Key Point
RIC with early alemtuzumab preconditioning results in favorable outcomes in HLH following allogeneic hematopoietic cell transplantation.
Abstract
Hemophagocytic lymphohistiocytosis (HLH) is a syndrome marked by a severe hyperinflammatory state characterized by aberrant T- and natural killer-cell activity leading to prolonged hypercytokinemia and can be rapidly fatal if not diagnosed and treated early. While upfront therapy is aimed at reducing hyperinflammation and controlling possible triggers, allogeneic hematopoietic stem cell transplantation (HSCT) is indicated for primary and relapsed/refractory cases to attain sustained remission. While this has been explored extensively in the pediatric population, there are limited data on adults undergoing HSCT for HLH. We analyzed transplant outcomes in an adult HLH population in the modern era who were transplanted at Dana-Farber Cancer Institute from 2010 onwards. Patients were uniformly transplanted on a reduced intensity platform incorporating early administration of alemtuzumab with standard infectious and graft-versus-host disease (GVHD) prophylaxis. Engraftment was documented for all patients. At 3 years after transplantation, overall survival (OS) was 75% (95% confidence interval [CI], 51-89) while 3-year progression-free survival (PFS) was 71% (95% CI, 46-86). The 3-year cumulative incidence of relapse was 15% (95% CI, 3.4-33). There were no isolated HLH relapses without relapse of malignancy. The cumulative incidence of nonrelapse mortality at 3 years was 15% (95% CI, 3.5-34). Infectious complications and GVHD outcomes were comparable to standard reduced-intensity conditioning (RIC) transplantation at our institute. Mixed chimerism was common but did not correlate with transplant outcomes. Our data suggest that the immune defect in HLH can be abrogated with allogeneic transplantation using a reduced intensity regimen with early administration of alemtuzumab as preconditioning, providing a potentially curative option for this difficult disease.
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is characterized by severe inflammation, hyperferritinemia, and a “cytokine storm,” leading to a clinical syndrome marked by fever, cytopenias, liver dysfunction, and a systemic inflammatory response which can culminate in multiorgan dysfunction.1 The aberrant activity of both macrophages and cytotoxic T lymphocytes,2 as well as natural killer cells, have been implicated in HLH. These aberrant cells cannot eliminate the trigger (eg, virus) and result in a prolonged hypercytokinemic state.3 The primary form of HLH (familial hemophagocytic lymphohistiocytosis) is marked either by genetic mutations such as PRF1, UNC13D, STX11, and STXBP2 that are involved in exocytosis of cytoplasmic granules and perforin-mediated cytotoxicity, or mutations including SH2D1A, XIAP, RAB27A, and LYST, which are associated with primary immune deficiency/dysregulatory states.4 The secondary form is typically triggered by infections (particularly Epstein-Barr virus [EBV]), malignancies (lymphomas, leukemia), immune dysregulatory states (bone marrow [BM] transplant, pregnancy), and autoimmune disorders such as Still’s disease and lupus.1
The severity of this syndrome necessitates prompt diagnosis and therapy, and indeed, HLH was associated with very poor outcomes until the 1990s and was almost universally fatal. The HLH-1994 study in a pediatric cohort of HLH patients established etoposide- and steroid-based algorithms for upfront therapy to break the immunologic cascade along, control the trigger if possible, and play a curative role for allogeneic hematopoietic stem cell transplantation (HSCT). A 3-year survival of 55% was seen for the whole cohort and 62% in those who underwent HSCT (66/113).5,6 Various observational studies have replicated these outcomes with survival in the 54% to 61% range, once again with better survival in those who were transplanted (50% to 70%).7 Patients with familial disease appeared to have more severe disease in the HLH-1994 study, with a 5-year survival of 50% ± 13%, and importantly, all those who did not undergo transplantation did not survive.5 Extrapolating from the pediatric experience and acknowledging that there may be important biologic differences between children and adults with HLH, consolidation with HSCT for primary as well as relapsed/refractory HLH is generally considered a standard approach. However, there have been few studies that have been published thus far in adult HLH patients undergoing HSCT, and no prospective data exist at this time. The largest retrospective study from the EBMT (European Blood and Marrow Transplant), using a number of heterogeneous regimens, demonstrated a 3-year OS of 41% (95% confidence interval [CI], 28% to 55%).8
In both the pediatric and adult settings, allogeneic transplantation has been historically associated with a high mortality rate (30%), particularly with myeloablative conditioning from complications such as veno-occlusive disease, infections, and graft-versus-host disease (GVHD). Once reduced-intensity conditioning (RIC) was adopted, initially in the pediatric population, overall survival (OS) was markedly improved, with one study demonstrating OS in the 75% range at a median follow-up of 30 months (n = 12).9 Typical RIC regimens incorporated fludarabine and melphalan10 with additional T-cell depletion in the form of alemtuzumab (CD-52–directed monoclonal antibody) or antithymocyte globulin.
