Key Points
A multidisciplinary SCD-pulmonary clinic is associated with improved pulmonary outcomes and reduced acute care use among children with SCD.
Abstract
People with sickle cell disease (pwSCD) are at risk of developing lung conditions that complicate their SCD but often face health care access barriers. An interdisciplinary clinic providing pulmonary care for pwSCD was created in 2014 at the Nationwide Children’s Hospital (NCH) to address access barriers that may prevent optimized treatment. We hypothesize that pwSCD and pulmonary disease would have fewer hospitalizations for acute chest syndrome (ACS), asthma, and vaso-occlusive episodes in the 2 years after their initial SCD-pulmonary clinic visit compared with the 2 years before. From 2014 to 2020, 119 pwSCD were evaluated in the SCD-pulmonary clinic and followed up at the NCH for at least 2 years before and after this initial visit. Acute care outcomes, pulmonary function, polysomnography, echocardiogram, laboratory, and medication prescribing data were collected and analyzed using the Wilcoxon signed ranked and McNemar tests. The median number of acute care visits for ACS (P < .001) and asthma (P = .006) were significantly lower during the 2 years after pwSCD’s initial SCD-pulmonary clinic evaluation compared with the 2 years before. Asthma and allergic rhinitis were more frequently diagnosed and prescriptions for hydroxyurea (P = .005) and inhaled corticosteroids (P = .005) were more common in the post–SCD-pulmonary clinic period. The median number of prescribed systemic corticosteroids was lower in the 2 years after SCD-pulmonary clinic evaluation (P < .0001). Lactate dehydrogenase and white blood cell counts also significantly decreased. Implementing a multidisciplinary SCD-pulmonary clinic is feasible and may allow improved management of pulmonary problems and lead to improvements in the usage of health and acute care.
Introduction
Sickle cell disease (SCD) is a genetic and chronic disease that primarily affects Black and Hispanic populations in the United States. It causes significant morbidity, including acute and chronic pain, decreased quality of life, end-organ damage, and reduces the average lifespan by 20 to 30 years compared with those without SCD.1-3 There are several well-documented factors that contribute to the morbidity and mortality in people with SCD (pwSCD), and acute and chronic pulmonary conditions are the most common ones. For example, asthma is seen in ∼12% of all children in the United States, but it is estimated that 20% to 25% of children with SCD have concomitant asthma.4 However, clear definitions of asthma in SCD versus wheezing from other chronic mechanisms, such as hemolysis-induced inflammation, are not well identified5 and typically require pulmonary subspecialty evaluation and management. People with asthma and SCD have an increased risk of all-cause mortality compared with those without asthma. This is likely because of their increased risk of developing acute vaso-occlusive pain episodes (VOEs), stroke, acute chest syndrome (ACS), and the increased need for blood transfusions compared with those with SCD alone.6,7
ACS itself is also a significant contributor to SCD morbidity and mortality. ACS is the second most common cause of hospitalization and the most common cause of death, with 25% of pwSCD succumbing to this complication.6 Recurrent ACS episodes lead to an increased risk of irreversible lung damage that can manifest as either a restrictive or an obstructive chronic lung disease pattern.7 In addition, obstructive sleep apnea (OSA) is another common pulmonary condition that can complicate SCD. Whereas 1% to 5% of children are diagnosed with OSA, the prevalence of OSA and other sleep disorders remains poorly defined, with past studies documenting that OSA affects between 5% and 59% of pwSCD.5,8 Sleep disorders are particularly problematic in pwSCD as airway obstruction during sleep leads to oxygen desaturations that can increase erythrocyte sickling and subsequent pathology, including cardiac dysfunction and pulmonary hypertension.9,10
Ensuring that pwSCD and coexistent pulmonary disease receive hematology and pulmonary preventive care has the potential to reduce SCD complications. However, it has been well-documented that pwSCD and their families report facing more barriers to accessing health care than other Black children without SCD, even when other demographic variables are controlled.11 They report increased difficulty attending appointments, arranging transportation, and waiting longer to see their providers compared with the general population. These issues may be related to poverty because many pwSCD live below the federal poverty line, which can limit the ability to attend appointments and/or be insured.11 Families of pwSCD also report a lack of communication between different parts of the health care system that can make accessing and navigating the health care system particularly challenging. Those with comorbid asthma report facing an even larger number of barriers to care, receiving more discordant care between their medical providers, and feeling even more marginalized than those with SCD alone. Families of pwSCD report having fewer opportunities to access quality comprehensive care than families of children with other chronic conditions and special health care needs12 and report feeling that this leads to an increased use of emergency department and increased hospitalizations.13
