Key Points
Bending stress exerted on the plasma membrane by sickle hemoglobin polymerization enhances sulfatide-mediated adhesion of mature erythrocytes in SCD.
Inflammatory sphingomyelinase activity could result in abnormal adhesion and membrane damage in sickle mature erythrocytes.
Abstract
Abnormal erythrocyte adhesion owing to polymerization of sickle hemoglobin is central to the pathophysiology of sickle cell disease (SCD). Mature erythrocytes constitute >80% of all erythrocytes in SCD; however, the relative contributions of erythrocytes to acute and chronic vasculopathy in SCD are not well understood. Here, we showed that bending stress exerted on the erythrocyte plasma membrane by polymerization of sickle hemoglobin under hypoxia, enhances sulfatide-mediated abnormal mature erythrocyte adhesion. We hypothesized that sphingomyelinase (SMase) activity, which is upregulated by accumulated bending energy, leads to elevated membrane sulfatide availability, and thus, hypoxic mature erythrocyte adhesion. We found that mature erythrocyte adhesion to laminin in controlled microfluidic experiments is significantly greater under hypoxia than under normoxia (1856 ± 481 vs 78 ± 23, mean ± SEM), whereas sickle reticulocyte (early erythrocyte) adhesion, high to begin with, does not change (1281 ± 299 vs 1258 ± 328, mean ± SEM). We showed that greater mean accumulated bending energy of adhered mature erythrocytes was associated with higher acid SMase activity and increased mature erythrocyte adhesion (P = .022, for acid SMase activity and P = .002 for the increase in mature erythrocyte adhesion with hypoxia, N = 5). In addition, hypoxia results in sulfatide exposure of the erythrocyte membrane, and an increase in SMase, whereas anti-sulfatide inhibits enhanced adhesion of erythrocytes. These results suggest that the lipid components of the plasma membrane contribute to SCD complications. Therefore, sulfatide and the components of its upregulation pathway, particularly SMase, should be further explored as potential therapeutic targets for inhibiting sickle erythrocyte adhesion.
Introduction
Sickle cell disease (SCD) is a genetic disorder associated with early mortality and is a significant global burden.1,2 Normal adult hemoglobin (HbA) binds to oxygen and does not polymerize, whereas sickle hemoglobin (HbS) forms extensive polymers when deoxygenated.3,4 During circulation, Hb progressively becomes deoxygenated as a result of oxygen delivery into the surrounding tissues, and deoxygenation is greatest in the prealveolar pulmonary vasculature. Polymerized HbS alters the normal morphology and physiology of the erythrocytes.5 HbS-containing erythrocytes undergo deleterious membrane changes and become abnormally adhesive, dense, and less deformable.5-9
HbS polymerization affects both reticulocytes (nucleic acid-bearing immature red blood cells) and mature erythrocytes. Most well studied sickle red blood cell adhesion mechanisms involve adhesion through proteins including integrins and BCAM/LU,10-14 which are more relevant to reticulocyte phenotype rather than mature erythrocyte phenotype.15 In contrast, most of the contributions made by membrane lipids to the sickle red blood cell adhesion remain unknown. Previously Zhou et al have demonstrated strong evidence that sulfated glycolipids (sulfatides) on sickle erythrocyte membrane mediate sickle red blood cell adhesion to histamine activated endothelial in vitro.16 Membrane lipids are ubiquitous to mammalian cell membranes17 and are not specific to either sickle reticulocytes or sickle mature erythrocytes.18,19 In fact, sphingomyelin (SM) constitutes 10% of the mammalian plasma membrane and sphingomyelinase (SMase) activity is shown to be proinflammatory in SCD.20 However, whether SMase contributes to sickle red blood cell adhesion remains unknown.
SMase activity has been linked to accumulated membrane bending energy in red blood cells, and sulfatides are synthesized from the hydrolysis of SM with SMase.21 We hypothesized that, with SMase upregulation in SCD, particularly mature erythrocytes would become abnormally adhesive through sulfatides owing to high levels of accumulated bending energy. Here, we tested this hypothesis by first probing the adhesion of sickle reticulocytes and mature erythrocytes to laminin (LN, isoform 1). LN serves as a surrogate for activated endothelium because it has a highly specific affinity for sulfatides,8,22,23 which are known to promote sickle red blood cell adhesion to the endothelium in SCD.16,18,24 In addition, we describe retrospective clinical investigations on LN adhesion in 41 patients with SCD. We then probed the morphologies of the adhered sickle reticulocytes and mature erythrocytes at the single cell level to estimate the accumulated bending energies. Finally, we describe SMase associated sulfatide-mediated adhesion of mature erythrocytes that can be inhibited. These results establish a new clinical and mechanistic perspective on the changing biophysical properties of the plasma membrane during HbS polymerization in SCD.
Methods
Reagents
Laminin (isoform 1, 90% purity, #L2020), anti-sulfatide (clone A2B5, #mab312), sodium metabisulfite (NBS, Na2S2O5,161519) were obtained from Sigma-Aldrich (St. Louis, MO). IgM negative control (#MCA692) was obtained from BioRad (Hercules, CA). Poly-d-lysine (#5049) was obtained from Advanced Biomatrix (San Diego, CA). Acridine orange (#A3568) and FACS buffer (#FC001) were obtained from Fisher Scientific (Hampton, NH). Unless otherwise indicated, reagents were reconstituted and diluted in phosphate-buffered saline (PBS).
Enzyme activity quantification
Enzyme activation was quantified using a human acid SMase activity kit (ab252889) obtained from Abcam (Cambridge, United Kingdom). Acid SMase activity in 10 μL of plasma was measured according to the manufacturer’s protocol.
Blood sample collection and clinical study
All 60 subjects had phenotypic homozygous sickle hemoglobin (HbSS or HbSβ0), were at clinical baseline without recent (within the last 2 weeks) history of vaso-occlusive crisis (VOC) during sample attainment, under an institutional review board–approved protocol, and most had undergone saline contrast echocardiography within 5½ years. This study was conducted following the Declaration of Helsinki. Surplus EDTA-anticoagulated whole blood was obtained and was tested for adhesion and Hb composition within 24 hours. Adhesion experiments were performed using whole blood without dilution or processing. Hb composition (fetal, HbF; and sickle, HbS, and adult, HbA [from transfusion]) was analyzed via high performance liquid chromatography with a Bio-Rad Variant II Instrument (Bio-Rad, Montreal, QC, Canada) in the core clinical laboratory of the University Hospitals Cleveland Medical Center (UHCMC).
