Key Points
Clofarabine added to standard treatment of adults with newly diagnosed ALL does not improve event-free and overall survival.
Clofarabine is associated with more toxicity and more patients going off protocol, which might have blunted a better MRD response.
Abstract
Clofarabine (CLO) is a nucleoside analog with efficacy in relapsed/refractory acute lymphoblastic leukemia (ALL). This randomized phase 3 study aimed to evaluate whether CLO added to induction and whether consolidation would improve outcome in adults with newly diagnosed ALL. Treatment of younger (18-40 years) patients consisted of a pediatric-inspired protocol, and for older patients (41-70 years), a semi-intensive protocol was used. Three hundred and forty patients were randomized. After a median follow-up of 70 months, 5-year event-free survival (EFS) was 50% and 53% for arm A and B (CLO arm). For patients ≤40 years, EFS was 58% vs 65% in arm A vs B, whereas in patients >40 years, EFS was 43% in both arms. Complete remission (CR) rate was 89% in both arms and similar in younger and older patients. Minimal residual disease (MRD) was assessed in 200 patients (60%). Fifty-four of 76 evaluable patients (71%) were MRD− after consolidation 1 in arm A vs 75/81 (93%) in arm B (P = .001). Seventy (42%) patients proceeded to allogeneic hematopoietic stem cell transplantation in both arms. Five-year overall survival (OS) was similar in both arms: 60% vs 61%. Among patients achieving CR, relapse rates were 28% and 24%, and nonrelapse mortality was 16% vs 17% after CR. CLO-treated patients experienced more serious adverse events, more infections, and more often went off protocol. This was most pronounced in older patients. We conclude that, despite a higher rate of MRD negativity, addition of CLO does not improve outcome in adults with ALL, which might be due to increased toxicity. This trial was registered at www.trialregister.nl as #NTR2004.
Introduction
Outcome in adult patients with acute lymphoblastic leukemia (ALL) has substantially improved to ∼50% event‐free survival (EFS) during the last decades.1-3 However, a substantial proportion of patients, especially above 40 years of age, will develop a relapse, despite efforts to intensify established treatment approaches, including allogeneic hematopoietic stem cell transplantation (alloHSCT). Outcome after relapse is still very unsatisfactory in adult patients.4,5 Therefore, prevention of relapse is still the major goal of frontline treatment in ALL. Several clinical studies have shown that patients with ALL and measurable residual disease (MRD)− complete remission (CR) who are consolidated with chemotherapy or alloHSCT had better survival than patients with MRD+ CR.5-9 Therefore, the need to achieve MRD negativity before proceeding with consolidation treatment is currently considered a major treatment goal.
Clofarabin (CLO) is a second generation, halogenated, nucleoside analog that combines the positive activities of the 2 first-generation purine nucleotides fludarabine and cladribine but with less toxic and less neurological side effects.10-12 It was approved in 2004 for relapsed or refractory ALL in patients 1 to 21 years old. CLO proved to be well tolerated and effective as an antileukemic drug when used as monotherapy or in combination with other DNA-damaging drugs.13-16 In a phase 2 study reported by Kantarjian et al, 62 adult patients with relapsed or refractory acute leukemia received CLO for 5 days with an overall response rate of 48%, whereas in ALL, this this was only 2/12 (17%).15 Similar low response rates were shown in studies where CLO was combined with cytarabin.17 CLO combined with cylophosphamide was more promising in adults, especially at first salvage.18-22 The combination of CLO with cyclophosphamide and etoposide in relapsed or refractory ALL showed remarkable remission rates but at the expense of substantial toxicities.16,19,23 In addition, toxicity appeared also considerable in acute myeloid leukemia (AML), as was observed by the HOVON-SAKK study group, which performed a phase 3 study demonstrating that CLO integrated in standard treatment regimens did reduce relapse rate but without improving survival.24 So far, the efficacy and toxicity of CLO in upfront treatment of ALL in combination with induction and consolidation chemotherapy has not been addressed in adults. Here, we report a large randomized phase 3 trial by the Dutch-Belgian HOVON study group in adult patients with ALL, comparing induction and consolidation therapy with vs without CLO added to prephase and as an extra consolidation course.
Methods
Patients
This study was conducted in 29 centers in the Netherlands, Belgium, and France from 23 October 2009 till 7 November 2016. Eligible patients were 18 to 70 years old and had a diagnosis of previously untreated precursor B- or T-ALL, mixed phenotype acute leukemia, or T-lymphoblastic lymphoma. Patients with mature B-cell ALL and acute undifferentiated leukemia were not eligible. Adequate renal and hepatic function were required. The study protocol was approved by independent ethics committees at each participating center, and the study was conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent.