To mitigate access barriers, some have advocated for grouping health care visits together on the same day to reduce the transportation and time burden that appointments put on families.11 Previous studies suggest that implementing a multidisciplinary care clinic is associated with a significant decrease in acute care usage among those with SCD who have a history of high acute care usage, and an interdisciplinary SCD and pulmonary clinic may improve appointment adherence. To this end, in 2014, the Nationwide Children's Hospital (NCH) created an interdisciplinary clinic that provides pulmoray care for pwSCD.14,15 The clinic was offered to pwSCD at the NCH with a history of at least 1 pulmonary complication, such as asthma, OSA, hypoxia, and/or recurrent ACS. To evaluate this model of care, we aim to compare the outcomes of pwSCD during the 2 years before their initial SCD-pulmonary visit with the 2 years after this visit. We hypothesize that pwSCD would have fewer hospitalizations for ACS, asthma, and VOEs in the 2 years after their initial SCD-pulmonary clinic visit.
Methods
Description of the interdisciplinary SCD-pulmonary comprehensive clinic
The SCD-pulmonary interdisciplinary team at the NCH included 2 pediatric hematologists, 2 pediatric pulmonologists, a respiratory therapist with portable pulmonary function testing (PFT) equipment, 3 SCD nurse practitioners, 2 SCD nurse clinicians, a social worker, a psychologist, a genetic counselor, and a school liaison. This bimonthly clinic is located on the main NCH campus and includes all the testing and counseling that are provided in the standard SCD comprehensive clinic, with additional evaluation by 1 of the pediatric pulmonologists, respiratory therapist teaching, and specific pulmonary testing including PFT, pulse oximetry, sleep and tobacco smoke exposure screening, and an option for plethysmography, polysomnography (PSG), and 6-minute walk test as clinically indicated. PwSCD were followed up biannually in this clinic if they had ongoing pulmonary needs or were transitioned back to the standard comprehensive SCD clinic after managing the pulmonary problem. All PFTs and plethysmography were performed in accordance with the third National Health and Nutrition Examination Survey (NHANES III) standards and the American Thoracic Society guidelines.
Study design and population
We conducted an institutional review board–approved retrospective chart review of all pwSCD who visited the NCH SCD-pulmonary interdisciplinary clinic between 13 February 2014 (initial SCD-pulmonary clinic) and 10 December 2020 (last SCD-pulmonary clinic of 2020). This study was conducted in accordance with the Declaration of Helsinki. The NCH SCD database and search function of the electronic medical record (EMR) were used to manually identify pwSCD who had been seen for their initial SCD-pulmonary clinic visit during the study period. From here, EMRs were examined to identify those who were followed up at the NCH for at least 2 years before and 2 years after their initial SCD-pulmonary visit. PwSCD were excluded from the study if they underwent stem cell transplant during the study period.
Data collection
Demographics (eg, age, gender, SCD genotype) and insurance type (eg, private, public) at the time of their initial SCD-pulmonary visit were recorded. Hospitalizations for VOE and/or ACS that occurred during the 4 years that an individual was followed were identified using the international classification of disease 10th revision codes (ICD10) listed within the EMR. PFTs, plethysmography, PSGs, and echocardiograms (ECHO) that were ordered and obtained during the 4-year period were also reviewed. PFT parameters included forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1). PSG parameters included the presence of OSA, apnea-hypopnea index (AHI), sleep efficiency, total rapid eye movement (REM) sleep, oxygen saturations, and the presence of hypoventilation. OSA was defined as an apnea/hypopnea index (AHI) of >5 during PSG. ECHO parameters included the presence of left ventricular hypertrophy (LVH), left atrial hypertrophy (LAH), diastolic or systolic dysfunction, and tricuspid regurgitant jet velocity (TRJV). A TRJV ≥2.5 m/s was considered abnormal.