The incorporation of alemtuzumab in the transplantation strategy for HLH may have several advantages, including depletion of recipient T cells resulting in better engraftment and depletion of donor T cells helping with GVHD reduction, in addition to primary control of HLH itself.11 The potential pitfalls of using alemtuzumab could be increased infectious mortality and morbidity and higher prevalence of mixed chimerism, particularly the deleterious effect on T-cell chimerism,12 which could compromise long-term outcomes. We sought to incorporate alemtuzumab with RIC as an effective strategy for allogeneic transplantation of adult HLH and study transplant outcomes with this strategy. We present here a retrospective outcomes analysis of patients with HLH (primary and secondary) who were transplanted at the Dana-Farber Cancer Institute since 2010.
Statistical method
Patient baseline characteristics were analyzed primarily descriptively. OS was defined as the time from stem cell infusion to death from any cause. Progression-free survival (PFS) was defined as the time from stem cell infusion to disease relapse, progression, or death from any cause, whichever occurred first. Patients who were alive without disease relapse or progression were censored at the time last seen alive and relapse or progression-free. OS and PFS were estimated using the Kaplan-Meier method, and the log-rank test was used for group comparison. Cumulative incidences of NRM, relapse, and GVHD were constructed in the competing risks framework considering relapse for NRM, NRM for relapse, relapse, or death without developing GVHD for GVHD as a competing event. All tests were 2-sided at the significance level of 0.05. Statistical analysis was performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC) and R version 3.3.2 (the CRAN [Comprehensive R Archive Network] project). The study was approved by the Dana-Farber/Harvard Cancer Center Institutional Review Board and conducted in accordance with the Declaration of Helsinki.
Results
Patient, donor, and transplant characteristics
Patient characteristics
In this cohort of adult patients with HLH, we identified 21 patients who underwent reduced-intensity allogeneic transplantation at Dana-Farber Cancer Institute between 2010 and 2019. The median age for the cohort was 45 years (21,72). Fifty-two percent of the patients were male. Of these 21 patients, 6 (29%) had a documented malignancy, 3 (14.3%) had a rheumatologic diagnosis, and 3 (14.3%) had a viral precipitant, while the remaining did not have a clear precipitating trigger. Eighteen of 21 patients (87.5%) underwent genetic testing; 61% had ≥1 variant in HLH-associated or immunologically-relevant genes. Specifically, 2 patients had an STXBP2 mutation, 6 patients had a perforin mutation, 1 patient had a Lyst/MUNC mutation, and 1 patient was found to have a GATA-2 mutation. Regarding cytomegalovirus (CMV) serostatus, the majority of patients (71%) were seropositive before transplant, and the remaining were seronegative.
Initial therapy was largely etoposide/dexamethasone-based (n = 16) or malignancy-directed chemotherapy (n = 5), with multiple agents, including alemtuzumab, as salvage/bridging therapy.
Donor characteristics
The majority of patients (57%) had matched unrelated donors, while a single patient had a 9/10 single-antigen mismatched unrelated donor. Thirty-three percent had matched related donors, while 10% had haploidentical related donors. The median donor age was 34 years (21,66) and 52% of the donors were male. In terms of donor–recipient gender match, 19% of patients were female with a male donor.
Transplant characteristics
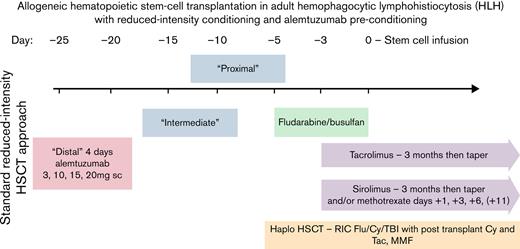
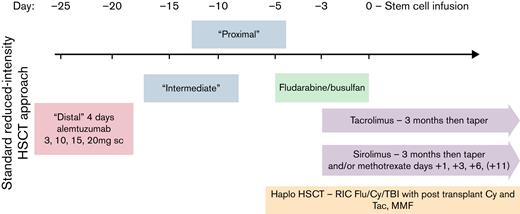
All patients underwent reduced-intensity allogeneic stem cell transplantation. Eighteen of 21 (87.5%) patients received alemtuzumab as “preconditioning” for HSCT, intentionally stopped at a median of 25 days (19-32) before cell infusion. The conditioning regimen comprised fludarabine/busulfan (n = 19) in 7 related and 12 unrelated HLA-matched HSCTs (fludarabine 30 mg/m2 for 4 doses, busulfan 0.8 mg/kg once daily for 4 doses) or fludarabine/cyclophosphamide/total body irradiation in 2 haploidentical HSCTs (Hopkins regimen). Eleven of 21 patients (52%) received peripheral blood stem cells (PBSCs), while the remaining received BM. HSCT occurred at a median of 8 months following diagnosis of HLH (range, 3-13 months). This reduced-intensity transplant strategy with early (“distal”) administration of alemtuzumab is illustrated in Figure 1.
