Abstract
Immune thrombotic thrombocytopenic purpura (iTTP) is an acquired, fatal microangiopathy if untreated. Randomized controlled trials (RCTs) demonstrated faster time to response with addition of caplacizumab to standard of care (SOC). However, concerns about RCT selection bias and the high cost of caplacizumab warrant examination of all evidence, including real-world observational studies. In this systematic review and meta-analysis, we searched for comparative studies evaluating SOC with or without caplacizumab for the treatment of iTTP. We assessed risk of bias using the Cochrane risk-of-bias-2 tool (RCTs) and the Newcastle-Ottawa Scale (observational studies). The primary efficacy and safety outcomes were all-cause mortality and treatment-emergent bleeding, respectively. Secondary outcomes included exacerbation and relapse, refractory iTTP, and time to response. We included 2 high-quality RCTs and 3 observational studies at high risk of bias comprising 632 total participants. Compared with SOC, caplacizumab was associated with a nonsignificant reduction in the relative risk [RR] of death in RCTs (RR, 0.21; 95% confidence interval [CI], 0.05-1.74) and observational studies (RR, 0.62; 95% CI, 0.07-4.41). Compared with SOC, caplacizumab was associated with an increased bleeding risk in RCTs (RR, 1.37; 95% CI, 1.06-1.77). In observational studies, bleeding risk was not significantly increased (RR, 7.10; 95% CI, 0.90-56.14). Addition of caplacizumab was associated with a significant reduction in refractory iTTP and exacerbation risks and shortened response time but increased relapse risk. Frontline addition of caplacizumab does not significantly reduce all-cause mortality compared with SOC alone, although it reduces refractory disease risk, shortens time to response, and improves exacerbation rates at the expense of increased relapse and bleeding risk.
Introduction
Immune thrombotic thrombocytopenic purpura (iTTP) is caused by an acquired, autoantibody-mediated deficiency of the von Willebrand factor (vWF)–cleaving protease ADAMTS13.1 It is characterized by platelet-rich microthrombi causing microvascular obstruction, thrombocytopenia, mechanical hemolysis, and end-organ ischemia.2 iTTP is a medical emergency that is almost universally fatal if untreated.3 Standard of care (SOC) treatment has evolved to include therapeutic plasma exchange (TPE), corticosteroids, and rituximab.4 These treatments improved overall survival rates to >95%.3 However, refractory disease and relapse risk remain concerns.2,4
Caplacizumab is a humanized monoclonal antibody that binds to vWF, blocking its interaction with platelet glycoprotein Ib-IX-V and reducing microthrombi formation.5 From 2018 to 2019, it was approved by the European Union and the US Food and Drug Administration for the initial treatment of iTTP in combination with SOC.5,6 This approval followed the completion of 2 randomized-controlled trials (RCTs) comparing SOC with or without caplacizumab: TITAN (2016) and HERCULES (2019).7,8 These studies both demonstrated faster resolution of an acute iTTP episode and fewer exacerbations with caplacizumab compared with SOC alone at the expense of increased bleeding. Based on this evidence, the International Society on Thrombosis and Haemostasis (ISTH) guidelines on iTTP treatment include a conditional recommendation for the use of caplacizumab in the frontline treatment of iTTP.4
However, the incorporation of caplacizumab into clinical practice remains controversial and variable, and many hematologists reserve its use for severe or refractory disease.9 One concern is its high cost (a treatment course, according to current drug labeling, costs 270 000 USD at minimum) and cost-ineffectiveness according to 1 analysis.10 Enthusiasm for caplacizumab is also tempered by potential RCT selection bias from including patients with less severe disease, raising concern that the results may not apply to real-world practice.4 The RCTs also did not show a mortality benefit with caplacizumab, although this was not their primary end point. Several observational studies have reported outcomes following the use of caplacizumab in the real-world setting.11-16 To date, the totality of evidence exploring all comparative studies evaluating SOC with vs without caplacizumab remains unknown. We performed a systematic review and meta-analysis of studies evaluating the effectiveness of SOC with vs without caplacizumab for the treatment of iTTP.
Methods
We registered the protocol for this systematic review with PROSPERO (#CRD42021274276).17
Search strategy
Using terms designed by a medical librarian (supplemental Data 1), we electronically searched PubMed, Embase, the Cochrane Library, and Scopus databases from inception through 19 July 2021. We scanned references of published reviews to identify studies not retrieved by our search. We hand-searched conference proceedings from 2018 (caplacizumab approval year in Europe) through 2021 from annual meetings of the American Society of Hematology, ISTH, and European Hematology Association.
Study selection
We removed duplicates and imported all citations into Covidence.18 Two authors independently reviewed each citation and abstract according to a priori selection criteria and performed full-text reviews to confirm eligibility. We included >1 report on the same study if it reported additional outcomes or extended follow-up not published in the original trial. We did not restrict to English language.
We included all studies that enrolled nonpregnant adults (aged ≥18 years) with an acute episode of iTTP. We included RCTs and nonrandomized observational studies that compared SOC with or without caplacizumab. We included studies that enrolled children and adults if >90% of the studied population were adults. We excluded case reports, case series, and single-arm studies evaluating caplacizumab without a comparator group or studies that enrolled patients with known, congenital TTP.
