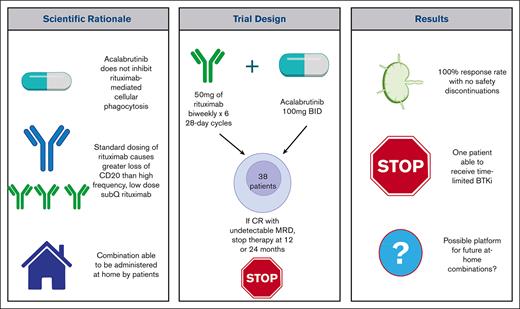
Key Points
Acalabrutinib and HFLD subcutaneous rituximab was assessed as a potential fixed-duration initial therapy for chronic lymphocytic leukemia.
This home-administered combination had a 100% response rate and can be a backbone for regimens aiming to achieve limited duration therapy.
Abstract
Bruton tyrosine kinase inhibitors are an effective therapeutic agent for previously untreated patients with chronic lymphocytic leukemia but require indefinite treatment that can result in cumulative toxicities. Novel combinations of agents that provide deep remissions could allow for fixed duration therapy. Acalabrutinib, unlike ibrutinib, does not inhibit anti-CD20 monoclonal antibody-dependent cellular phagocytosis, making it a suitable partner drug to rituximab. Using standard dosing (375 mg/m2) of rituximab causes loss of target membrane CD20 cells and exhaustion of the finite cytotoxic capacity of the innate immune system. Alternatively, using high-frequency, low-dose (HFLD), subcutaneous rituximab limits loss of CD20 and allows for self-administration at home. The combination of HFLD rituximab 50 mg administered twice a week for 6 cycles of 28 days with the addition of acalabrutinib starting in week 2 was evaluated in a phase II study of 38 patients with treatment naive chronic lymphocytic leukemia. Patients achieving a complete response with undetectable minimal residual disease after 12 or 24 cycles of acalabrutinib could stop therapy. All patient responded, including one with a complete response with undetectable minimal residual disease in the peripheral blood and bone marrow at 12 months who stopped therapy. At a median follow-up of 2.3 years 2 patients with high-risk features have progressed while on acalabrutinib monotherapy. We conclude that HFLD rituximab in combination with acalabrutinib is an effective and tolerable self-administered home combination that provides a platform to build upon regimens that may more reliably allow for fixed-duration therapy. This trial was registered at www.clinicaltrials.gov #NCT03788291.
Introduction
Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL) is the most prevalent mature B-cell neoplasm in the United States.1 Although the introduction of targeted small molecules has improved clinical outcomes, CLL remains incurable, and conventional therapies that are often continued as long as being tolerated and are effective. Further improvement in treatment outcomes will likely require development of multidrug targeted therapies with the potential to achieve deep remissions with undetectable minimal residual disease (MRD).2 These regimens could then be used for limited duration therapy and provide the basis for future development of potentially curative treatments.
The anti-CD20 monoclonal antibodies (mAb), rituximab and obinutuzumab, have previously been used with limited efficacy as monotherapy in patients with CLL.3 In contrast, addition of anti-CD20 mAb to chemotherapy significantly improves clinical outcomes.4 Regimens combining targeted therapies with anti-CD20 mAb have been developed and are widely used to treat CLL. The combination of Bruton tyrosine kinase inhibitors (BTKi) with anti-CD20 mAb has the possible advantage of combining the ability of BTKi to mobilize CLL cells from lymphoid tissue into the circulation where they are especially susceptible to clearance by anti-CD20 mAbs.5 However, 2 randomized clinical trials testing the addition of standard dose of rituximab to the inhibitor ibrutinib did not show any improvement in progression-free survival (PFS).6,7 On the basis of emerging data on the mechanisms of action of anti-CD20 mAb and the effect of ibrutinib on these mechanisms, we developed a novel combination of BTKi and rituximab regimen for patients with CLL.
Rituximab cytotoxicity in CLL is primarily mediated by antibody-dependent cellular phagocytosis (ADCP) with lesser roles for complement dependent cytotoxicity and antibody-dependent cellular cytotoxicity.8 The authors and others have previously shown in vitro that macrophage ADCP is significantly decreased by ibrutinib, but not by the more targeted BTKi, acalabrutinib.8,9 Subsequently, The ELEVATE-TN trial showed an improvement in PFS when obinutuzumab was added to acalabrutinib compared with acalabrutinib monotherapy (however, the study was not powered to formally compare these groups.)10 Our combination therapy regimen thus used acalabrutinib.
