Key Points
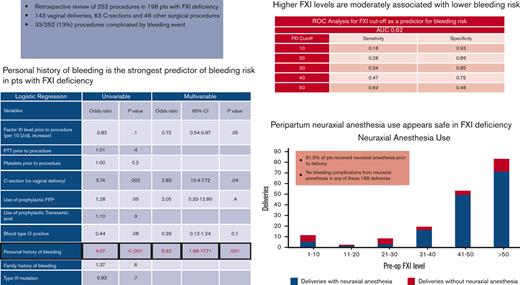
Personal history of bleeding strongly predicts perioperative or obstetric bleeding risk in patients with FXI deficiency.
Higher FXI levels may be associated with lower bleeding risk; however, there is no optimal sensitivity level to rule out bleeding risk.
Abstract
Factor XI (FXI) deficiency is an autosomal inherited, milder bleeding disorder that may predispose to a potential risk of life-threatening bleeding during childbirth or surgery. Unfortunately, data regarding obstetric and perioperative management of this condition are scarce, with limited cases reviewed in the last decade. Therefore, the present study aimed to expand this database and identify factors associated with increased bleeding risk. We performed a retrospective chart review of patients with FXI deficiency who underwent childbirth or other surgical procedures between August 2011 and April 2021 within a single academic health system and identified 198 patients who underwent 252 procedures, including 143 vaginal deliveries, 63 cesarean deliveries, and 46 other surgical procedures. Thirty-three of the 252 procedures resulted in bleeding complications. On multivariable logistic regression analysis, personal history of bleeding was the strongest predictor of perioperative or obstetric bleeding (odds ratio [OR], 5.92; P = .001). Higher FXI levels were correlated with lower odds of bleeding (OR, 0.72 with every 10 U/dL increase in FXI level; P = .05). On receiver operative characteristic analysis, FXI level of >40 U/dL predicted a lower bleeding risk with reasonable specificity (75%) but lacked sensitivity (47%). A family history of bleeding, ethnicity, genotype, preprocedural partial thromboplastin time, and platelet levels were not associated with bleeding risk. There were no cases of epidural or spinal hematoma associated with neuraxial anesthesia. FXI levels remain stable during pregnancy and repeated measurements may not be necessary.
Introduction
Factor XI (FXI) deficiency, also known as hemophilia C, is a rare bleeding disorder that Rosenthal and Dreskin first described in 1955. It was distinguished from the better-known hemophilia A and B by its presence in both genders due to its autosomal inheritance, a milder bleeding tendency, and the conclusion that the laboratory defect could be corrected by mixing tests with hemophilia A and B plasma.1
Although uncommon in the general population, with a frequency of ∼1 per million, it is more prevalent in Ashkenazi and Iraqi Jewish populations, with a rate of heterozygosity as high as 8% to 9%.2,3 Moreover, with the advent of expanded prenatal genetic profiling and direct-to-consumer genetic testing, people with or without a bleeding history and FXI deficiency are increasingly recognized, creating a need to understand this condition better.4
In the existing literature, severe deficiency in homozygous or compound heterozygous carriers is classified as FXI activity <20 international unit (IU)/dL and partial deficiency in heterozygous carriers is defined as FXI levels between 20 and 70 IU/dL. However, even in its most severe form, coagulation factor deficiency is not associated with a spontaneous bleeding tendency, unlike severe FVIII or FIX deficiency; therefore, it is not usually classified as a severe bleeding disorder.5 Instead, bleeding in FXI deficiency is usually injury or surgery related, particularly at sites with high local fibrinolytic activity, such as the genitourinary tract, nasal cavity, and oral cavity.
The management of patients with FXI deficiency is challenging for multiple reasons. There is a lack of correlation between plasma levels of FXI activity and the patient’s tendency to bleed. Multiple studies have attempted to establish an association between bleeding risk and FXI levels; however, ultimately the bleeding tendency remains unpredictable, with no clear correspondence to FXI levels, genotype, or consistency, even within an individual or family.6-8 This discordance leads to important questions about who needs to receive factor replacement in the perioperative or peripartum setting. Some evidence points to plasma replacement therapy during labor not being mandatory even for women with severe FXI deficiency, as observed by Salomon et al9 Furthermore, some anesthesiologists may be reluctant to administer neuraxial anesthesia to healthy women with FXI deficiency and no bleeding history despite growing evidence pointing toward its safety.10-13
Second, the management of bleeding episodes and prevention of bleeding related to surgery are not straightforward because of the risks of currently available therapies and the significant differences in the availability of FXI concentrates in different regions of the world. FXI concentrates carry a significant thrombotic risk and are not available for use in the United States. Lastly, as easily deduced by the rarity of this bleeding disorder and its underdiagnosis due to its intrinsically unpredictable clinical presentation, there is a paucity of data surrounding it, with <500 cases reviewed in the last decade. Therefore, this study aimed to expand this database to identify the factors associated with increased bleeding risk, assess neuraxial anesthesia safety, and analyze the impact of prophylactic administration of hemostatic agents on the incidence and severity of clinically significant bleeding.
