TO THE EDITOR:
Accurate patient ancestry determination is critical in the evaluation of candidates for allogeneic bone marrow transplantation (BMT) without suitable related donors, given that patients with non-European ancestry are much less likely to have an 8 of 8 HLA–matched unrelated donor (URD)1-4 and are more likely to suffer delays to transplant5 which can adversely affect transplant outcomes.6,7 It is known that recording patients with full or part non-European origins as White non-Hispanic can result in inaccurate and potentially dangerous overestimation of the likelihood of securing an 8 of 8 URD, thereby delaying pursuit of alternative–mismatched adult donors or cord blood grafts. To speed provision of allografts, our Center has developed a search prognosis tool that permits immediate estimation of the likelihood of securing an 8 of 8 URD in patients without an HLA-identical sibling.8,9 However, the tool’s accuracy is greatly influenced by the knowledge regarding a patient’s European or non-European origin.8,9
For these reasons, our BMT Program staff evaluated ancestry in all allograft candidates without HLA–identical sibling donors. The relevance of ancestry in donor searches is explained first, and then a kinship history is recorded including the patient’s country of origin, that of their maternal/paternal ancestors, and the patient’s consideration of themself as Black and/or Hispanic. Ancestry is then classified as European, African (including African American, Afro-Caribbean, and African immigrants), White Hispanic (including patients of Central/South American origin who self-identified as non-Black and Hispanic), Asian, Middle Eastern, or mixed non-European (patients who do not fit in these previous categories with full- or part-mixed non-European heritage). Utilization of this information optimizes patient triage to alternative donor allografts as necessary.
Consequently, we now have a large patient cohort whose ancestry is categorized as completely and accurately as possible (short of performing ancestral genotyping). The availability of these BMT-designated ancestry determinations now permits comparison with the race/ethnicity data collected at the registration of a new patient. At our center, patients are initially registered as being of White, Black, Asian, or other race, and of Hispanic or non-Hispanic ethnicity. The process used to obtain these data, however, has not been standardized. In this retrospective analysis, we compared hospital-registration race/ethnicity with BMT-designated ancestry in consecutive adult allograft candidates without HLA–identical sibling donors from October 2005 to July 2021. The study was approved by the institutional review board at Memorial Sloan Kettering Cancer Center and was conducted according to the Declaration of Helsinki.
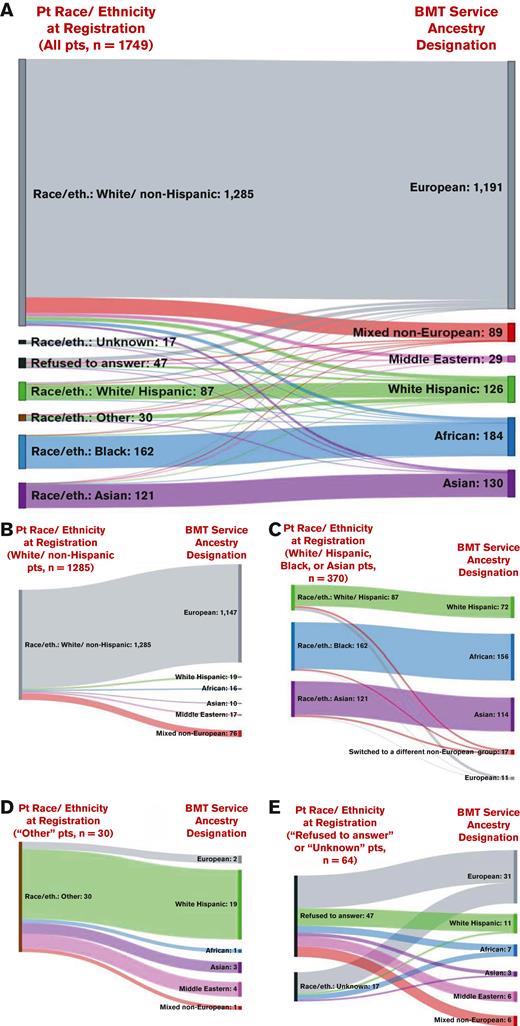
Of 1749 patients (median, 56 years; range, 18-80 years), approximately three-quarters (n = 1285, 73.5%) were registered as White/non-Hispanic, the remaining were recorded as White/Hispanic (n = 87, 5%), Black (n = 162, 10%), or Asian (n = 121, 7%), with 30 (2%) recorded as other, 17 (1%) as unknown, and 47 (3%) as refused to answer. However, dedicated BMT ancestral histories revealed a greater number with non-European ancestry, as shown in Figure 1A. Specifically, of 1285 patients originally recorded as White/non-Hispanic, 138 (11%) had non-European ancestry (19 White Hispanic, 16 African, 10 Asian, 17 Middle Eastern, and 76 mixed non-European; Figure 1B). An additional 17 of 370 patients (5%) registered as being from one non-White racial/ethnic group were from a different non-European ancestral group, and conversely 11 of 370 patients (3%) registered as having a race/ethnicity other than White/non-Hispanic had European ancestry (Figure 1C). Furthermore, of 30 patients initially registered as other, ancestry history revealed their heritage was European (n = 2), White Hispanic (n = 19), African (n = 1), Asian (n = 3), Middle Eastern (n = 4), or mixed non-European (n = 1) (Figure 1D). Consequently, dedicated ancestry interviews performed by BMT staff determined there were higher percentages of White Hispanic (7%, not 5%), African (11%, not 10%), and Asian (7.4%, not 6.9%) patients, and identified 29 patients with Middle Eastern (2%) and 89 patients with mixed non-European (5%) origins. Finally, of the 64 patients whose race/ethnicity was initially recorded as unknown or refused to answer, all shared their ancestry with transplant staff, and over half (n = 33) of these patients had non-European ancestry (11 White Hispanic, 7 African, 3 Asian, 6 Middle Eastern, and 6 mixed non-European; Figure 1E).
