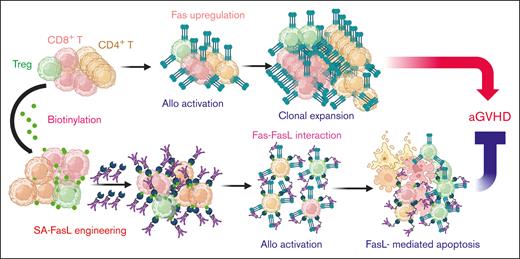
Key Points
Donor T cells engineered to display on their surface SA-FasL protein undergo apoptosis after activation by host alloantigens.
SA-FasL on donor lymphocytes effectively prevents acute GVHD.
Abstract
Alloreactive T-effector cells (Teffs) are the major culprit of acute graft-versus-host disease (aGVHD) associated with hematopoietic stem cell transplantation. Ex vivo nonspecific depletion of T cells from the donor graft impedes stem cell engraftment and posttransplant immune reconstitution. Teffs upregulate Fas after activation and undergo Fas ligand (FasL)–mediated restimulation-induced cell death (RICD), an important mechanism of immune homeostasis. We targeted RICD as a means to eliminate host-reactive Teffs in vivo for the prevention of aGVHD. A novel form of FasL protein chimeric with streptavidin (SA-FasL) was transiently displayed on the surface of biotinylated lymphocytes, taking advantage of the high-affinity interaction between biotin and streptavidin. SA-FasL–engineered mouse and human T cells underwent apoptosis after activation in response to alloantigens in vitro and in vivo. SA-FasL on splenocytes was effective in preventing aGVHD in >70% of lethally irradiated haploidentical mouse recipients after cotransplantation with bone marrow cells, whereas all controls that underwent transplantation with nonengineered splenocytes developed aGVHD. Prevention of aGVHD was associated with an increased ratio of CD4+CD25+FoxP3+ T regulatory (Tregs) to Teffs and significantly reduced transcripts for proinflammatory cytokines in the lymphoid organs and target tissues. Depletion of Tregs from the donor graft abrogated the protection conferred by SA-FasL. This approach was also effective in a xenogeneic aGVHD setting where SA-FasL–engineered human PBMCs were transplanted into NSG mice. Direct display of SA-FasL protein on donor cells as an effective means of eliminating alloreactive Teffs in the host represents a practical approach with significant translation potential for the prevention of aGVHD.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is an effective treatment for hematological malignancies, inherited hematological disorders, and immunodeficiencies.1-3 Acute graft vs host disease (aGVHD) orchestrated by mature donor T cells is one of the major barriers to the broad and successful application of HSCT.2,4,5 Exclusion of mature T cells by various ex vivo manipulations from the donor graft reduces the efficacy of engraftment and slows the pace of posttransplant immune recovery in recipients with myeloablative conditioning.6 More restricted approaches to deplete various donor T-cell subsets ex vivo have proven inefficient.6-8 A prevalent procedure is the posttransplant administration of cyclophosphamide at the peak of reciprocal donor-host sensitization to reduce both host-versus-graft and graft-versus-host reactions.9 However, cyclophosphamide impairs immune reconstitution, thereby necessitating delayed donor lymphocyte infusion.10 Regardless of recent advances, aGVHD remains the leading cause of morbidity and mortality associated with HSCT.2,5
We herein report a novel strategy to eliminate alloreactive mature donor Teffs in myeloablated graft recipients by taking advantage of Fas ligand (FasL)–mediated apoptosis as a critical homeostatic immune control mechanism.11-13 Fas pathway serves as a feedback mechanism to terminate an ongoing immune response and is critical to the maintenance of peripheral tolerance.12,14 Activated Teff cells upregulate Fas expression and become sensitive to FasL-mediated apoptosis after repeated interaction of T-cell receptor with the target antigen, defined as restimulation-induced cell death (RICD). The current study assessed RICD as a potential mechanism to eliminate alloreactive Teffs in the graft recipients for the prevention of aGVHD. We previously reported a novel form of FasL protein chimeric with streptavidin (SA-FasL). There are 2 unique features of this construct: (1) SA-FasL exists as tetramers and oligomers with robust apoptotic activity on Fas-expressing cells; and (2) SA-FasL can be positionally and transiently displayed on biotinylated biologic or nonbiologic surfaces with an in vivo half-life of ∼3.5 days with demonstrated immunomodulatory efficacy in various transplantation models.15-21 The underlying hypothesis of this study was that SA-FasL on donor alloreactive T cells will engage Fas upregulated on the same cell in response to host alloantigens, leading to autocrine apoptosis and prevention of aGVHD.
Methods
Engineering cells with the SA-FasL protein
Spleen cells were engineered with SA-FasL or streptavidin (SA) proteins per our previously reported protocols.16 Briefly, splenocytes were incubated in 5 μM EZ-Link Sulfo-NHS-LC biotin (hereafter referred as biotin) solution (Pierce) in sterile phosphate-buffered saline (PBS) at room temperature for 30 minutes. After washing, cells were incubated in PBS containing SA or SA-FasL proteins for 30 minutes, washed to remove unbound proteins, and then used for the indicated studies.
Collection of human PBMCs
Human peripheral blood mononuclear cells (PBMCs) were isolated as previously reported from healthy donors undersigned informed consent approved by the Institutional Review Board of University of Louisville. Peripheral blood was collected in heparin-containing vacutainer (BD Bioscience, catalog# 364606). PBMCs were isolated from buffy coats by Ficoll-paque (GE, catalog# 17-1440-03) density centrifugation and washed by sterile PBS before further use. Human PBMCs were engineered with SA-FasL, as described for the mouse cells.16
In vivo monitoring of adoptively transferred cells
4C.SJL or C57BL/6hCD2 splenocytes were engineered with various amounts of SA-FasL or SA proteins. Engineered cells were labeled with 2.5 μM CTV, and 4C.SJL (5 × 106 cells/mouse) or C57BL/6hCD2 (10 × 106 cells/mouse) cells were intravenously injected into F1 (C57BL/6xBALB/c; H-2b/d). Mice were euthanized 48 or 72 hours after cell infusion to monitor donor cell proliferation using flow cytometry. For xenogeneic settings, human neutrophil-deplete PBMCs were engineered with SA-FasL (25 ng/106 cells) or equimolar of SA protein as control. Engineered cells (5 × 106 cells) were injected IV into NSG mice 4 hours after total body irradiation (200 cGy). Animals were euthanized 5 days after cell infusion to assess the frequency and absolute numbers of various lymphoid cell populations using flow cytometry by gating on human CD45+ cells.
Haploidentical preclinical model of aGVHD and skin transplantation
F1 (C57BL/6xBALB/c; H-2b/d) mice (10-12 weeks old) were subjected to 1000 cGy total body irradiation (Gammacell 40 Extractor, 137Cs source) followed by IV infusion of 10 × 106 C57BL/6 nonengineered bone marrow cells admixed with 20 × 106 nonengineered or SA-FasL–engineered splenocytes. Animals were monitored twice weekly for general health and body weight, and acute GVHD clinical score was calculated per published literature.22 A clinical score of >6 and total body weight loss >25% were considered as experimental end point and animals were euthanized for various analysis. Long-term mice without aGVHD underwent transplantation with the skin from BALB/c and C3H third-party grafts (H-2k) and monitored for rejection twice weekly, as previously reported.23
Humanized NSG model of xenogeneic aGVHD
NSG females (8-10 weeks old) were subjected to 200 cGy total body irradiation and injected via tail vein 4 hours later with 10 × 106 SA-FasL–engineered or -nonengineered human PBMCs. Fresh human PBMCs were used in all experiments. Animals were monitored for body weight twice weekly, and the development of xenogeneic GVHD was assessed as published.24 Animals with >25% body weight loss and a clinical aGVHD score >6 were euthanized as an experimental end point.