Methods
We retrospectively evaluated the electronic medical records of patients with FXI deficiency who underwent childbirth or other surgical procedures between August 2011 and April 2021, within the Mount Sinai Health System in New York. We used the Mount Sinai Data Warehouse to collect the demographic and health-related data. This study was granted a waiver of informed consent and approved by our institutional review board (#IRB-20-03750). The criteria used to diagnose FXI deficiency in our patients included low FXI activity, that is, below the lower limit of the normal range in our laboratory (70-150 IU/dL) or the identification of a mutation in the FXI gene. Subsequently, a database of patients with a definitive diagnosis of FXI deficiency was created.
Health-related data included laboratory data on hematological parameters and clinical data, including surgical details, delivery, and bleeding complications. The non–health-related data included age, gender, and ethnicity. Laboratory data collected included FXI level, genotype, prothrombin time, partial thromboplastin time (PTT), hemoglobin, platelet count, and ABO Rh blood group. Clinical data included mode of delivery (vaginal or cesarean), surgical procedure, type of anesthesia (spinal, epidural, or combined spinal-epidural), periprocedural complications, estimated blood loss, administration of hemostatic products (fresh frozen plasma [FFP] or tranexamic acid ([TXA]), and packed red blood cell (RBC) transfusions. We collected available data on the personal history of bleeding and family history of bleeding. We used the 2017 American Congress of Obstetricians and Gynecologists guidelines that define postpartum hemorrhage (PPH) as a cumulative blood loss ≥1000 mL or associated with signs/symptoms of hypovolemia regardless of the route of birth.14 Early PPH occurred within 24 hours of delivery and late PPH occurred >24 hours after delivery.
History of bleeding phenotype was defined as ≥1 of the following: (1) unexplained easy bruisability, (2) epistaxis requiring medical attention, (3) heavy menstrual bleeding, (4) bleeding with dental procedures requiring intervention, (5) obstetrical bleeding, and (6) postoperative bleeding requiring intervention.15
Statistical methods
Categorical variables are presented as counts with percentages. Continuous variables are presented as means with standard deviations or medians with interquartile ranges. Categorical values were compared using the Fisher exact test. Continuous variables were compared using the paired t test or Mann-Whitey U test. We performed logistic regression to test the association between historical, laboratory, and procedural variables with the bleeding end point (defined as acute PPH or postoperative hemorrhage or any bleeding warranting nonprophylactic administration of packed RBCs, FFP, or TXA). Separate logistic regression analyses were performed for the entire population and only for obstetric patients. Receiver operative characteristic (ROC) curves were plotted for FXI levels to identify the cutoff with optimal sensitivity and specificity for the bleeding end point. Analyses were performed using SPSS software (SPSS Inc, Chicago, IL).
Results
Patient characteristics
We identified 198 patients who underwent 252 procedures including 143 vaginal deliveries, 63 cesarean deliveries, and 46 other surgical procedures. The mean age was 36 years, with 94% (186) females. Data on ethnicity were available for 177 of 198 patients. The majority (77%) were Ashkenazi Jewish, 8 patients identified as Hispanic, and 2 were African American. Only 12% of patients had severe FXI deficiency (defined as a FXI level of <20 U/dL). Median FXI level was 50 U/dL (2-190 U/dL). Median PTT was 33.1 seconds (20.5-47.3 seconds). Mutational analysis was available for 51% of the patients, with type II (c.403G>T p.E135X) and type III (c.901T>C p.F301L) being the most common mutations identified in 46 and 43 patients, respectively. A total of 16 different mutations were reported, with 1 patient harboring both type II and III mutations. There was no difference in FXI levels based on the underlying mutations (Table 1). Forty-three (21.7%) patients met at least 1 criterion for the bleeding phenotype, and 31 (15.7%) patients reporting prior surgical bleeding related to dental/obstetric or other procedures. Only 14 (7%) patients had a family history significant for bleeding.
Median FXI level as per genetic mutation
| Genetic mutation . | FXI level . |
|---|---|
| c.403G>T p.E135X (type II) | 49.5 [44.5-54.8] |
| c.901T>C p.F301L (type III) | 52.5 [45.0-61.0] |
| Other genotype | 43.0 [32.0-50.0] |
| Unknown | 44.0 [17.0-56.0] |
| Genetic mutation . | FXI level . |
|---|---|
| c.403G>T p.E135X (type II) | 49.5 [44.5-54.8] |
| c.901T>C p.F301L (type III) | 52.5 [45.0-61.0] |
| Other genotype | 43.0 [32.0-50.0] |
| Unknown | 44.0 [17.0-56.0] |
Data are presented as median [interquartile range].
Bleeding complications and factors predicting bleeding risk
Thirty-three procedures (13%) resulted in bleeding complications. Of these 33 patients, 21 had acute PPH and 2 had delayed PPH, whereas acute and delayed postsurgical bleeding (in nonpregnant patients) were reported in 3 and 2 cases, respectively. Surgical cases associated with bleeding included knee arthroplasty (n = 1), laparoscopy with proctopexy (n = 1), gastrectomy (n = 1), Hartmann procedure (n = 1), and laminectomy with posterior spinal fusion (n = 1). Five additional patients required nonprophylactic FFP transfusion to achieve hemostasis, which were also recorded as bleeding complications. Of the 21 patients with acute PPH, 8 patients had additional known risk factors for PPH besides FXI deficiency. Six patients had uterine atony, with 1 requiring hysterotomy with dilatation and curettage, 1 had retained placenta, and 1 sustained a second-degree perineal laceration.