Study design and treatment
Patients were randomly assigned to receive standard treatment without or with CLO 30 mg/m2 for 5 days given as monotherapy during prephase and after consolidation 1. All patients proceeded at day 8 after prephase with induction chemotherapy (± CLO), irrespective of hematological toxicity. The study was started as a randomized phase 2 feasibility study and continued as a randomized phase 3 study with CLO 30 mg/m2 after 60 patients had been randomized and feasibility of this dose was evaluated and approved by the Data Safety and Monitoring Board. We here report the results of the final analysis of the phase 3 part of the study. Randomization was stratified for age and immunophenotype (B- vs T-ALL). Treatment of younger (18-40 years) patients consisted of consecutive chemotherapy courses based upon a pediatric-inspired schedule as reported before, and older patients (41-70 years) were treated with a semi-intensive schedule25,26 (detailed in supplemental Tables 1 and 2). Central nervous system (CNS) prophylaxis was delivered intrathecally 12 to 18 times throughout the protocol. Before alloHSCT patients received at least 8 times intrathecally prophylaxis. No prophylaxis was given after alloHSCT. Cranial irradiation was only given in case of CNS localization provided the patient did not proceed to alloHSCT as these patients received their irradiation as part of the conditioning regimen. All patients were given daily low-dose trimethoprim/sulfamethoxazole for Pneumocystis jirovecii prophylaxis and valaciclovir for viral prophylaxis. Prevention of bacterial and fungal infections in patients with mucositis and neutropenia was recommended by use of penicillin, ciprofloxacin, and fluconazole. Granulocyte colony-stimulating factor was strongly recommended to all patients with neutropenia until recovery and was mandatory for patients ≤40 years old during remission induction course 1. The protocol was amended to include low molecular weight heparin-prophylaxis (nadroparin 5700 IU once daily) subcutaneously from start of prephase until 14 days after pegylated asparaginase for younger patients and until start of consolidation in older patients to reduce thrombo-embolic events in first course. Patients with t(9;22) positive ALL received the same regimen with the addition of a tyrosine kinase inhibitor therapy, preferably imatinib, in conjunction with chemotherapy. AlloHSCT with an HLA-identical sibling or HLA-identical (10/10) unrelated donor was offered to all CR1 patients after intensification 1 in patients ≤40 years and after consolidation 2 in patients >40 years of age; only in high-risk patients an alternative donor (cord blood, mismatched unrelated donor, or haploidentical related donor) was recommended in case of lack of a matched related or matched unrelated donor (10/10). AlloHSCT was not performed when there was no suitable donor or when the patient was not eligible for transplantation. Conditioning regimen before alloHSCT consisted of myeloablative conditioning (MAB) up to the age of 40. The regimens used were busulfan/cyclophosphamide or cyclophosphamide/total body irradiation (TBI). In patients >60 years old, a reduced-intensity conditioning (RIC) regimen was applied. The regimens used were fludarabin/TBI, fludarabin/melfalan/busulfan/fludarabine, or cyclophosphamide/fludarabin/TBI. In patients between 40 and 60 years old, the intensity of conditioning depended on physical fitness and centers policy.
Diagnostics
Baseline evaluation included evaluation of bone marrow (BM) morphology, and >20% leukemic cells were necessary for a diagnosis of ALL. Also, immunophenotyping was done, and these diagnostics were centrally reviewed. Cytogenetic analysis and molecular assessment for t(9;22) and 11q23 aberrations or BCR-ABL, including MLL-AF4 screening, were done. To detect extramedullary disease, a computed tomography scan was performed.
Criteria for response and endpoints
CR was defined by <5% leukemic blasts in a normocellular BM without peripheral leukemic cells and without extramedullary manifestations. Patients were considered as “CR on protocol” if CR was reached after at least 1 of the treatment cycles as planned. Relapse was defined by reappearance of disease either as unequivocal blasts in the BM (>5%), in the liquor, or at extramedullary sites after prior achievement of CR. Primary endpoint was EFS, which refers to the interval from randomization to the date of failure to enter a CR, death, or relapse, whichever occurs first. Secondary endpoints included CR rate, central assessment of MRD by real-time quantitative polymerase chain reaction (RQ-PCR) of rearranged immunoglobulin or T-cell receptor (TR) genes in the BM or by flow cytometry, disease-free survival (DFS; time from CR to relapse or death, whichever occurs first), relapse, nonrelapse mortality (NRM), overall survival (OS; time from randomization to death from any cause, patients still alive at last contact were censored), and adverse events (AEs).
Molecular minimal residual disease analyses
MRD levels were determined in BM by RQ-PCR of leukemia-specific rearranged immunoglobulin and TR genes with the use of clone-specific primers and a set of different germline TaqMan probes and germline primers.27-29 Quality control and standardized interpretation of RQ-PCR data were achieved following the guidelines of the European Study Group on MRD detection in ALL. Patients with MRD <10−4 were classified as MRD−. MRD evaluation took place after induction 1 and after consolidation 1 only in patients who were in CR. BM samples were sent to central reference laboratories at Erasmus MC (Rotterdam), Sanquin (Amsterdam) and VUB (Brussels). Results were classified as “molecular CR” in case of MRD negativity (defined as <10−4) at the respective time point with at least 1 RQ-PCR assay with a quantitative range of ≤104. Molecular MRD was the method of choice and was done centrally. If no material or targets were available for molecular analysis, local flow cytometric MRD data were used if available and if the applied assay allowed a sensitivity of at least 0.01%.