Clinical data, including white blood cell count (WBC), platelet count, absolute neutrophil count (ANC), absolute reticulocyte count (ARC), red blood cell count, total Hb, lactate dehydrogenase (LDH), mean corpuscular volume (MCV), creatinine (CRT), and serum ferritin, were obtained from the Adult SCD Clinic at UHCMC, on dates contemporaneous with blood sample collection. Saline-contrasted echocardiograms, obtained as part of the clinical evaluation of symptoms, were reviewed for intracardiac or intrapulmonary right to left shunts by echocardiography-trained cardiologists. Echocardiograms were obtained at a mean of 23 ± 6 months (mean ± SEM with a range of 95 days after to 64 months before); 1 patient was excluded (the study was >10 years previous). Right to left shunting was analyzed per convention, that is, bubbles appearing in the left ventricle ≥4 heartbeats following the appearance in the right ventricle were scored as representing intrapulmonary shunts (IPS) rather than intracardiac shunts.25 IPS occurs when blood flow is diverted around alveolar capillaries, impairing oxygen saturation of Hb.26 A “recent transfusion” threshold was defined as HbA% greater than 10%. In vivo Hb desaturation data were extracted from the medical records.
Microfluidic adhesion assays
The SCD Biochip is composed of functionalized microfluidic channels, as previously described.27 For studying the adhesion of HbA-containing erythrocytes, microchannels were functionalized with 0.1 g/mL poly-d-lysine for nonspecific adhesion. To study the adhesion of samples from subjects with HbSS SCD, microchannels were coated with LN to induce erythrocyte-specific adhesions. LN or poly-d-lysine functionalized microfluidic channels were injected with 15 μL of unprocessed whole blood at a shear stress of 0.1 Pa, corresponding to the average postcapillary shear stress. After nonadherent cells were removed by an equivalently SpO2-adjusted buffer wash at 0.1 Pa, adherent erythrocytes were analyzed by staining with acridine orange (thiazole orange), a fluorescent cationic dye selective for nucleic acids,28 at 0.1 mg/mL concentration within microchannels. Reticulocyte staining with acridine orange is a well-established technique with high detection accuracy.28 The experiments were performed under both normoxic and hypoxic conditions, with the latter achieving an SpO2 of ∼83% in the blood, using a micro-tube gas exchanger technology developed by us.29 Inhibition experiments with anti-sulfatide antibody were performed with sample aliquots from 10 unique patients with HbSS SCD. The treatments and microfluidic flow experiments were simultaneously performed at the same time. Adherent erythrocytes in the microchannels were visualized for quantification with the Olympus CellSens software using an Olympus IX83 inverted microscope and QImaging EXi Blue CCD camera at 20×. The scanned images covered an actual area of ∼32 mm2 on the microchannels.
Categorization of adherent red blood cells as reticulocytes and mature erythrocytes
Red blood cells were categorized as either reticulocytes, which contain nucleic acids, or mature erythrocytes, which do not (Figure 1) according to the Heilmeyer classification.30,31 More than 47 000 individual cells from 15 homozygous patients with SCD were analyzed systematically and categorized using a semiautomated workflow using JavaScript and a MATLAB algorithm (supplemental Notes 1).
Adhered erythrocytes are categorized as reticulocytes or mature erythrocytes based on the fluorescence intensity. Heilmeyer classification groups reticulocytes into 4 groups, group 1 with dense, clumped reticulum, group 2 extended network of loose reticulum, group 3 scattered granules with residual reticulum, and group 4 scattered granules. Mature erythrocytes without nucleic acids are not visible in the fluorescent channel. The same classification and notation are used for distinguishing adhered reticulocytes and mature erythrocytes.
Adhered erythrocytes are categorized as reticulocytes or mature erythrocytes based on the fluorescence intensity. Heilmeyer classification groups reticulocytes into 4 groups, group 1 with dense, clumped reticulum, group 2 extended network of loose reticulum, group 3 scattered granules with residual reticulum, and group 4 scattered granules. Mature erythrocytes without nucleic acids are not visible in the fluorescent channel. The same classification and notation are used for distinguishing adhered reticulocytes and mature erythrocytes.
Morphology analysis of red blood cells
The morphologies of individual cells were analyzed. More than 23 000 red blood cells from 15 homozygous subjects with SCD and more than 2000 red blood cells from 1 subject with non-sickle hemoglobin (HbAA) were included in the analysis. The images of adherent red blood cells in the microchannels were taken at a shear rate of 100 s–1. Briefly, the elongation and membrane curvature of single cells were calculated using computer vision methods. Membrane curvature was used to estimate the accumulated bending energy of each cell. Details of the elongation calculation and accumulated bending energy estimation can be found in supplemental Notes 1 and 2.
Statistical methods
Statistical analyses were performed using MATLAB. Data were tested for normality. Non-normally distributed data were analyzed using a non-parametric Mann-Whitney U test for 2 independent groups. One-way analysis of variance (ANOVA) test was performed to analyze the relationship between the IPS and other clinical variables. The chi-square method was used to test the associations between the history of IPS and sex, treatment type, and history of in vivo hypoxia. The Dixon Q ratio test was used to identify outliers. Box-Cox transformation was performed in R for normality approximation for outlier analysis of non-normal data. P < .05 was chosen to indicate a significant difference.
Results
Population
All samples were obtained from patients with HbSS. The HbA percentage in the 26 recently transfused patients ranged from 13.3% to 79.4% (mean ± SD, 41.4% ± 16.4%). A total of 62% (26/42) of all subjects had right to left shunting on echocardiography and 86% (22/26) had IPS. Among patients in the no-IPS group, 28% (5/18) were more precisely not evaluated for the right to left shunting during echocardiography. Men were more likely to have an associated diagnosis of IPS when compared with those without, or not evaluated (Table 1, 65% of the patients [15 male; 8 female] with IPS and 28% [5 male; 13 female] of those without or not evaluated; chi-square P = .017).