Original dose (375 mg/m2) IV rituximab has been previously shown to cause rapid onset and durable decreases in CD20 levels in circulating CLL cells which could limit treatment efficacy.11,12 The authors and others have previously shown that this potential cause of drug resistance could be obviated though use of a lower dose of rituximab (20 mg/m2) which can be administered every 48 hours without causing sustained loss of CD20 from circulating CLL cells (NCT00669318).12,13
On the basis of the above rationale that acalabrutinib might be a more ideal partner to rituximab than ibrutinib, our clinical trial used acalabrutinib in combination with high-frequency low-dose (HFLD) rituximab. Rituximab was selected over obinutuzumab because it is available for subcutaneous (SQ) at-home administration.14,15 Our primary goal was to determine if the use of this combination could allow for limited duration therapy in those who achieved a complete response (CR) with no detectable MRD after either 12 or 24 cycles of therapy.
Methods
Study design and objectives
The primary objective of this single center phase 2 trial was to estimate the rate of complete remissions after 1 year of therapy as defined by the 2018 iwCLL (International Workshop for Chronic Lymphocytic Leukemia) criteria.16 Secondary objectives were to assess the rate of undetectable measurable residual disease (defined as <1 in 10 000 clonal B cells via 6-color flow cytometry), PFS, and safety of the regimen. Correlative studies on the cytokine responses to the first infusion, effect of treatment on CLL-cell CD20 expression, and in vitro sensitivity of CLL cells to ADCP, antibody-dependent cellular cytotoxicity, and complement-dependent cytotoxicity will be reported separately. The institutional review board approved the protocol, and informed, written consent was obtained from all patients before enrollment. All authors had access to the primary clinical trial data. The study was registered before enrolling patients (ClinicalTrials.govNCT03788291).
Eligibility criteria
Patients aged ≥18 years were eligible if they had a diagnosis of CLL that was previously untreated and warranted therapy on the basis of the 2018 iwCLL criteria.16 An Eastern cooperative oncology group performance status of ≤2 was required, unless a performance status of ≤3 was attributable to their CLL. An absolute neutrophil count of ≥0.5 ×109/L, platelet count of ≥30 × 109/L, CrCl >30 mL per minute, and adequate hepatic function were required. Patients with Richter’s transformation, ongoing systemic infections or uncontrolled immune cytopenia were excluded. As per typical requirements to receive acalabrutinib, patients had to avoid strong CYP3A inhibitors, such as warfarin, and proton pump inhibitors before an August 2022 amendment, using the tablet formulation of acalabrutinib that allows for coadministration with acid-reducing agents.
Treatment protocol and clinical protocol assessments
Baseline evaluation included history and physical examination, laboratory evaluations, and imaging by computed tomography. Risk factor assessment was performed before treatment. Abnormalities detected by standard fluorescent in situ hybridization using probes for detection of deletion 17p13, 11q22.3, and 13q14 and trisomy 12 are reported using the standard hierarchical method.17 Next generation sequencing was used to detect deleterious mutations in TP53, NOTCH1, and SF3B1. IGHV somatic hypermutation status was measured with Sanger sequencing with those reads deviating from wild type by ≥2% reported as mutated.
Rituximab was given biweekly at 50 mg per dose on the same 2 days of the week with a 48-hour interval between doses for six 28-day cycles. The initial dose was given IV at an infusion rate of 25 mg per hour starting 30 minutes after premedication with oral diphenhydramine (50 mg) and acetaminophen (650 mg) to ensure that patients could tolerate the drug based on standard practice. If IV therapy was completed, all subsequent rituximab doses were administered SQ. After training, patients who tolerated SQ rituximab could then self-administer treatment at home with optional use of premedication (supplement 1). To study the effect of the first doses of IV and SQ rituximab on CLL cells, the trial was designed to give these initial doses in the first week of therapy before the initiation of acalabrutinib therapy. Acalabrutinib 100 mg oral twice daily was started on day 8 of the first cycle. Dose modifications were advised for grade 4 neutropenia or thrombocytopenia, and drug discontinuation for grade 4 infusion-related reactions or colitis.