Statistical analysis
The comparison of the survival curves was done using the log-rank (Mantel-Cox) test. Data are shown as individual data points or as mean ± standard error mean, as depicted in the figure legends. Unpaired, two-tailed or Mann-Whitney t test was performed as indicated. For multiple comparison, one-way analysis of variance with Tukey post hoc test was performed. Statistical significance was defined as P < .05 (GraphPad prism v.8).
Results
T cells engineered with SA-FasL are eliminated after allogeneic activation both ex vivo and in vivo
We first tested the impact of SA-FasL on T-cell proliferation in responses to alloantigens ex vivo. Splenocytes from 4C mice (H-2b), transgenic for a T-cell receptor that recognizes BALB/c MHC I-Ad antigen,25 were engineered with various amounts of SA-FasL or equimolar SA as a control protein and used as responders against irradiated BALB/c splenocytes in a standard [3H]-thymidine–based proliferation assay. There was a dose-dependent display of SA-FasL on the cell surface, as assessed using flow cytometry (supplemental Figure 1A). Nonengineered 4C and SA-engineered 4C T cells showed robust proliferation (Figure 1A). In marked contrast, there was an absence/minimal proliferation of SA-FasL–engineered 4C cells across all engineering levels. These observations were further confirmed using a carboxyfluorescein succinimidyl ester (CFSE)–based proliferation assay. Analysis of live cells at various times after culture using flow cytometry showed a significant reduction in the frequency of alloreactive CD4+ and CD8+ cells expressing the transgenic Vβ13 TCR as compared with control nonengineered 4C or SA-engineered 4C cells (Figure 1B; supplemental Figure 1B).
SA-FasL transiently displayed on the surface of T cells results in their elimination in response to alloantigens in vitro. (A) In vitro proliferation assay. SA-FasL– or SA-engineered 4C T cells (H-2Kb) were stimulated with irradiated BALB/c splenocytes (H-2Kd) for 48 or 72 hours. Cultures were pulsed with [3H]thymidine for the last 16 hours of incubation and harvested using a beta plate counter. DNA-incorporated radioactivity is plotted as an assessment of cell proliferation. Data were pooled from 2 independent experiments. (B) Frequencies of live total CD4+ and CD8+ T cells (top panels) and Vβ13+ transgenic CD4+ and CD8+ T cells (bottom panels) in mixed lymphocyte cultures. Experimental conditions are the same as in (A), except instead of pulsing with [3H]thymidine, cultures were harvested at 72 hours, stained with the Abs to indicated markers, and analyzed using flow cytometry. Data were pooled from 2 independent experiments. (C) SA-FasL induces autocrine death in alloreactive T cells. CTV-labeled nonengineered 4C cells were mixed 1:1 ratio with CFSE-labeled SA-FasL-4C cells and used as responders at the indicated ratios against a fixed number of irradiated BALB/c cells as stimulators. Cells were harvested after 72 hours of incubation and analyzed for live cells using flow cytometry (left panel). Representative flow dot plots of proliferating 4C cells (right panel). Data sets pooled from 2 independent experiments. One-way ANOVA with Tukey multiple comparison was used in panels A-B. Unpaired two-tailed t test was used in panel C. Data are shown as mean ± SEM. ANOVA, analysis of variance; SEM, standard error mean; cpm, counts per minute. ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001
SA-FasL transiently displayed on the surface of T cells results in their elimination in response to alloantigens in vitro. (A) In vitro proliferation assay. SA-FasL– or SA-engineered 4C T cells (H-2Kb) were stimulated with irradiated BALB/c splenocytes (H-2Kd) for 48 or 72 hours. Cultures were pulsed with [3H]thymidine for the last 16 hours of incubation and harvested using a beta plate counter. DNA-incorporated radioactivity is plotted as an assessment of cell proliferation. Data were pooled from 2 independent experiments. (B) Frequencies of live total CD4+ and CD8+ T cells (top panels) and Vβ13+ transgenic CD4+ and CD8+ T cells (bottom panels) in mixed lymphocyte cultures. Experimental conditions are the same as in (A), except instead of pulsing with [3H]thymidine, cultures were harvested at 72 hours, stained with the Abs to indicated markers, and analyzed using flow cytometry. Data were pooled from 2 independent experiments. (C) SA-FasL induces autocrine death in alloreactive T cells. CTV-labeled nonengineered 4C cells were mixed 1:1 ratio with CFSE-labeled SA-FasL-4C cells and used as responders at the indicated ratios against a fixed number of irradiated BALB/c cells as stimulators. Cells were harvested after 72 hours of incubation and analyzed for live cells using flow cytometry (left panel). Representative flow dot plots of proliferating 4C cells (right panel). Data sets pooled from 2 independent experiments. One-way ANOVA with Tukey multiple comparison was used in panels A-B. Unpaired two-tailed t test was used in panel C. Data are shown as mean ± SEM. ANOVA, analysis of variance; SEM, standard error mean; cpm, counts per minute. ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001
To investigate whether SA-FasL eliminates activated T cells via an autocrine mechanism by engaging with an upregulated Fas receptor on the same cell, CFSE-labeled SA-FasL–engineered 4C cells were comixed with equal numbers of CTV-labeled nonengineered cells and used as responders at various ratios to a fixed number of irradiated BALB/c stimulators. Flow cytometry analysis at 72 hours after culture showed ∼20% live CFSE+ vs ∼80% live CVT+ 4C cells across all stimulator/responder ratios used (Figure 1C). Although nonengineered 4C cells showed robust proliferation with accumulated daughter cells at each proliferation cycle, SA-FasL–engineered 4C cells showed minimal accumulation of proliferating cells. Similar results were found 48 hours after culture (supplemental Figure 1C). These observations implicate autocrine apoptosis as the primary mechanism of cell death, as paracrine apoptosis would have resulted in an increased level of death in naïve 4C T cells at the highest responder cell density (1:1) because of the greatest probability of cell-to-cell interaction. In fact, nonengineered 4C T cells in coculture with SA-FasL–engineered cells showed almost the same level of viability (∼80%) at all responder cell densities.
