TO THE EDITOR:
Coronavirus disease (COVID-19) is a severe viral illness that has resulted in significant morbidity and mortality worldwide. Several vaccines have been created that can prevent disease transmission as well as disease severity and mortality. All COVID-19 vaccines use the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein as the antigenic substrate. However, serious adverse reactions have been reported, including more than 150 cases of thrombocytopenia after vaccination.1 A precise mechanism linking COVID-19 vaccination and severe thrombocytopenia has yet to be confirmed. Identifying this mechanism could facilitate the development of a diagnostic test.
The serotonin release assay (SRA) is the gold standard diagnostic test for heparin-induced thrombocytopenia (HIT),2 which is characterized by severe thrombocytopenia and a risk of thrombosis. By using the SRA, we recently showed that a subset of critically ill patients with COVID-19 tested positive for platelet-activating immune complexes.3 Similarly, Althaus et al4 showed that immunoglobulin G (IgG) antibodies from critically ill patients who have COVID-19 can also activate platelets and lead to thrombotic events. Here, we used a modified SRA to demonstrate spike-dependent, platelet-activating immune complexes in a patient who had vaccine-induced thrombocytopenia (VIT) after receiving the Moderna COVID-19 mRNA vaccine. Blood samples were referred to the McMaster Platelet Immunology Laboratory for testing. Clinical data were obtained with patient consent, and additional testing was completed in keeping with ethics approval by the Hamilton Integrated Research Ethics Board.
Anti-platelet factor 4 (PF4)/heparin antibody testing was performed by using an anti-PF4/heparin enzymatic immunoassay (LIFECODES PF4 enhanced assay; Immucor Gti Diagnostics, Waukesha, WI) for IgG, IgM, and IgA PF4/heparin antibodies. Standard SRA testing was conducted in the absence and presence of heparin (0.1, 0.3, and 100 U/mL) or with exogenous PF4 added as previously described.2,5,6 A modified SRA (spike-SRA) was performed via exogenous administration of SARS-CoV-2 spike protein at various concentrations (0, 0.5, 5, 50, and 100 µg/mL). We also performed tests with various amounts of the COVID-19 Moderna vaccine or polyethylene glycol (PEG2000). Anti-human CD32 antibody (IV.3) or intravenous immunoglobulin (IVIg) were added to the SRA to confirm FcγRIIa signaling. Additional assays for drug-induced immune thrombocytopenia were also used as previously described, including the commercially available platelet glycoprotein-specific enzyme immunoassay (PakPlus, Immucor Gti Diagnostics), in-house flow cytometry, and radioimmunoprecipitation to identify platelet autoantibody binding with washed donor platelets.7,8
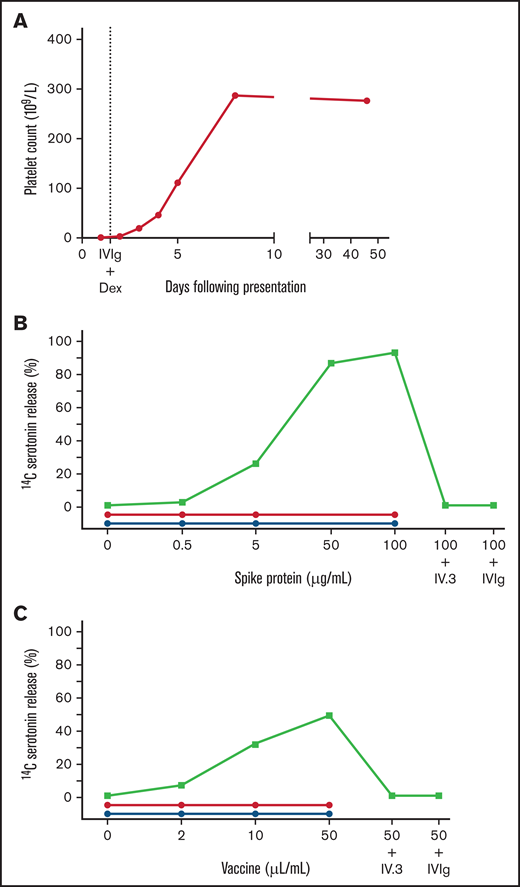
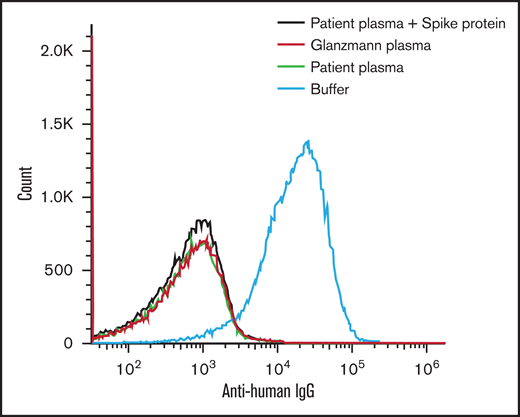
