 4 |
| Steroids, CHOP | 3 |
| Steroids, azathioprine | 2 |
| Steroids, chlorambucil | 2 |
| Antimicrobials, IVIG | 1 |
| Rituximab | 1 |
| Rituximab, fludarabine, cyclophosphamide, danazol | 1 |
| Steroids, androgen therapy | 1 |
| Steroids, antimicrobials, IVIG | 1 |
| Steroids, azathioprine, rituximab | 1 |
| Steroids, cyclophosphamide, rituximab | 1 |
| Steroids, danazol | 1 |
| Steroids, eculizumab, antimicrobials | 1 |
| Steroids, eculizumab, antimicrobials, TPE | 1 |
| Steroids, eculizumab, cyclophosphamide | 1 |
| Steroids, IVIG, antimicrobials | 1 |
| Steroids, IVIG, rituximab | 1 |
| Steroids, IVIG, TPE | 1 |
| Steroids, rituximab, cyclophosphamide | 1 |
| Steroids, TPE | 1 |
| TPE | 1 |
CHOP, cyclophosphamide, hydroxydaunorubicin, oncovin, prednisone; IVIG, intravenous immunoglobulin; TPE, therapeutic plasma exchange.
Therapy was not reported for 47 patients.
Among patients who were reported to have acute PCH and in whom it was reported whether they received corticosteroids (n = 109), there was no significant difference (P = .84) in the length of hospital admission between patients who received steroids (mean ± standard deviation, 15.1 ± 9.9 days) and those who did not receive steroids (14.7 ± 11.0 days). There was also no difference (P = .86) in the average age of patients treated (17.4 ± 25.4 years) and patients not treated (16.5 ± 24.2 years) with steroids. However, patients who received steroids, compared with those who did not receive steroids, had both a lower hemoglobin at presentation (6.0 vs 7.7 g/dL, P = .01) and a lower hemoglobin nadir (5.0 g/dL vs 6.7 g/dL, P = .001).
The hospital admission timeframe for patients with acute PCH was reported for 116 patients with a median of 13 days in the hospital (range, 1-72 days). A subset of cases (n = 23) had chronic or relapsing disease ranging from 2 to 132 months. Of patients with chronic/relapsing disease, 6 had syphilis and 3 had underlying hematologic neoplasms (CLL, MDS, multiple myeloma). Among the 230 patients reviewed, 213 were alive at the time of report, 14 were unknown or not reported, and 3 were deceased. The 3 deceased patients were a 3-year-old female with no medical comorbidities, a 60-year-old male with recent resolution of bacteremia, and a 77-year-old female with non-Hodgkin lymphoma; 2 of these patients received corticosteroid therapy before succumbing, and 1 patient died before initiation of therapy.
Discussion
Our results, consisting of the largest review of PCH cases to date, generally support previously described epidemiological findings, with more than half of cases in our study being reports of PCH in children aged ≤5 years who presented with a median hemoglobin of <5.5 g/dL. These findings illustrate that contemporary PCH most commonly presents as postinfectious hemolysis in children, and can be associated with severe anemia. Despite representing the largest compilation of cases to date, we only identified 230 reported cases of PCH in the English medical literature, highlighting the rarity of this condition. Prior authors have suggested that PCH is twice as common among males vs females.7,15-17 In contrast, our analyses, based on this large cohort, highlight a nearly equal male-to-female ratio (1.17:1) of PCH cases among all ages overall and in children aged ≤5 years (1.25:1).
Historically, PCH was frequently encountered in older patients with tertiary or congenital syphilis, illustrated by our finding that between 1950 and 1970, 53% of reported cases of PCH were diagnosed in patients with syphilis. During these 2 decades, the median age of all patients with PCH was 31 years, and 42 years for those with PCH secondary to syphilis. Conversely, our findings illustrate that contemporary PCH is essentially a different disease, generally afflicting children after acute infection, most commonly upper respiratory infections and gastroenteritis. The most common timeframe reported for infections preceding PCH onset was 2 weeks (range, 2 days to 5 weeks). Thus, clinicians should be cognizant of the fact that a diagnosis of PCH should not depend on the presence of an active infection, and an infection may not always precede the development of PCH. Notably, we identified no reported cases of SARS-CoV-2 infection associated with PCH despite mounting evidence that SARS-CoV-2 may be a predisposing factor for autoimmune hemolysis.18 Nevertheless, continued surveillance of post–SARS-CoV-2 PCH is warranted. In addition, it is worth highlighting the dramatic increase in the incidence of syphilis in the United States over the previous few years19; thus, healthcare providers should be aware of the potential for the re-emergence of chronic PCH in addition to the more commonly encountered acute form.