EMR data were obtained using automated data pull and manual collection. Automated data abstraction was used to collect demographics, clinical and acute care visits, intensive care unit (ICU) admissions, PFTs, medication prescribing, and diagnoses. For each of these automated variables, 10 charts were manually reviewed to verify that the automated data that were pulled were accurate. ECHO and PSG data were manually abstracted from studies that had been completed at any point during the 4-year study period, whereas prescription of SCD-modifying medication (eg, hydroxyurea), inhaled corticosteroid (ICS), or systemic corticosteroid, laboratory studies (as long as not transfused within the previous 3 months), PFT results, self-reported tobacco smoke exposure, and the ICD10 codes for diagnoses of asthma and/or allergic rhinitis were collected, if available, from SCD visits that occurred ∼2 years before their initial SCD-pulmonary visit and from the initial SCD-pulmonary visit and the SCD-pulmonary visit that occurred ∼2 years later.
Statistical analysis
Data were summarized with standard descriptive statistics: frequency and percentage for categorical variables, and median and interquartile range (IQR) for quantitative variables. Wilcoxon signed rank tests were used to compare quantitative variables from before with those after the initial SCD-pulmonary visit, such as the number of acute visits, number of systemic steroid courses, and laboratory values. McNemar test was used to compare categorical data between the 2 time points. P values were 2-sided and P < .05 were considered statistically significant. All statistical analyses were completed using SAS software, version 9.4 (SAS Institute, Cary, NC) or the base R package (R Foundation for Statistical Computing, Vienna, Austria).
Results
Participants
Of the 513 pwSCD followed at NCH during the study period, 145 had at least 1 SCD-pulmonary visit, and 119 were followed for at least 2 years before and 2 years after their initial SCD-pulmonary visit and included in the analyses (Table 1). Of the 119 pwSCD who were followed longitudinally, 77 (65%) were evaluated at a separate pulmonary clinic visit before their initial SCD-pulmonary care visit, but 58% had at least 1 instance of clinic nonattendance when they were scheduled for an appointment in the separate pulmonary clinic. In contrast, in the 2 years after their initial SCD-pulmonary clinic visit, pwSCD attended a median of 3 (IQR, 2-3) additional SCD-pulmonary visits, and only 19% had a least 1 instance of SCD-pulmonary clinic nonattendance (P < .001).
Participant demographics
| Characteristic . | pwSCD (n = 119) . |
|---|---|
| Median age, y (IQR) | 10.2 (6.7-14.9) |
| Male (n, %) | 60 (50) |
| Race | |
| African or African American (n, %) | 119 (100) |
| Insurance type (n, %) | |
| Public | 108 (91) |
| Private | 11 (9) |
| Smoke exposure (n, %) | 59 (50) |
| SCD genotype (n, %) | |
| SS | 79 (66) |
| SC | 30 (25) |
| Sβ+ | 10 (8) |
| Characteristic . | pwSCD (n = 119) . |
|---|---|
| Median age, y (IQR) | 10.2 (6.7-14.9) |
| Male (n, %) | 60 (50) |
| Race | |
| African or African American (n, %) | 119 (100) |
| Insurance type (n, %) | |
| Public | 108 (91) |
| Private | 11 (9) |
| Smoke exposure (n, %) | 59 (50) |
| SCD genotype (n, %) | |
| SS | 79 (66) |
| SC | 30 (25) |
| Sβ+ | 10 (8) |
Values shown as frequency (percentage) or median (IQR).