Our patient was a 25-year-old woman who presented to the hospital 10 days after receiving the Moderna mRNA COVID-19 vaccine with fatigue, petechiae, and wet purpura. The initial platelet count was 1 × 109/L 1000/mm3 without evidence of schistocytes on blood smear (Figure 1A). Coagulation studies were within the normal range, including prothrombin time of 13.6 seconds (normal, 10.7-15.6 seconds), international normalized ratio of 1.1 (normal, 0.8-1.3), and partial thromboplastin time of 30 seconds (normal, 22-35 seconds). The presence of a lupus anticoagulant was likely excluded, given the use of a lupus-sensitive reagent for partial thromboplastin time testing. Anti-PF4/heparin antibodies were not detected (optical density [OD], 0.221) and the SRA tests, with or without heparin or exogenous PF4, were negative. Assays for drug-induced immune thrombocytopenia with washed donor platelets as above were also negative for platelet binding with vaccine, PEG2000, or SARS-CoV-2 spike protein (eg, Figure 2).3 These results support an alternative diagnosis than drug-induced immune thrombocytopenia. The patient was treated with dexamethasone and IVIg for presumed immune thrombocytopenic purpura. The platelet count normalized by day 7 of treatment (Figure 1A).
Patient platelet count and functional activation in VIT. Patient platelet count (panel A) and investigation of platelet activation using a modified SRA with exogenous addition of spike protein (panel B) or vaccine (panel C). The platelet count fully recovered by day 7 of treatment with dexamethasone (Dex) and IVIg. Serum from the patient (green squares) caused dose-dependent platelet activation and serotonin release with spike protein (93%; 100 µg/mL) and with vaccine (53%; 50 µL/mL). This effect was not observed with plasma from patients who recovered from severe (n = 5; red circles) or mild infection (n = 3; blue circles) with COVID-19. The activation was inhibited with FcγRIIa blockade using the monoclonal antibody IV.3 (5 µg/mL) or IVIg (400 µg/mL).
Patient platelet count and functional activation in VIT. Patient platelet count (panel A) and investigation of platelet activation using a modified SRA with exogenous addition of spike protein (panel B) or vaccine (panel C). The platelet count fully recovered by day 7 of treatment with dexamethasone (Dex) and IVIg. Serum from the patient (green squares) caused dose-dependent platelet activation and serotonin release with spike protein (93%; 100 µg/mL) and with vaccine (53%; 50 µL/mL). This effect was not observed with plasma from patients who recovered from severe (n = 5; red circles) or mild infection (n = 3; blue circles) with COVID-19. The activation was inhibited with FcγRIIa blockade using the monoclonal antibody IV.3 (5 µg/mL) or IVIg (400 µg/mL).
Flow cytometry of IgG binding to the platelet surface. Platelets incubated with plasma sample from our patient (green) failed to show IgG binding with exogenous spike protein administration (red). Negative buffer control (black) and positive Glanzmann thrombasthenia (blue) are shown for comparison.
Flow cytometry of IgG binding to the platelet surface. Platelets incubated with plasma sample from our patient (green) failed to show IgG binding with exogenous spike protein administration (red). Negative buffer control (black) and positive Glanzmann thrombasthenia (blue) are shown for comparison.