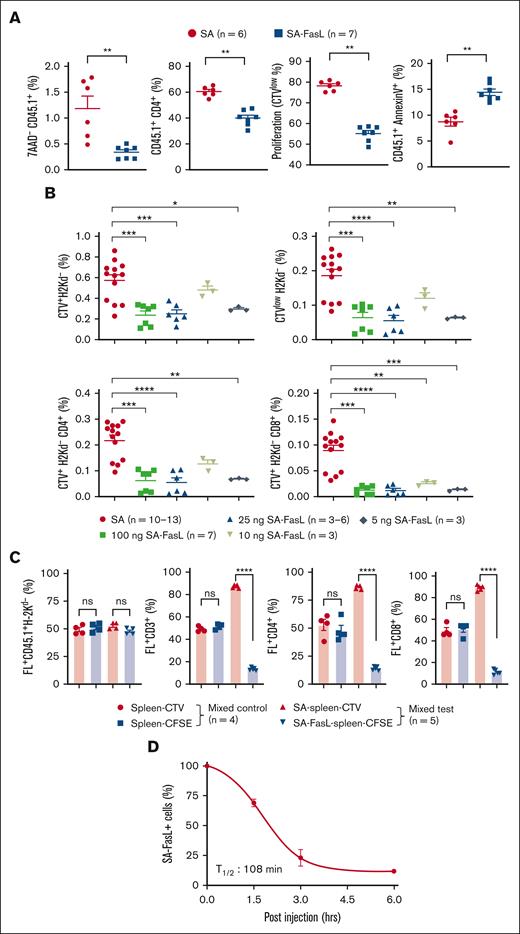
We next assessed the efficacy of SA-FasL in eliminating alloreactive cells in vivo by adoptive transfer of CTV-labeled 4C.SJL cells engineered with various doses of SA-FasL or SA control protein into F1 mice. SA-FasL significantly (P < .01) reduced the frequency of live donor (7AAD- CD45.1+) cells as well as CD45.1+CD4+ T cells as compared with SA-engineered controls (Figure 2A). Decrease in donor cells engineered with SA-FasL was because of apoptosis (CD45.1+AnnexinV+) that translated into significantly (P < .01) reduced frequency and absolute number of proliferating cells as compared with the SA control group (Figure 2A; supplemental Figure 2A-B). These differences were not unique to the 4C TCR transgenic model as in vivo cell tracking assay in a regular parent-to-F1 model [C57BL/6-to-F1(BALB/cxC57BL/6)] resulted in similar observations (Figure 2B; supplemental Figure 2C). To assess if alloreactive T cells undergo apoptosis in an autocrine fashion in vivo, donor splenocytes engineered with SA-FasL were labeled with CFSE and comixed at 1:1 ratio SA-engineered and CTV-labeled cells and transplanted into lethally irradiated F1 recipients. A second group of mice that underwent transplantation with nonengineered and CFSE- or CTV-labeled cells at 1:1 ratio served as controls. Analysis of donor cells 72 hours after transplantation demonstrated specific elimination of SA-FasL–engineered cells (Figure 2C; supplemental Figure 2D-E), further confirming autocrine apoptosis as the underlying mechanism of alloreactive T-cell elimination. Autocrine apoptosis was not because of engineering with SA-FasL altering the Fas expression as the level of this receptor was similar to that on nonengineered cells (supplemental Figure 3). The in vivo turnover kinetic studies established ∼108 minutes as the half-life of SA-FasL on donor T cells transplanted into lethally irradiated F1 recipients (Figure 2D). Collectively, these results demonstrate that engineering a donor graft with SA-FasL protein is an effective approach to physically eliminate alloreactive Teffs primarily through autocrine apoptosis both ex vivo and in vivo.
SA-FasL–engineered T cells are effectively eliminated in response to alloantigens in vivo. (A) Frequency, proliferation, and apoptosis of 4C cells in F1 recipients. 4C.SJL splenocytes were labeled with CTV, engineered with SA-FasL protein (100 ng/106 cells), and injected IV (5 × 106 cells/mouse) into F1 recipients (H2Kb/d). Cells engineered with an equimolar of SA (50 ng/106 cells) were used as controls. Splenic T cells were analyzed 48 hours after injection for the frequency of total donor live (7AAD-CD45.1+) cells, CD4+T (CD45.1+CD4+) cells, proliferating (CTVlow) cells, and apoptotic (CD45.1+AnnexinV+) cells. Data were pooled from 2 independent experiments, with 3 to 4 per group. (B) Tracking of C57BL/6hCD2 donor cells in F1 recipients. Donor splenocytes were labeled with CTV and engineered with the indicated doses of SA-FasL protein (ng/106 cells). Cells engineered with equimolar of SA (50 ng/106 cells) at the highest dose of SA-FasL were used as controls. Cells were adoptively transferred into F1 recipients (10 × 106 cells per mouse) that were euthanized 72 hours later to harvest the spleen. Splenocytes were analyzed in flow cytometry by gating on donor cells negative for H-2Kd for the frequency of total cells (CTV+H-2Kd-), CD4+ (CTV+H-2Kd-CD4+), CD8+ (CTV+H-2Kd-CD8+) cells, as well as proliferating donor cells (CTVlowH-2Kd-). Data were pooled from 3 independent experiments with n = 3 to 4 per group. (C) SA-FasL–engineered alloreactive T cells undergo autocrine apoptosis in vivo. CFSE-labeled and SA-FasL–engineered C57BL/6hCD2 (CD45.1+) splenocytes were comixed at 1:1 ratio with CTV-labeled and SA-engineered splenocytes and injected (IV) into F1 recipients 6 hours after irradiation (1000 cGy). Mice also received 10 × 106 nonlabeled and nonengineered bone marrow cells. F1 mice that underwent transplantation with nonengineered cells served as controls. Mice were euthanized 72 hours after cell infusion, and spleen cells were analyzed for the indicated donor cell types using flow cytometry. (D) Turnover kinetics of SA-FasL on engineered CD3+ T cells. CTV-labeled SA-FasL plenocytes (CD45.1) were injected into irradiated F1 (1000 cGy) animals. Mice were euthanized at various time points after infusion and analyzed for the presence of SA-FasL on donor CD3+ T cells using an antibody to the streptavidin portion of the molecule in flow cytometry. For comparison of mean, Mann Whitney test and 1-way ANOVA with Tukey post hoc test was used in panels A-C, respectively. Nonlinear regression analysis was done for panel D. Data are represented as mean ± SEM. ANOVA, analysis of variance; SEM, standard error mean. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
SA-FasL–engineered T cells are effectively eliminated in response to alloantigens in vivo. (A) Frequency, proliferation, and apoptosis of 4C cells in F1 recipients. 4C.SJL splenocytes were labeled with CTV, engineered with SA-FasL protein (100 ng/106 cells), and injected IV (5 × 106 cells/mouse) into F1 recipients (H2Kb/d). Cells engineered with an equimolar of SA (50 ng/106 cells) were used as controls. Splenic T cells were analyzed 48 hours after injection for the frequency of total donor live (7AAD-CD45.1+) cells, CD4+T (CD45.1+CD4+) cells, proliferating (CTVlow) cells, and apoptotic (CD45.1+AnnexinV+) cells. Data were pooled from 2 independent experiments, with 3 to 4 per group. (B) Tracking of C57BL/6hCD2 donor cells in F1 recipients. Donor splenocytes were labeled with CTV and engineered with the indicated doses of SA-FasL protein (ng/106 cells). Cells engineered with equimolar of SA (50 ng/106 cells) at the highest dose of SA-FasL were used as controls. Cells were adoptively transferred into F1 recipients (10 × 106 cells per mouse) that were euthanized 72 hours later to harvest the spleen. Splenocytes were analyzed in flow cytometry by gating on donor cells negative for H-2Kd for the frequency of total cells (CTV+H-2Kd-), CD4+ (CTV+H-2Kd-CD4+), CD8+ (CTV+H-2Kd-CD8+) cells, as well as proliferating donor cells (CTVlowH-2Kd-). Data were pooled from 3 independent experiments with n = 3 to 4 per group. (C) SA-FasL–engineered alloreactive T cells undergo autocrine apoptosis in vivo. CFSE-labeled and SA-FasL–engineered C57BL/6hCD2 (CD45.1+) splenocytes were comixed at 1:1 ratio with CTV-labeled and SA-engineered splenocytes and injected (IV) into F1 recipients 6 hours after irradiation (1000 cGy). Mice also received 10 × 106 nonlabeled and nonengineered bone marrow cells. F1 mice that underwent transplantation with nonengineered cells served as controls. Mice were euthanized 72 hours after cell infusion, and spleen cells were analyzed for the indicated donor cell types using flow cytometry. (D) Turnover kinetics of SA-FasL on engineered CD3+ T cells. CTV-labeled SA-FasL plenocytes (CD45.1) were injected into irradiated F1 (1000 cGy) animals. Mice were euthanized at various time points after infusion and analyzed for the presence of SA-FasL on donor CD3+ T cells using an antibody to the streptavidin portion of the molecule in flow cytometry. For comparison of mean, Mann Whitney test and 1-way ANOVA with Tukey post hoc test was used in panels A-C, respectively. Nonlinear regression analysis was done for panel D. Data are represented as mean ± SEM. ANOVA, analysis of variance; SEM, standard error mean. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
SA-FasL on donor cells prevents aGVHD without a significant impact on immune reconstitution and competency
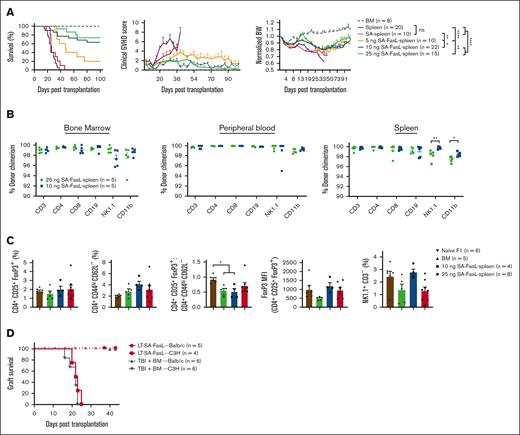
To investigate whether engineering donor T cells prevents aGVHD, we used a haploidentical, myeloablative transplant model. C57BL/6 splenocytes (2 × 107) engineered with various doses of SA-FasL protein comixed with whole bone marrow cells (1 × 107) were transplanted into lethally irradiated F1 recipients. Recipients of control splenocytes (nonengineered or engineered with SA protein) showed high-grade clinical GVHD scores and significant weight loss, expiring within 46 days (Figure 3A). In marked contrast, >70% of mice that underwent transplantation with splenocytes engineered with SA-FasL doses exceeding 10 ng/106 cells survived (P < .0001) with improved clinical aGVHD scores and body weight (Figure 3A). The efficacy of SA-FasL was dose-dependent, with the highest dose (25 ng/106 cells) showing ∼73% survival, whereas the lowest dose (5 ng/106 cells) resulted in ∼20% survival (Figure 3A) for a 100-day experimental end point.