Clinical phenotype of the study population with and without a history of intrapulmonary shunting
| . | Subjects with h/o IPS (mean ± SD) . | Subjects without known h/o IPS (mean ± SD) . | P value . |
|---|---|---|---|
| Number of subjects, N | 23 | 18 | |
| Age (y) | 34.5 ± 10.1 | 31.3 ± 9.4 | .216∗ |
| Sex | M = 15 / F = 8 | M = 5 / F = 13 | .017† |
| Total (mature erythrocyte + reticulocyte) Adhesion in normoxia | 965 ± 742 | 426 ± 796 | .005∗ |
| Total (mature erythrocyte + reticulocyte) Adhesion in hypoxia | 3321 ± 3785 | 1183 ± 1250 | .010∗ |
| Hgb (g/dL) | 8.3 ± 1.8 | 8.4 ± 1.1 | .864∗ |
| WBC (109/L) | 11.2 ± 2.8 | 10.1 ± 3.8 | .275‡ |
| ANC (106/L) | 6719 ± 2567 | 5937 ± 2815 | .359‡ |
| Platelet count (109/L) | 356.9 ± 123.3 | 316.0 ± 108.9 | .275‡ |
| MCV | 92.1 ± 9.9 | 93.6 ± 11.8 | .864∗ |
| Reticulocyte count (109/L) | 441.3 ± 190.1 | 294.2 ± 202.7 | .022‡ |
| LDH (U/L) | 495.4 ± 301.0 | 299.0 ± 147.1 | .004∗ |
| Ferritin (μg/L) | 2298 ± 2975 | 2272 ± 2608 | .747∗ |
| HbS (%) | 62.2 ± 22.2 | 55.2 ± 21.6 | .265∗ |
| HbA (%) | 25.8 ± 21.5 | 30.6 ± 24.5 | .532∗ |
| HbF (%) | 4.4 ± 4.6 | 6.7 ± 6.5 | .211∗ |
| Recently transfused (HbA > 10%) | 14/23 | 12/18 | .702† |
| h/o “in vivo” Hb desaturation (nocturnal or exertional) | 17/23 | 7/18 | .014† |
| . | Subjects with h/o IPS (mean ± SD) . | Subjects without known h/o IPS (mean ± SD) . | P value . |
|---|---|---|---|
| Number of subjects, N | 23 | 18 | |
| Age (y) | 34.5 ± 10.1 | 31.3 ± 9.4 | .216∗ |
| Sex | M = 15 / F = 8 | M = 5 / F = 13 | .017† |
| Total (mature erythrocyte + reticulocyte) Adhesion in normoxia | 965 ± 742 | 426 ± 796 | .005∗ |
| Total (mature erythrocyte + reticulocyte) Adhesion in hypoxia | 3321 ± 3785 | 1183 ± 1250 | .010∗ |
| Hgb (g/dL) | 8.3 ± 1.8 | 8.4 ± 1.1 | .864∗ |
| WBC (109/L) | 11.2 ± 2.8 | 10.1 ± 3.8 | .275‡ |
| ANC (106/L) | 6719 ± 2567 | 5937 ± 2815 | .359‡ |
| Platelet count (109/L) | 356.9 ± 123.3 | 316.0 ± 108.9 | .275‡ |
| MCV | 92.1 ± 9.9 | 93.6 ± 11.8 | .864∗ |
| Reticulocyte count (109/L) | 441.3 ± 190.1 | 294.2 ± 202.7 | .022‡ |
| LDH (U/L) | 495.4 ± 301.0 | 299.0 ± 147.1 | .004∗ |
| Ferritin (μg/L) | 2298 ± 2975 | 2272 ± 2608 | .747∗ |
| HbS (%) | 62.2 ± 22.2 | 55.2 ± 21.6 | .265∗ |
| HbA (%) | 25.8 ± 21.5 | 30.6 ± 24.5 | .532∗ |
| HbF (%) | 4.4 ± 4.6 | 6.7 ± 6.5 | .211∗ |
| Recently transfused (HbA > 10%) | 14/23 | 12/18 | .702† |
| h/o “in vivo” Hb desaturation (nocturnal or exertional) | 17/23 | 7/18 | .014† |
F, female; Hgb, hemoglobin; HbF, fetal hemoglobin; h/o, history of; M, male.
Calculated based on non-parametric Mann-Whitney test.
Calculated based on chi-square.
Calculated based on one-way ANOVA.
Hypoxia enhances adhesion by mature erythrocytes, but not by reticulocytes
We identified reticulocytes and mature erythrocytes using a fluorescent acridine orange dye. Reticulocytes were visible in the fluorescent channel image, whereas mature erythrocytes remained invisible (Figure 1A). We compared the reticulocyte percentages from the complete blood count panel to those from whole blood smears processed with acridine orange and found an R-squared value of 0.98 (supplemental Figure 1, N = 8, P = .005, linear regression). Notably, the actual reticulocyte numbers may be less because of false positives caused by mitochondrial DNA retention of mature erythrocytes in SCD.32
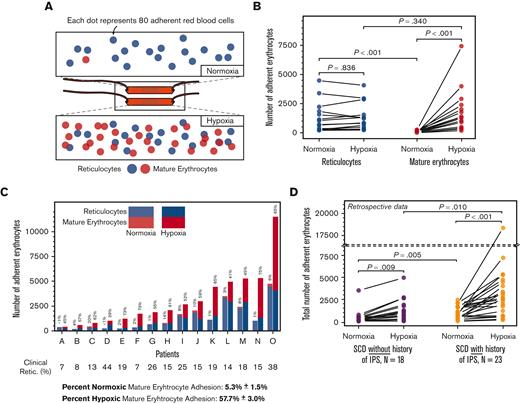
Schematic representations of SCD Biochip and differential populations of reticulocytes and mature erythrocytes under normoxic and hypoxic conditions are shown in Figure 2A. Reticulocyte adhesion to LN under normoxia (Figure 2B; mean ± SEM, 1258 ± 328; N = 15) was significantly greater than that of mature erythrocyte adhesion under normoxia (Figure 2B; mean ± SEM, 78 ± 23; N = 15; Mann Whitney P < .001). However, reticulocyte adhesion to LN under hypoxia or normoxia did not differ (Figure 2B; mean ± SEM, 1281 ± 299; N = 15, under hypoxia vs mean ± SEM, 1258 ± 328; N = 15, under normoxia; Mann Whitney P = .836). The reticulocyte adhesion under hypoxia was directly associated with ARC (P = .039, PCC = 0.54, linear regression and Pearson correlation, data not shown). Mature erythrocyte adhesion to LN was significantly greater under hypoxia than under normoxia (Figure 2B, mean ± SEM, 1856 ± 481; vs mean ± SEM, 78 ± 23; Mann Whitney P < .001). In addition, the percentage of adherent mature erythrocytes in each sample greatly increased under hypoxia (Figure 2C; mean ± SEM, 5.3% ± 1.5% vs mean ± SEM, 57.7 ± 3.0; N = 15, 1-way ANOVA; P < .001). In summary, the adhesion of mature erythrocytes, but not reticulocytes, was significantly and rapidly enhanced by hypoxia in whole blood samples from patients with HbSS, in line with the hypothesis of this study.