Immunophenotyping MRD analyses
BM samples were processed, bulk-lysed, and subsequently stained using 6 or 8 color stainings according to locally used protocols. One million to 4 million cells (if available) were acquired, and MRD positivity was defined if at least 20 ALL cells could be detected. MRD negativity was defined as MRD < 0.01% using an assay with a sensitivity of at least 0.01%.
Risk classification
Patients were classified as having high-risk disease if they met 1 of the following criteria: white blood cell count at diagnosis > 30 × 109/L for B-ALL and > 100 × 109/L in T-ALL, no CR after induction, or specific cytogenetic/molecular abnormalities (Ph chromosome or BCR-ABL, 11q23 aberrations, hypodiploidy, or complex karyotype). All other patients were classified as intermediate risk disease.
Statistical analysis
The study was powered on the randomization of patients that could be randomized to the final dose level of CLO for the phase 3 part of the study. Main endpoint for the comparison of the 2 treatment arms was EFS from registration. In order to detect with 80% power (2-sided significance level α = 0.05; 1:1 randomization) an improvement of EFS with hazard ratio (HR) = 0.65, which corresponds to an improvement of the CR rate from 85% to 90%, and 2-year EFS from 40% to 55%, 174 events had to be observed. This would require 316 patients to be accrued in 3.5 to 4 years, with an expected accrual of about 90 patients per year, and 2 years of follow-up after the last registered patient. In order to overcome possible dropout, 340 patients would be registered in the phase 3 part. Randomization between standard treatment without or with CLO was done with a minimization procedure, stratified by age (18-40 vs 41-70 years), precursor B-ALL vs T-ALL immunophenotype and center, ensuring balance within each stratum and overall. The formal test for the difference in EFS between the 2 treatment arms would be done with a multivariate Cox regression analysis with adjustment for the stratification factors age (18-40 vs 41-70 years) and immunophenotype (B cell vs T cell). As a sensitivity analysis, we also performed a nonmodeling-based stratified logrank test for difference in EFS between the 2 treatment arms.
All analyses would be according to the intention-to-treat principle (ie, patients would be analyzed according to the treatment arms they were assigned to). However, patients initially randomized but considered ineligible afterward based on information that should have been available before randomization would be excluded from all analyses (modified–intention-to-treat).
Secondary efficacy endpoints were response rate (hematological, as well as molecular), DFS from CR, OS from randomization, and DFS and OS from allogeneic transplantation and from start maintenance. Actuarial probabilities of EFS, DFS, and OS at appropriate timepoints including 95% confidence intervals (CIs) were calculated using the actuarial method of Kaplan and Meier. Kaplan-Meier survival curves were constructed to illustrate survival. Response rates were compared between the 2 arms using logistic regression analysis or the Fisher exact test, whichever appropriate.
Adverse events and infections were scored according to the National Cancer Insitute Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. A serious adverse event (SAE) was defined as any untoward medical occurrence that resulted in death; was life threatening; lead to (prolongation of) hospitalization; or resulted in disability, a congenital anomaly/birth defect, or any other medically important condition. SAEs had to be reported from the first study-related procedure until 30 days following the last protocol treatment or until the start of subsequent therapy for the disease under study. SAEs occurring after 30 days also had to be reported if considered to be at least possibly related to CLO by the investigator. All analyses were performed using Stata (StataCorp. 2019. Stata: Release 16. Statistical Software; StataCorp LLC, College Station, TX).
Results
Patient characteristics
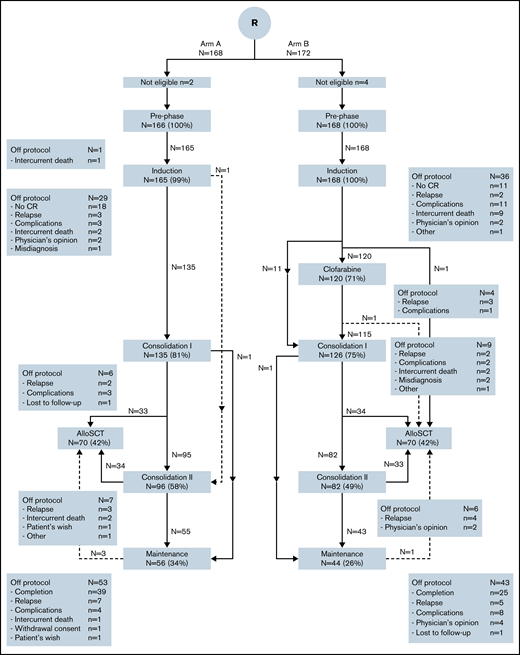
Three hundred and forty patients were randomized between standard treatment without or with CLO. One hundred and sixty-eight patients were randomized to standard treatment and 172 patients to the CLO arm. Six patients were ineligible: 5 patients due to misdiagnosis and 1 due to breast cancer within 5 years of diagnosis of ALL (CONSORT diagram, Figure 1; subgroups in supplemental Figures 1-3). Table 1 summarizes the main baseline characteristics of eligible patients. Both groups were balanced in terms of age, World Health Organization (WHO) performance status, immunophenotype, risk group, and BCR-ABL positivity. Two hundred and ten patients were considered high risk, with 102 (61%) and 108 (64%) in the standard and CLO-arm, respectively.