Allogeneic HSCT strategy for adult HLH with RIC and incorporation of early (“distal”) administration of alemtuzumab. GVHD prophylaxis comprised calcineurin inhibitor (tacrolimus) along with methotrexate or sirolimus for HLA-matched donors and posttransplant cyclophosphamide along with tacrolimus and mycophenolate mofetil for haploidentical donors. Alemtuzumab was administered at a median of 25 days before stem cell infusion.
Allogeneic HSCT strategy for adult HLH with RIC and incorporation of early (“distal”) administration of alemtuzumab. GVHD prophylaxis comprised calcineurin inhibitor (tacrolimus) along with methotrexate or sirolimus for HLA-matched donors and posttransplant cyclophosphamide along with tacrolimus and mycophenolate mofetil for haploidentical donors. Alemtuzumab was administered at a median of 25 days before stem cell infusion.
Patient, donor, and transplant characteristics are summarized in Table 1.
Patient, disease, and transplant characteristics
| Demographics . | Median (range) . | Number (n) . | Percentage . |
|---|---|---|---|
| Total | 21 | 100 | |
| Age | 45 (21-72) | — | — |
| Patient sex | |||
| Female | — | 10 | 48 |
| Male | — | 11 | 52 |
| Donor age | 34 (21-66) | — | — |
| Donor sex | — | — | — |
| Female | — | 10 | 48 |
| Male | — | 11 | 52 |
| Patient donor sex | |||
| Male patient/female donor | — | 4 | 19 |
| Patient CMV serostatus | |||
| Positive | — | 15 | 71 |
| Cell source | |||
| BM | — | 10 | 48 |
| PBSCs | — | 11 | 52 |
| Donor type | |||
| Matched related | — | 7 | 33 |
| Matched unrelated | — | 12 | 57 |
| Mismatched related (haplo) | — | 2 | 10 |
| HLA type (A,B,C, DR1, and DQB1) | |||
| 5 of 10 | — | 2 | 10 |
| 9 of 10 | — | 2 | 10 |
| 10 of 10 | — | 17 | 80 |
| Conditioning intensity | |||
| RIC | — | 21 | 100 |
| Etiology HLH | |||
| ALPS | — | 1 | 4.8 |
| Acute myeloid leukemia | — | 1 | 4.8 |
| Blastic plasmacytoid dendritic cell neoplasm | — | 1 | 4.8 |
| Diffuse large B-cell lymphoma | — | 2 | 9.5 |
| EBV | — | 1 | 4.8 |
| Juvenile rheumatoid arthritis | — | 2 | 9.5 |
| MCTD/SLE | — | 1 | 4.8 |
| T-cell lymphoma | — | 1 | 4.8 |
| No trigger | — | 10 | 47.7 |
| Mutations | — | 10 | 47.6 |
| STXBP2 | — | 2 | — |
| Perforin | — | 6 | — |
| Lyst/MUNC | — | 1 | — |
| GATA-2 | — | 1 | — |
| Last alemtuzumab to transplant (n = 18), d | 26 (19-32) | — | — |
| Time of diagnosis to transplant, mo | 8 (3-17) | — | — |
| Demographics . | Median (range) . | Number (n) . | Percentage . |
|---|---|---|---|
| Total | 21 | 100 | |
| Age | 45 (21-72) | — | — |
| Patient sex | |||
| Female | — | 10 | 48 |
| Male | — | 11 | 52 |
| Donor age | 34 (21-66) | — | — |
| Donor sex | — | — | — |
| Female | — | 10 | 48 |
| Male | — | 11 | 52 |
| Patient donor sex | |||
| Male patient/female donor | — | 4 | 19 |
| Patient CMV serostatus | |||
| Positive | — | 15 | 71 |
| Cell source | |||
| BM | — | 10 | 48 |
| PBSCs | — | 11 | 52 |
| Donor type | |||
| Matched related | — | 7 | 33 |
| Matched unrelated | — | 12 | 57 |
| Mismatched related (haplo) | — | 2 | 10 |
| HLA type (A,B,C, DR1, and DQB1) | |||
| 5 of 10 | — | 2 | 10 |
| 9 of 10 | — | 2 | 10 |
| 10 of 10 | — | 17 | 80 |
| Conditioning intensity | |||
| RIC | — | 21 | 100 |
| Etiology HLH | |||
| ALPS | — | 1 | 4.8 |
| Acute myeloid leukemia | — | 1 | 4.8 |
| Blastic plasmacytoid dendritic cell neoplasm | — | 1 | 4.8 |
| Diffuse large B-cell lymphoma | — | 2 | 9.5 |
| EBV | — | 1 | 4.8 |
| Juvenile rheumatoid arthritis | — | 2 | 9.5 |
| MCTD/SLE | — | 1 | 4.8 |
| T-cell lymphoma | — | 1 | 4.8 |
| No trigger | — | 10 | 47.7 |
| Mutations | — | 10 | 47.6 |
| STXBP2 | — | 2 | — |