Data extraction and risk-of-bias assessment
For each included study, 2 authors independently extracted data using a standardized form. We extracted data on study design, setting, inclusion criteria, enrollment period, baseline characteristics of study participants and treatments provided, and outcomes of interest. We discussed discrepancies in data extraction, and a third author arbitrated when needed. Our primary efficacy outcome was all-cause mortality, and our primary safety outcome was bleeding. We initially intended to evaluate iTTP-specific mortality, but cause of death was inconsistently reported, particularly in retrospective studies. Instead, we focused on all-cause mortality. Secondary outcomes included time to platelet count recovery, iTTP exacerbation or relapse (clinical recurrence within or after 30 days of TPE cessation, respectively), refractory iTTP, duration of TPE, hospital length of stay (LOS), treatment-emergent thrombosis, and treatment-emergent bleeding (any bleeding, major bleeding, and intracranial bleeding). We extracted event rates for binary outcomes and means or medians for continuous outcomes. We used the event rates from the longest duration of follow-up if multiple rates were reported. If studies reported different refractory iTTP rates, we chose the rate that reflected the International TTP Working Group’s definition of refractory iTTP.2 Because time to platelet count recovery was not reported for the entire TITAN cohort, we used aggregate data reported for the subgroup of patients with a baseline ADAMTS13 activity <10% (n = 58).
To assess risk of bias, we used the Cochrane risk-of-bias tool (RoB-2) for RCTs19 and the Newcastle-Ottawa Scale (NOS) for nonrandomized, observational studies.20,21 Two authors independently performed study-level and outcome-based quality appraisals for each study. We answered all RoB-2 signaling questions but reported an aggregate summary of the domains of interest; the overall risk of bias was generated by the RoB-2 algorithm. For NOS, we report study-level selection and comparability domains and cohort follow-up adequacy from the outcome domain; we also report the average of outcome-level appraisal of outcome assessment, reporting, and follow-up adequacy. We also report the combined qualitative assessments performed by the 2 reviewers for each observational study. We judged the overall risk of bias using the NOS star system as follows: very high risk of bias (0-3 NOS points), high risk of bias (4-6 NOS points), and low risk of bias (7-9 NOS points).22
Data synthesis and primary analysis
We performed meta-analysis using intention-to-treat analysis for efficacy outcomes. We used the safety population (all patients who received at least 1 dose of the study drug) for the safety (bleeding) outcomes.
For binary outcomes, we used a binary generalized linear mixed effects model23,24 to estimate the population-averaged relative risk (RR) and used an exact inference procedure to estimate risk difference.25 These approaches avoid arbitrary continuity corrections to sparse binary outcomes.26 We report all binary outcomes as the absolute risk difference percentage and RR with 95% confidence intervals (CIs) generated using exact method procedures25 and the R package, altmeta.25,27
We estimated random effects models to generate the mean difference of continuous outcomes. If studies reported ranges, we calculated means and standard deviations based on the available aggregate statistics.28,29
We pooled (meta-analyzed) RCTs separately from observational studies and did not perform crossdesign synthesis owing to disparate risk of bias among the 2 study designs.30,31 Following the guideline on crossdesign evidence synthesis,30 we qualitatively describe differences in the effect estimates in RCTs vs observational studies. We performed a sensitivity analysis, excluding studies that were abstract-only publications. We quantified between-study heterogeneity and the corresponding significance level using the Cochran Q statistic and estimated between-study variance using τ2. We calculated Higgins and Thompson I2, interpreted as the percentage of variability in the treatment estimates attributable to heterogeneity between studies rather than to sampling error.32 We performed all analyses using R version 4.33 We followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) checklist and guidelines for all aspects of this review.34
Results
Literature search
Of the 852 citations identified, we performed full-text review on 72 and identified 5 eligible studies that collectively enrolled 632 patients (Figure 1); 2 were RCTs and 3 were nonrandomized observational studies. All observational studies were retrospective; the French11 and United Kingdom12 studies compared the caplacizumab cohort to historical controls from the precaplacizumab era. We report the characteristics of each included study in Table 1 and supplemental Data 2. The demographics of the included patients were representative of the average iTTP population,35 except that the HERCULES and TITAN trials excluded patients at high-bleeding risk. Most patients presented with an initial (rather than recurrent) episode of iTTP and most patients had an ADAMTS13 activity <10% (Table 1). All patients received TPE, but corticosteroid and rituximab use varied across trials and study groups (supplemental Table 2C). The timing of caplacizumab initiation also varied across trials, ranging from before TPE started to 3 days after TPE (supplemental Table 2C); however, all patients continued caplacizumab for an additional 30 days after completion of TPE. Median follow-up was also variable (Table 1). There was no treatment contamination except in HERCULES; patients with recurrent disease (after 28 days since last treatment) switched to receiving open-label caplacizumab, but initial trial assignments remained concealed. Studies also used different definitions of major bleeding. The United Kingdom and French studies used the ISTH definition,36 whereas the HERCULES, TITAN, and Barcelona studies did not report their definitions (supplemental Table 2D).
PRISMA flow diagram of study selection for systematic review. ASH, American Society of Hematology; EH, European Hematology Association.
PRISMA flow diagram of study selection for systematic review. ASH, American Society of Hematology; EH, European Hematology Association.