Reticulocyte and mature erythrocyte adhesion to laminin (LN) in normoxic and hypoxic microscale flow. (A) Staining of sickle erythrocytes for nucleic acids with acridine orange reveals the reticulocytes. Varying levels of reticulocyte (blue) and mature erythrocyte (red) adhesion under different physiologically relevant oxygenation conditions are shown. The partial oxygen saturation (SpO2) is greater than 95% under (i) normoxia and approximately 83% under (ii) hypoxia. (B) Comparison of adherent sickle erythrocytes under normoxia and hypoxia. Under normoxic conditions, adherent sickle erythrocytes are predominantly reticulocytes. Reticulocyte adhesion to LN under hypoxia was not significantly different from reticulocyte adhesion under normoxia. However, the contribution to overall adhesion by mature erythrocytes increased significantly under hypoxia. (C) Stacked and grouped column bars represent the adherent sickle erythrocyte types individually for patients. Shown above each column is the mature erythrocyte adhesion percentage. Minimal normoxic mature erythrocyte adhesion notably increased because of hypoxia and became the predominant contributor to total erythrocyte adhesion under hypoxia in 12/15 samples. Shown below patient identifiers are the reticulocyte percentages obtained from a clinical laboratory, and clinical reticulocyte percentages are not statistically associated (P=.162, linear regression) (D) Hypoxia enhanced total erythrocyte adhesion is associated with intrapulmonary shunting in subjects with HbSS SCD. Erythrocytes from both subject groups display a significant increase in adhesion under hypoxia compared with normoxia, and normoxic and hypoxic adhesion of erythrocyts are significantly different between the IPS and IPS unknown groups. Nno-IPS=18, NIPS=23. P values were calculated using the Mann-Whitney test and 1-way ANOVA for non-normal and normal data, respectively. Data are presented as mean ± SEM.
Reticulocyte and mature erythrocyte adhesion to laminin (LN) in normoxic and hypoxic microscale flow. (A) Staining of sickle erythrocytes for nucleic acids with acridine orange reveals the reticulocytes. Varying levels of reticulocyte (blue) and mature erythrocyte (red) adhesion under different physiologically relevant oxygenation conditions are shown. The partial oxygen saturation (SpO2) is greater than 95% under (i) normoxia and approximately 83% under (ii) hypoxia. (B) Comparison of adherent sickle erythrocytes under normoxia and hypoxia. Under normoxic conditions, adherent sickle erythrocytes are predominantly reticulocytes. Reticulocyte adhesion to LN under hypoxia was not significantly different from reticulocyte adhesion under normoxia. However, the contribution to overall adhesion by mature erythrocytes increased significantly under hypoxia. (C) Stacked and grouped column bars represent the adherent sickle erythrocyte types individually for patients. Shown above each column is the mature erythrocyte adhesion percentage. Minimal normoxic mature erythrocyte adhesion notably increased because of hypoxia and became the predominant contributor to total erythrocyte adhesion under hypoxia in 12/15 samples. Shown below patient identifiers are the reticulocyte percentages obtained from a clinical laboratory, and clinical reticulocyte percentages are not statistically associated (P=.162, linear regression) (D) Hypoxia enhanced total erythrocyte adhesion is associated with intrapulmonary shunting in subjects with HbSS SCD. Erythrocytes from both subject groups display a significant increase in adhesion under hypoxia compared with normoxia, and normoxic and hypoxic adhesion of erythrocyts are significantly different between the IPS and IPS unknown groups. Nno-IPS=18, NIPS=23. P values were calculated using the Mann-Whitney test and 1-way ANOVA for non-normal and normal data, respectively. Data are presented as mean ± SEM.
Mature erythrocyte adhesion associates with a history of intrapulmonary shunt
We retrospectively investigated the possible effects of enhanced mature erythrocyte adhesion under hypoxia, and found that hypoxia-enhanced mature erythrocyte adhesion may be relevant to SCD comorbidities. IPS and right to left shunts are increasingly being recognized in patients with HbSS.25,33 The combined total red blood cell adhesion of mature erythrocytes and reticulocytes increased under hypoxia compared with normoxia in all patients with HbSS (Figure 2D; mean ± SEM, 1183 ± 295 vs 426 ± 188; N = 18, P=.009 for subjects without known IPS; and 3321 ± 789 vs 965 ± 165; N = 23, P=.001 for subjects with known IPS; Mann-Whitney). However, total red blood cell adhesion to LN was higher in subjects whose echocardiogram showed IPS, compared with those without or not examined, under both normoxia and hypoxia (Figure 2D; mean ± SEM, 965 ± 165, N = 23 vs 426 ± 188, N = 18; P = .005 for normoxic conditions; and 3321 ± 789, N = 23 vs 1183 ± 295, N = 18, P = .010 for hypoxic conditions, Mann-Whitney). Patients with HbSS and an associated diagnosis of IPS were more likely to have evidence of hemoglobin desaturation (exertional and/or nocturnal Hb desaturation, Table 1; 17/22 in subjects with IPS and 7/18 in those without IPS, chi-square, P = .014). Because the enhanced adhesion of mature erythrocytes rather than reticulocytes were responsible for the increase in total red blood cell adhesion to LN, enhanced hypoxic mature erythrocyte adhesion could be associated with IPS in SCD.
Adherent sickle erythrocytes have greater accumulated bending energy than reticulocytes
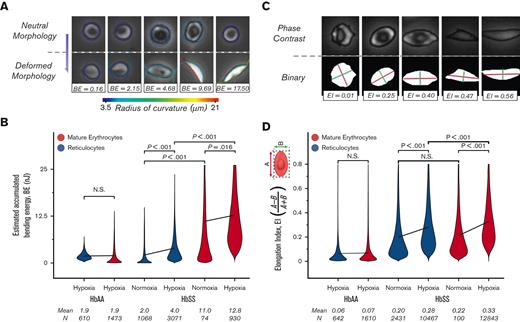
We postulated a role for SMase in the enhanced adhesion of mature erythrocytes because of its association with sulfatide and LN. Accumulated bending energy in the erythrocyte membrane elicits SMase activity21 and the radius of curvature of the red blood cell membrane can be directly linked to the accumulated membrane energy. Therefore, analysis of the morphological changes imposed by polymerization of sickle hemoglobin could provide indirect insights into SMase activity. We investigated the bending energy associated with mature erythrocytes and reticulocytes from HbAA and HbSS donors under hypoxia and normoxia. Figure 3A illustrates the relationship between the change in radius of curvature during deformation and the estimated accumulated bending energy. The shear rate of 100 s-1 induced mechanical stress on the HbAA red blood cell membrane, which corresponds to an estimated bending energy of 1.9 nanojoules (nJ), and the bending energy of the reticulocytes and mature erythrocytes in the HbAA sample was comparable (Figure 3B; mean ± 95% CI, 1.9 ± 0.09, N = 1473 for mature erythrocytes; and 1.9 ± 0.03, N = 610 for reticulocytes; P=.357, 1-way ANOVA). The accumulated bending energy of reticulocytes and mature erythrocytes differed significantly for HbSS samples under both normoxia and hypoxia (Figure 3B; mean ± 95% CI, 2.0 ± 0.08, N = 1068 vs 11.0 ± 0.41, N = 74 and P < .001 under normoxia and 4.0 ± 0.13, N = 3071 vs 12.8 ± 0.27, N = 930, P < .001 under hypoxia, 1-way ANOVA).