Adverse events to evaluate safety as a secondary outcome were assessed at baseline, throughout treatment, and during the 30-day period after treatment discontinuation and were graded by the National Cancer Institute Common Terminology Criteria for Adverse Events Version 5.0.
Response assessments, including computed tomography and MRD testing by flow cytometry, were performed just before the completion of cycle 12 and 24. If patients achieved a CR by iwCLL criteria and had undetectable MRD in the blood and bone marrow, they were able to terminate therapy and be followed until disease progression. Because the intent of the study was only to stop acalabrutinib if patients had undetectable MRD disease status, bone marrow biopsies were not routinely performed in patients who had detectable MRD in the peripheral blood. Patients who did not achieve a CR with undetectable MRD remained on acalabrutinib and had repeat response assessments as above, performed after 24 cycles of therapy. In the absence of a CR with undetectable MRD, acalabrutinib could be continued until disease progression or unacceptable toxicity at the patient and physician discretion.
Statistical analysis
Patient and disease characteristics were summarized using count and proportions for categorical variables and medians and ranges for continuous variables. The primary objective was to estimate the CR rate of acalabrutinib and HFLD rituximab in patients with previously untreated CLL. The planned sample size of 40 participants had produced a 95% 2-sided exact binomial confidence interval (CI) with a maximum width of 32.4%. The final evaluable sample size of 38 participants did not appreciably reduce the precision of the CR rate estimate. The resulting CI based on 38 participants had a maximum width of 33.2%.
CR rate was defined as the proportion of patients with a best overall response of CR with or without marrow recovery. Associated 95% 2-sided exact binomial CIs were calculated for all response rates. PFS was defined as the time from treatment initiation until the date of first documentation of definitive disease progression or date of death from any cause, whichever occurred first. Patients who did not progress, die, or were failed to follow-up were censored at the day of their last clinical trial clinic visit. All analyses were descriptive in nature, and no hypothesis testing was performed. SAS v9.4 was used for all analyses (SAS Institute Inc, Cary, NC).
Results
Patients
Thirty-nine patients were enrolled from April 2019 to July 2021. One patient was found to have a non-CLL B-cell non-Hodgkin lymphoma and was excluded from analyses. Demographics for the 38 evaluable patients are included in Table 1. The median age was 67 years, 60.5% were male, and 92.1% had an ECOG (Eastern Cooperative Oncology Group) performance status of ≤1. At time of treatment, most patients were Rai stage 2. Most (68.4%) patients had at least 1 high-risk feature: TP53 mutation, 17p13 deletion, 11q22.3 deletion, NOTCH1 mutation, or IGHV unmutated disease (Table 1).