Characteristics of the included studies and patients enrolled
| Study name (y) . | Setting . | Study design . | Enrollment period . | Drug assign-ment . | No. of partici-pants . | Age (y), mean (range) . | No. (%) female . | No. (%) non-White . | Cardiac troponin I (μg/L), median (range) . | No. (%) with neuro involvement on presentation‡ . | Median follow-up (mo) . | No. (%) presenting with recurrent TTP . | No. (%) with confirmed ADAMTS13 <10% . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HERCULES (2019)9 | International (Australia, Europe, Canada, US): 92 sites worldwide | RCT | November 2015 - April 2017 | Caplacizumab + SOC | 72 | 45 (18-77) | 49 (68) | 22 (31) | 0.09 (0.01-75.96) | 6 (8) | 28 d after discontinuation of treatment | 24 (33) | 58 (81) |
| SOC | 73 | 47 (21-79) | 51 (70) | 14 (19) | 0.07 (0.01-7.28) | 5 (7) | 39 (53) | 65 (89) | |||||
| TITAN (2016)8 | International (Europe, US, Australia): 56 sites | RCT | October 2010 - January 2014 | Caplacizumab + SOC | 36 | 41 (19-72) | 24 (67) | 4 (11) | Not reported | Not reported | 1 (mortality); 12 (relapse) | 12 (33) | 28 (78) |
| SOC | 39 | 42 (21-67) | 20 (51) | 5 (13) | Not reported | Not reported | 12 (31) | 30 (77) | |||||
| France (2021)12 | France (multiple centers) | Observational study with historical control | September 2018 - December 2019 (intervention group); June 2015 - September 2018 (historical control) | Caplacizumab + SOC | 90 | 45 (34-57)† | 63 (70) | 16 (18) | Not reported; 51 (56%) with cardiac involvement | 55 (61) | 127 | 12 (13) | 90 (100) |
| SOC | 180 | 43 (30-57)† | 127 (70) | 31 (17) | Not reported; 86 (47%) with cardiac involvement | 111 (62) | 21 (12) | 180 (100) | |||||
| United Kingdom (2021)13 | United Kingdom (England, Scotland, and Wales): 22 sites | Observational study with historical control | May 2018 - January 2020 (intervention arm); 2014 - 2018 (historical control) | Caplacizumab + SOC | 85 | 45 (15-93) | 56 (66) | 28 (33) | Not reported | 56 (66) | 80 | Not reported | 84 (99) |
| SOC | 39 | 46 (3-82) | 31 (80) | Not reported | Not reported | 29 (74) | Not reported | Not reported | Not reported | ||||
| Barcelona∗ (2020)14 | Single center | Observational study with concurrent control | May 2014 - May 2020 | Caplacizumab + SOC | 9 | 43 (39-55)† | 8 (89) | Not reported | Not reported | 3 (33) | 6.8 | Not reported | Not reported |
| SOC | 9 | 41 (33-52)† | 6 (67) | Not reported | Not reported | 6 (66) | 51.8 | Not reported | Not reported |
| Study name (y) . | Setting . | Study design . | Enrollment period . | Drug assign-ment . | No. of partici-pants . | Age (y), mean (range) . | No. (%) female . | No. (%) non-White . | Cardiac troponin I (μg/L), median (range) . | No. (%) with neuro involvement on presentation‡ . | Median follow-up (mo) . | No. (%) presenting with recurrent TTP . | No. (%) with confirmed ADAMTS13 <10% . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HERCULES (2019)9 | International (Australia, Europe, Canada, US): 92 sites worldwide | RCT | November 2015 - April 2017 | Caplacizumab + SOC | 72 | 45 (18-77) | 49 (68) | 22 (31) | 0.09 (0.01-75.96) | 6 (8) | 28 d after discontinuation of treatment | 24 (33) | 58 (81) |
| SOC | 73 | 47 (21-79) | 51 (70) | 14 (19) | 0.07 (0.01-7.28) | 5 (7) | 39 (53) | 65 (89) | |||||
| TITAN (2016)8 | International (Europe, US, Australia): 56 sites | RCT | October 2010 - January 2014 | Caplacizumab + SOC | 36 | 41 (19-72) | 24 (67) | 4 (11) | Not reported | Not reported | 1 (mortality); 12 (relapse) | 12 (33) | 28 (78) |
| SOC | 39 | 42 (21-67) | 20 (51) | 5 (13) | Not reported | Not reported | 12 (31) | 30 (77) | |||||
| France (2021)12 | France (multiple centers) | Observational study with historical control | September 2018 - December 2019 (intervention group); June 2015 - September 2018 (historical control) | Caplacizumab + SOC | 90 | 45 (34-57)† | 63 (70) | 16 (18) | Not reported; 51 (56%) with cardiac involvement | 55 (61) | 127 | 12 (13) | 90 (100) |
| SOC | 180 | 43 (30-57)† | 127 (70) | 31 (17) | Not reported; 86 (47%) with cardiac involvement | 111 (62) | 21 (12) | 180 (100) | |||||
| United Kingdom (2021)13 | United Kingdom (England, Scotland, and Wales): 22 sites | Observational study with historical control | May 2018 - January 2020 (intervention arm); 2014 - 2018 (historical control) | Caplacizumab + SOC | 85 | 45 (15-93) | 56 (66) | 28 (33) | Not reported | 56 (66) | 80 | Not reported | 84 (99) |
| SOC | 39 | 46 (3-82) | 31 (80) | Not reported | Not reported | 29 (74) | Not reported | Not reported | Not reported | ||||
| Barcelona∗ (2020)14 | Single center | Observational study with concurrent control | May 2014 - May 2020 | Caplacizumab + SOC | 9 | 43 (39-55)† | 8 (89) | Not reported | Not reported | 3 (33) | 6.8 | Not reported | Not reported |
| SOC | 9 | 41 (33-52)† | 6 (67) | Not reported | Not reported | 6 (66) | 51.8 | Not reported | Not reported |
We report the inclusion and exclusion criteria, additional differences in baseline characteristics, and differences in treatments received across studies in the supplemental Data 2.