| Perforin | — | 6 | — |
| Lyst/MUNC | — | 1 | — |
| GATA-2 | — | 1 | — |
| Last alemtuzumab to transplant (n = 18), d | 26 (19-32) | — | — |
| Time of diagnosis to transplant, mo | 8 (3-17) | — | — |
Initial and salvage bridging therapies before transplantation
Initial therapy for HLH was done primarily with etoposide/dexamethasone per the HLH-1994 protocol (17 patients) or with malignancy-directed chemotherapy (eg, R-CHOEP [rituximab cyclophosphamide doxorubicin vincristine etoposide prednisolone] with the inclusion of etoposide given HLH) in cases where an underlying malignancy was detected (6 patients). For refractory disease, alemtuzumab salvage therapy, 30 mg weekly for 3 weeks, was used (4 patients). Ruxolitinib (3 patients) and tacrolimus (5 patients) were used as steroid-free bridging therapies before transplantation. One patient received tocilizumab, and 2 patients received anakinra for additional control of hyperinflammation. One patient received rituximab for EBV reactivation.
Clinical outcomes following transplantation
Engraftment and chimerism
All patients (100%) engrafted neutrophils (achieved absolute neutrophil count >500) at a median of 15.5 days and platelets (median, 19.5 days); 14.3% of patients did not nadir in terms of neutrophils, while 11% of patients did not have a drop in platelet count below 20 000 before engraftment (Table 2).
Neutrophil and platelet engraftment
| . | n (%) . |
|---|---|
| Neutrophil engraftment (500) | |
| Did not nadir | 3 (14.3) |
| Reached nadir | 18 (85.7) |
| Median, d (range) | 16 (11-27) |
| Platelets >20 000 engraftment | |
| Did not nadir | 11 (52) |
| Reached nadir | 10 (48) |
| Median in days (range) | 20 (15-40) |
| Platelets >50 000 engraftment | |
| Did not nadir | 7 (33.3) |
| Reached nadir | 14 (66.7) |
| Median, d (range) | 21 (13-40) |
| . | n (%) . |
|---|---|
| Neutrophil engraftment (500) | |
| Did not nadir | 3 (14.3) |
| Reached nadir | 18 (85.7) |
| Median, d (range) | 16 (11-27) |
| Platelets >20 000 engraftment | |
| Did not nadir | 11 (52) |
| Reached nadir | 10 (48) |
| Median in days (range) | 20 (15-40) |
| Platelets >50 000 engraftment | |
| Did not nadir | 7 (33.3) |
| Reached nadir | 14 (66.7) |
| Median, d (range) | 21 (13-40) |
Donor total leukocyte chimerism (TLC), T-cell, and granulocyte chimerism are shown in supplemental Table 1A. Mixed patterns of chimerism were common. At 30 days, median TLC was 92% (range, 43-100), with only 1 patient having a TLC <50%. Although TLC was improved at 1 year (median, 99%; range, 64-100), mixed chimerism was seen in some patients (30% of patients had TLC <90%). Regarding T-cell chimerism, 24% of patients had T-cell chimerism <30% at 30 days, while 33% had T-cell chimerism <50% at 30 days, and 29.4% of patients had T-cell chimerism of <90% at 1 year after transplant. In terms of granulocyte chimerism, 36.8% of patients had granulocyte chimerism <90% at 30 days, while only 18.8% of patients had granulocyte chimerism <90% at 1 year.
Two patients received CD34-selected stem cell boosts for cytopenias with full donor chimerism; 2 patients received donor lymphocyte infusions (DLIs) for T-cell chimerism ≤20% in hopes of sustaining remission. None of the patients required second transplantation for primary or secondary graft failure. There was no correlation between alemtuzumab timing (ie, the time elapsed between alemtuzumab administration and transplant) and chimerism (total leukocyte, T-cell, or granulocyte chimerism) in this cohort (supplemental Table 1B).
Neither TLC, T-cell, nor granulocyte chimerism were associated with OS or PFS in this cohort.