Demographics
| Characteristic . | Treated patients (n = 38), n (%) . |
|---|---|
| Age (y) | |
| Median | 66.5 (40-78) |
| Range | |
| Male | 23 (60.5) |
| ECOG PS | |
| 0 | 18 (47.4) |
| 1 | 17 (44.7) |
| 2 | 3 (7.9) |
| Rai stage | |
| 0 | 0 |
| I | 3 (7.9) |
| II | 19 (50.0) |
| III | 11 (28.9) |
| IV | 5 (13.2) |
| β2 microglobulin (mg/L) | |
| Median | 3.7 (2, 11.4) |
| Range | |
| Hemoglobin | |
| Median | 11.9 (7.8, 15.4) |
| Range | |
| Platelet count | |
| Median | 138 (69, 438) |
| Range | |
| Cytogenetics | |
| Del (17p) | 5 (13.2) |
| Del (11q) | 6 (15.8) |
| Trisomy 12 | 6 (15.8) |
| Del (13q) | 15 (39.5) |
| No cytogenetic abnormalities | 6 (15.8) |
| IGHV mutation status | |
| Mutated | 15 (39.5) |
| Unmutated | 23 (60.5) |
| Mutated TP53 | |
| Yes | 8 (21.1) |
| No | 30 (78.9) |
| Mutated NOTCH1 | |
| Yes | 9 (23.7) |
| No | 29 (76.3) |
| Mutated SF3B1 | |
| Yes | 4 (10.5) |
| No | 34 (89.5) |
| At least 1 high-risk feature (TP53mut/del(17p)/del(11q)/ NOTCH1 mut/IGHV unmutated) | |
| Yes | 26 (68.4) |
| No | 12 (31.6) |
| Characteristic . | Treated patients (n = 38), n (%) . |
|---|---|
| Age (y) | |
| Median | 66.5 (40-78) |
| Range | |
| Male | 23 (60.5) |
| ECOG PS | |
| 0 | 18 (47.4) |
| 1 | 17 (44.7) |
| 2 | 3 (7.9) |
| Rai stage | |
| 0 | 0 |
| I | 3 (7.9) |
| II | 19 (50.0) |
| III | 11 (28.9) |
| IV | 5 (13.2) |
| β2 microglobulin (mg/L) | |
| Median | 3.7 (2, 11.4) |
| Range | |
| Hemoglobin | |
| Median | 11.9 (7.8, 15.4) |
| Range | |
| Platelet count | |
| Median | 138 (69, 438) |
| Range | |
| Cytogenetics | |
| Del (17p) | 5 (13.2) |
| Del (11q) | 6 (15.8) |
| Trisomy 12 | 6 (15.8) |
| Del (13q) | 15 (39.5) |
| No cytogenetic abnormalities | 6 (15.8) |
| IGHV mutation status | |
| Mutated | 15 (39.5) |
| Unmutated | 23 (60.5) |
| Mutated TP53 | |
| Yes | 8 (21.1) |
| No | 30 (78.9) |
| Mutated NOTCH1 | |
| Yes | 9 (23.7) |
| No | 29 (76.3) |
| Mutated SF3B1 | |
| Yes | 4 (10.5) |
| No | 34 (89.5) |
| At least 1 high-risk feature (TP53mut/del(17p)/del(11q)/ NOTCH1 mut/IGHV unmutated) | |
| Yes | 26 (68.4) |
| No | 12 (31.6) |
Cytogenetics are reported via Döhner hierarchical method.17
ECOG, Eastern Cooperative Oncology Group; PS, performance status.
Efficacy
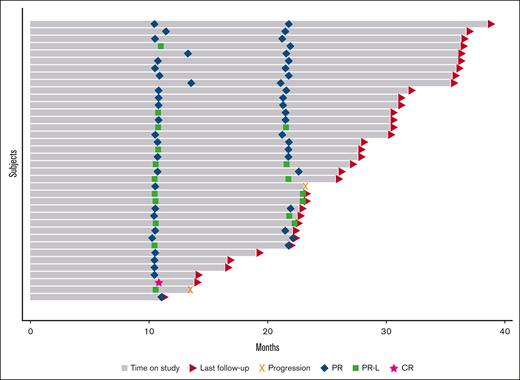
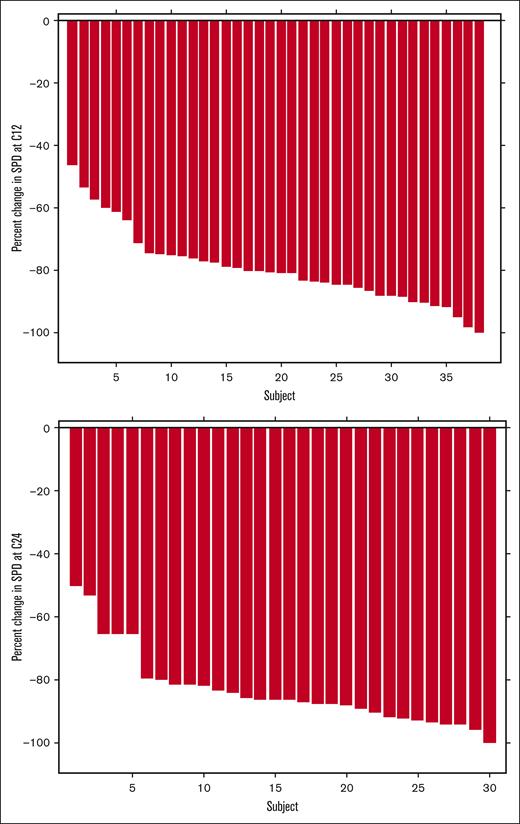
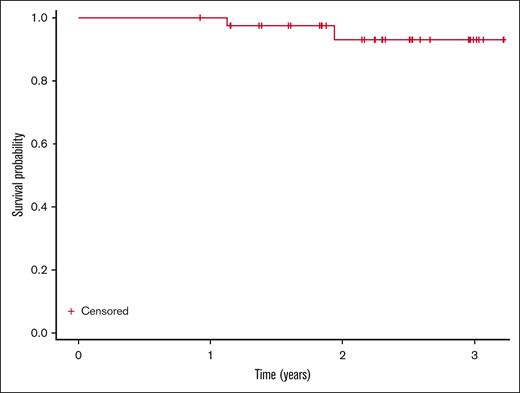