We performed data extraction and quality appraisal based on abstract proceedings only. This study did not have corresponding full-text publications in peer-reviewed journals.
Median (interquartile range).
Defined in HERCULES as Glasgow Coma Scale ≤1; defined in the French study as headache, confusion, seizure, coma, or focal deficit; defined in the United Kingdom study as any neurologic symptom (including headaches, forgetfulness, limb weakness, paresthesia, agitation, encephalopathy, depressed mood, and anxiety); and defined in the Barcelona study as focal deficits, convulsion, headache, or dizziness.
Risk-of-bias assessment
We judged the HERCULES and TITAN trials to be at an overall low risk of bias. For both studies, there were some concerns regarding blinding procedures and outcome reporting, but missing outcome data were well described (supplemental Table 3A). The concern for RCT selection bias raised by the ISTH4 was not reinforced by our risk-of-bias assessment because the RoB-2 tool’s signaling questions focus on methods of randomization and treatment allocation rather than study population representativeness.
We judged the observational studies to be at a high risk of bias because of lack of matching between experimental and control groups, not addressing confounders, and not addressing loss to follow-up or missing outcome data (supplemental Table 3B). In addition, the Barcelona study was a non–peer-reviewed, abstract-only publication that reported minimal methodological details.13
Outcomes
All-cause mortality
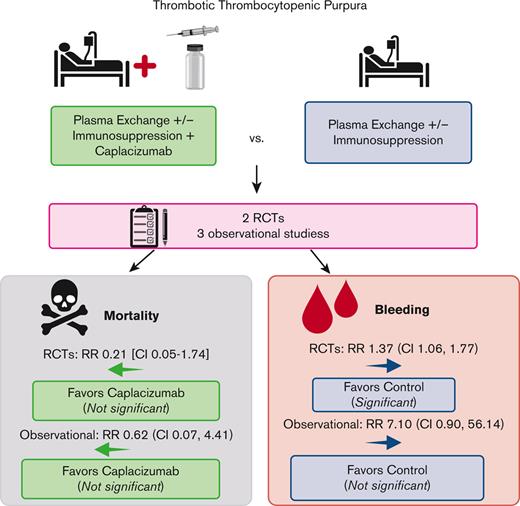
Among the 220 patients randomized in the HERCULES and TITAN trials, 1 of 108 died in the caplacizumab arms, whereas 5 of 112 died in the SOC arms. Compared with SOC, the addition of caplacizumab was associated with a nonsignificant reduction in the RR (0.21; 95% CI, 0.05-1.74) and absolute risk (−0.04; 95% CI, −0.08 to 0.01) of death from any cause (Figure 2A). Therefore, there were 40 fewer deaths (ranging from 80 fewer to 10 more deaths) per 1000 patients treated with caplacizumab (compared with those treated with SOC alone). Among the 412 patients enrolled in the observational studies, there were 6 deaths (of 184 participants) in the caplacizumab arms (5 deaths were from the United Kingdom study12) and 13 deaths (of 228 participants) in the control arm (12 deaths were from the French study11), yielding a nonsignificant reduction in the RR (0.62; 95% CI, 0.07-4.41) and absolute risk (−0.02; 95% CI, −0.11 to 0.08) of death with caplacizumab compared with SOC (Figure 2A). Therefore, there were 20 fewer (ranging from <110 to >80) deaths per 1000 patients treated with caplacizumab compared with SOC alone.
Summary of primary efficacy and safety outcomes across eligible trials. We present the outcomes reported in each individual trial as well as meta-analysis according to study design. Control refers to SOC alone without caplacizumab (refer to supplemental Table 2C “Details of treatments received”). Event rates refer to the number of patients with the event of interest. Below the pooled estimate of the risk difference, we present the same finding as the difference in absolute risk of the outcome among patients treated with caplacizumab plus SOC (compared with those treated with SOC alone) per 1000 patients treated with caplacizumab. Df, degrees of freedom; SD, standard deviation.
Summary of primary efficacy and safety outcomes across eligible trials. We present the outcomes reported in each individual trial as well as meta-analysis according to study design. Control refers to SOC alone without caplacizumab (refer to supplemental Table 2C “Details of treatments received”). Event rates refer to the number of patients with the event of interest. Below the pooled estimate of the risk difference, we present the same finding as the difference in absolute risk of the outcome among patients treated with caplacizumab plus SOC (compared with those treated with SOC alone) per 1000 patients treated with caplacizumab. Df, degrees of freedom; SD, standard deviation.
Bleeding
In the safety populations of the RCTs, 65 of 106 patients in the caplacizumab arms vs 49 of 110 patients in the SOC arms experienced any treatment-emergent bleeding. Compared with SOC, the addition of caplacizumab was associated with a statistically significant increase in the RR (1.37; 95% CI, 1.06-1.77) and absolute risk (0.17; 95% CI, 0.04-0.30) of any bleeding (Figure 2B). Across the Barcelona and United Kingdom studies, 15 of 94 patients in the caplacizumab arms vs 0 of 48 patients in the SOC arms experienced any bleeding; the French study did not report this outcome. This was associated with an increased RR (7.10; 95% CI, 0.90-56.14) and absolute risk (0.16; 95% CI, 0.08-0.24) of any bleeding (Figure 2B).