Acute care utilization
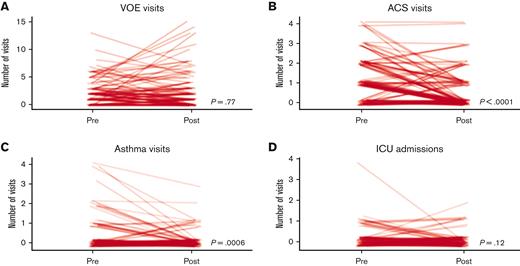
The median number of acute care visits by this cohort for ACS (P < .001) and asthma (P = .006) were significantly lower, and the number of unique pwSCD who had at least 1 visit for ACS or for asthma were significantly lower during the 2 years after they were examined the SCD-pulmonary clinic than during the 2 years before that (ACS, 66% vs 34%; P < .001; asthma, 24% vs 12%; P = .014) (Figure 1B-C). There were no statistically significant differences in the median number of hospital admissions for VOE nor for receipt of ICU care (Figure 1A-D).
The number of acute visits for VOE, ACS, and asthma, and ICU admissions before and after initial SCD-pulmonary clinic visit. Each line represents 1 of the 119 pwSCD.
The number of acute visits for VOE, ACS, and asthma, and ICU admissions before and after initial SCD-pulmonary clinic visit. Each line represents 1 of the 119 pwSCD.
Diagnoses and prescription of medication
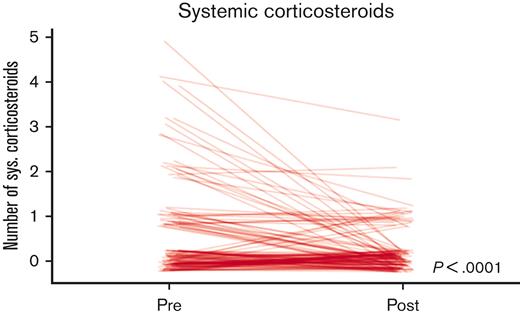
Diagnoses of asthma and allergic rhinitis were more frequently observed after SCD-pulmonary clinic evaluation, along with an increase in prescriptions issued for hydroxyurea therapy and ICS (Table 2). There was a significant reduction in the number of pwSCD for whom systemic corticosteroids were prescribed (36% before vs 18% after, P < .001) and in the overall number of systemic corticosteroid courses that were prescribed in the 2 years after visiting the SCD-pulmonary clinic (P < .001) (Figure 2).
Coexistent asthma and/or allergic rhinitis diagnosis and prescribed medication among patients examined 2 years before and 2 years after the initial SCD-pulmonary clinic visit
| Characteristic . | Before . | After . | P values . |
|---|---|---|---|
| Diagnoses | |||
| Asthma | 75 (64) | 88 (75) | .0123 |
| Allergic rhinitis | 27 (23) | 43 (36) | .0124 |
| Medications | |||
| Hydroxyurea | 62 (52) | 77 (65) | .0051 |
| ICS | 50 (42) | 68 (57) | .0046 |
| ICS/LABA∗ | 14 (12) | 18 (15) | .34 |
| Characteristic . | Before . | After . | P values . |
|---|---|---|---|
| Diagnoses | |||
| Asthma | 75 (64) | 88 (75) | .0123 |
| Allergic rhinitis | 27 (23) | 43 (36) | .0124 |
| Medications | |||
| Hydroxyurea | 62 (52) | 77 (65) | .0051 |
| ICS | 50 (42) | 68 (57) | .0046 |
| ICS/LABA∗ | 14 (12) | 18 (15) | .34 |
Values shown as frequency (percentage).
ICS/LABA: combined inhaled corticosteroid + long-acting beta agonist.
The number of prescribed systemic corticosteroid courses decreased during the 2 years before and after initial SCD-pulmonary clinic visit. Each line represents 1 of the 119 pwSCD.
The number of prescribed systemic corticosteroid courses decreased during the 2 years before and after initial SCD-pulmonary clinic visit. Each line represents 1 of the 119 pwSCD.