Only 8 of these 33 bleeding events were observed in patients with FXI levels <20 U/dL. Baseline characteristics such as age, gender, ethnicity, genotype, and blood group were equally distributed among patients with and without bleeding events (Table 2). Table 3 compares the laboratory parameters, procedure type, and hemostatic interventions in patients with and without bleeding complications. The median preprocedural PTT value was higher for those with bleeding complication (32.8 vs 31.0 U/dL, P = .03). Median FXI activity was significantly different in patients with a bleeding complication compared to those without (41.5 vs 49.0 U/dL, P = .03). A significantly higher number of cesarean deliveries were associated with bleeding events (23.8%) than vaginal deliveries (7.6%, P = .006). Use of prophylactic FFP was significantly higher in the group with bleeding events (24.2% vs 5.9%, P = .002).
Baseline characteristics according to the presence of bleeding complication
| . | Bleeding complication, N (%)∗ . | No bleeding complication, N (%) . | P . |
|---|---|---|---|
| 33 | 219 | ||
| Obstetrical | 26 (78.8) | 180 (82.2) | .6 |
| Nonobstetrical | 7 (21.2) | 39 (17.8) | |
| Age ± standard deviation, y | 37.9 (26-49) | 36.1 (25-47) | .4 |
| Female | 28 (84.8) | 207 (94.5) | .06 |
| Ethnicity | .5 | ||
| Ashkenazi Jewish | 22 (66.7) | 154 (70.3) | |
| Hispanic | 4 (12.1) | 11 (5.0) | |
| African American | 0 | 3 (1.4) | |
| Other White | 3 (9.1) | 20 (9.1) | |
| Other non-White | 2 (6.1) | 6 (2.7) | |
| Unknown | 2 (6.1) | 25 (11.4) | |
| Blood group | .6 | ||
| A | 17 (51.5) | 82 (38.1) | |
| B | 8 (24.2) | 45 (20.9) | |
| AB | 2 (6.1) | 13 (6.0) | |
| O | 6 (18.2) | 75 (34.9) | |
| Genotype | .08 | ||
| c.403G>T p.E135X (type II) | 6 (18.2) | 51 (23.3) | |
| c.901T>C p.F301L (type III) | 7 (21.2) | 54 (24.7) | |
| Other genotype | 4 (12.1) | 13 (5.9) | |
| Unknown | 16 (48.5) | 101 (46.1) | |
| Personal history of bleeding | <.001 | ||
| 1 type† | 14 (42.4) | 34 (15.5) | |
| ≥2 types | 2 (6.3) | 7 (3.4) | |
| Family history of bleeding | 3 (9.4) | 14 (7.0) | .6 |
| . | Bleeding complication, N (%)∗ . | No bleeding complication, N (%) . | P . |
|---|---|---|---|
| 33 | 219 | ||
| Obstetrical | 26 (78.8) | 180 (82.2) | .6 |
| Nonobstetrical | 7 (21.2) | 39 (17.8) | |
| Age ± standard deviation, y | 37.9 (26-49) | 36.1 (25-47) | .4 |
| Female | 28 (84.8) | 207 (94.5) | .06 |
| Ethnicity | .5 | ||
| Ashkenazi Jewish | 22 (66.7) | 154 (70.3) | |
| Hispanic | 4 (12.1) | 11 (5.0) | |
| African American | 0 | 3 (1.4) | |
| Other White | 3 (9.1) | 20 (9.1) | |
| Other non-White | 2 (6.1) | 6 (2.7) | |
| Unknown | 2 (6.1) | 25 (11.4) | |
| Blood group | .6 | ||
| A | 17 (51.5) | 82 (38.1) | |
| B | 8 (24.2) | 45 (20.9) | |
| AB | 2 (6.1) | 13 (6.0) | |
| O | 6 (18.2) | 75 (34.9) | |
| Genotype | .08 | ||
| c.403G>T p.E135X (type II) | 6 (18.2) | 51 (23.3) | |
| c.901T>C p.F301L (type III) | 7 (21.2) | 54 (24.7) | |
| Other genotype | 4 (12.1) | 13 (5.9) | |
| Unknown | 16 (48.5) | 101 (46.1) | |
| Personal history of bleeding | <.001 | ||
| 1 type† | 14 (42.4) | 34 (15.5) | |
| ≥2 types | 2 (6.3) | 7 (3.4) | |
| Family history of bleeding | 3 (9.4) | 14 (7.0) | .6 |
Data are presented as count (percentage) or mean ± standard deviation.
Bleeding complication defined as acute bleeding or nonprophylactic transfusion of RBCs, FFP, or TXA.
Types of bleeding include heavy menstrual bleeding, dental bleeding, easy bruising, epistaxis, obstetrical bleeding, or postoperative bleeding.