Caplacizumab receipt was associated with a nonsignificant increase in the RR (1.73; 95% CI, 0.39-7.07) and absolute risk (0.02; 95% CI, −0.02 to 0.07) of major bleeding compared with SOC in RCTs (Table 2; supplemental Figure 4E). Among observational studies, only the Barcelona study reported major bleeding (0 events in both arms). In the RCTs’ safety population, caplacizumab receipt was associated with an equivocal RR (1.04; 95% CI, 0.15-7.25) and absolute risk (0.00; 95% CI, −0.03 to 0.03) of intracranial bleeding (Table 2; supplemental Figure 4F). No observational studies reported intracranial bleeding.
Secondary outcomes
| Binary outcomes . | Randomized controlled trials . | Observational studies . | ||||
|---|---|---|---|---|---|---|
| Risk difference (95% CI) . | Risk difference interpretation per 1000∗ . | RR (95% CI) . | Risk difference (95% CI) . | Risk difference interpretation per 1000∗ . | RR (95% CI) . | |
| TTP exacerbation | −0.29 (−0.42 to −0.14)† | 290 fewer (from 420 fewer to 140 fewer)† | 0.16 (0.07-0.47)† | −0.35 (−0.43 to −0.27)† | 350 fewer (from 430 fewer to 270 fewer)† | 0.10 (0.04-0.42)† |
| TTP relapse | 0.14 (−0.00 to 0.27) | 140 more (from equal to 270 more) | 3.81 (1.58-14.28)† | 0.00 (−0.29 to 0.29)∗∗ | equal (from 290 fewer to 290 more) | 1.00 (0.07-13.64)∗∗ |
| Refractory TTP | −0.08 (−0.13 to −0.02)† | 80 fewer (from 130 fewer to 20 fewer)† | 0.11 (0.01-0.81)† | −0.22 (−0.45 to −0.03)† | 220 fewer (from 450 fewer to 30 fewer)† | 0.12 (0.02-0.61)† |
| Thrombosis | 0.00 (−0.07 to 0.07) | equal (from 70 fewer to 70 more) | 1.02 (0.40-2.63) | 0.00 (−0.05 to 0.06) | equal (from 50 fewer to 60 more) | 1.07 (0.57-2.03) |
| Major bleeding | 0.02 (−0.02 to 0.07) | 20 more (from 20 fewer to 70 more) | 1.73 (0.39-7.07) | 0.00 (−0.19 to 0.19)∗∗ | equal (from 190 fewer to 190 more)∗∗ | 1.00 (0.02-45.13) ∗∗ |
| Intracranial bleeding | 0.00 (−0.03 to 0.03) | equal (from 30 fewer to 30 more) | 1.04 (0.15-7.25) | Not reported | ||
| Binary outcomes . | Randomized controlled trials . | Observational studies . | ||||
|---|---|---|---|---|---|---|
| Risk difference (95% CI) . | Risk difference interpretation per 1000∗ . | RR (95% CI) . | Risk difference (95% CI) . | Risk difference interpretation per 1000∗ . | RR (95% CI) . | |
| TTP exacerbation | −0.29 (−0.42 to −0.14)† | 290 fewer (from 420 fewer to 140 fewer)† | 0.16 (0.07-0.47)† | −0.35 (−0.43 to −0.27)† | 350 fewer (from 430 fewer to 270 fewer)† | 0.10 (0.04-0.42)† |
| TTP relapse | 0.14 (−0.00 to 0.27) | 140 more (from equal to 270 more) | 3.81 (1.58-14.28)† | 0.00 (−0.29 to 0.29)∗∗ | equal (from 290 fewer to 290 more) | 1.00 (0.07-13.64)∗∗ |
| Refractory TTP | −0.08 (−0.13 to −0.02)† | 80 fewer (from 130 fewer to 20 fewer)† | 0.11 (0.01-0.81)† | −0.22 (−0.45 to −0.03)† | 220 fewer (from 450 fewer to 30 fewer)† | 0.12 (0.02-0.61)† |
| Thrombosis | 0.00 (−0.07 to 0.07) | equal (from 70 fewer to 70 more) | 1.02 (0.40-2.63) | 0.00 (−0.05 to 0.06) | equal (from 50 fewer to 60 more) | 1.07 (0.57-2.03) |
| Major bleeding | 0.02 (−0.02 to 0.07) | 20 more (from 20 fewer to 70 more) | 1.73 (0.39-7.07) | 0.00 (−0.19 to 0.19)∗∗ | equal (from 190 fewer to 190 more)∗∗ | 1.00 (0.02-45.13) ∗∗ |
| Intracranial bleeding | 0.00 (−0.03 to 0.03) | equal (from 30 fewer to 30 more) | 1.04 (0.15-7.25) | Not reported | ||
| Continuous outcomes . | Mean difference (95% CI) . | Mean difference interpretation . | Mean difference (95% CI) . | Mean difference interpretation . |
|---|---|---|---|---|
| Time to platelet count recovery (d) | −0.66 (−0.90 to −0.43)† | 0.66 d fewer (from 0.90 fewer to 0.43 fewer)† | −4.84 (−7.91 to −1.76)† | 4.84 d fewer (from 7.91 fewer to 1.76 fewer)† |
| Hospital LOS (d) | −6.00 (−8.92 to −3.08)† | 6 d fewer (from 8.92 fewer to 3.08 fewer)† | −9.06 (−18.96 to 0.84) | 9.06 d fewer (from 18.96 fewer to 0.84 fewer) |
| Duration of TPE (d) | −3.60 (−3.82 to −3.38)† | 3.60 d fewer (from 3.82 fewer to 3.38 fewer)† | −6.63 (−11.83 to −1.43)† | 6.63 d fewer (from 11.83 fewer to 1.43 fewer)† |
| Continuous outcomes . | Mean difference (95% CI) . | Mean difference interpretation . | Mean difference (95% CI) . | Mean difference interpretation . |
|---|---|---|---|---|
| Time to platelet count recovery (d) | −0.66 (−0.90 to −0.43)† | 0.66 d fewer (from 0.90 fewer to 0.43 fewer)† | −4.84 (−7.91 to −1.76)† | 4.84 d fewer (from 7.91 fewer to 1.76 fewer)† |
| Hospital LOS (d) | −6.00 (−8.92 to −3.08)† | 6 d fewer (from 8.92 fewer to 3.08 fewer)† | −9.06 (−18.96 to 0.84) | 9.06 d fewer (from 18.96 fewer to 0.84 fewer) |
| Duration of TPE (d) | −3.60 (−3.82 to −3.38)† | 3.60 d fewer (from 3.82 fewer to 3.38 fewer)† | −6.63 (−11.83 to −1.43)† | 6.63 d fewer (from 11.83 fewer to 1.43 fewer)† |
All-cause mortality and any bleeding are reported separately in Figure 2. We present the event rates and corresponding forest plots for each outcome in supplemental Data 4.