CONSORT diagram of study. Arm B included CLO in prephase. In this diagram, the 2 age groups (younger and older than 40 years of age) are combined (for CONSORT diagrams in subgroups, see supplemental Figures 1-3). Treatment protocols are detailed in supplemental Tables 1 and 2. In this figure, induction includes induction course and consolidation A and B for patients ≤40 years and remission induction 1 and consolidation 1 for patients >40 years of age. Consolidation 1 consists of intensification course 1A and 1B and remission induction course 2 for younger and older patients, respectively, and consolidation 2 contains interphase and intensification 2 for younger and consolidation 2 for older patients.
CONSORT diagram of study. Arm B included CLO in prephase. In this diagram, the 2 age groups (younger and older than 40 years of age) are combined (for CONSORT diagrams in subgroups, see supplemental Figures 1-3). Treatment protocols are detailed in supplemental Tables 1 and 2. In this figure, induction includes induction course and consolidation A and B for patients ≤40 years and remission induction 1 and consolidation 1 for patients >40 years of age. Consolidation 1 consists of intensification course 1A and 1B and remission induction course 2 for younger and older patients, respectively, and consolidation 2 contains interphase and intensification 2 for younger and consolidation 2 for older patients.
Patient characteristics at baseline according to randomization with or without CLO
| . | Standard treatment (arm A) . | CLO + standard treatment (arm B) . |
|---|---|---|
| Total, n | 166 | 168 |
| Age | ||
| Median (range), y | 42 (18-70) | 43 (18-70) |
| 18-40 y, n (%) | 79 (48) | 80 (48) |
| 41-70 y, n (%) | 87 (52) | 88 (52) |
| Performance status, n (%) | ||
| WHO 0 | 69 (42) | 72 (43) |
| WHO 1 | 69 (42) | 67 (40) |
| WHO 2 | 12 (7) | 13 (8) |
| WHO 4 | 1 (1) | — |
| Unknown | 15 (9) | 16 (10) |
| Sex, n (%) | ||
| Male | 96 (58) | 100 (60) |
| Female | 70 (42) | 68 (40) |
| Immunophenotype, n (%) | ||
| B-ALL | 118 (71) | 119 (71) |
| T-ALL | 29 (17) | 28 (17) |
| Mixed phenotype | 5 (3) | 4 (2) |
| T-LBL | 14 (8) | 17 (10) |
| WBC | ||
| Median ×109/L, range | 10.8 (0.5-524) | 11.0 (0.5-540) |
| >30 × 109/L for B-lineage | 33/120 (27) | 30/123 (24) |
| >100x109/L for T-lineage | 6/46 (13) | 2/45 (4) |
| % blast count in BM, median (range) | 88 (0-100) | 88 (0-100) |
| Cytogenetics and/or molecular analysis, n (%) | 7 (4) | 2 (1) |
| Not done/failure | 38/158 (24) | 30/166 (18) |
| t(9;22)/BCR-ABL | 10/156 (6) | 18/164 (11) |
| 11q23 abnormality/MLL fusion | 16/151 (11) | 15/156 (10) |
| Hypodiploidy | 23/151 (15) | 32/156 (21) |
| Complex karyotype | ||
| CNS involvement, n (%) | 5 (3) | 7 (4) |
| Risk group, n (%) | ||
| High risk | 102 (61) | 108 (64) |
| Standard risk | 64 (39) | 60 (36) |
| . | Standard treatment (arm A) . | CLO + standard treatment (arm B) . |
|---|---|---|
| Total, n | 166 | 168 |
| Age | ||
| Median (range), y | 42 (18-70) | 43 (18-70) |
| 18-40 y, n (%) | 79 (48) | 80 (48) |
| 41-70 y, n (%) | 87 (52) | 88 (52) |
| Performance status, n (%) | ||
| WHO 0 | 69 (42) | 72 (43) |
| WHO 1 | 69 (42) | 67 (40) |
| WHO 2 | 12 (7) | 13 (8) |
| WHO 4 | 1 (1) | — |
| Unknown | 15 (9) | 16 (10) |
| Sex, n (%) | ||
| Male | 96 (58) | 100 (60) |
| Female | 70 (42) | 68 (40) |
| Immunophenotype, n (%) | ||
| B-ALL | 118 (71) | 119 (71) |
| T-ALL | 29 (17) | 28 (17) |
| Mixed phenotype | 5 (3) | 4 (2) |
| T-LBL | 14 (8) | 17 (10) |
| WBC | ||
| Median ×109/L, range | 10.8 (0.5-524) | 11.0 (0.5-540) |
| >30 × 109/L for B-lineage | 33/120 (27) | 30/123 (24) |
| >100x109/L for T-lineage | 6/46 (13) | 2/45 (4) |
| % blast count in BM, median (range) | 88 (0-100) | 88 (0-100) |
| Cytogenetics and/or molecular analysis, n (%) | 7 (4) | 2 (1) |
| Not done/failure | 38/158 (24) | 30/166 (18) |
| t(9;22)/BCR-ABL | 10/156 (6) | 18/164 (11) |
| 11q23 abnormality/MLL fusion | 16/151 (11) | 15/156 (10) |
| Hypodiploidy | 23/151 (15) | 32/156 (21) |
| Complex karyotype | ||
| CNS involvement, n (%) | 5 (3) | 7 (4) |
| Risk group, n (%) | ||
| High risk | 102 (61) | 108 (64) |
| Standard risk | 64 (39) | 60 (36) |
LBL, lymphoblastic lymphoma; N, number of patients; WBC, white blood cell count at diagnosis.