Sankey diagrams comparing the hospital registration race/ethnicity information (left) vs the BMT Service ancestry designation (right). Panels show comparisons for all patients in the cohort (n = 1749) (A), and those initially classified as White/non-Hispanic (n = 1285) (B), White/Hispanic, Black, or Asian (n = 370) (C), other (n = 30) (D), and refused to answer or unknown (n = 64) (E). Patient race/ethnicity information was recorded during patient initial registration at the hospital. Ancestry designation was determined from dedicated ancestral histories performed by transplant staff at the time of allograft evaluation, after a detailed explanation of the relevance of these data to patient care. Differences and resultant inaccuracies in race/ethnicity vs ancestry designations are demonstrated. eth, ethnicity; Pt, patient.
Sankey diagrams comparing the hospital registration race/ethnicity information (left) vs the BMT Service ancestry designation (right). Panels show comparisons for all patients in the cohort (n = 1749) (A), and those initially classified as White/non-Hispanic (n = 1285) (B), White/Hispanic, Black, or Asian (n = 370) (C), other (n = 30) (D), and refused to answer or unknown (n = 64) (E). Patient race/ethnicity information was recorded during patient initial registration at the hospital. Ancestry designation was determined from dedicated ancestral histories performed by transplant staff at the time of allograft evaluation, after a detailed explanation of the relevance of these data to patient care. Differences and resultant inaccuracies in race/ethnicity vs ancestry designations are demonstrated. eth, ethnicity; Pt, patient.
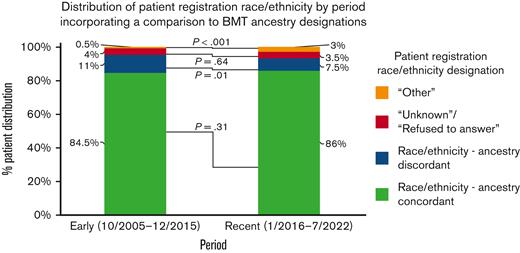
To evaluate changes over time, we analyzed the distribution of the registered race/ethnicity of patients, incorporating a comparison with BMT-adjudicated ancestry, early in the study period (October 2005 to December 2015, n = 906) vs recently (January 2016 to July 2021, n = 843; Figure 2). We defined race/ethnicity-ancestry discordance as patients registered as White/non-Hispanic who had non-European ancestry, or vice versa, or patients of any racial/ethnic group other than White/non-Hispanic whose ancestry was from a different non-European group. Notably, although the proportion of discordant patients decreased over time (101/906, 11% vs 65/843, 7.5%; P = .01), those initially registered as other increased (6/906, 0.5% vs 24/843, 3%; P < .001), and those registered as unknown or who refused to answer remained unchanged. Consequently, the overall concordance rate of race/ethnicity vs ancestry in the 2 eras (764/906, 84.5% and 725/843, 86%, respectively; P = .31) was the same.
Distribution of patient registration race/ethnicity, incorporating a comparison with BMT ancestry designations, in the early (October 2005 to December 2015) versus recent (January 2016 to July 2021) analysis periods. Race/ethnicity-ancestry discordance was defined as patients initially recorded as White/non-Hispanic who had non-European ancestry, or vice versa, or patients from a racial/ethnic group other than White/non-Hispanic who had a different non-European ancestry. Recently, the discordance decreased, whereas the proportion of patients initially registered as other increased. Race/ethnicity-ancestry concordance and proportions of patients whose race/ethnicity at registration was unknown or refused to answer were unchanged. P values were generated using Pearson χ2 tests.