Engineering donor graft with SA-FasL abrogates lethal aGVHD and shows efficient immune reconstitution. (A) Survival of lethally irradiated (1000 cGy) F1(C57BL/6xBALB/c) recipients grafted with a mixture of C57BL/6 allogeneic bone marrow cells (1 × 107) and splenocytes (2 × 107). F1 animals underwent transplantation with nonengineered C57BL/6 bone marrow cells with or without GVHD-causing spleen cells engineered with the indicated doses of SA-FasL protein (ng/106 cells) or a SA dose (12.5 ng/106) equimolar to the highest dose of SA-FasL. Animals were monitored for survival, clinical GVHD scores, and body weight. (B) Donor chimerism (H-2Kb+H-2Kd-) in the indicated tissues and frequency of CD4+ Treg (CD4+CD25+FoxP3+), Teff (CD4+CD44hiCD62L-), and NK (NK1.1+CD3-) cells. (C) Frequency of CD4+ Treg (CD4+CD25+FoxP3+), Teff (CD4+CD44hiCD62L-), and NK (NK1.1+CD3-) cells, FoxP3 MFI, and Treg/Teff ratios in the spleen of long-term (>100 days) animals compared with that of bone marrow only recipients and unmanipulated naïve F1 animals. (D) Skin graft survival. Long-term survivors (100 days after transplantation) with bone marrow cells only and bone marrow cells along with SA-FasL–engineered splenocytes were challenged simultaneously with donor-matched (BALB/c, H-2d) and third-party (C3H, H-2k) skin grafts. All long-term recipients accepted donor-matched skin allografts while rejecting third-party grafts in an acute fashion. For comparison of survival curves, log-rank (Mantel-Cox) test was used in panels A,D. Mann Whitney test in panel B and 1-way ANOVA with Tukey post hoc test in panel C was used for mean comparison. Data are represented as mean ± SEM. ANOVA, analysis of variance; SEM, standard error mean. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Engineering donor graft with SA-FasL abrogates lethal aGVHD and shows efficient immune reconstitution. (A) Survival of lethally irradiated (1000 cGy) F1(C57BL/6xBALB/c) recipients grafted with a mixture of C57BL/6 allogeneic bone marrow cells (1 × 107) and splenocytes (2 × 107). F1 animals underwent transplantation with nonengineered C57BL/6 bone marrow cells with or without GVHD-causing spleen cells engineered with the indicated doses of SA-FasL protein (ng/106 cells) or a SA dose (12.5 ng/106) equimolar to the highest dose of SA-FasL. Animals were monitored for survival, clinical GVHD scores, and body weight. (B) Donor chimerism (H-2Kb+H-2Kd-) in the indicated tissues and frequency of CD4+ Treg (CD4+CD25+FoxP3+), Teff (CD4+CD44hiCD62L-), and NK (NK1.1+CD3-) cells. (C) Frequency of CD4+ Treg (CD4+CD25+FoxP3+), Teff (CD4+CD44hiCD62L-), and NK (NK1.1+CD3-) cells, FoxP3 MFI, and Treg/Teff ratios in the spleen of long-term (>100 days) animals compared with that of bone marrow only recipients and unmanipulated naïve F1 animals. (D) Skin graft survival. Long-term survivors (100 days after transplantation) with bone marrow cells only and bone marrow cells along with SA-FasL–engineered splenocytes were challenged simultaneously with donor-matched (BALB/c, H-2d) and third-party (C3H, H-2k) skin grafts. All long-term recipients accepted donor-matched skin allografts while rejecting third-party grafts in an acute fashion. For comparison of survival curves, log-rank (Mantel-Cox) test was used in panels A,D. Mann Whitney test in panel B and 1-way ANOVA with Tukey post hoc test in panel C was used for mean comparison. Data are represented as mean ± SEM. ANOVA, analysis of variance; SEM, standard error mean. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Long-term survivors (>100 days) displayed full donor chimerism in the peripheral blood, bone marrow, and spleen (Figure 3B). These mice also showed efficient reconstitution of Treg (CD4+CD25+FoxP3+), Teff (CD4+CD44hiCD62L–), and NK (NK1.1+CD3–) cells, with the Treg/Teff cell ratio being comparable to that of control GVHD-free mice receiving only BM cells (Figure 3C; supplemental Figure 4A-B). Long-term survivors were immunocompetent with established tolerance to parental antigens as they accepted BALB/c (H-2d) skin graft while promptly rejecting C3H (H-2k) third-party grafts within 24 days, mice that underwent transplantation with only bone marrow cells showed a similar rejection tempo (Figure 3D). These results demonstrate that transplantation with SA-FasL–engineered allogeneic cells is effective in preventing aGVHD without a detectable negative impact on immune tolerance and reconstitution.