OS
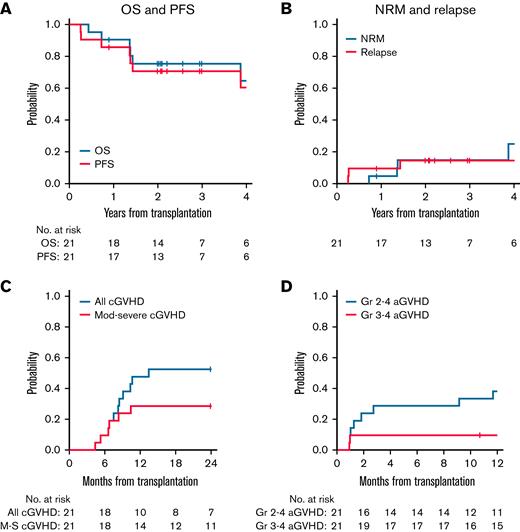
At 3 years after transplantation, OS was 75% (95% CI, 51-89) while 3-year PFS was 71% (95% CI, 46-86).
Relapse and nonrelapse mortality
The 3-year cumulative incidence of relapse was 15% (95% CI, 3.4-33). Two of 21 patients relapsed with their original malignancy (diffuse large B-cell lymphoma) early posttransplant (3-4 months), while 1 patient relapsed with an unusual monoclonal B-cell proliferation that may have been present before transplant. One of these patients also relapsed with concurrent HLH. There were no isolated HLH relapses without relapse of malignancy in this cohort. The cumulative incidence of nonrelapse mortality at 3 years was 15% (95% CI, 3.5-34).
Clinical outcomes following allogeneic transplantation for HLH
| Clinical Outcome . | Estimate, % (95% CI) . |
|---|---|
| aGVHD | |
| Grade 2-4 at 6 mo | 29 (11-49) |
| Grade 2-4 at 1 y | 38 (18-58) |
| Grade 3-4 at 6 mo | 9.5 (1.5-27) |
| cGVHD | |
| All cGVHD at 2 y | 52 (29-71) |
| Moderate/severe cGVHD at 2 y | 29 (11-49) |
| Relapse incidence at 3 y | 15 (3.4-33) |
| Nonrelapse mortality at 3 y | 15 (3.5-34) |
| PFS at 3 y | 71 (46-86) |
| OS at 3 y | 75 (51-89) |
| Clinical Outcome . | Estimate, % (95% CI) . |
|---|---|
| aGVHD | |
| Grade 2-4 at 6 mo | 29 (11-49) |
| Grade 2-4 at 1 y | 38 (18-58) |
| Grade 3-4 at 6 mo | 9.5 (1.5-27) |
| cGVHD | |
| All cGVHD at 2 y | 52 (29-71) |
| Moderate/severe cGVHD at 2 y | 29 (11-49) |
| Relapse incidence at 3 y | 15 (3.4-33) |
| Nonrelapse mortality at 3 y | 15 (3.5-34) |
| PFS at 3 y | 71 (46-86) |
| OS at 3 y | 75 (51-89) |
(A and B) OS, PFS, and relapse and nonrelapse mortality at 3 years after allogeneic HSCT for HLH. OS at 3 years after HSCT was 75% (95% CI, 51-89), while PFS at 3 years after HSCT was 71% (95% CI, 46-86). The cumulative incidence of relapse at 3 years after HSCT was 15% (95% CI, 3.4-33), while the cumulative incidence of NRM at 3 years after HSCT was 15% (95% CI, 3.5-34). Relapse indicates relapse of underlying malignancy (lymphoproliferative disorder) with or without HLH. There were no cases of isolated HLH relapse. (C and D) GVHD outcomes following allogeneic HSCT for HLH. Cumulative incidence of grade 2 to 4 aGVHD at 6 months was 29% (95% CI, 11-49) and 38% (95% CI, 18-58) at 1 year after HSCT. The cumulative incidence of grade 3 to 4 aGVHD at 6 months was 9.5% (95% CI, 1.5-27). The cumulative incidence of cGVHD at 2 years was 52% (95% CI, 29-71), while the cumulative incidence of moderate/severe cGVHD at 2 years was 29% (95% CI, 11-49).
(A and B) OS, PFS, and relapse and nonrelapse mortality at 3 years after allogeneic HSCT for HLH. OS at 3 years after HSCT was 75% (95% CI, 51-89), while PFS at 3 years after HSCT was 71% (95% CI, 46-86). The cumulative incidence of relapse at 3 years after HSCT was 15% (95% CI, 3.4-33), while the cumulative incidence of NRM at 3 years after HSCT was 15% (95% CI, 3.5-34). Relapse indicates relapse of underlying malignancy (lymphoproliferative disorder) with or without HLH. There were no cases of isolated HLH relapse. (C and D) GVHD outcomes following allogeneic HSCT for HLH. Cumulative incidence of grade 2 to 4 aGVHD at 6 months was 29% (95% CI, 11-49) and 38% (95% CI, 18-58) at 1 year after HSCT. The cumulative incidence of grade 3 to 4 aGVHD at 6 months was 9.5% (95% CI, 1.5-27). The cumulative incidence of cGVHD at 2 years was 52% (95% CI, 29-71), while the cumulative incidence of moderate/severe cGVHD at 2 years was 29% (95% CI, 11-49).