Additional serum testing identified SARS-CoV-2 spike protein antibodies of the IgG (OD, 2.847), IgM (OD, 1.168), and IgA (OD, 3.130) classes.9 Antibodies against SARS-CoV-2 nucleocapsid protein were absent, confirming that the vaccine induced production of spike antibodies and there was no previous infection. To further investigate the mechanism of thrombocytopenia, we tested the patient’s serum by using a modified SRA (spike-SRA) with added recombinant SARS-CoV-2 spike protein. We observed dose-dependent platelet activation with increasing SARS-CoV-2 spike protein (0, 0.5, 5, 50, and 100 µg/mL; Figure 1B). The reaction was inhibited by an FcγRIIa blocker (IV.3; 5 µg/mL) and IVIg (400 µg/mL), confirming FcγRIIa-dependent platelet activation. Platelet activation was also demonstrated to a lesser degree with increasing amounts of Moderna COVID-19 vaccine (0, 2, 10, and 50 µL/mL; Figure 1C) and the excipient PEG2000. Spike-SRA platelet activation was not observed in patients with high-titer anti-spike antibodies who had recovered from severe (n = 5) or mild (n = 3) COVID-19. Furthermore, platelet activation was not detected in a negative control sample from a healthy subject who had received the Moderna COVID-19 vaccine and had not developed thrombocytopenia; this was measured by P-selectin expression using flow cytometry (data not shown). Circulating spike protein was detected in our patient’s serum using a commercial enzyme immunoassay testing kit ( 10.4 ng/mL; positive control , 17.8 ng/mL ; negative control 0.4 ng/mL, COVID-19 Spike Protein Elisa Kit; Abcam, Cambridge, UK). Together, these results suggest that the thrombocytopenia in this patient was secondary to FcγRIIa-mediated platelet activation by SARS-CoV-2 spike immune complexes.
Our serologic investigations highlight a potential mechanism for COVID-19 VIT involving SARS-CoV-2 spike-dependent FcγRIIa-mediated platelet activation. Similar immune complex–mediated platelet activation has also been observed with severe COVID-19 infection.2,4 The mechanism described here resembles platelet activation seen in HIT but does not involve anti-PF4/heparin antibodies. HIT serves as a useful analogy, but certain key differences were noted in our patient. Notably, our patient presented with bleeding symptoms as opposed to thrombosis; however, in parallel to HIT, not all patients with platelet-activating antibodies develop thrombosis.10 Finally, it is unclear why only a minority of patients with anti-spike antibodies exhibit thrombocytopenia and platelet activation. One hypothesis is that platelet activation is dependent on unique spike protein epitopes, which are recognized by only a minority of identified antibodies, as seen in HIT.11 Therefore, using our knowledge of platelet activation from studying HIT, we propose this mechanism for COVID-19 VIT involving SARS-CoV-2 anti-spike antibodies.
It is important to recognize that the mechanism described here is different from the recently reported, extremely rare HIT-like syndrome known as vaccine induced thrombotic thrombocytopenia (VITT). In VITT, patients present with life-threatening thrombosis in the context of strongly positive, platelet activating anti-PF4/heparin antibodies that present following adenoviral-based COVID-19 vaccination.12 The mechanism of VIT proposed here is independent of anti-PF4/heparin antibodies seen in VITT and presents differently.
Our patient also highlights the applicability of the SRA for detecting platelet activation disorders aside from HIT. Although the SRA is classically performed in the presence of heparin, it can be modified to include various antigens to elicit immune complex formation and identify platelet activation. In our patient, the addition of spike protein led to significant platelet activation that was inhibited by IV.3 and IVIg, which suggests immune complex signaling. The SRA may thus prove useful in a variety of other clinical scenarios involving platelet activation, as has been shown here.
Ultimately, the role of SARS-CoV-2 spike protein requires further clarification in regard to platelet activation and its role in vaccine- and PEG-dependent platelet activation. We postulate that the small subset of antibodies against the spike protein that formed after vaccination can activate platelets and cause thrombocytopenia. The prevalence of this phenomenon still needs to be clinically determined. Regardless, the modified SRA presented here may be a useful diagnostic test as more cases of VIT are recognized.
Acknowledgments: The authors thank the laboratory of Matthew Miller (PhD), at McMaster University for providing SARS-CoV-2 spike protein.
This work was supported by grants from the COVID-19 Rapid Research Fund (C-191-2426729-NAZY), the Canadian Institute of Health Research-COVID-19 Immunity Task Force (VR2-173204), the Ontario Research Fund (2426729) (I.N.), and the Academic Health Sciences Organization (HAH-21-02) (D.M.A.).
Contribution: J.A. and T.G. provided clinical care for the patient, analyzed and interpreted data, and helped write the manuscript; D.M.A. and J.G.K. designed the research and helped write the manuscript; S.D.J. analyzed and interpreted data and helped write the manuscript; N.I. performed additional experiments and analyses; J.W.S. performed the described studies, analyzed data, and helped write the manuscript; I.N. designed the research, analyzed and interpreted data, and helped write the manuscript; and all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ishac Nazy, Michael G. DeGroote School of Medicine, McMaster University, HSC 3H53, 1280 Main St West, Hamilton, ON L8S 4K1, Canada; e-mail: nazyi@mcmaster.ca.
References
Author notes
For data sharing, please contact Ishac Nazy via email at nazyi@mcmaster.ca.