Recipients of SA-FasL–engineered splenocytes show significantly reduced number of Teff and increased numbers of Treg cells
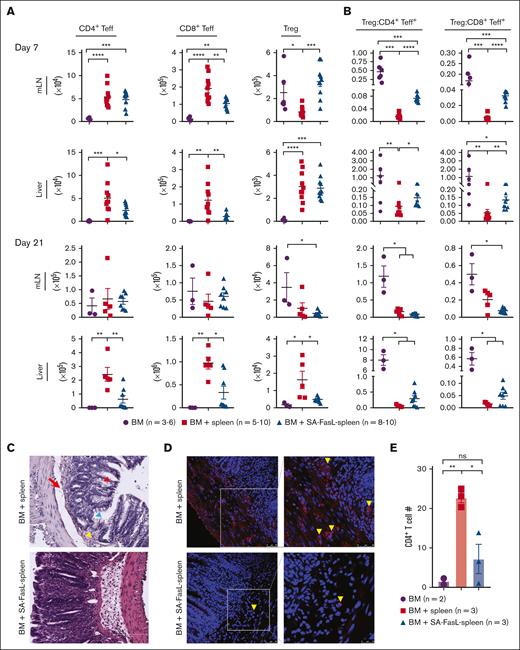
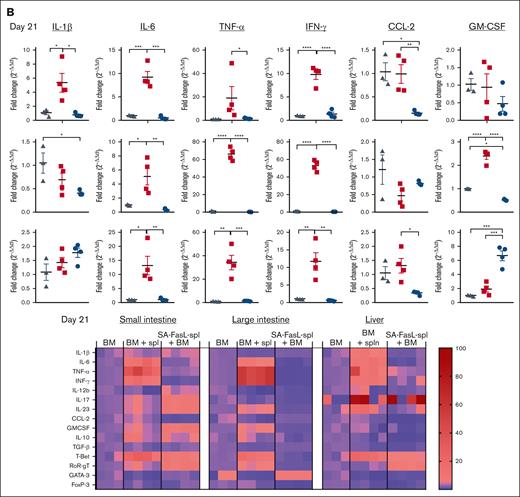
SA-FasL may prevent aGVHD by 2 interlinked mechanisms; apoptosis of alloreactive Teffs and the release of apoptotic bodies from dying cells initiating a feed-forward immunoregulatory pathway resulting in the generation/expansion of Treg cells.26 Activation and extensive proliferation of naïve alloreactive T cells in secondary lymph organs early after HSCT followed by homing to target tissues, skin, liver, intestine, are hallmarks of aGVHD.27,28 When analyzed on day 7 after transplantation, recipients of SA-FasL–engineered splenocytes had reduced numbers of CD4+ and CD8+ Teffs in the liver and mesenteric lymph nodes as compared with mice grafted with nonengineered splenocytes (Figure 4A). Importantly, the SA-FasL group also had increased absolute numbers of CD4+CD25+FoxP3+ Tregs in the lymph nodes (P < .001), which translated into an increased Treg/Teff ratio (P < .0001, Figure 4B). Analysis on day 21 after transplantation revealed no significant differences in T cells subsets in the mesenteric lymph nodes of the SA-FasL and control groups, but the absolute intrahepatic numbers of CD4+ and CD8+ Teff cells were significantly reduced (P < .05) in the recipients of SA-FasL–engineered splenocytes. The absolute number of CD4+CD25+FoxP3+ Tregs was also increased (P < .05) in the liver of control group as compared with the liver of the SA-FasL group, which did not translate into a significantly increased ratio of Tregs/Teffs because of the higher numbers of Teffs (Figure 4B). The T-cell subset frequencies showed a similar pattern to absolute cell numbers in the lymph nodes and liver, with some variations (supplemental Figure 5). Analysis of the large intestine on day 21 after transplantation using immunohistochemistry showed extensive tissue damage and significantly higher numbers of CD4+ T cells in the control group than in the SA-FasL–engineered group, which showed normal tissue structure with minimal CD4+ T-cell infiltration (Figure 4C-D; supplemental Figure 5B-C). Taken together, these data demonstrate that SA-FasL eliminates Teffs in lymphoid tissues with a null effect on Tregs during the induction phase of the disease, shifting the balance of immune responses toward aGVHD prevention.
Recipients of SA-FasL–engineered splenocytes have reduced levels of T effectors and increased T-regulatory cells. (A) Absolute number of CD4+ Teff (CD4+FoxP3-CD44+CD62L-PD1+), CD8+ Teff (CD8+CD44+CD62L-PD1+), and Treg (CD4+CD25+FoxP3+) cells in mesenteric lymph nodes (mLN) and the liver (cells per gram). Intrahepatic immune cells and mesenteric lymph nodes were harvested 7 and 21 days after transplantation from F1 recipients of C57BL/6 bone marrow cells (BM) and BM cells cotransplanted with SA-FasL–engineered (BM + SA-FasL-spleen) or nonengineered splenocytes (BM + spleen). Cells were analyzed for activated CD4+ and CD8+ Teff and CD4+ Treg cells using flow cytometry. (B) Ratios of CD4+ Treg cells to CD4+ and CD8+ Teff cells. (C) Representative images of H&E staining of large intestine from F1 recipients (n = 3) at day 21 after transplantation showing cellular infiltration (yellow arrowheads), epidermal cell vacuolar degeneration (red arrowheads), mucosal epithelia degeneration (blue arrowhead), and disruption of mucosal-submucosal junction (red arrow) in nonengineered splenocytes recipients (BM + spleen) as compared with that of SA-FasL-engineered splenocytes (BM + SA-FasL-spleen) recipients (n = 3). (D) Representative images of 2-color immunofluorescence staining for CD4 (red) and nucleus (blue) with infiltrated CD4+ T-cell counts per section per animal. Recipients of nonengineered splenocytes have significantly higher frequencies of CD4+ T cells within crypts and lamina propria of large intestine than the recipients of SA-FasL–engineered splenocytes. Tissues from recipients of BM cells without donor splenocytes served as control (n = 2). Data point represent averaged cell numbers from 5 random fields per section per animal. Scale bar: 50 μm (left); 25 μm (right). Data are shown as mean ± SEM. For comparisons, 1-way ANOVA with Tukey post hoc test was used in panels A,D and Mann Whitney test in panel B. ANOVA, analysis of variance; SEM, standard error mean. ∗P < .05∗∗; P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Recipients of SA-FasL–engineered splenocytes have reduced levels of T effectors and increased T-regulatory cells. (A) Absolute number of CD4+ Teff (CD4+FoxP3-CD44+CD62L-PD1+), CD8+ Teff (CD8+CD44+CD62L-PD1+), and Treg (CD4+CD25+FoxP3+) cells in mesenteric lymph nodes (mLN) and the liver (cells per gram). Intrahepatic immune cells and mesenteric lymph nodes were harvested 7 and 21 days after transplantation from F1 recipients of C57BL/6 bone marrow cells (BM) and BM cells cotransplanted with SA-FasL–engineered (BM + SA-FasL-spleen) or nonengineered splenocytes (BM + spleen). Cells were analyzed for activated CD4+ and CD8+ Teff and CD4+ Treg cells using flow cytometry. (B) Ratios of CD4+ Treg cells to CD4+ and CD8+ Teff cells. (C) Representative images of H&E staining of large intestine from F1 recipients (n = 3) at day 21 after transplantation showing cellular infiltration (yellow arrowheads), epidermal cell vacuolar degeneration (red arrowheads), mucosal epithelia degeneration (blue arrowhead), and disruption of mucosal-submucosal junction (red arrow) in nonengineered splenocytes recipients (BM + spleen) as compared with that of SA-FasL-engineered splenocytes (BM + SA-FasL-spleen) recipients (n = 3). (D) Representative images of 2-color immunofluorescence staining for CD4 (red) and nucleus (blue) with infiltrated CD4+ T-cell counts per section per animal. Recipients of nonengineered splenocytes have significantly higher frequencies of CD4+ T cells within crypts and lamina propria of large intestine than the recipients of SA-FasL–engineered splenocytes. Tissues from recipients of BM cells without donor splenocytes served as control (n = 2). Data point represent averaged cell numbers from 5 random fields per section per animal. Scale bar: 50 μm (left); 25 μm (right). Data are shown as mean ± SEM. For comparisons, 1-way ANOVA with Tukey post hoc test was used in panels A,D and Mann Whitney test in panel B. ANOVA, analysis of variance; SEM, standard error mean. ∗P < .05∗∗; P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
SA-FasL–mediated protection is associated with decreased levels of transcripts in target tissues for cytokines/chemokines implicated in the development of aGVHD
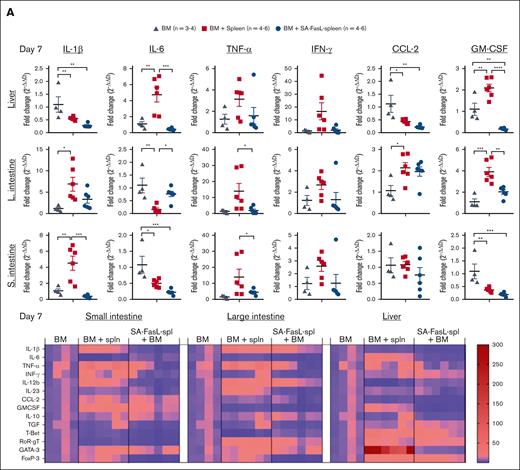
Proinflammatory cytokines and chemokines, released from activated donor immune cells as well as damaged host tissues owing to conditioning, play a critical role in the development of aGVHD. The transcript profile of cytokines and chemokines in aGVHD target tissues was assessed using quantitative reverse transcription polymerase chain reaction (qRT-PCR). We observed decreased levels of transcripts for IL-1β, IL-6, TNF-α, IFN-γ, and IL-23 proinflammatory cytokines implicated in GVHD29,30 in the liver and small and large intestine of animals that underwent transplantation with SA-FasL–engineered splenocytes as compared with the recipients of nonengineered splenocytes on day 7 after transplantation, except for IL-6, which showed increased expression in large intestine (Figure 5A). The increases in the transcripts for proinflammatory cytokines were much more pronounced and significant (P < .05) on day 21 effector phase as compared with day 7 initiation phase of GVHD (Figure 5B). Transcripts for CCL2 were significantly reduced in the liver (P < .01) and small intestine (P < .05) of the SA-FasL-engineered splenocyte group on day 21 after transplantation, consistent with the role of this chemokine in the recruitment of CD8+ Teff cells into target tissues for GVHD.31 The transcripts for granulocyte macrophage colony stimulating factor, involved in the pathology of aGVHD,32 were also reduced in the liver (P < .001; day 7) of the SA-FasL-engineered splenocyte group as compared with the nonengineered splenocyte group, with the exception of the small intestine on day 21 that showed a significant increase (P < .001).