Therapy for PCH is generally supportive. In the era of PCH mediated by acute infectious processes, the disease is generally transient and self-limited. This is highlighted by the finding that over the last 2 decades, 88% (52/59) of patients with data reported had acute PCH that resolved after a median of 14 days. Despite this, some healthcare practitioners have suggested that corticosteroids may be beneficial in certain patients,20 and this belief seems quite prevalent given the number of patients administered steroids in our review, with 52% of patients for whom data were reported having received corticosteroid therapy. Although this study was not designed to assess effectiveness of various therapies, we found no difference in the age or the hospital stay length of patients that received steroids compared with those who did not. However, patients who were administered steroids had both a lower hemoglobin level at presentation and a lower hemoglobin nadir, suggesting steroids were preferentially given to patients with more severe disease. Although the most effective therapy for PCH remains under investigation, recent reports have proposed complement inhibitors for patients with severe or refractory disease,21 and we identified 3 reports of patients administered eculizumab.
Although best practice regarding medical therapy remains inconclusive, our analysis indicates that blood transfusion is frequently performed in patients with PCH, because the hemoglobin can decrease precipitously, and to markedly low levels. Because the DL antibody almost invariably has specificity for the P-antigen on the surface of RBCs, provision of RBCs lacking the P-antigen (ie, P-null) has been suggested. However, P-null RBCs (pp phenotype) are extraordinarily rare, occurring in between ∼5 and 6 per million persons,22 and transfusing P-antigen–positive cells has not been shown to lead to increased hemolysis.23 Similarly, 1 modification that has been considered previously is washing units to remove the complement-containing plasma, because hemolysis in PCH is primarily complement mediated. Today, washing RBCs for patients with PCH is not necessary, because of the additive solution that RBCs are stored in as well as previous evidence demonstrating transfusion of unwashed products did not exacerbate hemolysis.23
When encountering a patient with laboratory evidence of hemolytic anemia, 1 of the first considerations of PCH may occur after the results of a DAT. Because the IgG antibody dissociates from RBCs at warmer temperatures and leaves only complement bound, the classic finding is a DAT positive for C3 only. However, this is also seen in the more common cAIHA mediated by IgM autoantibodies. Distinguishing PCH from cAIHA can be further complicated by the presence of concomitant cold agglutinins (CA) in patients with PCH. If the CA has a broad thermal amplitude, it may cause a false positive DL antibody test despite being a monophasic hemolysin.24 Although the thermal amplitude was rarely assessed in the reports analyzed, 57 of 72 patients in whom CA titers were measured had detectable CAs, ranging from a titer of 1:1 to 1:2048. In addition, although most patients had a DAT positive only for C3, the DAT was negative in 24 patients (10.4%), IgG and C3 were present in 18 (7.8%), and 3 (1.3%) had IgG but not C3 bound to RBCs. These findings underscore the importance of not relying solely on the DAT to include or exclude PCH in the differential diagnosis of a patient with evidence of hemolysis. Instead, if a pediatric patient presents with signs and symptoms of hemolytic anemia, particularly in the context of a recent infection, PCH should be considered, and DL testing should be performed regardless of DAT results.
We acknowledge that this review may not be exhaustive because cases that may have been clinically compatible with PCH but lacked confirmatory laboratory testing were excluded. Furthermore, there are known reporting biases in the medical literature. For example, patients with mild disease may not have been diagnosed or hospitalized, and thus these results may be skewed to patients with more severe findings. This analysis comprised the largest cohort of patients with PCH described in the English language to date, and its implications include the importance of recognizing the most common patient population, use of the most appropriate diagnostic modality, and illustrating that additional research is necessary to elucidate whether corticosteroids and/or other therapeutic measures are indeed effective. In summary, we highlight several important clinical, laboratory, and epidemiologic features of patients with PCH, demonstrating the importance of maintaining this condition in the differential for patients with hemolysis. This point is aptly illustrated by Heddle,25 who described 1 hospital that had diagnosed no cases of PCH over a 6-year period yet after a single PCH diagnosis, 4 additional cases were identified in the ensuing 9 months.
Although the pathophysiology of PCH is well described, diagnosis and management remain challenging. Hematologists and transfusion medicine specialists should be familiar with the clinical and laboratory findings of this disease. This review highlights the temporal association with infectious agents, immunohematologic details, and outcomes. Our findings help demonstrate the characteristic features of PCH, illustrate the limitations associated with confirmatory testing, and may help guide further investigation and management of this rare disease.
Acknowledgment
The authors are grateful to Mark Smith for creating the DL test graphic and the visual abstract.
Authorship
Contribution: J.W.J., C.A.F.V., B.D.A., J.S.W., and L.D.S. collected the data and performed the analysis; J.W.J. and B.D.A. drafted the manuscript; G.S.B. and B.D.A. provided supervision; and all authors critically revised the manuscript and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jeremy Jacobs, Department of Laboratory Medicine, Yale School of Medicine, 55 Park St, New Haven, CT 06520; e-mail: Jeremy.jacobs@yale.edu.
References
Author notes
Data are available on request from the corresponding author, Jeremy W. Jacobs (Jeremy.jacobs@yale.edu).
The full-text version of this article contains a data supplement.