Adhered mature erythrocytes accumulate greater membrane deformation than adhered reticulocytes. (A) Representative images of red blood cells for various accumulated bending energy (BE) estimates are presented. The change in the radius of curvature along the periphery during deformation provides an estimate of the accumulated BE. (B) Accumulated BE are not different between HbAA mature erythrocytes and reticulocytes. However, it is estimated that HbSS mature erythrocytes that adhere to LN under hypoxia have 3 times more accumulated BE than reticulocytes. (C) Representative images of red blood cells with various EIs are presented. Major (A, red line) and minor (B, green line) axes are obtained by fitting an ellipsis to a binary mask (bottom row) generated for each cell (upper row). The EI is 0 for perfectly circular cells. (D) EI of adherent reticulocytes (blue) and mature erythrocytes (red) for HbAA and HbSS are presented. A shear rate of 100 s–1 causes a slight elongation of HbAA; however, the EI of mature erythrocytes is not different from that of reticulocytes in HbAA. In HbSS, hypoxia induces greater elongation of mature erythrocytes and reticulocytes. HbS-containing erythrocytes have significantly greater mean EI than HbS-containing reticulocytes under hypoxia, but not under normoxia. Lines connect means, and P values were calculated using a 1-way ANOVA test.
Adhered mature erythrocytes accumulate greater membrane deformation than adhered reticulocytes. (A) Representative images of red blood cells for various accumulated bending energy (BE) estimates are presented. The change in the radius of curvature along the periphery during deformation provides an estimate of the accumulated BE. (B) Accumulated BE are not different between HbAA mature erythrocytes and reticulocytes. However, it is estimated that HbSS mature erythrocytes that adhere to LN under hypoxia have 3 times more accumulated BE than reticulocytes. (C) Representative images of red blood cells with various EIs are presented. Major (A, red line) and minor (B, green line) axes are obtained by fitting an ellipsis to a binary mask (bottom row) generated for each cell (upper row). The EI is 0 for perfectly circular cells. (D) EI of adherent reticulocytes (blue) and mature erythrocytes (red) for HbAA and HbSS are presented. A shear rate of 100 s–1 causes a slight elongation of HbAA; however, the EI of mature erythrocytes is not different from that of reticulocytes in HbAA. In HbSS, hypoxia induces greater elongation of mature erythrocytes and reticulocytes. HbS-containing erythrocytes have significantly greater mean EI than HbS-containing reticulocytes under hypoxia, but not under normoxia. Lines connect means, and P values were calculated using a 1-way ANOVA test.
It would be supportive if the sickle mature erythrocytes were also more elongated than the reticulocytes; thus, we investigated the elongation of adhered red blood cells. The elongation index (EI) equation was set such that the EI was bound between 0 to 1, and the more elongated the cells, the greater the EI (Figure 3A). The EI of erythrocytes and reticulocytes from patients with HbAA or HbSS was analyzed under physiologically relevant hypoxic flow. The mean EI of erythrocytes and reticulocytes in the HbAA sample under hypoxic flow was similar (Figure 3B; mean ± 95% CI, 0.069 ± 0.003, N = 1610 for erythrocytes; and 0.064 ± 0.005, N = 642 for reticulocytes, P =.120, 1-way ANOVA). This similarity was present in HbSS samples under normoxia, albeit at a different level (Figure 3B; mean ± 95% CI, 0.216 ± 0.026, N = 100 for erythrocytes and 0.198 ± 0.005, N = 2431 for reticulocytes, P = .210, Mann-Whitney). Under hypoxic flow, the mean EI of HbSS erythrocytes was greater than the mean EI of HbSS reticulocytes (Figure 3B; mean ± 95% CI, 0.327 ± 0.002, N = 12 843 for erythrocytes; and 0.282 ± 0.003, N = 10 467 for reticulocytes, P < .001, 1-way ANOVA). The distribution of EI for each patient is shown in supplemental Figure 2. Taken together, these data support the hypothesis that SMase enhances mature erythrocyte adhesion via a sulfatide-dependent mechanism.
Accumulated energy of adhered mature erythrocytes associates with their adhesion and acid SMase activity
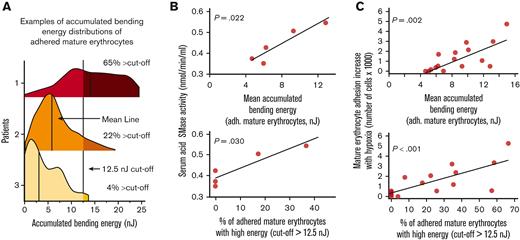
Previously, López et al reported that erythrocyte membrane bending elicits SMase activity.21 We investigated the relationship between accumulated bending energy and acid SMase activity. We performed a set of linked experiments that included the measurement of acid SMase activity using a colorimetric enzymatic activity kit and mature erythrocyte adhesion to LN using SCD Biochip. Specifically, the accumulated bending energy distributions of adhered mature erythrocytes were represented using 2 parameters for statistical analysis: mean accumulated bending energy and percentage of cells above 12.5 nJ cutoff (Figure 4A). It should be noted that these results belonged to 5 samples from patients with SCD that were not recently transfused to ensure the retainment of patient-specific sera (HbS %: mean ± SD, 76 ± 15). We found that both bending energy parameters were positively associated with acid SMase activity (Figure 4B; P = .022, for mean accumulated bending energy; and P = .030 for percent cells above the 12.5 nJ cutoff, N = 5, linear regression). We also investigated whether the bending energy parameters were associated with the adhesion of mature erythrocytes and found that the difference in mature erythrocyte adhesion under hypoxia vs normoxia was also positively associated with both bending parameters (Figure 4C; P = .002, for mean accumulated bending energy, and P < .001 for percent cells above the 12.5 nJ cutoff, N = 16, linear regression). These results indicate that the bending energy changes to mature erythrocyte adhesion increase with hypoxia.