GVHD
Cumulative incidence of grade 2 to 4 acute GVHD (aGVHD) at 6 months was 29% (95% CI, 11-49), while it was slightly higher at 1 year at 38% (95% CI, 18-58). The cumulative incidence of grade 3 to 4 aGVHD at 6 months was 9.5% (95% CI, 1.5-27).
The cumulative incidence of chronic GVHD (cGVHD) at 2 years was 52% (95% CI, 29-71), and the cumulative incidence of moderate/severe cGVHD at 2 years was 29% (95% CI, 11-49).
Infectious complications
Infectious complications were documented both before HSCT as well as following HSCT. Before HSCT, there were 4 cases of EBV reactivation, 3 cases of CMV reactivation, 2 cases of herpes simplex virus reactivation, and 1 case each of respiratory syncytial virus and influenza B virus infection. After HSCT, there were no cases of EBV reactivation, 3 cases of CMV reactivation, and 1 case each of adenovirus and parainfluenza virus infection. The last 6 consecutive patients in the cohort received letermovir prophylaxis based on the data for enhanced CMV prophylaxis in patients receiving additional T-cell depletion (alemtuzumab).13
Before HSCT, there were 3 patients with documented fungal infections (candidemia, aspergilloma, mucor). There was a single documented case of fungal infection in the form of candidemia after HSCT. Four patients in the cohort received antifungal prophylaxis.
Before HSCT, there were 6 documented bacterial infections, namely 4 cases of staphylococcal bacteremia, 1 case of vancomycin-resistant enterococcal bacteremia, 1 case of pseudomonal bacteremia, and 2 cases of pneumonia. After HSCT, there were 7 documented bacterial infections, namely 1 case each of staphylococcal, enterococcal, and Klebsiella bacteremia, 2 cases of urinary tract infections, 2 cases of clostridium difficile diarrhea, and 1 case of a bacterial abscess.
Other significant transplant-related complications
After HSCT, 1 case of thrombotic microangiopathy was seen, and 2 patients developed posterior reversible encephalopathy syndrome. Three patients were found to have pulmonary infiltrates consistent with cryptogenic organizing pneumonia, necessitating high-dose steroid administration.
Prognostic factors for survival
There was no correlation between survival outcomes and any of the following factors: donor HLA match, type of malignancy, cell source (PBSCs or BM), donor sex, donor age, or 30-day T-cell chimerism. The only factor that was associated with OS was age. The 3-year OS was 100% for patients aged <45 years compared with 55% (95% CI, 23-78) for those aged ≥45 (P = .01).
PFS was also not associated with any of the abovementioned variables except male recipients with female donors. The 3-year PFS was 25% (95% CI, 0.9-67) for male patients with female donors compared with 82% (95% CI, 54-94) for sex-matched or female recipients with male donors (P = .026). Patients aged <45 years had better PFS, but this was not statistically significant (90% for age <45 vs 55% for age ≥45; P = .056).
PFS was also not associated with any of the abovementioned variables except male recipients with female donors. The 3-year PFS was 25% (95% CI, 0.9-67) for male patients with female donors compared with 82% (95% CI, 54-94) for sex-matched or female recipients with male donors (P = .026). Patients aged <45 years had better PFS, but this was not statistically significant (90% for age <45 vs 55% for age ≥45; P = .056).
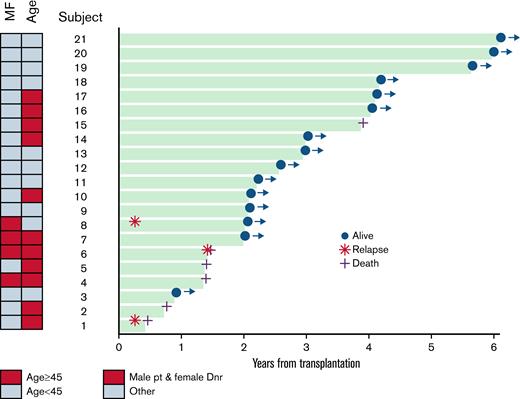
Figure 3 demonstrates a swimmer’s plot with individual outcomes for 21 patients for up to 6 years following transplantation. The association of survival with patient age and female donor/male recipient is also shown in this plot. However, given the small number of patients, it is difficult to draw definitive conclusions regarding risk factors for survival in this cohort, and these analyses are exploratory only.
Swimmer’s plot demonstrating outcomes of 21 patients for up to 6 years following transplantation for HLH. Relapse indicates relapse of underlying malignancy (lymphoproliferative disorder) with or without HLH. There were no cases of isolated HLH relapse.