Median follow-up was 2.3 years. At the time of cycle 12 response assessment, all 38 evaluable patients had responded with 1 patient (2.6%; 95% CI, 0.07-13.8) having a CR based on iwCLL 2018 criteria with undetectable MRD in the blood and bone marrow. This patient was able to terminate the use of acalabrutinib. Twenty-six patients (68.4%; 95% CI, 51.4-82.5) had a partial response and 11 (28.9%; 95% CI, 15.4-45.9) had a partial response with lymphocytosis (Table 2). Of the 32 patients who had gone through a cycle 24 response assessment, 23 patients (71.9%; 95% CI, 53.3-86.3) had a partial response, and 7 (21.9%; 95% CI, 9.3-40.0) had a partial response with lymphocytosis (Table 2; Figure 1). Nodal responses are described in Figure 2. Two patients with TP53 disruption (del17p and TP53 mutation) had progressive disease at 1.1 and 1.9 years. Median PFS was not reached (Figure 3). No patients had died during the study period; 6 patients had not yet reached their cycle 24 response assessment at time of data cutoff. Notably, no patients had COVID-19 infection or received COVID-19 vaccines, which have the potential to affect absolute lympocyte count or adenopathy, proximal to efficacy assessments.
Response by 2018 iwCLL criteria and MRD status via flow cytometry undetectable is defined as <1 in 10 000 CLL cells detected via flow cytometry
| Response . | Cycle 12 (n = 38) . | Cycle 24 (n = 32) . | MRD status . |
|---|---|---|---|
| CR | 1 (2.6%; 95% CI, 0.07-13.8) | - | Undetectable in PB and BM |
| PR | 26 (68.4%; 95% CI, 51.4-82.5) | 23 (71.9%; 95% CI, 53.3-86.3) | |
| PR lymphocytosis | 11 (28.9%; 95% CI, 15.4-45.9) | 7 (21.9%; 95% CI, 9.3-40.0) | |
| Progressive disease | - | 2 (6.3%; 95% CI, 0.8-20.8) |
| Response . | Cycle 12 (n = 38) . | Cycle 24 (n = 32) . | MRD status . |
|---|---|---|---|
| CR | 1 (2.6%; 95% CI, 0.07-13.8) | - | Undetectable in PB and BM |
| PR | 26 (68.4%; 95% CI, 51.4-82.5) | 23 (71.9%; 95% CI, 53.3-86.3) | |
| PR lymphocytosis | 11 (28.9%; 95% CI, 15.4-45.9) | 7 (21.9%; 95% CI, 9.3-40.0) | |
| Progressive disease | - | 2 (6.3%; 95% CI, 0.8-20.8) |
Detectable is ≥1 in 10 000 CLL cells.
BM, bone marrow; CI, confidence interval; PB, peripheral blood; PR, partial response.
Swimmer plot: bars represent each study patient’s time on treatment through time of last follow-up (triangle) or progression (x). Response to therapy was assessed at cycle 12 and 24 (diamonds represent partial responses, squares partial response with lymphocytosis, and stars CRs).
Swimmer plot: bars represent each study patient’s time on treatment through time of last follow-up (triangle) or progression (x). Response to therapy was assessed at cycle 12 and 24 (diamonds represent partial responses, squares partial response with lymphocytosis, and stars CRs).
Waterfall plots representing change in the sum of the products of the dimensions of target lymph nodes from baseline following cycle 12 (top) and cycle 24 (bottom). Cycle 24 data is shown for the 30 subjects who had a cycle 24 assessment without previous progression.