Greater accumulated bending energy is associated with higher acid SMase activity and increased mature erythrocyte adhesion. (A) Accumulated bending energy distributions of mature erythrocytes under hypoxia vary widely between patients. Three example distributions are shown, with varying distributions for the demonstration. Distributions are represented by 2 parameters for statistical analysis: mean accumulated bending energy and percent cells above 12.5 nJ cutoff. (B) Accumulated bending energy distribution parameters associated with serum acid SMase activity of patients with SCD that are not recently been transfused (P = .022, for mean accumulated bending energy, and P = .030 for percent cells above 12.5 nJ cutoff, N = 5). (C) An increase in mature erythrocyte adhesion with hypoxia is associated with mean accumulated bending energy (P = .002, N = 16) and the percentage of adhered mature erythrocytes with relatively higher accumulated bending energy (P < .001, N = 16). P values are calculated using linear regression.
Greater accumulated bending energy is associated with higher acid SMase activity and increased mature erythrocyte adhesion. (A) Accumulated bending energy distributions of mature erythrocytes under hypoxia vary widely between patients. Three example distributions are shown, with varying distributions for the demonstration. Distributions are represented by 2 parameters for statistical analysis: mean accumulated bending energy and percent cells above 12.5 nJ cutoff. (B) Accumulated bending energy distribution parameters associated with serum acid SMase activity of patients with SCD that are not recently been transfused (P = .022, for mean accumulated bending energy, and P = .030 for percent cells above 12.5 nJ cutoff, N = 5). (C) An increase in mature erythrocyte adhesion with hypoxia is associated with mean accumulated bending energy (P = .002, N = 16) and the percentage of adhered mature erythrocytes with relatively higher accumulated bending energy (P < .001, N = 16). P values are calculated using linear regression.
Hypoxia exposes sulfatides and sulfatide antibody inhibits SMase enhanced erythrocyte adhesion
SMase activity increases the hydrolysis rate of sphingomyelins into ceramides in the erythrocyte membrane, which stochastically suggests a greater number of sulfatide domains on the plasma membrane. Because hypoxia induces membrane bending in sickle mature erythrocytes and greater accumulated bending energy is associated with SMase, we postulate that inducing hypoxia results in elevated sulfatide availability on the erythrocyte membrane. Therefore, we performed flow cytometry to show the effect of hypoxia on membrane sulfatide availability. We used sodium metabisulfite to scavenge oxygen before the staining step and measured membrane sulfatide availability with and without sodium metabisulfite. The cytometry results from the 3 HbSS samples with relatively high HbS content are presented in Figure 5A. The data demonstrated that the size and membrane sulfatide increased together with hypoxia (20% vs 0.24% sulfatide-positive erythrocytes under hypoxia vs normoxia). In addition, sulfatide availability under normoxia showed no difference from unstained control samples (0.24% vs 0.20% sulfatide-positive erythrocytes), which is in line with the low adhesion of mature erythrocytes under normoxia shown in Figure 2.
Hypoxia results in sulfatide exposure on the erythrocyte membrane and SMase incubation adds to the hypoxic adhesion of sickle red blood cells and anti-sulfatide antibody can inhibit SMase enhanced adhesion. (A) RBCs isolated from peripheral blood of 3 patients with SCD were treated with sodium metabisulfite for 1 hour. Legend denotes the HbS percentage of each sample and percentage of sulfatide-positive cells based on the gating strategy. Erythrocyte size and sulfatide availability increase when oxygen is scavenged by sodium metabisulfite, suggesting that sulfatide is exposed to the membranes of elongated sickle erythrocytes. (B) Sickle red blood cell adhesion to laminin following either 1 hour PBS or SMase (2.5 mU/mL) incubation at 37°C. Antibodies (1:200 v/v) or PBS were added for incubation after 30 minutes for the remaining 30 minutes. Hypoxia enhances the adhesion of sickle red blood cells, and SMase further increases this adhesion. Enzymatic activity of SMase could lead to the outer surface of the sickle red cell membrane with greater sulfatide availability, which is known to promote adhesion to activated endothelial cells. NBS, sodium metabisulfite, Na2S2O5.
Hypoxia results in sulfatide exposure on the erythrocyte membrane and SMase incubation adds to the hypoxic adhesion of sickle red blood cells and anti-sulfatide antibody can inhibit SMase enhanced adhesion. (A) RBCs isolated from peripheral blood of 3 patients with SCD were treated with sodium metabisulfite for 1 hour. Legend denotes the HbS percentage of each sample and percentage of sulfatide-positive cells based on the gating strategy. Erythrocyte size and sulfatide availability increase when oxygen is scavenged by sodium metabisulfite, suggesting that sulfatide is exposed to the membranes of elongated sickle erythrocytes. (B) Sickle red blood cell adhesion to laminin following either 1 hour PBS or SMase (2.5 mU/mL) incubation at 37°C. Antibodies (1:200 v/v) or PBS were added for incubation after 30 minutes for the remaining 30 minutes. Hypoxia enhances the adhesion of sickle red blood cells, and SMase further increases this adhesion. Enzymatic activity of SMase could lead to the outer surface of the sickle red cell membrane with greater sulfatide availability, which is known to promote adhesion to activated endothelial cells. NBS, sodium metabisulfite, Na2S2O5.
A previous study has indicated that antibody A2B5 can detect sulfatides.34 By using this antibody and bacterial origin SMase we designed a set of controlled adhesion experiments to demonstrate the involvement of SMase and sulfatide in mature erythrocyte adhesion. 2.5 mU/mL corresponds to 2.5 nmol/minute per mL and although this value can be considered high compared with plasma levels of SMase in SCD, we wanted to observe the functional effects of SMase similarly to the work of Dinkla et al 35 Notably, we did not perform acridine orange staining in this set of experiments as we have already shown the role of mature erythrocytes in hypoxia enhanced adhesion. We tested samples in a paired manner and found that enhanced sickle red blood cell adhesion under hypoxia increased significantly with SMase treatment (Figure 5B; mean ± 95% CI, 917 ± 186 for normoxia control, 3480 ± 715 for hypoxia control, and 4845 ± 944 for hypoxia SMase, N = 10, P=.003 between normoxia and hypoxia, and P = .035 between hypoxia and hypoxia SMase groups, paired t-test). We then compared these findings with samples incubated with negative and anti-sulfatide antibodies, in addition to SMase. We found that the anti-sulfatide antibody, but not the control antibody, inhibited red blood cell adhesion to LN under hypoxia (Figure 5B; mean ± 95% CI, 5161 ± 989 for control antibody, 2783 ± 594 for anti-sulfatide antibody, N = 10, P = .155 between SMase and control antibody with SMase, and P = .011 between SMase and sulfatide antibody with SMase, paired t-test). These data demonstrate that sulfatide mediates the hypoxia-enhanced adhesion of sickle erythrocytes.