Swimmer’s plot demonstrating outcomes of 21 patients for up to 6 years following transplantation for HLH. Relapse indicates relapse of underlying malignancy (lymphoproliferative disorder) with or without HLH. There were no cases of isolated HLH relapse.
Discussion
The approach to the therapy of HLH has become more standardized over the last 2 decades, and consolidative HSCT has become a mainstay for those with primary HLH or relapsed/refractory disease in cases of secondary HLH.6 While encouraging data in the pediatric setting has been seen with HSCT for HLH, there are very little data published on outcomes following transplantation in adults in the modern era. Here we present the largest single-center experience reported thus far of allogeneic transplantation in adults with HLH from Dana-Farber Cancer Institute, using a reduced intensity regimen with the incorporation of alemtuzumab in the transplantation strategy.
We found that patients had a very encouraging OS of 75% at 3 years. The largest previous study on allogeneic transplantation in adult patients with HLH, a retrospective registry analysis from the EBMT society, looked at 67 patients from 1995 to 2016 and found a much poorer 3-year OS of 41% (95% CI, 28% to 55%). Death was predominantly due to relapse/progression (28%), infection (25%), and GVHD (17%).8 There was significant heterogeneity in donor type, conditioning, and GVHD prophylaxis in this cohort, and hence it is difficult to extrapolate these outcomes to transplantation practice today with much confidence. A more homogenous single-center analysis of patients undergoing HSCT for HLH in the current era may be more informative.8
The improved OS in our cohort is a result of both relatively low rates of NRM (15%) as well as low rates of relapse (15%). It is worth noting that there were no isolated HLH relapses in this cohort; relapses were associated with relapse of primary malignancy, indicating that reduced-intensity allogeneic transplantation can be curative for HLH in adult patients in a large proportion of cases.
In the relatively novel transplant strategy used in our cohort, only RIC regimens were used for both matched and mismatched donors with the addition of alemtuzumab (CD52-directed monoclonal antibody) in the majority of patients. The concept of using RIC in transplantation for HLH is not a new one. A small 12-patient study from 2006 in pediatric patients reported favorable engraftment in all patients who underwent RIC transplantation, with an OS of 75% at a median of 30 months. It is worth noting that mixed chimerism was seen in 3/9 survivors, which did not appear to impact their overall outcome.9 The authors hypothesized that given HLH is an immune dysregulation disorder, a reduced intensity regimen might be sufficient to abrogate the defect while reducing transplant-related mortality. A phase 2 study in a pediatric population also showed that RIC was associated with a 66.7% OS at 18 months. However, there was a very high rate of graft failure in this study. At 1 year, the proportion of patients alive with sustained engraftment without DLI or second transplant was 39.1% (95% CI, 25.2% to 54.6%), and that of being alive and engrafted (with or without DLI) was 60.9% (95% CI, 45.4% to 74.9%).14 Another experience from Cincinnati Children’s Hospital with 40 patients showed similar findings with a dramatic difference in survival between reduced intensity (92%) and myeloablative (43%) transplants at 3 years.12 The results from our study seem to more closely mimic the reduced intensity experience in the pediatric population and are an improvement over the numbers in the EBMT study referenced earlier.8 Hence, given the excellent OS in our cohort, we propose that a reduced intensity platform with alemtuzumab preconditioning should be the preferred conditioning regimen in adult HLH patients undergoing allogeneic transplantation.
While alemtuzumab preconditioning depletes both recipient and donor T cells, potentially helping with engraftment and prevention of GVHD, there has been a legitimate concern that depletion of donor T cells could compromise the achievement of full donor chimerism, ultimately affecting transplant outcomes. The minimum donor chimerism threshold necessary to prevent reactivation of HLH has not been established, although studies have suggested that donor chimerism ≥20% to 30% may be necessary to maintain remission.15 This conundrum has also led to some debate regarding the optimal timing of alemtuzumab administration; while earlier administration (days −22 to −19 before stem cell infusion or day 0) may have a greater impact on recipient T cells, later administration of alemtuzumab (days −12 to −8 before day 0) may affect the donor graft more.11 Indeed, earlier administration did lead to less mixed chimerism in 2 small single-center studies.12,16 BMT-CTN 1204, a landmark phase 2 study in children and young adults with HLH and primary immunodeficiency, used an “intermediate” alemtuzumab dosing strategy with OS of 67% at 18 months, but an impressive rate of graft failure/need for another cellular intervention (>50%).14 In our cohort, the alemtuzumab was stopped at a median of 25 days before the start of conditioning to minimize toxicity to the donor graft. We found no association between the timing of alemtuzumab and donor chimerism thresholds. Furthermore, patients had acceptable outcomes in terms of graft function, with only 2 patients requiring CD34-selected stem cell boosts and another 2 patients requiring T-cell boosts in the form of donor lymphocyte infusion for T-cell chimerism <20%. This suggests that the early administration of alemtuzumab is a viable strategy. Importantly, mixed chimerism did not affect OS or PFS in our cohort, as discussed in greater detail below.