Waterfall plots representing change in the sum of the products of the dimensions of target lymph nodes from baseline following cycle 12 (top) and cycle 24 (bottom). Cycle 24 data is shown for the 30 subjects who had a cycle 24 assessment without previous progression.
PFS. Kaplan-Meier curve of PFS from treatment initiation (N = 38). Two subjects progressed (1.1 and 1.9 years), with no deaths observed.
PFS. Kaplan-Meier curve of PFS from treatment initiation (N = 38). Two subjects progressed (1.1 and 1.9 years), with no deaths observed.
Safety
The most common all-grade, all cause adverse events (Table 3) included headache (68.4%), myalgias (50%), COVID-19 infection (39.5%), arthritis or arthralgias (39.5%), bruising (39.5%), fatigue (36.8%) and rash (23.7%). IV rituximab infusion reactions occurred in 63.2% of the patients (all grade 2), and 31.6% of the patients experienced low-grade injection site reactions related to SQ rituximab.
All-causality adverse events, listed by organ system, including all-grade events occurring in ≥20% of patients, and grade 3 or 4 adverse events which occurred in 1 or more patients
| Adverse event (all causality) N = 38 . | All grades, (≥20%) n (%) . | Grade 3/4 (≥ 1 patient) n (%) . |
|---|---|---|
| Hematologic toxicities | ||
| Anemia | 2 (5.3) | 2 (5.3) |
| Neutropenia | 1 (2.6) | 1 (2.6) |
| Infections | ||
| COVID-19 | 15 (39.5) | 5 (13.2) |
| Cryptococcus lymphadenitis | 1 (2.6) | 1 (2.6) |
| Febrile neutropenia | 1 (2.6) | 1 (2.6) |
| Joint infection | 1 (2.6) | 1 (2.6) |
| Pneumonia (non-COVID) | 5 (13.2) | 1 (2.6) |
| Dental infection | 2 (5.3) | 2 (5.3) |
| Cellulitis | 3 (7.9) | 0 |
| Upper respiratory infection | 12 (31.6) | 0 |
| Urinary tract infection | 8 (21.1) | 3 (7.9) |
| Musculoskeletal/Skin | ||
| Arthritis/arthralgias | 15 (39.5) | 0 |
| Bruising | 15 (39.5) | 0 |
| Hematoma | 4 (10.5) | 1 (2.6) |
| Myalgias | 19 (50) | 0 |
| Rash | 9 (23.7) | 0 |
| Gastrointestinal | ||
| Constipation | 8 (21.1) | 0 |
| Diarrhea | 9 (23.7) | 0 |
| Nausea | 11 (28.9) | 0 |
| Rectal bleeding | 1 (2.6) | 1 (2.6) |
| Other | ||
| A-fib | 1 (2.6) | 1 (2.6) |
| Cough | 10 (26.3) | 0 |
| Dizziness | 8 (21.1) | 0 |
| Edema | 12 (31.6) | 0 |
| Fatigue | 14 (36.8) | 0 |
| Headache | 26 (68.4) | 0 |
| Infusion reaction | 24 (63.2) | 0 |
| Injection site reaction | 12 (31.6) | 0 |
| Sinus bradycardia | 2 (5.3) | 2 (5.3) |
| CVA | 1 (2.6) | 1 (2.6) |
| Urinary tract obstruction | 1 (2.6) | 1 (2.6) |
| Adverse event (all causality) N = 38 . | All grades, (≥20%) n (%) . | Grade 3/4 (≥ 1 patient) n (%) . |
|---|---|---|
| Hematologic toxicities | ||
| Anemia | 2 (5.3) | 2 (5.3) |
| Neutropenia | 1 (2.6) | 1 (2.6) |
| Infections | ||
| COVID-19 | 15 (39.5) | 5 (13.2) |
| Cryptococcus lymphadenitis | 1 (2.6) | 1 (2.6) |
| Febrile neutropenia | 1 (2.6) | 1 (2.6) |
| Joint infection | 1 (2.6) | 1 (2.6) |
| Pneumonia (non-COVID) | 5 (13.2) | 1 (2.6) |
| Dental infection | 2 (5.3) | 2 (5.3) |
| Cellulitis | 3 (7.9) | 0 |
| Upper respiratory infection | 12 (31.6) | 0 |
| Urinary tract infection | 8 (21.1) | 3 (7.9) |
| Musculoskeletal/Skin | ||
| Arthritis/arthralgias | 15 (39.5) | 0 |
| Bruising | 15 (39.5) | 0 |
| Hematoma | 4 (10.5) | 1 (2.6) |
| Myalgias | 19 (50) | 0 |
| Rash | 9 (23.7) | 0 |
| Gastrointestinal | ||
| Constipation | 8 (21.1) | 0 |