Transplantation with SA-FasL–engineered splenocytes results in reduced levels of anti-inflammatory and increased levels of regulatory cytokines in GVHD target tissues. Total RNA was isolated on day 7 (A) and 21 (B) from the liver, small intestine, and large intestine of F1 recipients of C57BL/6 bone marrow cells (BM) and BM cells cotransplanted with SA-FasL–engineered (BM + SA-FasL-spleen) or nonengineered (BM + spleen) splenocytes. The transcripts for the indicated cytokines and chemokines were analyzed using TaqMan RT-qPCR. Fold change expression (2–ΔΔCt) was calculated with respect to GAPDH as a house-keeping gene and BM-only recipients. Data are representative of 2 independent experiments and shown as mean ± SEM. For comparisons, 1-way ANOVA with Tukey post hoc test was used in panels A-C. ANOVA, analysis of variance; SEM, standard error mean. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Transplantation with SA-FasL–engineered splenocytes results in reduced levels of anti-inflammatory and increased levels of regulatory cytokines in GVHD target tissues. Total RNA was isolated on day 7 (A) and 21 (B) from the liver, small intestine, and large intestine of F1 recipients of C57BL/6 bone marrow cells (BM) and BM cells cotransplanted with SA-FasL–engineered (BM + SA-FasL-spleen) or nonengineered (BM + spleen) splenocytes. The transcripts for the indicated cytokines and chemokines were analyzed using TaqMan RT-qPCR. Fold change expression (2–ΔΔCt) was calculated with respect to GAPDH as a house-keeping gene and BM-only recipients. Data are representative of 2 independent experiments and shown as mean ± SEM. For comparisons, 1-way ANOVA with Tukey post hoc test was used in panels A-C. ANOVA, analysis of variance; SEM, standard error mean. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
SA-FasL–engineered splenocyte group also showed reduced expression of T-bet transcription factor, a master regulator of Th1 differentiation,33 in all GVHD target organs, reaching significance (P < .05) on day 21 after transplantation (supplemental Figure 6). The level of transcripts for day 7 were not significant between the 2 groups, except small intestine where SA-FasL–engineered splenocyte group showed a significant (P < .05) increase. RORγt, transcriptional regulator of Th17, showed a mixed pattern of expression with reduced level of transcripts in the small intestine (P < .01, day 7) and large intestine (P < .01, day 21) of SA-FasL–engineered splenocyte group with increased levels (P < .01) on day 21 in the liver and small intestine as compared with those of the nonengineered splenocyte group (supplemental Figure 6). The SA-FasL–engineered splenocyte group also showed decreased levels of transcripts for FoxP3 in all 3 tissues on day 7 and only the large intestine on day 21 after transplantation, which is consistent with the paucity of CD4+ T cells detected using immunohistochemistry (Figure 4C-D; supplemental Figure 5C). Taken together, SA-FasL blunts the inflammatory responses implicated in the pathogenesis of aGVHD.
Donor T regulatory cells are indispensable to SA-FasL–mediated protection against aGVHD
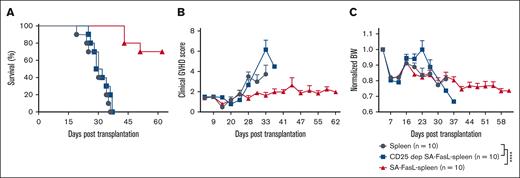
Given the demonstrated role of donor CD4+FoxP3+ Treg cells in the prevention of aGVHD34,35 and increased ratio of Treg-to-Teffs in lymph nodes and liver of SA-FasL group on day 7 after transplantation, we investigated the role of donor Treg cells in protection against aGVHD in our model. C57BL/6 splenocytes were engineered with SA-FasL and subjected to negative selection of CD4+CD25+FoxP3+. Donor splenocytes depleted for CD25+ cells had marked reduction of FoxP3+ Treg cells as compared with unmanipulated splenocytes (0.33 vs 7.12%; supplemental Figure 7). Transplantation of SA-FasL–engineered whole splenocytes into a lethally irradiated haploidentical GVHD model resulted in the long-term survival of ∼70% mice (>60-day observation period; Figure 6A), characterized by a marked reduction in clinical scores (Figure 6B) and preserved body weight (Figure 6C). In contrast, all recipients of Treg depleted SA-FasL–engineered splenocytes developed severe GVHD, presenting mortality and disease indices similar to recipients of nonengineered allogeneic splenocytes. These studies implicate donor Treg cells as an important regulatory mechanism that confers SA-FasL–mediated prevention of aGVHD in our model.
CD25+ donor cells are indispensable for the efficacy of SA-FasL in preventing aGVHD. Lethally irradiated F1 mice received bone marrow cells admixed with nonengineered or SA-FasL–engineered splenocytes or SA-FasL–engineered CD25-deplete splenocytes. Animals were followed for development of lethal acute GVHD and survival. For survival curve comparison, log-rank (Mantel-Cox) test was used. ∗P < .05; ∗∗P < .01
CD25+ donor cells are indispensable for the efficacy of SA-FasL in preventing aGVHD. Lethally irradiated F1 mice received bone marrow cells admixed with nonengineered or SA-FasL–engineered splenocytes or SA-FasL–engineered CD25-deplete splenocytes. Animals were followed for development of lethal acute GVHD and survival. For survival curve comparison, log-rank (Mantel-Cox) test was used. ∗P < .05; ∗∗P < .01
Transient display of SA-FasL on human PBMCs prevents aGVHD in a xenogeneic setting
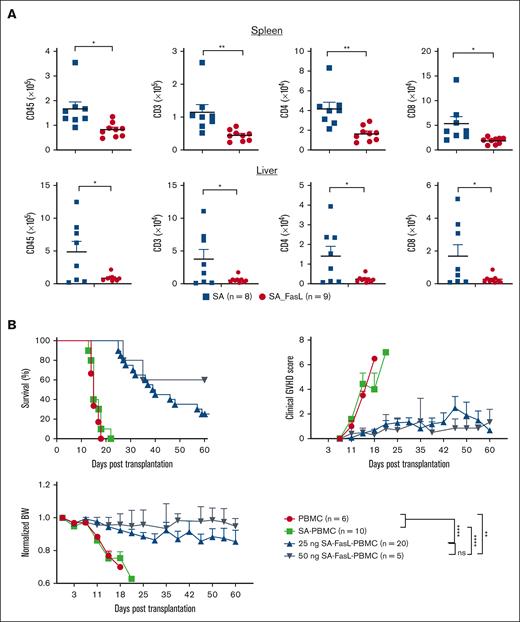
We next assessed the efficacy of SA-FasL in eliminating human T cells infused into immunocompromised NOD-scid-IL2γRnull (NSG) mice as an important step to clinical translation. When analyzed 5 days after infusion, the spleen of SA-engineered PBMC recipients had significantly higher numbers (Figure 7A) and frequencies (supplemental Figure 8) of human total CD45+ cells as well as CD4+ and CD8+ T cells as compared with the recipients of SA-FasL–engineered cells. A similar pattern was also observed in the liver (Figure 7A; supplemental Figure 8), a major target for human T-cell–mediated xenogeneic aGVHD. Infusion of 107 SA-engineered human PBMC into sublethally irradiated NSG mice resulted in aGVHD with severe clinical scores and a median survival time of 15 days (Figure 7B). In marked contrast, recipients of SA-FasL–engineered PBMCs showed improved survival along with a significant decline in clinical GVHD scores and preserved body weight. Notably, the improved survival was dose-dependent, as a higher concentration of SA-FasL protein (50 ng/106 cells) was more effective in preventing aGVHD than a lower concentration (25 ng/106 cells), 60% vs 25%, over the 60-day experimental end point. Thus, it remains to be investigated if higher concentrations of SA-FasL result in further improved efficacy in abrogating aGVHD in this preclinical model of the human disease.