Hematological and surgical data according to presence of bleeding complication
| . | Bleeding complication . | No bleeding complication . | P . |
|---|---|---|---|
| 33 | 219 | ||
| Laboratory values before delivery/procedure | |||
| FXI (U/dL) | 41.5 [14.0-52.0] | 49.0 [40.0-57.3] | .03 |
| Activated PTT (sec) | 32.8 [29.9-41.8] | 31.0 [28.7-34.2] | .03 |
| Hemoglobin (g/dL) | 12.0 [11.3-13.0] | 12.3 [11.6-13.3] | .2 |
| Platelets (109/L) | 186 [152-240] | 199 [173-232] | .3 |
| Blood loss during delivery/procedure (mL) | 659 [213-1000] | 286 [124-400] | <.001 |
| Blood loss following delivery/procedure (mL) | 58 [0-809] | 0 [0-36] | <.001 |
| Hemoglobin decrease following delivery/procedure (g/dL) | 2.8 [1.3-3.5] | 1.6 [1.0-2.6] | .003 |
| Procedure type | .006 | ||
| Vaginal delivery | 11 (33.3) | 132 (60.3) | |
| Cesarean delivery | 15 (45.5) | 48 (21.9) | |
| Other surgical procedure | 7 (21.2) | 39 (17.8) | |
| Hemostatic interventions | |||
| Prophylactic FFP | 8 (24.2) | 13 (5.9) | .002 |
| Prophylactic TXA | 1 (3.0) | 6 (2.8) | .9 |
| Prophylactic RBC | 0 | 2 (0.1) | .8 |
| Nonprophylactic FFP | 10 (30.3) | 0 | <.001 |
| Nonprophylactic TXA | 9 (27.3) | 0 | <.001 |
| Nonprophylactic RBC | 8 (24.2) | 0 | <.001 |
| . | Bleeding complication . | No bleeding complication . | P . |
|---|---|---|---|
| 33 | 219 | ||
| Laboratory values before delivery/procedure | |||
| FXI (U/dL) | 41.5 [14.0-52.0] | 49.0 [40.0-57.3] | .03 |
| Activated PTT (sec) | 32.8 [29.9-41.8] | 31.0 [28.7-34.2] | .03 |
| Hemoglobin (g/dL) | 12.0 [11.3-13.0] | 12.3 [11.6-13.3] | .2 |
| Platelets (109/L) | 186 [152-240] | 199 [173-232] | .3 |
| Blood loss during delivery/procedure (mL) | 659 [213-1000] | 286 [124-400] | <.001 |
| Blood loss following delivery/procedure (mL) | 58 [0-809] | 0 [0-36] | <.001 |
| Hemoglobin decrease following delivery/procedure (g/dL) | 2.8 [1.3-3.5] | 1.6 [1.0-2.6] | .003 |
| Procedure type | .006 | ||
| Vaginal delivery | 11 (33.3) | 132 (60.3) | |
| Cesarean delivery | 15 (45.5) | 48 (21.9) | |
| Other surgical procedure | 7 (21.2) | 39 (17.8) | |
| Hemostatic interventions | |||
| Prophylactic FFP | 8 (24.2) | 13 (5.9) | .002 |
| Prophylactic TXA | 1 (3.0) | 6 (2.8) | .9 |
| Prophylactic RBC | 0 | 2 (0.1) | .8 |
| Nonprophylactic FFP | 10 (30.3) | 0 | <.001 |
| Nonprophylactic TXA | 9 (27.3) | 0 | <.001 |
| Nonprophylactic RBC | 8 (24.2) | 0 | <.001 |
Data are presented as count (percentage) or median [interquartile range].
Among the entire population, cesarean delivery, prophylactic FFP administration, and personal history of bleeding were associated with higher odds of bleeding in the univariable logistic regression (Table 4). On multivariate logistic regression, a personal history of bleeding (odds ratio [OR], 5.92; P = .001), FXI levels (OR, 0.72 per 10 U/dL increase; P = .05), and cesarean deliveries (OR, 2.83; P = .04) remained significantly associated with the bleeding end point. When confined to obstetric patients alone (Table 5), only cesarean deliveries (OR, 3.40; P = .03) and personal history of bleeding (OR, 6.10; P = .002) remained significantly associated with the bleeding end point in the multivariable analysis. Age, ethnicity, genotype, family history of bleeding, preprocedural PTT, and platelet levels were not associated with bleeding risk.
Factors predicting bleeding complication among entire population
| . | Univariable logistic regression . | Multivariable logistic regression . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P value . | OR . | 95% CI . | P value . | |
| Pre-procedure FXI level (per 1 U/dL increase) | 0.98 | 0.96-1.00 | .1 | 0.97 | 0.95-1.00 | .05 |
| Pre-procedure FXI level (per 10 U/dL increase) | 0.83 | 0.68-1.02 | .1 | 0.72 | 0.54-0.97 | .05 |
| Pre-procedure PTT | 1.01 | 0.99-1.04 | .4 | |||
| Pre-procedure platelet count | 1.00 | 0.99-1.00 | .2 | |||
| Cesarean delivery (compared to vaginal delivery) | 3.74 | 1.61-8.70 | .002 | 2.83 | 10.4-7.72 | .04 |
| Use of prophylactic FFP | 1.28 | 1.00-1.65 | .05 | 2.05 | 0.33-12.80 | .4 |
| Use of prophylactic TXA | 1.10 | 0.13-9.43 | .9 | |||
| Blood type O+ | 0.44 | 0.17-1.12 | .08 | 0.39 | 0.12-1.24 | .1 |
| Personal history of bleeding | 4.07 | 1.88-8.82 | <.001 | 5.92 | 1.98-17.71 | .001 |
| Family history of bleeding | 1.37 | 0.37-5.05 | .6 | |||
| Type III mutation | 0.83 | 0.30-2.33 | .7 | |||
| . | Univariable logistic regression . | Multivariable logistic regression . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P value . | OR . | 95% CI . | P value . | |
| Pre-procedure FXI level (per 1 U/dL increase) | 0.98 | 0.96-1.00 | .1 | 0.97 | 0.95-1.00 | .05 |
| Pre-procedure FXI level (per 10 U/dL increase) | 0.83 | 0.68-1.02 | .1 | 0.72 | 0.54-0.97 | .05 |
| Pre-procedure PTT | 1.01 | 0.99-1.04 | .4 | |||
| Pre-procedure platelet count | 1.00 | 0.99-1.00 | .2 | |||
| Cesarean delivery (compared to vaginal delivery) | 3.74 | 1.61-8.70 | .002 | 2.83 | 10.4-7.72 | .04 |
| Use of prophylactic FFP | 1.28 | 1.00-1.65 | .05 | 2.05 | 0.33-12.80 | .4 |
| Use of prophylactic TXA | 1.10 | 0.13-9.43 | .9 | |||
| Blood type O+ | 0.44 | 0.17-1.12 | .08 | 0.39 | 0.12-1.24 | .1 |
| Personal history of bleeding | 4.07 | 1.88-8.82 | <.001 | 5.92 | 1.98-17.71 | .001 |
| Family history of bleeding | 1.37 | 0.37-5.05 | .6 | |||
| Type III mutation | 0.83 | 0.30-2.33 | .7 | |||
CI, confidence interval.