Treatment and response
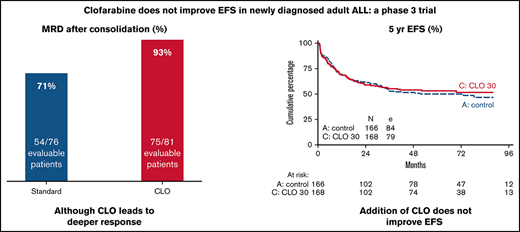
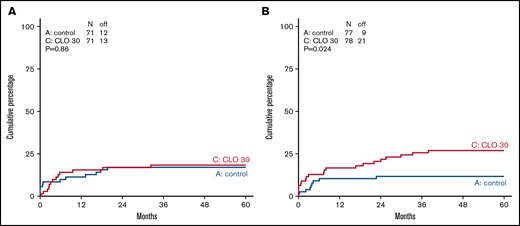
Overall, 236/334 (71%) patients completed protocol treatment, (ie, received alloHSCT or started with maintenance treatment). Patients randomized for standard arm completed treatment in 74%, and 67% of patients completed treatment in CLO arm. According to age category, 78% and 71% (younger patients ≤40 years) and 70% and 64% (older patients >40 years) completed treatment in the standard and CLO arm, respectively. Because CLO might induce early BM toxicity and therefore might prolong regeneration after induction 1, the time between start of induction 1 and start of CLO-consolidation 1 in both arms and both age groups was calculated. For patients 18 to 40 years old, median duration between start of induction 1 and start of CLO-consolidation 1 was 111 days (interquartile range [IQR], 102-122) in arm A vs 108 (IQR, 100-119) days in arm B. For older patients, median duration was 67 days (IQR, 61-75) in arm A vs 65 (IQR, 54-74) days in arm B. So, neither in the younger nor in the older patients was there a statistically significant difference. In total, 12 patients (see Figure 1) did not receive CLO consolidation while still on protocol. In 10/12 (83%) cases this seemed due to toxicity during CLO in prephase. Two patients refused to proceed. More CLO-treated patients who were in CR went off protocol due to other reasons (mainly toxicities) than relapse, death, or normal completion, which was statistically significant in older patients (P = .024, Figure 2). Overall, 89% of patients achieved a CR during protocol treatment, which proved similar in both study arms (Table 2). Quantification of MRD by RQ-PCR analysis of rearranged immunoglobulin/TR genes (n = 167) or by flow cytometry (n = 33) was performed in 200 (60%) patients, with 99 and 101 in arm A and B, respectively. The main reason for not having a MRD status was no available material. Fifty-four out of 76 evaluated patients (71%) were MRD− (defined as <10−4) after the first consolidation course in the standard arm vs 75 out of 81 (93%) in arm B (P = .001) (Table 2). The MRD response after the first consolidation was 75% and 94% in younger patients for the standard vs the CLO-arm, respectively. For older patients, these percentages were 68% and 91%, respectively. Overall, as presented in Table 2, 78 out of 297 CR patients developed a relapse (26%), which were evenly distributed among the study arms: 42 of 148 patients in the standard arm (28%) vs 36 out of 149 CLO-treated patients (24%). The relapse rate in younger patients was 32/142 (23%), with no significant difference between both arms (27% in arm A vs 18% in arm B). For older patients, these data were 30% vs 29%, respectively. Patients who were MRD− after the first consolidation course developed a relapse in 23 out of 126 patients (18%), whereas CR1 patients who were MRD+ developed a relapse in 11/25 (44%) of cases (Table 2). Thus, MRD negativity after consolidation was associated with a lower relapse rate (HR, 0.35; 95% CI, 0.17-0.71).
Cumulative incidence of going off protocol not due to completion, relapse, or death in CR patients in standard arm and CLO-arm. Cumulative incidence for going of off protocol not due to completion, relapse, or death is shown in patients ≤40 years of age (A) and patients >40 years of age (B) in control (blue) vs CLO group (red).