Presented as the difference in absolute risk of the outcome among patients treated with caplacizumab plus SOC (compared with those treated with SOC alone) per 1000 patients treated with caplacizumab.
Statistically significant (P < .05).
Based on only 1 study (see supplemental Data for details).
iTTP exacerbation (clinical recurrence within 30 days of stopping TPE)
In the RCTs, receipt of caplacizumab was associated with a significantly reduced RR (0.16; 95% CI, 0.07-0.47) and absolute risk (−0.29; 95% CI, −0.42 to −0.14) of iTTP exacerbation compared with SOC (Table 2; supplemental Figure 4A). In the meta-analysis of the French and Barcelona studies, caplacizumab was associated with a statistically significant reduction in the RR (0.10; 95% CI, 0.04-0.42) and absolute risk (−0.35; 95% CI, −0.43 to −0.27) of exacerbations compared with SOC (Table 2; supplemental Figure 4A).
iTTP relapse (clinical recurrence >30 days after stopping TPE)
In the RCTs compared with SOC, caplacizumab receipt was associated with a significant increase in the RR (3.81; 95% CI, 1.58-14.28) and absolute risk (0.14; 95% CI, −0.00 to 0.27) of relapse (Table 2; supplemental Figure 4B). Among observational studies, only the Barcelona study reported relapse (1 participant in each arm) (Table 2; supplemental Figure 4B).
Refractory iTTP
Compared with SOC, receipt of caplacizumab was associated with a significant reduction in the RR (0.11; 95% CI, 0.01-0.81) and absolute risk (−0.08; 95% CI, −0.13 to −0.02) in refractory iTTP (Table 2; supplemental Figure 4C). In the meta-analysis of the French and Barcelona trials, there was a significant reduction in the RR (0.12; 95% CI, 0.02-0.61) and absolute risk (−0.22; 95% CI, −0.45 to −0.03) of refractory iTTP compared with SOC (Table 2; supplemental Figure 4C).
Thrombosis
In the RCTs, receipt of caplacizumab was associated with an equivocal RR (1.02; 95% CI, 0.40-2.63) and absolute risk (0; 95% CI, −0.07 to 0.07) of thrombosis compared with SOC (Table 2; supplemental Figure 4D). Across the French and United Kingdom studies, there was an RR (1.07; 95% CI, 0.57-2.03) and absolute risk (0.00; 95% CI, −0.05 to 0.06) of thrombosis with caplacizumab compared with SOC (Table 2; supplemental Figure 4D).
Time to platelet count recovery/platelet response
This outcome was defined and reported differently across studies. The HERCULES study defined this as time from starting caplacizumab or placebo to a platelet count of ≥150 000/μL with discontinuation of TPE within 5 days.8 The TITAN and Barcelona trials defined platelet response as the second consecutive day with a platelet count ≥150 000/μL.7 Across RCTs, the pooled mean difference in time to response was significantly shorter by 0.7 days (95% CI, 0.4-0.9) in the caplacizumab arms (Table 2; supplemental Figure 4G). Response was defined as a platelet count >150 000/μL in the French and United Kingdom studies, the latter starting from the initiation of TPE. In the meta-analysis of observational studies, time to response was significantly shorter in the caplacizumab arms by an average of 4.8 days (95% CI, 1.8-7.9) compared with SOC (Table 2; supplemental Figure 4G).
Duration of TPE (days)
Across RCTs, the pooled difference in mean TPE duration was significantly shorter in the caplacizumab arms with an average of 3.6 fewer days (95% CI, 3.4-3.8) of TPE compared with SOC (Table 2; supplemental Figure 4H). Across the observational studies, there was also a statistically significant reduction in TPE duration with an average of 6.6 fewer days (95% CI, 1.4-11.8) of TPE in the caplacizumab arms than in the SOC arms (Table 2; supplemental Figure 4H).