Diagnostic testing findings
Of the 86 pwSCD who had PFTs completed at their initial SCD-pulmonary visit, 29 showed either an obstructed or restrictive pattern, with no significant increase in observed abnormal patterns over time (P = .25). In addition, 47% had improvement in post-clinic FEV1, with stable longitudinal trends in FVC and FEV1 (supplemental Figure 1). Of the 62 participants who had bronchodilator testing, 27 (44%) had response. ECHO findings demonstrated a high frequency of LVH and LAH in pwSCD who had these studies completed before and after their initial SCD-pulmonary clinic evaluation (Table 3). Thirty-six individuals completed a PSG before attending the SCD-pulmonary clinic and 36 completed a PSG after attending the clinic (n = 15 matched studies). Sleep abnormalities were common during both study observation periods, with reduced REM sleep, reduced sleep efficiency, hypoxia, OSA, and prolonged sleep onset latency observed (Table 4). Ninety-four pwSCD had laboratory values that were obtained 2 years before and 2 years after their initial SCD-pulmonary clinic visit (Table 5).
Unmatched ECHO and matched PFT patterns of pwSCD before and after the initial SCD-pulmonary clinic evaluation
| . | Before . | After . |
|---|---|---|
| ECHO performed (n) | 57 | 64 |
| LVH (n, %) | 20 (50) | 21 (53) |
| LAH (n, %) | 12 (30) | 10 (25) |
| Diastolic dysfunction (n, %) | 0 (-) | 0 (-) |
| Systolic dysfunction (n, %) | 0 (-) | 0 (-) |
| TRJV (n, %) | ||
| <2.5 | 28 (49) | 32 (50) |
| ≥2.5 | 11 (19) | 7 (11) |
| Not reported | 18 (32) | 25 (39) |
| PFTs (n) | 86 | 86 |
| Patterns | ||
| Normal (n, %) | 57 (66) | 61 (71) |
| Obstructed (n, %) | 20 (23) | 17 (20) |
| Restricted (n, %) | 9 (10) | 8 (9) |
| . | Before . | After . |
|---|---|---|
| ECHO performed (n) | 57 | 64 |
| LVH (n, %) | 20 (50) | 21 (53) |
| LAH (n, %) | 12 (30) | 10 (25) |
| Diastolic dysfunction (n, %) | 0 (-) | 0 (-) |
| Systolic dysfunction (n, %) | 0 (-) | 0 (-) |
| TRJV (n, %) | ||
| <2.5 | 28 (49) | 32 (50) |
| ≥2.5 | 11 (19) | 7 (11) |
| Not reported | 18 (32) | 25 (39) |
| PFTs (n) | 86 | 86 |
| Patterns | ||
| Normal (n, %) | 57 (66) | 61 (71) |
| Obstructed (n, %) | 20 (23) | 17 (20) |
| Restricted (n, %) | 9 (10) | 8 (9) |
Values shown as frequency (percentage)
Unmatched PSG results before and after the initial SCD-pulmonary clinic evaluation
| PSG . | Before . | After . |
|---|---|---|
| Number of unique patients | 36 | 36 |
| REM AHI | 1 (0-4) | 1 (0-4.65) |
| AHI | 0.6 (0-2) | 0.5 (0.1-1.2) |
| Sleep efficiency reduced | 15 (42) | 10 (29)∗ |
| Sleep onset latency prolonged | 8 (22) | 7 (20)∗ |
| REM sleep decreased | 25 (69) | 24 (69)∗ |
| Mean O2 (n, %) | 96 (95-98) | 96.5 (94.8-98) |
| Hypoxia | 13 (36) | 16 (44) |
| OSA | 10 (28) | 8 (22) |
| Hypoventilation | 1 (3) | 1 (3) |
| PSG . | Before . | After . |
|---|---|---|
| Number of unique patients | 36 | 36 |
| REM AHI | 1 (0-4) | 1 (0-4.65) |
| AHI | 0.6 (0-2) | 0.5 (0.1-1.2) |
| Sleep efficiency reduced | 15 (42) | 10 (29)∗ |
| Sleep onset latency prolonged | 8 (22) | 7 (20)∗ |
| REM sleep decreased | 25 (69) | 24 (69)∗ |
| Mean O2 (n, %) | 96 (95-98) | 96.5 (94.8-98) |
| Hypoxia | 13 (36) | 16 (44) |
| OSA | 10 (28) | 8 (22) |
| Hypoventilation | 1 (3) | 1 (3) |
Values shown as frequency (percentage) or median (IQR).