Factors predicting bleeding complication among obstetric population
| . | Univariable logistic regression . | Multivariable logistic regression . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P value . | OR . | 95% CI . | P value . | |
| Pre-procedure FXI level (per 1 U/dL increase) | 0.97 | 0.95-1.00 | .02 | 0.99 | 0.96-1.02 | .5 |
| Pre-procedure FXI level (per 10 U/dL increase) | 0.73 | 0.57-0.94 | .02 | 0.84 | 0.60-1.19 | .5 |
| Pre-prcedure PTT | 1.11 | 1.03-1.19 | .004 | 1.14 | 1.00-1.31 | .06 |
| Pre-procedure platelet count | 1.00 | 0.99-1.00 | .8 | |||
| Cesarean delivery (compared to vaginal delivery) | 3.75 | 1.61-8.73 | .002 | 3.40 | 1.15-10.03 | .03 |
| Use of prophylactic FFP | 1.76 | 1.06-2.95 | .03 | 0.32 | 0.03-3.38 | .3 |
| Blood type O+ | 0.61 | 0.23-1.60 | .3 | |||
| Personal history of bleeding | 3.42 | 1.36-8.62 | .009 | 6.10 | 1.90-19.57 | .002 |
| Family history of bleeding | 1.21 | 0.25-5.81 | .9 | |||
| Type III mutation | 0.67 | 0.28-2.25 | .7 | |||
| . | Univariable logistic regression . | Multivariable logistic regression . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P value . | OR . | 95% CI . | P value . | |
| Pre-procedure FXI level (per 1 U/dL increase) | 0.97 | 0.95-1.00 | .02 | 0.99 | 0.96-1.02 | .5 |
| Pre-procedure FXI level (per 10 U/dL increase) | 0.73 | 0.57-0.94 | .02 | 0.84 | 0.60-1.19 | .5 |
| Pre-prcedure PTT | 1.11 | 1.03-1.19 | .004 | 1.14 | 1.00-1.31 | .06 |
| Pre-procedure platelet count | 1.00 | 0.99-1.00 | .8 | |||
| Cesarean delivery (compared to vaginal delivery) | 3.75 | 1.61-8.73 | .002 | 3.40 | 1.15-10.03 | .03 |
| Use of prophylactic FFP | 1.76 | 1.06-2.95 | .03 | 0.32 | 0.03-3.38 | .3 |
| Blood type O+ | 0.61 | 0.23-1.60 | .3 | |||
| Personal history of bleeding | 3.42 | 1.36-8.62 | .009 | 6.10 | 1.90-19.57 | .002 |
| Family history of bleeding | 1.21 | 0.25-5.81 | .9 | |||
| Type III mutation | 0.67 | 0.28-2.25 | .7 | |||
Hemostatic interventions
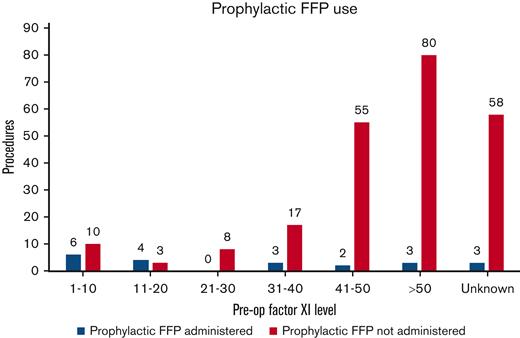
Prophylactic FFP was administered before 21 (8.3%) procedures in 17 patients. Mean FXI level for patients receiving prophylactic FFP was 25.6 U/dL (range, 1-71 U/dL) (Figure 1). Eleven of the 17 patients had severe FXI deficiency, whereas 6 patients had a partial deficiency. All patients with partial deficiency who were given empiric FFP were known to have a bleeding phenotype except 2, 1 with an FXI level of <40 and no prior surgical history, and another woman who was treated with a heparin infusion for a history of factor V Leiden mutation and was given a unit of FFP before urgent cesarean delivery.
Distribution of procedures in which prophylactic FFP was administered to patients across various FXI levels. Empiric FFP was administered before 47.6% vs 4.7% of the procedures performed in patients with severe FXI and partial FXI deficiency, respectively.