Distribution of patient registration race/ethnicity, incorporating a comparison with BMT ancestry designations, in the early (October 2005 to December 2015) versus recent (January 2016 to July 2021) analysis periods. Race/ethnicity-ancestry discordance was defined as patients initially recorded as White/non-Hispanic who had non-European ancestry, or vice versa, or patients from a racial/ethnic group other than White/non-Hispanic who had a different non-European ancestry. Recently, the discordance decreased, whereas the proportion of patients initially registered as other increased. Race/ethnicity-ancestry concordance and proportions of patients whose race/ethnicity at registration was unknown or refused to answer were unchanged. P values were generated using Pearson χ2 tests.
Our analysis demonstrates that lack of dedicated evaluation can result in an inaccurate (or absent) record of patient ancestry (as well as race/ethnicity). It is known that race/ethnicity data are more accurate when elicited from self-report rather than from healthcare staff assignment.10 However, our findings support the practice of explanation of the relevance of this information to donor searches, because this enhances patient participation in accurate data capture. Importantly, misclassifications resulting from lack of dedicated evaluation, which could adversely affect patient care, can be avoided.
To the best of our knowledge, this analysis is the first of its kind in the transplant field. We acknowledge that self-reported ancestry may not always correspond to genetic ancestry.11 This could only be further resolved by ancestral genotyping, which is not practical and may not be appropriate. However, for alternative donor allograft candidates, we are currently investigating whether ancestral genotyping can further refine the accuracy of their URD search prognosis. This is especially important for patients with a fair search prognosis, for whom an HLA-matched URD may not be identified in the time required for optimal patient care.8,9
Our results also have implications for the capture and interpretation of registry data, including that submitted to the Center for International Blood and Marrow Transplant Research, if extracted from the hospital patient registration database. Furthermore, race/ethnicity/ancestry inaccuracies could impact assessments of whether clinical trial populations reflect the diversity of patients with that disease.12 Here, misclassifications could impact the validity of results and analyses of racial/ethnic/ancestral differences in treatment access and responses. Accurate race/ethnicity/ancestry data are also critical for analyses of correlative laboratory science,13 valid reporting of patient composition to funding agencies,14 and analyses of attempts to mitigate health care delivery disparities.15 Overall, mistakenly recording patients from marginalized or disadvantaged racial/ethnic groups as White or other, or failing to record them at all, could mask disparities, thereby preventing them from being addressed. This is especially troubling given the recent Abraham et al report that demonstrated structural racism as a primary mediator of disparities in the provision of curative therapy for patients with acute myeloid leukemia.16 Our findings also have broader implications from the standpoint of equity across cancer care and medicine.
In a recent New England Journal of Medicine perspective,17 Deyrup and Graves argue that extensive training is needed concerning human biologic variation and social definitions of race. Our data emphasize the need for training of staff on how to broach race/ethnicity/ancestry questions. When supported by leadership with the required attention and resources, coordinated improvement in the race/ethnicity data collection process, such as the multi-institutional We Ask Because We Care campaign (Shapiro et al18), is feasible, effective, and advances health equity. Such efforts are essential, given the rapidly increasing diversity of the US population.19
Acknowledgments: The authors thank the Memorial Sloan Kettering Cancer Center transplant coordinators and other staff for performing detailed ancestral evaluations at the time of donor evaluation.
This work was supported in part by a grant from the National Cancer Institute (grant P30 CA008748).
Contribution: W.B.F. and J.N.B designed the study, assembled and analyzed the data, and wrote the manuscript; E.D., S.C., and K.N. maintained the patient database and provided the data; and all authors interpreted the data, reviewed and edited the manuscript, and have approved the final version of the manuscript for submission.
Conflict-of-interest disclosure: I.P. has received research funding from Merck and serves as a member of Data and Safety Monitoring Board for ExCellThera. A.S. serves as a consultant at the Scientific Advisory Board of ExCellThera. J.N.B. has received consultancy payments from Gamida Cell and the New York Blood Center and a research funding from Merck. The remaining authors declare no competing financial interests.
Correspondence: Juliet N. Barker, Adult Bone Marrow Transplantation Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: barkerj@mskcc.org; and Warren B. Fingrut, Adult Bone Marrow Transplantation Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: fingrutw@mskcc.org.
References
Author notes
Data are available on request from the corresponding authors, Juliet N. Barker (barkerj@mskcc.org) and Warren B. Fingrut (fingrutw@mskcc.org).