Hospital LOS
The median LOS in the HERCULES trial was 9.0 days (range, 2.0-37.0) vs 12.0 days (range, 4.0-53.0) in the caplacizumab vs SOC arms, respectively (Table 2; supplemental Figure 2I). The TITAN trial did not report this outcome. Across the observational studies, LOS was also shorter in the caplacizumab groups, but the difference was not significant (mean difference, −9.06 days [95% CI, −18.96 to 0.84], favoring caplacizumab) (Table 2; supplemental Figure 2I).
Heterogeneity
For all-cause mortality, we calculated I2 as 0% across the RCTs, indicating that there is no evidence of heterogeneity, and 46% across the observational studies, suggesting moderate between-study heterogeneity (Figure 2). For any treatment-emergent bleeding, we calculated I2 as 0% across both the RCTs and observational studies (Figure 2), indicating no evidence of between-study heterogeneity. We report the Cochran Q test statistic with P value and between-study variance for all secondary outcomes in supplemental Data 4.
Sensitivity analysis
Pooled analyses of peer-reviewed publications (exclusion of the abstract-only Barcelona study) yielded similar outcomes across observational studies as in the primary analyses. The meta-analyses of all-cause mortality, treatment-emergent bleeding, and refractory TTP each demonstrated similar point estimates with wider CIs after excluding the Barcelona trial; the point estimate and CI for TTP exacerbation were essentially superimposable between the primary and sensitivity analyses. We report the sensitivity analyses of all outcomes in supplemental Data 5.
Discussion
In this systematic review and meta-analysis of studies evaluating SOC with or without caplacizumab for the treatment of iTTP, we identified 2 RCTs and 3 observational studies for inclusion. There does not appear to be evidence of an all-cause mortality benefit with caplacizumab, as our estimates are notable for very wide CI. Caplacizumab appears to reduce exacerbation and refractory iTTP risk and shorten time to response, duration of TPE, and LOS, but at the expense of an increased risk of bleeding and relapse.
We found that caplacizumab shortened the time to platelet normalization (and hence, duration of TPE and hospital LOS). This is explained by the drug inhibiting platelet binding to VWF within hours of administration, accelerating the resolution of microangiopathy.37 These findings were consistent across our meta-analysis of RCTs and with a 2021 integrated analysis of patient-level data from both RCTs.38 We also found that compared with SOC, receipt of caplacizumab is associated with a reduced risk of iTTP exacerbation and refractory iTTP. However, we redemonstrated that receipt of caplacizumab was associated with an increased risk of relapsed iTTP.38 Increased relapse risk may be explained by caplacizumab not correcting the underlying ADAMTS13 deficiency, potentially leading to disease recurrence after drug discontinuation in patients with persistent, severe ADAMTS13 deficiency.4 As caplacizumab was continued for 30 days after the last TPE across all studies, disease recurrence was classified as a relapse rather than an exacerbation. Future studies on caplacizumab should report short- and long-term relapse rates.2,39 These future studies should also consider the variation in duration of caplacizumab administration in real-world practice and its effect on relapse rates; some hematologists stop or modify caplacizumab dosing at time of ADAMTS13 recovery if this occurs before 30 days.15
Despite some compelling, short-term benefits of caplacizumab, it does not appear to affect mortality. We found that the pooled estimate of all-cause mortality across observational studies was similar to the RCTs—a point estimate favoring reduced overall mortality in the caplacizumab arms but a nonsignificant finding with wide CI. The all-cause mortality risk difference across RCTs can be interpreted as follows: for every 1000 patients treated with caplacizumab, an average of 40 deaths are prevented compared with SOC alone, with a CI of this estimate ranging from 80 fewer to 10 more deaths with caplacizumab. This questionable mortality benefit may be explained by low event rates. Our analysis differs from prior reports that concluded a mortality benefit with caplacizumab use; a 2017 report following the publication of TITAN and a 2021 integrated analysis of both RCTs.38,40 There are several explanations for the discrepant conclusions. First, we focused on mortality during the overall study period, whereas the integrated analysis evaluated mortality during the blinded treatment period (excluding deaths that occurred after discontinuation of caplacizumab). Second, the integrated analysis combined data from TITAN and HERCULES without preserving the randomization of the original trials, whereas we performed meta-analysis, which is considered superior to simple pooling.41 Third, although each individual trial evaluated mortality as a secondary outcome, both reports38,40 published the mortality benefit by way of a post hoc composite outcome comprising iTTP-specific mortality, iTTP exacerbations, and major thromboembolic events. Although the composite outcome was used to overcome the low statistical power for these secondary outcomes of a rare disease, the conclusion implies that the results apply to each individual component of the composite rather than the overall composite and can be misleading.39,42,43 As such, the use of composite outcomes in clinical trials is controversial.39,42,43 Finally, we qualitatively compared meta-analysis of RCTs to real-world, observational studies. Therefore, we conclude that there is insufficient evidence that caplacizumab reduces overall mortality. Notably, the inclusion of patients without severe ADAMTS13 deficiency, whose diagnosis of iTTP could be questioned, may have biased these findings toward the null. Studies did not report outcomes stratified by baseline ADAMTS13 activity so we were unable to assess for differential treatment effect in this subgroup. The pooled estimate across the observational studies may have been blunted by the United Kingdom study, the only study with a higher death rate in the caplacizumab arm, which the authors attribute to higher severity of illness compared with the historical controls. Finally, there were differences in the SOC treatments in the experimental and control groups across the observational studies (supplemental Data 2), such as differential prescribing of corticosteroids and rituximab, potentially confounding this analysis.