Distribution of procedures in which prophylactic FFP was administered to patients across various FXI levels. Empiric FFP was administered before 47.6% vs 4.7% of the procedures performed in patients with severe FXI and partial FXI deficiency, respectively.
Eight of these 21 (38%) procedures resulted in bleeding complications, despite prophylactic FFP use. Three of these 8 bleeding events occurred in patients who had previously undergone surgery, with prophylactic FFP without any hemorrhagic complications, and 3 events occurred in patients who received <10 mL/kg FFP dose periprocedurally. Only 7 patients in the study received prophylactic TXA. One of them experienced a bleeding complication. Postprocedural FFP and TXA were administered in 10 and 9 patients, respectively, to arrest bleeding or follow high-risk surgeries. Eight patients also required packed RBC transfusions for significant PPH or postprocedural hemorrhage.
Neuraxial anesthesia
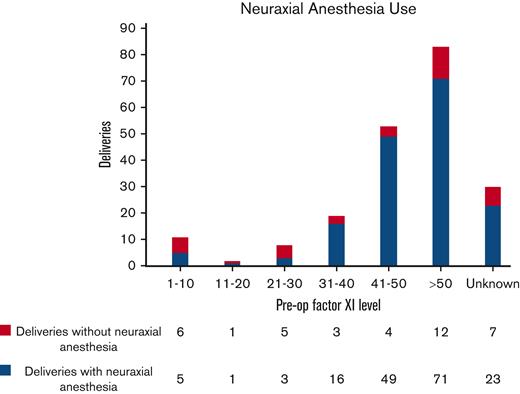
Neuraxial anesthesia was administered before 174 (70%) procedures. Mean FXI level for patients receiving neuraxial anesthesia was 50 U/dL (3-118 U/dL). There were no cases of epidural or spinal hematoma associated with neuraxial anesthesia in our cohort. Five patients with a negative bleeding history despite surgical challenges received neuraxial anesthesia at FXI levels of <10 U/dL without any complications. Only one of them received prophylactic FFP before surgery. In general, the majority of procedures in patients (78.7%) with FXI levels >30 U/dL were performed under neuraxial anesthesia, as compared to only 32.2% of procedures in patients with levels ≤30 U/dL. Among the obstetric procedures alone, most deliveries (168/206 or 81.5%) were performed under neuraxial anesthesia. Eighty-six percent of patients with preoperative FXI levels >30 U/dL received spinal/epidural anesthesia. Figure 2 illustrates the distribution of deliveries in which neuraxial anesthesia was administered to patients across various FXI levels.
Distribution of deliveries in which neuraxial anesthesia was administered to patients across various FXI levels. Eighty-six percent of patients with preoperative (pre-op) FXI levels >30 U/dL received spinal/epidural anesthesia before delivery.
Distribution of deliveries in which neuraxial anesthesia was administered to patients across various FXI levels. Eighty-six percent of patients with preoperative (pre-op) FXI levels >30 U/dL received spinal/epidural anesthesia before delivery.
ROC analysis
ROC curves were plotted for FXI levels to identify the cutoff with optimal sensitivity and specificity for predicting bleeding risk. The results were similar for all patients and obstetric patients only (Table 6). For the entire cohort, the area under the curve was 0.62, with a sensitivity of 47% and specificity of 75%, using an FXI cutoff value of 40 U/dL.
ROC analyses for FXI level cutoffs as a predictor of bleeding risk
| All patients . | Obstetrical patients only . | ||||
|---|---|---|---|---|---|
| Area under the curve, 0.62 . | Area under the curve, 0.64 . | ||||
| Cutoff . | Sensitivity . | Specificity . | Cutoff . | Sensitivity . | Specificity . |
| 10 | 0.16 | 0.93 | 10 | 0.16 | 0.96 |
| 20 | 0.28 | 0.89 | 20 | 0.24 | 0.95 |
| 30 | 0.34 | 0.85 | 30 | 0.32 | 0.91 |
| 40 | 0.47 | 0.75 | 40 | 0.44 | 0.80 |
| 50 | 0.69 | 0.46 | 50 | 0.68 | 0.48 |
| All patients . | Obstetrical patients only . | ||||
|---|---|---|---|---|---|
| Area under the curve, 0.62 . | Area under the curve, 0.64 . | ||||
| Cutoff . | Sensitivity . | Specificity . | Cutoff . | Sensitivity . | Specificity . |
| 10 | 0.16 | 0.93 | 10 | 0.16 | 0.96 |
| 20 | 0.28 | 0.89 | 20 | 0.24 | 0.95 |
| 30 | 0.34 | 0.85 | 30 | 0.32 | 0.91 |
| 40 | 0.47 | 0.75 | 40 | 0.44 | 0.80 |
| 50 | 0.69 | 0.46 | 50 | 0.68 | 0.48 |
FXI trend in pregnancy
At least 2 FXI levels measured at different timepoints during pregnancy were available for the 81 women. The mean FXI level did not change between these 2 measurements (49.7 U/dL at first measurement vs 48.3 U/dL later in pregnancy, P = .3).