Outlier analysis
We performed outlier tests to determine the possible outliers in the adhesion data of the subjects. One data point was removed from the IPS and non- or not tested IPS groups (supplemental Notes 4). Re-analysis showed no differences (supplemental Table 1; supplemental Figure 3).
Discussion
Chronic inflammation causes dysregulation of sphingolipid metabolism which manifests itself with altered SMase activity which is proinflammatory in SCD.20 Altered SMase activity afflicts erythrocytes that are rich in membrane lipids including SM which is the precursor of sulfatide.36 Here we demonstrated a contribution of SMase to SCD pathology by providing the first evidence of accumulated bending energy-associated sulfatide mediated mature erythrocyte adhesion to a microvasculature-mimicking LN.
In SCD, reticulocytes have traditionally been regarded as more adhesive to the endothelium than erythrocytes because of the numerous adhesion proteins they have.10,11,37-39 In contrast, mature erythrocytes constitute 80% of all red blood cells in anemic patients on average, which adds to the likelihood of erythrocyte adhesion events. In addition, HbS is likely to polymerize faster in mature erythrocytes owing to greater Hb concentration compared with reticulocytes.40 The proposed hypothesis suggests that mature erythrocyte adhesion is likely to be more affected by chronic inflammation because of greater accumulated membrane damage. In line with this, we found that hypoxia did not lead to a significant increase in reticulocyte adhesion, whereas mature erythrocyte adhesion was markedly enhanced owing to hypoxia, often surpassing the basal contribution attributable to reticulocytes (Figure 2C). This finding, along with the association of the IPS phenotype with sickle erythrocyte adhesion to LN, emphasizes the significance of chronic inflammation and SMase action in SCD.
It is important to note that the increase in mature erythrocyte adhesion illustrated in Figure 2C is unlikely to be associated with either clinical reticulocyte percentage or ARC (P = .162 and P = .096, respectively, linear regression). This rejects the hypothesis that mature erythrocytes and reticulocytes are adhesive because of the common mechanisms. In contrast, membrane damage accumulation over time owing to cyclic HbS polymerization could separate mature erythrocytes from reticulocytes, as corroborated by the data shown in Figure 3. These data demonstrate that SMase, which is known to contribute to extracellular vesicle generation in SCD, could impair the adhesion and deformability of sickle mature erythrocytes as well. Therefore, chronic inflammation and SMase warrant significant attention because of their central role in affecting the biophysical features of erythrocytes.
We demonstrated a strong positive relationship between bending energy changes and an increase in mature erythrocyte adhesion to LN under hypoxia (Figure 4C). Because LN has a high affinity for sulfatides that are downstream of hydrolysis of SM by SMase, we directly measured the acid SMase activity using patient sera to investigate whether SMase potentially participates in this relationship. We demonstrated that acid SMase activity was also positively associated with bending parameters (Figure 4B), supporting the sulfatide-mediated adhesion hypothesis.
We investigated whether sulfatides become available on the sickle erythrocyte membrane surface under hypoxia. The cytometry results demonstrated that erythrocyte size and sulfatide availability on the membrane increased when sickle erythrocytes were exposed to hypoxia (Figure 5A). Furthermore, it should be noted that sulfatide availability did not differ significantly from the unstained control without hypoxia. This finding is in line with the low adhesion of mature erythrocytes to LN, despite the relatively high accumulated bending energy. Awojoodu et al previously reported elevated erythrocyte microparticle generation under 24 hours, albeit the effect was not present after 1 hour.20 It can be postulated that adhesion to LN could be reflecting the more immediate effects of hypoxia before the excessive sulfatide is lost via vesiculation, which warrants further testing.
We showed that SMase further increased hypoxic adhesion of red blood cells with an additional set of controlled microfluidic adhesion assay experiments. In addition, we showed that the contribution of sulfatides to SMase and hypoxia enhanced adhesion, using an A2B5 clone monoclonal antibody as a blocker (Figure 5B). Sulfatide antibody blocking is feasible because of the high specific affinity of LN for sulfatides; however, there are many other lipids downstream of SM, including phosphatidylserine, which is also known to mediate sickle red blood cell adhesion to the endothelium. Furthermore, previously it has been shown that adhesion of reticulocytes to LN (isoform-10) is triggered by oxidative stress through Lu/BCAM phosphorylation in reticulocytes but not in mature erythrocytes.41 Nevertheless, given that reticulocytes spend much less time in circulation compared with mature erythrocytes, the differences that we demonstrated between adhesion of mature erythrocytes and reticulocytes can be explained by the mechanisms that are relevant to oxidative stress. Therefore, further comprehensive studies should be performed to elucidate the scale of the effects of SMase on sickle red blood cell adhesion.
We examined previously reported clinical phenotypes associated with hypoxia and increased hemolysis, in preparation for future prospective clinical trials.25,33 We found that total red blood cell adhesion to LN under both normoxia and hypoxia was significantly greater in subjects with a more severe hemolytic disease phenotype, as marked by higher LDH and ARC (supplemental Figure 4), primarily owing to the binding of reticulocytes. However, as we showed, reticulocyte and mature erythrocyte adhesion to LN depends on the oxygen saturation of Hb; these differences may be relevant to understanding the pathophysiology of SCD.
In this study, we showed that subjects with SCD and an associated IPS exhibited higher total red blood cell adhesion in normoxia than those without, or not evaluated for, an IPS (Figure 2D) driven mostly by reticulocytes. However, subjects with SCD and a history of IPS also showed increased total red blood cell adhesion under hypoxia (Figure 2D), which was driven mostly by mature erythrocytes. These findings highlighted a tenable pathophysiological association. In SCD, alveolar bypass may be especially detrimental because sickle Hb polymerizes more extensively when desaturated. It is plausible that abnormal erythrocyte adhesion mediated by hypoxia, may lead to microvascular damage in the pulmonary arteries (where red blood cells are most deoxygenated), which may further enhance the development of IPS. Definitively establishing a causal relationship between abnormal mature erythrocyte adhesion and IPS development warrants a dedicated prospective effort, which we are undertaking.