We also found that caplacizumab increases the risk of any bleeding, but not in major or intracranial bleeding, compared with SOC. The integrated analysis similarly concluded that mild, mucocutaneous bleeding was the main safety finding. Although this increase in any bleeding, but not of major or intracranial bleeding, may be considered a reasonable tradeoff for reducing the risk of iTTP exacerbation or refractory disease (potentially more severe clinical entities than mild bleeding), these findings should be interpreted cautiously, as the studies were not powered to detect differences in rare safety events. According to our analysis, there was no effect on thrombosis risk, which differs from both post hoc reports38,40 that concluded, based on a composite outcome, that caplacizumab reduces major thromboembolic events.
Our analysis has several strengths. First, we focused on outcomes reported at the longest follow-up. Second, we qualitatively compared RCT outcomes to real-world, observational studies. The direction (and often magnitude) of treatment effect were remarkably similar across the 2 study designs, further supporting the quality of the evidence. Third, our findings are consistent with a prior analysis concluding lack of cost-effectiveness of caplacizumab because of its high cost and increased risk of iTTP relapse.10
This analysis questions the ISTH conditional recommendation to use front line caplacizumab in all patients presenting with acute iTTP given that it does not reduce the risk of death or relapse.4 Our analysis did show a decreased risk of refractory disease, exacerbations, and shorter time to platelet recovery with caplacizumab, suggesting it may have a role in patients with refractory disease or in patients who need rapid resolution of thrombotic microangiopathy, such as those with severe disease on presentation (eg, neurologic involvement or elevated troponin). Future research should focus on developing a risk prediction tool that identifies patients who are at risk of severe or refractory disease who may benefit from caplacizumab, a currently unmet need. The studies included in this analysis did not evaluate caplacizumab without TPE, a strategy that has only been reported in case reports thus far.44,45 If caplacizumab is demonstrated to be safe and efficacious without TPE, this could change the cost-benefit analysis and is an important question for future studies. The role of caplacizumab in the treatment of iTTP requires further study with longer follow-up and a focus on relapse and mortality in both high- and low-risk populations.
Limitations
Our study has several limitations. First, our analysis likely reflects caplacizumab use exclusively in the frontline setting (supplemental Data 2). However, studies evaluating the effectiveness of caplacizumab as salvage therapy in refractory disease may not be possible owing to illness severity and high mortality. Second, we were unable to conduct a subgroup analysis among patients with diagnosis-defining ADATMS13 activity <10%; future studies that enroll patients without severe ADAMTS13 deficiency should report outcomes stratified by activity <10% and ≥10%. Third, we were also limited by incomplete outcome reporting in some studies, different definitions of the same outcome across studies, and that 1 observational study was reported as a non–peer-reviewed abstract (supplemental Data 3). Fourth, the included studies assessed exacerbation and relapse according to definitions of these outcomes from the precaplacizumab era: clinical recurrence within or after 30 days of TPE cessation, respectively. Given the temporizing effects of caplacizumab, the definition of exacerbation has since been revised by the International TTP Working Group as clinical recurrence within 30 days of stopping TPE or caplacizumab.2 Future studies should report outcomes according to this revised definition. Finally, there was variable and often only short-term follow-up across the studies (Table 1).
Conclusions
Based on best available evidence, frontline caplacizumab does not significantly reduce all-cause mortality compared with SOC alone. However, it appears to reduce risk of refractory disease, shorten time to response, and improve exacerbation rates at the expense of increased relapse rate and bleeding risk. Therefore, this analysis questions the ISTH conditional recommendation to use frontline caplacizumab in all patients presenting with acute iTTP. Future studies should report outcomes at extended follow-up and avoid using composite outcomes.
Acknowledgments
The authors thank Maylene Qiu for her assistance with creating the searching terms.
This work was supported by grants from the National Heart, Lung, and Blood Institute, National Institutes of Health (T32HL007971) (M.D.) and in part by grants R01LM013519, R01AG073435, R56AG074604, and R56AG069880 (J.T. and Y.C.).
The authors used Biorender to create the visual abstract.
Authorship
Contribution: M.D., A.C., and A.M.P. developed the study protocol; J.T., A.X., and J.Y. worked with a medical librarian to create the search terms and perform the systematic search; M.D., A.X., and J.Y. screened the references and performed full-text review; M.D. and A.M.P. independently performed data extraction and risk-of-bias assessment; A.C. arbitrated discrepancies when needed; J.Y. and Y.C. performed the statistical analysis; J.T. and M.D. prepared the figures and tables; M.D. prepared the initial draft of the manuscript; and all authors provided critical feedback and facilitated iterative revisions of manuscript and approved the final version.
Conflict-of-interest disclosure: A.C. has served as a consultant for Synergy and has received authorship royalties from UpToDate. A.M.P. has received grant funding on behalf of her institution from Sanofi Genzyme. The remaining authors declare no competing financial interests.
Correspondence: Mia Djulbegovic, 3400 Civic Center Blvd, Perelman South Pavilion 12th Floor, Philadelphia, PA 19104; e-mail: mia.djulbegovic@pennmedicine.upenn.edu.
References
Author notes
This work is a secondary analysis of previously published data17.
The data that support the findings of this study are available on request from the corresponding author, Mia Djulbegovic (mia.djulbegovic@pennmedicine.upenn.edu).
This research was conducted through secondary analysis of previously published, aggregated data and therefore did not meet criteria for human subject research.
The full-text version of this article contains a data supplement.