Transient display of SA-FasL protein on human PBMCs is effective in preventing xenogeneic aGVHD. (A) Absolute number of human cells recovered from NSG recipients. NSG mice were subjected to 200 cGy total body irradiation followed by IV infusion of neutrophil-depleted human PBMCs (5 × 106) engineered with SA-FasL (25 ng/106 cells) or equimolar of SA protein as control. Splenocytes and liver infiltrates harvested 5 days after infusion were analyzed using flow cytometry gating on total human cells (CD45+), CD4+, or CD8+ T cells. Data were pooled from 3 independent experiments. (B) Prevention of xenogeneic aGVHD using SA-FasL–engineered PBMC. Irradiated NSG mice underwent transplantation with 10 × 106 human PBMCs left unmodified or engineered with SA or the indicated doses of SA-FasL. Animals were monitored for the signs and development of xenogeneic aGVHD. For comparison of means, unpaired two-tailed t test was used in panel A. Survival curve comparison was done by using log-rank (Mantel Cox). Data are represented as mean ± SEM. SEM, standard error mean. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Transient display of SA-FasL protein on human PBMCs is effective in preventing xenogeneic aGVHD. (A) Absolute number of human cells recovered from NSG recipients. NSG mice were subjected to 200 cGy total body irradiation followed by IV infusion of neutrophil-depleted human PBMCs (5 × 106) engineered with SA-FasL (25 ng/106 cells) or equimolar of SA protein as control. Splenocytes and liver infiltrates harvested 5 days after infusion were analyzed using flow cytometry gating on total human cells (CD45+), CD4+, or CD8+ T cells. Data were pooled from 3 independent experiments. (B) Prevention of xenogeneic aGVHD using SA-FasL–engineered PBMC. Irradiated NSG mice underwent transplantation with 10 × 106 human PBMCs left unmodified or engineered with SA or the indicated doses of SA-FasL. Animals were monitored for the signs and development of xenogeneic aGVHD. For comparison of means, unpaired two-tailed t test was used in panel A. Survival curve comparison was done by using log-rank (Mantel Cox). Data are represented as mean ± SEM. SEM, standard error mean. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Discussion
Ex vivo pan T-cell depletion from the donor inoculum to mitigate aGVHD is associated with compromised engraftment and delayed posttransplant immune reconstitution.6 Alloreactive T cells upregulate the Fas receptor after activation and become susceptible to Fas-mediated apoptosis.12,36,37 Thus, the Fas pathway provides a unique opportunity to preferentially eliminate alloreactive Teffs as a prophylactic approach for aGVHD. Here, we tested the efficacy of SA-FasL transiently displayed on donor lymphocytes to induce apoptosis in alloreactive Teffs and prevent aGVHD. SA-FasL on T cells was effective in inhibiting their proliferation in response to alloantigens in vitro and in vivo primarily through autocrine apoptosis, where SA-FasL engages with Fas on the same cell. Mice receiving SA-FasL–engineered allogeneic splenocytes showed significantly lower frequency and absolute number of donor cells as compared with the control group adoptively transferred with SA-engineered cells. The reduction in donor cells correlated with increased apoptosis and occurred at all SA-FasL protein doses used for engineering. These observations are in line with studies reporting elimination of alloreactive T cells in ex vivo cultures treated with soluble FasL protein1,38,39 and single-cell studies demonstrating autocrine apoptosis as the major driver of FasL-mediated cell death.40,41 However, we cannot rule out paracrine apoptosis that may occur in vivo because of the close physical proximity of alloreactive Teffs in the target tissues.
In a haploidentical mouse model, transient display of SA-FasL on donor lymphocytes effectively prevented GVHD in over 70% of graft recipients. This immunomodulatory scheme was also effective in mitigating aGVHD in a humanized mouse model of xenogeneic aGVHD. The efficacy of SA-FasL was dose-dependent, as higher doses of the protein resulted in better protection. These observations are consistent with several studies using soluble FasL for ex vivo elimination of alloreactive T cells as an approach to mitigate aGVHD.1,38,39 Our strategy targeting the elimination of alloreactive Teffs in the graft recipient has 3 distinct advantages over studies using soluble FasL for ex vivo treatment of the donor graft. First, in vitro cultures represent a contrived system that lacks the intricate cellular and molecular networks occurring in a 3-dimensional space that dictate Teff activation requirements, effector function, expansion, and establishment of long-term memory. Considerable differences in T-cell activation between in vitro and in vivo systems have been reported.42,43 Particularly, there is activation-independent significant T-cell death in vitro,43 which may restrict the T-cell repertoire and immune reconstitution after transplantation into allogeneic host subjected to myeloablative conditioning. The direct display of SA-FasL on donor graft overcomes these limitations and has the potential to specifically eliminate Teffs responding to host alloantigens after transplantation, leaving alloantigen-unrelated repertoire unaltered. In support of this notion, long-term mice showed full donor chimerism, efficient immune engraftment and reconstitution, and accepted parental skin grafts while rejecting third-party grafts, demonstrating immune tolerance and competency. Thus, long-term animals are expected to generate an effective immune response to tumors in line with studies demonstrating ex vivo treatment of donor cells with soluble FasL as a prophylactic approach preserves the graft-vs-tumor effect.7,8 In marked contrast, the efficacy of treating donor HSC ex vivo with soluble FasL is limited to the elimination of donor T memory cells in the graft, irrespective of their antigen specification, without an impact on naïve alloreactive T cells that have been shown to be the major driver of aGVHD.8,44,45 Second, apoptosis of alloreactive T cells in vivo may initiate a feed-forward regulatory pathway involving immunoregulatory cytokines and Tregs20,46-48 that contributes to the prevention of aGVHD. Furthermore, apoptotic bodies can alter the maturation of antigen-presenting cells (APCs), which is critical to the priming and initiation of lethal aGVHD, and polarize them toward tolerance induction.49-51 In line with these reports, we observed a high ratio of Tregs to Teffs and significantly reduced levels of inflammatory cytokines, particularly granulocyte macrophage colony stimulating factor involved in the activation and maturation of APCs,32 in the SA-FasL group. Third, pluripotent hematopoietic stem cells are shown to be resistant to Fas-mediated apoptosis and respond to the FasL trophic effect for improved engraftment.52,53 Thus, the presence of SA-FasL on the donor graft may also facilitate hematopoietic stem cell engraftment, an additional advantage over the ex vivo treatment of the graft with soluble FasL.