Because of the presence of transfused subjects, we investigated normoxic adhesion of stored HbA-containing red blood cells and found that average normoxic adhesion to LN of sickle red blood cells from whole blood was substantially higher than that of stored HbA-containing red blood cells (supplemental Figure 5A). In addition, the measured HbS percentage (from the examined sample) did not correlate with total red blood cell adhesion under normoxia or hypoxia (supplemental Figure 5B). Finally, we assessed the effect of excluding transfused subjects on total red blood cell adhesion under both normoxia and hypoxia relative to a history of IPS and found a negligible impact (data not shown).
We have previously shown that adhesion of sickle red blood cells to LN was significantly enhanced under hypoxia, in a subject-specific manner, using an in vitro microfluidic assay.27,29 We demonstrated that although the majority of adherent red blood cells consisted of reticulocytes in normoxia, reticulocyte adhesion did not increase under hypoxia. In contrast, relatively minimal adhesion by mature erythrocytes was significantly enhanced under hypoxia, which can be explained by the proposed mechanism (Figure 6). These findings suggest that therapies that primarily target sickling of deoxy-Hb are likely to have a significant impact on mature erythrocytes, which are affected by hypoxia, but not on the adhesion of reticulocytes, which are not. Furthermore, SMase and membrane lipids could be new therapeutic targets for improving multiple facets of SCD pathology, including abnormal adhesion, microparticle generation, and membrane damage in mature erythrocytes.
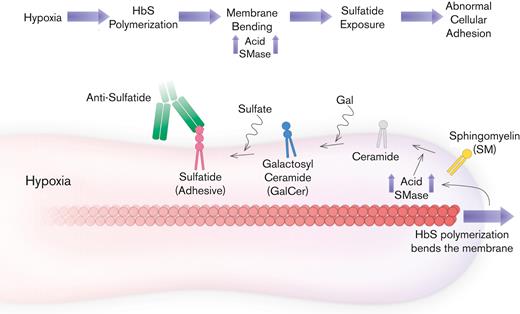
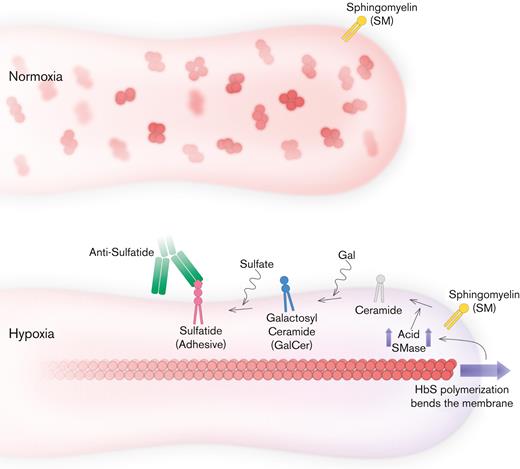
Proposed mechanism for blocking mechanical bending-associated mature erythrocyte adhesion. Low-level adhesion and high deformability of mature erythrocytes are favorable for SCD. HbS does not disrupt SMase activity under normoxia. However, under hypoxia, HbS polymerization increases the accumulation of bending energy and initiates a cascade that leads to sulfatide exposure via SMase upregulation. Therefore, mature erythrocytes become highly adhesive, suggesting poor biophysical properties. Inhibition of mature erythrocyte adhesion is possible with antibodies against sulfatide; however, inhibition of SMase activity could also improve other biophysical properties of mature erythrocytes, such as deformability, while preventing abnormal adhesion as well.
Proposed mechanism for blocking mechanical bending-associated mature erythrocyte adhesion. Low-level adhesion and high deformability of mature erythrocytes are favorable for SCD. HbS does not disrupt SMase activity under normoxia. However, under hypoxia, HbS polymerization increases the accumulation of bending energy and initiates a cascade that leads to sulfatide exposure via SMase upregulation. Therefore, mature erythrocytes become highly adhesive, suggesting poor biophysical properties. Inhibition of mature erythrocyte adhesion is possible with antibodies against sulfatide; however, inhibition of SMase activity could also improve other biophysical properties of mature erythrocytes, such as deformability, while preventing abnormal adhesion as well.
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute grants R01HL133574, OT2HL152643, K25HL159358, R42HL160384, and R56HL165946, and the National Science Foundation CAREER Award 1552782. The authors acknowledge with gratitude the contributions of the subjects and clinicians at the Seidman Cancer Center (University Hospitals, Cleveland, OH).
Authorship
Contribution: U.G., U.A.G., and J.A.L. developed the idea and designed the study; U.G., F.W., E.K., N.A., E.Q., C.Y., and A.H. performed the experiments; U.G., E.K., F.W., Y.M., and R.A. analyzed the data; U.G., E.K., A.B., U.A.G., and J.A.L. discussed and interpreted the data; B.C.H. and M.G. reviewed some echo results; U.G. and E.K. wrote the manuscript; U.G. prepared the figures and table; and N.A., A.H., U.A.G., and J.A.L. edited the manuscript.
Conflict-of-interest disclosure: R.A., J.A.L., U.A.G., and Case Western Reserve University have financial interests in Hemex Health Inc. J.A.L., E.K., U.A.G., and Case Western Reserve University have financial interests in BioChip Labs Inc. U.A.G. and Case Western Reserve University have financial interests in Xatek Inc. U.A.G. has financial interests in DxNow Inc. Financial interests include licensed intellectual property, stock ownership, research funding, employment, and consulting. Hemex Health Inc offers point-of-care diagnostics for hemoglobin disorders, anemia, and malaria. BioChip Labs Inc offers commercial clinical microfluidic biomarker assays for inherited or acquired blood disorders. Xatek Inc offers point-of-care global assays to evaluate the hemostatic process. DxNow Inc offers microfluidic and bio-imaging technologies for in vitro fertilization, forensics, and diagnostics. Competing interests of Case Western Reserve University employees are overseen and managed by the Conflict of Interests Committee according to a Conflict-of-Interest Management Plan. The remaining authors declare no competing financial interests.
Correspondence: Umut A. Gurkan, Departments of Mechanical and Aerospace Engineering and Biomedical Engineering, Case Western Reserve University, 10900 Euclid Ave, Cleveland, OH 44106; e-mail: umut@case.edu.
References
Author notes
Data are available on request from the corresponding author, Umut A. Gurkan (umut@case.edu).
The full-text version of this article contains a data supplement.