O2, oxygen.
Data missing for 1 participant, making the denominator 35 instead of 36. OSA is defined as AHI > 5.
Laboratory results of the 94 pwSCD for whom clinical data were available before and after the initial SCD-pulmonary visit
| Characteristic (median, IQR) . | Before . | After . | P value . |
|---|---|---|---|
| WBCs (103/μL) | 10.8 (8.4-13.2) | 9.2 (7.0-12.2) | .0105 |
| Hemoglobin (g/dL) | 9.5 (7.8-10.8) | 9.6 (8.3-10.9) | .0260 |
| Platelets (103/μL) | 343 (251-446) | 378 (243-475) | .16 |
| Reticulocyte (%) | 7.5 (3.5-14.2) | 6.1 (3.5-12.7) | .77 |
| RBCs (106/μL) | 3.2 (2.7-4.2) | 3.0 (2.6-4.0) | .0099 |
| MCV (fL) | 79.1 (71.8-85.0) | 83.5 (74.2-91.0) | <.0001 |
| ANC (103/mm3) | 4.5 (3.3-5.9) | 4.6 (3.1-5.6) | .62 |
| LDH (U/L) | 1011 (841-1251) | 780 (607-1026) | .0077 |
| Creatinine (mg/dL) | 0.37 (0.30-0.44) | 0.43 (0.35-0.51) | <.0001 |
| Hemoglobin F (%) | 18.5 (12.7-32.4) | 17.6 (9.2-23.4) | .0059 |
| Characteristic (median, IQR) . | Before . | After . | P value . |
|---|---|---|---|
| WBCs (103/μL) | 10.8 (8.4-13.2) | 9.2 (7.0-12.2) | .0105 |
| Hemoglobin (g/dL) | 9.5 (7.8-10.8) | 9.6 (8.3-10.9) | .0260 |
| Platelets (103/μL) | 343 (251-446) | 378 (243-475) | .16 |
| Reticulocyte (%) | 7.5 (3.5-14.2) | 6.1 (3.5-12.7) | .77 |
| RBCs (106/μL) | 3.2 (2.7-4.2) | 3.0 (2.6-4.0) | .0099 |
| MCV (fL) | 79.1 (71.8-85.0) | 83.5 (74.2-91.0) | <.0001 |
| ANC (103/mm3) | 4.5 (3.3-5.9) | 4.6 (3.1-5.6) | .62 |
| LDH (U/L) | 1011 (841-1251) | 780 (607-1026) | .0077 |
| Creatinine (mg/dL) | 0.37 (0.30-0.44) | 0.43 (0.35-0.51) | <.0001 |
| Hemoglobin F (%) | 18.5 (12.7-32.4) | 17.6 (9.2-23.4) | .0059 |
Data represented as median (IQR). P values result from Wilcoxon signed rank test.
ANC, absolute neutrophil count; LDH, lactate dehydrogenase; RBCs, red blood cells; WBCs, white blood cells.
Discussion
Despite the well-established burden of pulmonary disease and difficulties with access to care in pwSCD, optimal treatment paradigms are not yet well established. Studies have demonstrated that pwSCD and their families often have health care access barriers.16 The combined SCD-pulmonary clinic at the NCH was established with the intent of easing health care access barriers by allowing children with SCD to be examined for their underlying pulmonary complications at the same time that they were receiving their hematology care. We observed that receiving care in this clinic was associated with a variety of improved outcomes, including reduced acute health care visits for ACS and asthma and prescriptions for systemic corticosteroid courses as well as an increased recognition of underlying asthma and prescribing of hydroxyurea and ICS. Future studies are warranted to understand the mechanisms that drive these improvements because our study design does not allow definitive conclusions about this model of care and whether it improves outcomes. Furthermore, the frequency of combined assessment should be studied to determine whether combined assessments, performed more frequently than biannual assessments, affect outcomes or, conversely, contribute to care burden. Our findings do suggest, however, that this innovative care model is feasible and may be a strategy to reduce access barriers, allow for timely communication between subspecialty providers, and facilitate optimized care among children with SCD who are at high risk.