Immunophenotyping of the mice that underwent transplantation revealed several interesting findings. First, the numbers of both CD4+ and CD8+ Teff subsets were significantly increased in the mesenteric lymph nodes and the liver of the GVHD control group as compared with the SA-FasL group 1 week after transplantation. After 3 weeks, the increase in the number of these cell types leveled off in the lymph nodes while becoming more amplified in the liver of the control group than in the SA-FasL group. Second, the reduction in the numbers of Teff cells in the SA-FasL group translated into significantly reduced levels of proinflammatory cytokines (IL-1β, IL-6, TNF-α, and IFN-γ) implicated in pathogenicity of aGVHD.2,29,54 These findings are consistent with the progression of aGVHD, which begins with the activation of donor T cells in response to alloantigens presented by host APCs in local lymph nodes and culminates in a cascade of molecular and cellular interactions followed by immune effector cell trafficking to various target tissues to inflict damage.27,55 Immunohistochemical analysis of the large intestine on day 21 showed intense infiltration with CD4+ T cells and significant tissue damage in the control group, whereas the SA-FasL group had minimal infiltration and tissue damage. Third, the increased Treg numbers in the lymph nodes of the SA-FasL group early after transplantation is consistent with the demonstrated sensitivity of activated alloreactive T cells, but not Treg cells, to FasL-mediated apoptosis, which is regulated by cytokines in an inflammatory milieu.56,57 Increased proliferation in response to stimulatory cytokines and/or conversion of naïve T cells may also contribute to the increased frequency of Treg cells in our model. This notion is consistent with our recent studies demonstrating that SA-FasL–mediated tolerance to allogeneic islets involves apoptosis of Teff cells that culminates in a cascade of regulatory mechanisms involving phagocyte uptake of apoptotic bodies, secretion of TGF-β, and increased frequency of Treg cells that are critical for induction and maintenance of long-term graft survival.20 Human Treg cells use FasL to induce apoptosis in both APCs and Teff cells as a mechanism of immune suppression.58-60 Importantly, Treg cells engineered to display SA-FasL on their surface are resistant to apoptosis and have enhanced immunoregulatory function.61,62 Exclusion of Treg from the parental donor splenocyte inoculum mitigated the protective efficacy of SA-FasL, consistent with published studies reporting a key role for donor Tregs in the prophylaxis of aGVHD.35,63 Our findings are also consistent with studies using cyclophosphamide to prevent aGVHD by targeting Teffs for physical and functional elimination. Donor Tregs were shown to be required for the efficacy of cyclophosphamide in preventing aGVHD in various mouse models.5,6 We previously shown that SA-FasL–engineered Tregs show significantly improved efficacy as compared with nonengineered cells in preventing aGVHD in an adoptive transfer preclinical model.64 Thus, the direct display of SA-FasL on T cells in donor inoculum provides 2 distinct advantages: preferential elimination of alloreactive Teffs and functional enhancement of Tregs.
We herein demonstrate that transient display of a novel form of FasL protein, SA-FasL, on the donor lymphocytes is effective in eliminating alloreactive Teffs posttransplantation, leading to the prevention of aGVHD in haploidentical and humanized mouse models (visual abstract). The process of donor graft engineering is straightforward and efficient, providing an attractive platform for clinical translation. SA-FasL does not persist in the system because of its short half-life ∼108 minutes on CD3+ T cells, thereby minimizing potential off-target effects. Our approach harbors significant potential as monotherapy or in combination with various clinical regimens to improve both the efficacy and safety of mismatched T-cell–replete hematopoietic cell transplants. This approach may also improve donor lymphocyte infusion to manage the relapse of hematological malignancies after HSCT.
Authorship
Contribution: H.S., E.S.Y., and P.S. conceived and designed studies; P.S., L.B., A.T., A.E.G., Z.S., A.E.G., and H.T. performed the study and collected and analyzed the data; and P.S., A.T., E.S.Y., H.S., and N.A. interpreted the analysis and wrote the manuscript.
Conflict-of-interest disclosure: H.S., E.S.Y., and P.S. have a provisional patent on using SA-FasL–engineered cells as a prophylactic approach for aGVHD. H.S. is CEO of Fascure Therapeutics, LLC, and the scientific cofounder, stockholder, and member of SAB for iTolerance, Inc. E.S.Y. is a consultant for iTolerance. The remaining authors declare no competing financial interests.
Correspondence: Esma S. Yolcu, University of Missouri, Columbia Child Health and Molecular Microbiology and Immunology NextGen Precision Health Bldg, Columbia, MO 65211; e-mail: esma.yolcu@health.missouri.edu; and Haval Shirwan, University of Missouri, Columbia Child Health and Molecular Microbiology and Immunology NextGen Precision Health Bldg, Columbia, MO 65211; e-mail: haval.shirwan@health.missouri.edu.
References
Author notes
SA-FasL and SA proteins are available through a material transfer agreement with the University of Missouri, Columbia, MO.
Data and reagents are available on request from the corresponding authors, Esma S. Yolcu (esma.yolcu@health.missouri.edu) and Haval Shirwan (haval.shirwan@health.missouri.edu).
The full-text version of this article contains a data supplement.
The current affiliation for P.S. is the Department of Pediatrics-Research, The University of Texas MD Anderson Cancer Center, Houston, TX.

![SA-FasL transiently displayed on the surface of T cells results in their elimination in response to alloantigens in vitro. (A) In vitro proliferation assay. SA-FasL– or SA-engineered 4C T cells (H-2Kb) were stimulated with irradiated BALB/c splenocytes (H-2Kd) for 48 or 72 hours. Cultures were pulsed with [3H]thymidine for the last 16 hours of incubation and harvested using a beta plate counter. DNA-incorporated radioactivity is plotted as an assessment of cell proliferation. Data were pooled from 2 independent experiments. (B) Frequencies of live total CD4+ and CD8+ T cells (top panels) and Vβ13+ transgenic CD4+ and CD8+ T cells (bottom panels) in mixed lymphocyte cultures. Experimental conditions are the same as in (A), except instead of pulsing with [3H]thymidine, cultures were harvested at 72 hours, stained with the Abs to indicated markers, and analyzed using flow cytometry. Data were pooled from 2 independent experiments. (C) SA-FasL induces autocrine death in alloreactive T cells. CTV-labeled nonengineered 4C cells were mixed 1:1 ratio with CFSE-labeled SA-FasL-4C cells and used as responders at the indicated ratios against a fixed number of irradiated BALB/c cells as stimulators. Cells were harvested after 72 hours of incubation and analyzed for live cells using flow cytometry (left panel). Representative flow dot plots of proliferating 4C cells (right panel). Data sets pooled from 2 independent experiments. One-way ANOVA with Tukey multiple comparison was used in panels A-B. Unpaired two-tailed t test was used in panel C. Data are shown as mean ± SEM. ANOVA, analysis of variance; SEM, standard error mean; cpm, counts per minute. ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/7/10/10.1182_bloodadvances.2022008495/2/m_blooda_adv-2022-008495-gr1.jpeg?Expires=1767849309&Signature=J9C~m~-TrA2KITkCobg6jxp7TI7riIhjZnTkqXa3ggtzShEbBV8EYtNLFteyWo1-lPYvQbJAWxvGw5GxIBmykhkPVRfg9qi-5GtE1RjpSlHnGWRC-a8sapouewzaYUJRFEJZojmpVL9HFZ8kCDZ7NCzcYiIkFh5L1InL6a8QRzf~oV4XwOyXKwLDOyh0az3Kx33zBvzASVxW1Ijen3Njai0ikLArRnnq8altVdR3RGdDV4bA-hpF-3idQhHzxI3uS28q1c3fyS4lX~5rmyCqYaO-oIZVs9bOqj~t5TZjleKUjmneb8A6ZtWuhlxu8JQIUkiIlgq4K8ZhzhVig92aPw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)