Discussion
Our study assessed a large cohort of patients and investigated the risk factors of bleeding in patients with FXI deficiency. In a mixed cohort of parturients and other surgical patients, 13% of procedures were complicated by bleeding events. The most common bleeding event was PPH, which was found to be almost twice as high for the parturients with FXI deficiency as in the general population of women undergoing labor (11% vs 6%).16,17 PPH rates of 17% and 18% have been reported in recent systematic reviews including 490 and 372 deliveries in the studies by Davies et al and Wiewel-Verschueren et al, respectively.5,18 Similarly, postoperative bleeding was reported in ∼11% (5/46) of the other surgical procedures in our study as compared to 21% in the study by Santoro et al.19 On the other hand, the use of prophylactic interventions such as FFP or TXA was lower than the aforementioned studies (11% vs 20%).5,18
A personal history of bleeding was found to be the strongest risk factor for perioperative or obstetric bleeding events in this population (OR, 5.9; P = .001). This finding is consistent with the current literature that indicates a significant correlation between bleeding history and an increased risk of PPH.9,17-22 Moreover, a case-control study conducted by Stoeckle et al not only argued in favor of this correlation, but also pinpointed an increase in PPH only among cesarean deliveries compared with the control cohort.4 Similarly, in our study, cesarean deliveries were associated with a significantly increased risk of PPH as compared with vaginal deliveries (OR, 3.4; P = .003). Other analyses have also highlighted the importance of reviewing a patient’s personal history in conjunction with a family history of bleeding events while also evaluating the proposed intervention to be performed, as seen in the retrospective study conducted by Santoro et al.19 Family history of bleeding was not found to be a significant risk factor in our study.
Irrespective of an early series published in 199720 demonstrating a strong negative correlation between bleeding and FXI levels (r = −0.36, P = .0001), more recent studies have failed to establish such an association.9,19,21 Our data do not provide an unequivocal answer as to how to interpret FXI levels in this context. We found a statistically significant but weaker association between FXI concentration and bleeding risk (OR, 0.72 for 10 U/dL of FXI level increase). Although an FXI level cutoff of 40 U/dL can predict a lower bleeding risk with reasonable specificity (75%), no clear FXI level is adequately sensitive to detect all patients who are at an increased risk of bleeding. The lack of a reliable correlation between FXI levels and bleeding risk renders the management of patients with mild FXI deficiency without a bleeding history particularly challenging when screening patients for prophylactic interventions. This calls for the development of a disease specific bleeding score (BS) that can assist in standardizing this assessment. A recent Dutch study assessing bleeding severity in rare bleeding disorders showed no correlation between the International Society of Thrombosis and Hemostasis Bleeding Assessment Tool (BAT) score and FXI activity.23 A specific Rare Bleeding Disorders BAT developed by the European Network of Rare Bleeding Disorders group was shown to have a higher predictive power when compared to International Society of Thrombosis and Hemostasis BAT for rare bleeding diathesis, however, this remains to be validated in FXI deficiency.24 As of now, the only reliable BS standardized for bleeding disorders is the one published for von Willebrand disease by Tosetto et al,25 which was successfully employed by Guéguen et al26 to quantitatively evaluate the bleeding tendency in a small cohort of patients with FXI deficiency. Future studies attempting to develop and validate a clinical BS customized for FXI deficiency in a larger patient population would be particularly useful.
There is no established straightforward correlation between the genotype, FXI levels, and bleeding tendency. The most common FXI gene mutations described are type I mutations involving a splice site; type II, a Glu117stop mutation; and type III, a Phe283Leu substitution. In our study, there was no difference between FXI levels based on the underlying mutation, although it should be noted that mutational analysis was available for only half of the cases. Similarly, in the study by Salomon et al, type II/III mutation was associated with the highest frequency of PPH, although homozygotes with type II/II mutation had the lowest FXI levels.9 However, these differences were not statistically significant.9 In another cohort study, the genotype II/II had a stronger association with injury and surgery related bleeding than II/III, whereas another study did not find any difference in bleeding tendency in relation to genotype.2,15
Traditionally blood group O has been associated with a higher bleeding risk attributed to lower baseline von Willebrand factor (VWF) levels, with a suboptimal rise of VWF levels during pregnancy and a higher risk of PPH.27 More recently, a large observational study on the association of ABO blood group with bleeding severity in patients with bleeding of unknown cause showed that blood group O was associated with increased bleeding severity, independently from VWF and FVIII levels.28 As per the authors, the lack of an explanation for the increased bleeding severity in patients with blood group O was the greatest limitation of the study. In our study, no association between blood group type and the risk of bleeding was identified.
In an attempt to further identify and describe the most commonly encountered clinical dilemmas in this patient population, we specifically assessed patients undergoing neuraxial anesthesia. In our analysis, no significant adverse events resulted from this procedure, and there were no instances of epidural or spinal hematoma in 174 cases. These data are in line with growing evidence supporting the safety of epidural/spinal anesthesia during delivery in this population. In our study, a substantial majority (81.5%) of deliveries were performed under neuraxial anesthesia. This is higher than that previously reported in other studies on patients with FXI deficiency. The systematic review conducted by Davies et al in 2016, which looked at the use of labor analgesia in 6 different studies totaling 236 deliveries, showed no reported complications in any of the 73 procedures (31% of the deliveries) where regional block was administered.5 Similarly, Gerber et al appreciated in their study, more frequent use of neuraxial anesthesia (up to 59% of the deliveries) with no bleeding complications related to the analgesia even in women who experienced concomitant PPH.21 The latter also described 5 patients with severe FXI deficiency undergoing neuraxial anesthesia without bleeding complications, even though only 2 received FFP. Likewise, a small subset of 5 patients in our study with a negative bleeding history despite surgical challenges received neuraxial anesthesia at an FXI level <10 U/dL without any complications, and only one of them received prophylactic FFP before surgery. Yet, a considerable number of patients with severe deficiency, 50% in the recent study by Gerber et al and 70% in our cohort, did not receive epidural injections due to concerns for adverse reaction.21 On the other hand, patients with partial deficiency have consistently been demonstrated to not be at risk for more severe complications originating from this anesthesia modality. In a single-center report from Israel where >30% of women who delivered were of Ashkenazi Jewish ancestry, FXI levels were checked on a random sample of 261 Ashkenazi women, and 15% of these women were found to have partial deficiency with levels <70 U/dL. Because no epidural/spinal hematomas were documented among 57 722 women who received neuraxial anesthesia during the span of a decade at this institute, the authors extrapolated the numbers to estimate that at least 2150 women with unknown partial FXI deficiency have safely undergone labor analgesia at their center.10 In sum, these data challenge the practice of withholding neuraxial anesthesia solely based on low FX1 levels.