Cumulative incidence of going off protocol not due to completion, relapse, or death in CR patients in standard arm and CLO-arm. Cumulative incidence for going of off protocol not due to completion, relapse, or death is shown in patients ≤40 years of age (A) and patients >40 years of age (B) in control (blue) vs CLO group (red).
Response according to randomization for CLO
| . | Standard treatment (arm A), n = 166 . | CLO + standard treatment (arm B), n = 168 . |
|---|---|---|
| CR | ||
| After induction cycle 1, n (%) | 131 (79) | 131 (78) |
| After consolidation, n (%) | 143 (86) | 145 (86) |
| CR on protocol, n (%) | 148 (89) | 149 (89) |
| MRD negativity* | ||
| After RI 1 | 45/83 (54) | 62/88 (70) |
| After consolidation 1 | 54/76 (71) | 75/81 (93) |
| On protocol | 76/99 (77) | 88/101 (87) |
| Relapsed disease | ||
| Relapse after CR | 42/148 (28) | 36/149 (24) |
| Relapse in MRD− patients after consolidation 1 | 10/54 (19) | 13/72 (18) |
| Relapse in MRD+ patients after consolidation 1 | 8/20 (40) | 3/5 (60) |
| Nonrelapse mortality in CR, n (%) | ||
| ≤40 y | 5/71 (7) | 7/71 (10) |
| >40 y | 19/77 (25) | 17/78 (22%) |
| . | Standard treatment (arm A), n = 166 . | CLO + standard treatment (arm B), n = 168 . |
|---|---|---|
| CR | ||
| After induction cycle 1, n (%) | 131 (79) | 131 (78) |
| After consolidation, n (%) | 143 (86) | 145 (86) |
| CR on protocol, n (%) | 148 (89) | 149 (89) |
| MRD negativity* | ||
| After RI 1 | 45/83 (54) | 62/88 (70) |
| After consolidation 1 | 54/76 (71) | 75/81 (93) |
| On protocol | 76/99 (77) | 88/101 (87) |
| Relapsed disease | ||
| Relapse after CR | 42/148 (28) | 36/149 (24) |
| Relapse in MRD− patients after consolidation 1 | 10/54 (19) | 13/72 (18) |
| Relapse in MRD+ patients after consolidation 1 | 8/20 (40) | 3/5 (60) |
| Nonrelapse mortality in CR, n (%) | ||
| ≤40 y | 5/71 (7) | 7/71 (10) |
| >40 y | 19/77 (25) | 17/78 (22%) |
CR, complete remission; MRD, minimal residual disease; N, number of patients; RI, remission induction course.
Indicates the number of patients for whom a sample was obtained.
Subgroup analysis
No convincing indication was found that subgroups (Ph+/Ph−, B-/T-ALL, T-ALL/T-LyLy, different age groups, intermediate-/high-risk group) would selectively benefit from addition of CLO (compared with the control treatment) (supplemental Table 3 and 4). Also, in Ph− patients, without a transplant, no advantage of CLO was found, but younger patients (Ph− and Ph+) who were not consolidated by alloHSCT showed a lower relapse rate if they had received CLO during induction and consolidation (36% vs 14%, in standard vs CLO arm, respectively) (supplemental Table 4). Of interest, 19 patients >60 years of age showed similar CR and MRD negativity as the 68 patients of 41 to 60 years of age (supplemental Table 3) in arm A. In arm B, CR and MRD rate were also similar between the 2 age groups, but MRD negativity rate was significantly higher in the CLO arm for both age groups than patients treated in standard arm. EFS after 5-year follow-up was 47 vs 50% in arm A vs arm B in patients aged 41 to 60 years old and 26 vs 28% for patients over 61 years of age.
Maintenance treatment and alloHSCT
Among all patients who completed consolidation and intensification courses, 100 patients (41%) received maintenance treatment and 140 (58%) proceeded to alloHSCT, without significant differences between study arms nor in the time to maintenance or the time to alloHSCT, taking the extra time for the CLO consolidation cycle in arm B into account (CONSORT diagram, Figure 1). AlloHSCT was applied in 68 younger patients 56 (82%) after MAB and 12 (18%) after RIC conditioning regimen, and 72 older patients (17 [24%] MAB, 55 [76%] RIC). Donor type was a matched sibling in 55 patients, matched unrelated donors in 76 patients, and a mismatched unrelated donor in 1 patient, and 8 patients received cord blood stem cells. AlloHSCT was evaluated as a time-dependent covariate to address the question of whether alloHSCT was associated with better outcome. However, no such effect was observed. Results are shown in the supplemental Table 5, indicating that multivariable analysis with alloHSCT as a time-dependent covariate firmly showed that age 41 to 70 years was the predominant variable associated with DFS (HR, 2.13; 95% CI, 1.47-3.09; P < .001).