| Diarrhea | 9 (23.7) | 0 |
| Nausea | 11 (28.9) | 0 |
| Rectal bleeding | 1 (2.6) | 1 (2.6) |
| Other | ||
| A-fib | 1 (2.6) | 1 (2.6) |
| Cough | 10 (26.3) | 0 |
| Dizziness | 8 (21.1) | 0 |
| Edema | 12 (31.6) | 0 |
| Fatigue | 14 (36.8) | 0 |
| Headache | 26 (68.4) | 0 |
| Infusion reaction | 24 (63.2) | 0 |
| Injection site reaction | 12 (31.6) | 0 |
| Sinus bradycardia | 2 (5.3) | 2 (5.3) |
| CVA | 1 (2.6) | 1 (2.6) |
| Urinary tract obstruction | 1 (2.6) | 1 (2.6) |
CVA, cerebral vascular accident,
Grade 3 or 4 events occurring in ≥1 patient included COVID-19 infection (13.2%) with no patients requiring intubation for respiratory failure, urinary tract infection (7.9%), dental infections (5.3%), anemia (5.3%), and sinus bradycardia (5.3%) (Table 3). No patients had a new diagnosis of atrial fibrillation during the trial and 1 patient had a new diagnosis of hypertension. One patient had paraesophageal Cryptococcus neoformans lymphadenitis requiring short-term interruption of the acalabrutinib during diagnostic evaluation and subsequent acalabrutinib dose was decreased to 100 mg daily while on treatment with isavuconazole, which resolved the infection. Acalabrutinib administration was briefly paused during a COVID-19 infection, a rectal hemorrhage, and a urinary tract obstruction that required hospitalization. Rituximab administration was delayed for patients with COVID-19 and urinary tract infections. No patients discontinued therapy owing to adverse events.
Discussion
Acalabrutinib and HFLD rituximab was an effective initial treatment for progressive CLL. The response rate was 100%, and with a median follow-up of 2.3 years, only 2 high-risk patients with TP53 disruptions have progressed. With 1 patient achieving a CR with undetectable MRD, the combination did not allow for early treatment discontinuation for the majority of the patients. However, this combination could serve as a platform for the addition of other agents in an attempt to deepen responses in fixed duration therapy, as has been demonstrated with the combination of ibrutinib and venetoclax in treatment-naïve CLL.18
Although previous trials have shown no improvement in PFS when rituximab is added to ibrutinib vs ibrutinib alone,6 there was an improvement in depth of response in patients who received the combination,7 which is relevant in a field that is moving toward MRD-guided therapy. The results of these trials of ibrutinib based regimens are not necessarily informative as to the combination of acalabrutinib and rituximab. Ibrutinib is known to inhibit antibody-dependent cellular cytotoxicity and ADCP8,19 as an off-target effect9 and also downregulates expression of CD20.20,21 In contrast, acalabrutinib does not significantly decrease ADCP in vitro suggesting that this important mechanism of action of rituximab remains intact.8,9,22 In our study, the overall response rate of 100% compares favorably to the response rate to acalabrutinib monotherapy (86%) or acalabrutinib in combination with obinutuzumab (94%)10 as well as ibrutinib in combination with rituximab,23 (95.8%). We could not compare the quality of responses in our trial with the ECOG 1912 trial because bone marrow biopsies were deferred in our study in patients with detectable MRD in the peripheral blood.