The abovementioned results, in conjunction with the evidence that emerged from our study, are significant and should also be considered when evaluating the indications and actual benefits of prophylactic treatment in patients with FXI deficiency in the peripartum setting. Although clinically relevant, indications have not yet been objectively and consistently determined. It has been shown that prophylaxis may reduce the absolute number of bleeding events and complications in parturient patients, most importantly in those with severe deficiency or those with a personal history of bleeding.9,18
Other studies have directly investigated and discussed the available modalities for prophylaxis. These include antifibrinolytic agents, plasma-derived products, and recombinant FVIIa. Patients who are heterozygous, with a less prominent history of bleeding in the past, may safely be treated with TXA.17 Meanwhile, FFP has traditionally been indicated for patients with severe deficiency. The indication for FFP use, although beneficial, is not devoid of risks, especially in patients with cardiovascular conditions, because the large volume of plasma required for treatment may lead to fluid overload. Furthermore, the additional use of FFP is sometimes predicated on the follow-up of postinfusion FXI levels, which often cannot be obtained in a timely fashion.5,29 Given the lack of institutional or national guidelines on the use of prophylactic FFP for FXI deficiency, the practice of FFP prescription varied among providers within our institution. In general, the doses ranged from 10 to 20 mL/kg, with the lower end of the range being used for patients with an FXI >20 U/dL or no significant bleeding history, whereas a dose of 20 mL/kg was prescribed for those with levels <20 U/dL or prior bleeding history. No specific FXI was targeted, given the time lag in laboratory assessments.
Finally, we observed again that FXI activity levels remain stable during pregnancy. This indicates that trending these levels throughout pregnancy may be unnecessary, further sparing expenses and expediting clinical decision making in these cases. The consistent FXI levels before and during pregnancy have also been demonstrated by other authors in different settings.5,30-32
Given its retrospective design, our study has some limitations. Although we analyzed a large population, this study may have lacked the power to fully establish an FXI level cutoff for meriting prophylaxis. Setting up an international registry with an even larger cohort would allow for different results. This may help determine the impact of different mutations by analyzing a more diverse population from a genotypic perspective. Prospective studies aimed at investigating when to prescribe preoperative prophylactic treatment to patients with FXI deficiency would help to draw definitive conclusions. Nevertheless, such a study may not be feasible, considering the eventual severity of side effects from bleeding in these settings, particularly in obstetrical cases. Another important limitation is that TXA was not consistently administered in the setting of PPH in our study cohort despite TXA use being a standard practice among obstetricians since the WOMAN study established its efficacy in 2017.33 This reflects an important real-world scenario in which the standard of care measures may be overlooked due to a lack of familiarity with their applicability in rare bleeding disorders.
In summary, our study highlights the importance of the careful evaluation of individual patients. Personal history of bleeding was the strongest predictor of bleeding risk. In the absence of prior surgical challenges, FXI levels play a role in clinical decision making, as higher levels confer some degree of protection against hemorrhagic complications. Although an optimally sensitive cutoff remains to be established, an FXI level of 40 U/dL is reasonably specific for a nonbleeding end point. Our study reinforces the safety of neuraxial anesthesia in carefully selected patients with severe FXI deficiency. FXI levels remain stable during pregnancy.
Authorship
Contribution: E.F. and K.H. conceived and designed the study; S.H. and M.S. collected data; D.S.F. performed statistical analyses and interpretation of results; S.H., M.S., and G.S.D.C.C. performed the literature review and prepared the first draft of the manuscript; Y.B., L.C., D.S.F., K.H., and E.F. reviewed and revised the manuscript; and all authors reviewed the results and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shivani Handa, Division of Hematology-Oncology, Department of Medicine, Icahn School of Medicine at Mount Sinai, 1 Gustave Levy Pl, New York, NY 10029; e-mail: shivani.handa@mountsinai.org.
References
Author notes
Presented in abstract form at the 63rd annual meeting of the American Society of Hematology, Atlanta, GA, 12 December 2021.
Partial data from our patient cohort were also presented in poster form (#869), Factor XI Deficiency in Pregnant Women: A Case-Series from a New York City Hospital, at the American Society of Hematology 2020 conference.
Data from this study were previously published as oral abstract.34
Data can be made available in deidentified Excel sheets on request to the corresponding author, Shivani Handa (shivani.handa@mountsinai.org).