Event-free, overall, and disease-free survival
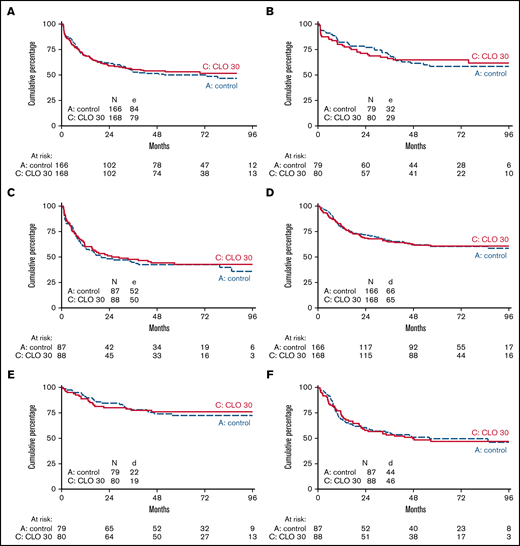
The median follow-up of 203 patients alive was 70 months (IQR, 55-88 months). EFS at 5 years from randomization was 50% (95% CI, 42-57) in patients receiving standard treatment and 53% (95% CI, 45-60) for CLO (HR, 0.93; 95% CI, 0.69-1.27; P = .67 adjusted for age and phenotype). For patients ≤40 years, 5-year EFS was 58% (95% CI, 47-69) vs 65% (95% CI, 53-74) in arm A and B, respectively, whereas in patients >40 years, 5-year EFS was 43% (95% CI, 32-53) in both arms (Figure 3A-C). In addition, OS was not significantly different. Five-year OS was 60% (95% CI, 52-68) in arm A vs 61% (95% CI, 53-68) in arm B (HR, 0.98; 95% CI, 0.70-1.38; P = .92). Patients ≤40 years old showed an OS of 72% at 5 years vs 76% in arm A and B, respectively. Patients >40 years old showed an OS of 50% and 47% in arm A vs B, respectively (Figure 3D-F). Five-year DFS was 56% in arm A vs 60% in arm B (HR, 0.90; 95% CI, 0.64-1.28; P = .57) and proved similar in younger and older patients in either study arm.
EFS and overall survival of patients treated according to standard arm vs additional CLO. EFS (A-C) and OS (D-F) of patients treated according to standard arm vs additional CLO are shown. Panels A and D show survival of all age groups together. Panels B and E are patients ≤40 years of age. Panels C and F are patients >40 years of age.
EFS and overall survival of patients treated according to standard arm vs additional CLO. EFS (A-C) and OS (D-F) of patients treated according to standard arm vs additional CLO are shown. Panels A and D show survival of all age groups together. Panels B and E are patients ≤40 years of age. Panels C and F are patients >40 years of age.
Safety and tolerability
The 2 treatment arms were compared with respect to AEs, NRM in CR patients, and number of patients in each treatment arm stopping protocol treatment not due to completion, relapse, or death. Percentages of grades 3 to 4 AE were 89% and 93% in arms A and B, respectively. CLO-treated patients experienced more SAEs, 70% vs 82% in younger patients (≤40 years of age) and 52% vs 75% in patients >40 years of age (Table 3). Grade 3 and 4 infections occurred in both groups but were significantly more present in the CLO-treated patients (45% vs 66% in arm A and B, respectively [P < .001]), with respiratory tract infections, sepsis, and abdominal infections being the most prevalent types of infection. In younger as well as in older patients, this significant difference between arms was similar (≤40 years of age, 53% vs 66%, and in patients >40 years of age, 37% vs 67% in arm A vs B, respectively [Table 3; detailed in supplemental Table 6]). Thrombo-embolic events were frequently reported, but no difference was seen between arms in both age groups (34% vs 25% in patients ≤40 years in arm A and B, respectively, and 26% in patients >40 years on both arms).
Adverse events occurring during treatment (CTCAE grade ≥3)
| . | Standard treatment (arm A) . | CLO + standard treatment (arm B) . | ||
|---|---|---|---|---|
| ≤40 y, n = 79 . | >40 y, n = 87 . | ≤40 y, n = 80 . | >40 y, n = 88 . | |
| Any AE grade ≥3, n (%) | 73 (92) | 75 (86) | 74 (93) | 81 (92) |
| Infection | 42 (53) | 32 (37) | 52 (65) | 59 (67) |
| Gastro-intestinal | 29 (37) | 25 (29) | 29 (36) | 29 (33) |
| Neurological event | 12 (15) | 10 (11) | 15 (19) | 13 (15) |
| Thrombo-embolic events (CTCAE grade ≥2) | 27 (34) | 23 (26) | 20 (25) | 22 (25) |
| Any serious adverse event, n (%) | 55 (70) | 45 (52) | 66 (82) | 66 (75) |
| Fatal serious adverse event, n (%) | 2 (3) | 11 (13) | 6 (8) | 12 (14) |
| . | Standard treatment (arm A) . | CLO + standard treatment (arm B) . | ||
|---|---|---|---|---|
| ≤40 y, n = 79 . | >40 y, n = 87 . | ≤40 y, n = 80 . | >40 y, n = 88 . | |
| Any AE grade ≥3, n (%) | 73 (92) | 75 (86) | 74 (93) | 81 (92) |
| Infection | 42 (53) | 32 (37) | 52 (65) | 59 (67) |
| Gastro-intestinal | 29 (37) | 25 (29) | 29 (36) | 29 (33) |
| Neurological event | 12 (15) | 10 (11) | 15 (19) | 13 (15) |
| Thrombo-embolic events (CTCAE grade ≥2) | 27 (34) | 23 (26) | 20 (25) | 22 (25) |
| Any serious adverse event, n (%) | 55 (70) | 45 (52) | 66 (82) | 66 (75) |
| Fatal serious adverse event, n (%) | 2 (3) | 11 (13) | 6 (8) | 12 (14) |
Toxicity is graded according to National Cancer Insitute Common Terminology Criteria for Adverse Events (CTCAE, version 3).