Given our “real-world” approach to deferring marrow biopsies that would not change management, we sought to compare our data to a recently presented real-world analysis of patients who received a BTKi in the frontline setting.24 This analysis included 373 patients who had received acalabrutinib as first-line treatment and set out to define the time to next treatment as a surrogate for PFS. Patients who had switched to a different BTKi were excluded to account for intolerability owing to adverse effects and attempt to focus solely on patients in which treatment was altered because of progression of disease. They found that at 12 months, 91.2% of patients remained on acalabrutinib therapy, compared with our study in which all patients remained on treatment at 12 months. With the caveats of comparing real-world and clinical trial populations, we perceive that this does provide a reasonable estimate of the efficacy of our combination regimen compared with acalabrutinib monotherapy. These efficacy data support further investigation of the acalabrutinib and HFLD rituximab regimen as a component of future multidrug therapy regimens.
The treatment regimen was tolerable. Despite slow infusion of a small dose of rituximab, most patients experienced an infusion reaction, but none were more than that of grade 2, and all were able to complete the infusion. Although 12 patients had grade 1 or 2 injection site reactions to the SQ rituximab, all were able to continue. This study demonstrated that HFLD SQ rituximab can be safely and effectively self-administered. No patients needed to terminate treatment owing to drug toxicities.
This study design is feasible as a fully home-administered regimen that would limit the cost and inconvenience of infusion centers for patients. Access to infusion chair time has become increasing more limited with the current healthcare staffing crisis, and our method of patient delivered anti-CD20 mAb could help address this. This regimen also has implications for administration of rituximab in rural areas, or global administration of rituximab in areas with limited resources. This combination also used less dose of rituximab than the standard dose, which also affects total drug cost to patients and healthcare systems. When one considers the vast number of patients who receive rituximab,25 use of home dosing with lower cumulative amount of drug has major implications for these patients.
Limitations of the study include relatively short follow-up duration because with longer assessment there may be additional undetectable MRD remissions. Future endeavors could include comparing this combination to acalabrutinib and rituximab with standard dose of rituximab, given our hypothesis that HFLD rituximab might be more effective in CLL, and discussions of a randomized trial to address this are ongoing. This can serve as a platform for adding additional agents such as BCL2 inhibitors which might result in more undetectable MRD responses, while retaining the ability of at-home administration.
In summary, we present a single-center experience using HFLD SQ rituximab in combination with acalabrutinib in previously untreated patients with CLL. This effective and tolerable regimen allowed patients to receive anti-CD20 monoclonal antibody treatment at home amidst the COVID-19 pandemic and could be the basis for designing future multidrug regimens aimed at achieving more effective targeted therapy combinations.
Acknowledgments
The authors thank the patients for participating in this clinical trial. This work was supported by AstraZeneca (P.M.B).
Visual abstract adapted from “Liposome Based Drug Delivery” template by BioRender.com (2023).
Authorship
Contribution: D.S.W. conducted the study, collected and analyzed results, and wrote the manuscript; C.S.Z. and P.M.B. designed and supervised the research, enrolled patients, and wrote the manuscript; A.M.B. performed statistical analysis; J.W.F, P.M.R., and C.C. performed the research; and G.R. analyzed and interpreted the data.
Conflict-of-interest disclosure: C.S.Z. discloses research funding from Acerta/AstraZeneca and TG Therapeutics; P.M.R. discloses research funding from Roche/Genentech, consulting for Kite Pharma/Gilead and Caribou Biosciences; C.C. discloses research funding from SecuraBio, Gilead, Genentech, and Bristol Myers Squibb (BMS); P.M.B. discloses consulting for AstraZeneca, AbbVie, Gilead, Genentech, BMS, Adaptive, BeiGene, Janssen, and GSK. The remaining authors declare no competing financial interests.
Correspondence: Danielle S. Wallace, Wilmot Cancer Institute and Department of Medicine, University of Rochester, 601 Elmwood Ave, Rochester, NY 14624; e-mail: danielle_wallace@urmc.rochester.edu.
References
Author notes
Presented in abstract form at the 63rd annual meeting of the American Society of Hematology, Atlanta, GA, 11 December 2021.
Deidentified participant data including demographics, response assessments, and adverse events are available to interested parties for 5 years following publication and are available on request from the corresponding author, Danielle S. Wallace (danielle_wallace@urmc.rochester.edu).
The full-text version of this article contains a data supplement.