Engraftment of both neutrophils and platelets was achieved in all 21 patients in our cohort. Total donor leukocyte and T-cell chimerism ranged widely (supplemental Table 1A). As mentioned above, donor lymphocyte infusions were required in 2 patients to boost T-cell chimerism (using a threshold of <20%), while CD34-selected boosts were required in an additional 2 patients who had poor counts despite having full donor chimerism. This was an improvement in primary graft failure rates compared with historical data14; however, the need for CD34-selected boosts for graft exhaustion/secondary graft failure should be anticipated in these patients. Irrespective of the timing of administration, the effect of alemtuzumab on chimerism is generally deleterious and likely explains the phenomenon seen in our cohort. Total leukocyte, T-cell, and granulocyte chimerism did not impact survival outcomes in our cohort as mentioned above, although this would require study in a much larger number of patients to be definitive.
In terms of GVHD, results were similar to those seen in non-HLH patients undergoing reduced-intensity transplantation at our institution with calcineurin-inhibitor–based prophylaxis. Grade 2 to 4 aGVHD at 1 year was in the 40% range, with severe aGVHD in the 10% range while moderate/severe cGVHD at 2 years was 29%. The rates of cGVHD are slightly higher in our cohort compared with historical data in transplants incorporating alemtuzumab. There could be several reasons for this observation. The numbers could have been influenced by the use of PBSC grafts (used in approximately 50% of the cohort) and may have been improved by the use of marrow products when there is no underlying hematologic malignancy. It is not clear if the day −25 limit on alemtuzumab administration contributed to this, but this could, in theory, lead to less in-vivo T-cell depletion of the donor graft. Conversely, it may have helped with better tolerance to infectious complications. Although infections were noted, they did not result in significant morbidity despite the use of alemtuzumab preconditioning and a vulnerable patient population. Antifungal prophylaxis is not mandated at our institution for transplant patients (except for cord blood transplants) due to the low rates of invasive fungal infections historically (1% to 3%) and the use of fungal prophylaxis was not uniform. Fungal infections in this cohort were manageable with this strategy with a single documented case of candidemia after transplant; however, we encourage the use of institutional antifungal prophylaxis strategies when this regimen is used for transplantation in HLH. Overall, NRM was very modest, and the frailty of this population is not necessarily a contraindication for transplantation if they meet adequate testing before transplant.
In the pediatric population, mutations associated with HLH are typically homozygous null mutations. Conversely, in the adult HLH population, mutations are almost always heterozygous and are hypomorphic alleles, not null alleles.17 Of the 21 patients in this study, approximately half did not have any identifiable trigger; in the remaining patients, malignancy was the dominant trigger, while rheumatologic diagnoses and infection accounted for the remaining cases. Eighteen patients were evaluated for a genetic predisposition to HLH; 61% of those tested had ≥1 mutation, recognized as hypomorphic alleles for familial HLH genes. Six of 21 patients did have a perforin mutation, which was not unexpected. The association between such mutations and the clinical syndrome of HLH has never been robustly established in the adult HLH population. As an example, a paper from Miller and colleagues on 88 adult HLH patients identified 7 unique “disruptive variants” (ie, variants implicated in HLH thought to have an impact on protein function, with the most common variant being PRF1 A91V). Interestingly, these disruptive variants were not enriched in adult HLH compared with a control population when adjusted for ancestry, particularly when considering the less common non-PRF1 A91V variants.18 There was also no clinical difference between those with disruptive variants and those without. Within the limitations of this study, the data suggested that disruptive germline variants do not drive HLH in adults; however, where clinical suspicion for germline HLH is high, testing should still be done. This is an important concept highlighting that the decision to proceed with transplantation should be based on the clinical course of the patient rather than the presence or absence of mutations.
In conclusion, although our analysis has a relatively small sample size, this is the largest and most homogenous series examining transplant outcomes in adult HLH patients in the modern era and certainly with the most encouraging survival outcomes published thus far. Reduced-intensity allogeneic transplantation incorporating early alemtuzumab preconditioning in adults with primary or relapsed/refractory secondary HLH appears to be very well-tolerated and effective, leading to excellent transplant outcomes irrespective of the type of HLH or mutational status and offering a curative option in this difficult disease.
Authorship
Contribution: M.G. and S.N. designed the research, analyzed data, and wrote the manuscript; H.T.K. performed the statistical analysis and analyzed the data; and E.J., D.C.F., A.L., V.T.H., C.S.C., J.K., R.J.S., J.H.A., and N.B. made significant contributions/edits to the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mahasweta Gooptu, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02115; e-mail: Mahasweta_gooptu@dfci.harvard.edu.
References
Author notes
The full-text version of this article contains a data supplement.