Notably, we observed a significant reduction in the number of hospitalizations for ACS and asthma and in the prescription of systemic corticosteroid courses in the 2 years after the initial SCD-pulmonary clinic visit compared with the 2 years before the visit. These findings are consistent with a recent study on pulmonology involvement in a nonintegrated clinic and support the notion that improved preventive care reduces usage of acute health care.17,18 For example, attaining better control of asthma or inflammatory airway disease from improved ICS use may lead to reduced asthma and ACS admissions and associated prescription of systemic corticosteroid.19 Limiting systemic corticosteroids is particularly important for pwSCD because these medications are known to increase the risk of subsequent hospitalization for VOE.20 Frequency of VOE was not significantly different before and after the initial SCD-pulmonary clinic visit. This could be related to the fact that frequency of VOEs increases with age.21 Our study period may have also been too short to detect an impact of other factors, beyond pulmonary care, that may influence the frequency of VOE, because VOEs have many possible triggers. We also observed improved hematologic parameters including a significantly lower white blood cells and lactate dehydrogenase., indicative of less hemolysis and release of inflammatory cell byproducts. Although fetal hemoglobin significantly declined, and creatinine and mean corpuscular volume increased, we suspect that these changes were related to the increase in the age of our cohort.
Similar to what was observed in a prior study evaluating an integrated clinic,15 we saw a reduction in clinic nonattendance. By combining pulmonology care with routine hematology care, pwSCD were able to make fewer trips to receive necessary care. This has potential to considerably reduce the amount of time and resources needed to receive high quality care. The collaborative clinic also allowed for easy communication between pulmonary and hematology providers, which may reduce discordance in care. Finally, we observed that prescription of medication for pwSCD and asthma increased after their initial SCD-pulmonary visit. The increase in prescription of ICS was predicted because asthma diagnoses increased. Increased prescription of hydroxyurea, however, might suggest that management by an interdisciplinary team that emphasizes the use of this important disease-modifying therapy could be a strategy to optimize its use.
Our study has a few limitations. Because of its retrospective nature, we were unable to determine causality, and future research is needed to dissect which components of this interdisciplinary clinic may be key to improving outcomes and care. Also, although prescription of ICS and hydroxyurea increased, given our data source, we were unable to reliably evaluate whether medication nonadherence may have limited the impact of these therapies on outcomes. In addition, many of the diagnostic testing variables, such as mean corpuscular volume, creatinine, FEV1, and FVC increase with age, making it challenging to determine whether the interdisciplinary clinic was associated with improvements in these parameters. Finally, our relatively small sample size and limited paired PSG and ECHO data may have hindered our ability to determine the full impact of this model of care. Future multicenter prospective studies are needed to better determine the impact of an interdisciplinary care model on long-term changes in cardiopulmonary and sleep variables. These studies could also elucidate the characteristics of patients who are at high risk of adverse outcomes and could receive the largest potential benefit from interdisciplinary care.
In conclusion, introducing a multidisciplinary SCD-pulmonary clinic may allow improved management of common pulmonary problems observed in pwSCD and may lead to improvements in overall health and acute care utilization. Additional studies are warranted to test whether this care model is sustainable, scalable, and definitively improves outcomes for pwSCD.
Acknowledgments
The authors thank the Research Information Solutions and Innovation team at the Nationwide Children’s Hospital for assistance with electronic data pulls. The authors thank the The Ohio State University's ASPIRE Medical Research Program for the support toward C.P.
Authorship
Contribution: B.T.K. and S.C. designed the study and were coprincipal investigators; R.N.Z., C.P., B.T.K., and S.C. coordinated the project and analyzed the data; R.N.Z., J.S., B.T.K., and S.C. contributed to writing the first draft; and all authors contributed to editing and revision of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Susan Creary, 700 Children’s Dr., Columbus, OH 43205; e-mail: susan.creary@nationwidechildrens.org.
References
Author notes
∗B.T.K. and S.C. are joint senior authors.
Data are available on request from the corresponding author, Susan Creary (susan.creary@nationwidechildrens.org).
The full-text version of this article contains a data supplement.