CLO, clofarabine; N, number of patients.
The rates of treatment discontinuations due to any adverse event were 6% (10/166) in the control group and 11% (22/168) in the CLO group (<40 years, 8% vs 11%, and >40 years, 5% vs 14% in arm A and B, respectively) (Figure 1; supplemental Figures 1-3). Figure 2 shows that CLO-treated patients more often went off protocol due to other reasons than completion, relapse, or death while being in CR, especially in older patients (12% vs 27% in arm A and B). These other reasons consisted of toxicity (20), lost to follow-up (2), and alternative treatment (1) and therefore is a convenient parameter for cumulative toxicity (Figure 1; supplemental Figures 1-3). Fatal SAEs were reported in 2 vs 6 patients (3% vs 8%) in patients <40 years old and in 11 vs 12 patients (13% vs 14%) of patients >40 years of age. The most common reason for a fatal AE was infection. Five-year NRM in CR patients was 16% (standard error, 3%) in arm A vs 17% (standard error, 3%) in arm B.
Discussion
CLO is an effective antineoplastic drug that proved efficacious in several phase 2 studies in younger and older patients with relapsed or refractory ALL.12,13,15,30,31 Furthermore, combining CLO with conventional chemotherapeutic drugs including alkylating drugs and anthracyclines was suggested to result in synergistic activity.17,22,32 Combining CLO with conventional chemotherapy in an upfront treatment setting of pediatric very high-risk precursor B-ALL resulted in unacceptable toxicity.33 Its additive value in adult patients in the context of intensified chemotherapy, however, was not studied before. This paper reports the first large phase 3 study with mature follow-up on the use of CLO as an integrated drug in intensified chemotherapeutic schedules for both younger (≤40 years of age) and older adult patients (>40 years of age) with ALL as part of first-line treatment. The results of this study failed to reveal an improvement of EFS or OS, both in the subgroups of younger and older patients. Although hematological response rates were similar in both study arms, the addition of CLO resulted in a significantly better MRD response. In a subset of patients (60% of patients were evaluable), CLO was more effective than standard treatment in terms of eradication of residual disease. Nevertheless, the overall relapse rate appeared similar in either study arm as well as DFS and OS, which might be explained by a higher number of patients not completing full protocol treatment due to prematurely going off protocol for toxicity reasons.
The prognostic value of MRD early in the course of treatment has been shown in pediatric patients34-39 but also extensively in adult patients.8,40-43 Therefore, the question why the depth of the response did not translate into an improved outcome is relevant. The most likely explanation for the lack of efficacy is the finding that an increased rate of AEs, and possibly also death in CR patients, may have counterbalanced an early advantage of CLO. However, although more AEs were observed in CLO-treated patients, NRM appeared not significantly different between both study arms. Of interest, we did find a significantly higher proportion of CLO-treated patients going off protocol, especially in elderly patients, while being in CR. Going off protocol for other reasons than normal completion, relapse, or death most often implies a continuation with a less intensive course of succeeding chemotherapy or an earlier switch to maintenance chemotherapy. Thereby, an initial beneficial effect of CLO may have been blunted by succession of insufficient intensified consolidation chemotherapy. That explanation would compare well to earlier findings in AML patients, in whom incorporation of CLO into intensified induction and consolidation chemotherapy also proved associated with a better MRD response but no improved outcome.24 The current results also confirm earlier concerns about toxicity with an increase in SAEs and infection rate related to CLO treatment. Because earlier studies exploring CLO in combination with cyclophosphamide for heavily treated and relapsing leukemia patients led to prohibitive toxicity,19,21 the CLO dose used in our study was reduced.20,22 In addition, the Children’s Oncology Group performed a randomized phase 3 study evaluating CLO upfront in children and adolescents with very high-risk ALL. Intensification of chemotherapy with CLO in this subgroup did not improve survival and appeared associated with considerable toxicity.33
This study has some limitations that must be considered when interpreting the results. First, adult ALL consists of many subgroups nowadays and much larger numbers of patients would be needed to address the value of CLO in each subgroup. This study was powered to detect a difference in the entire combined group of both younger and older and B-cell and T-cell ALL, in which a difference in favor of CLO was not found. We cannot exclude that CLO might be beneficial in a specific subset. Moreover, CLO was used at a lower dose (30 mg/m2) than initially used in CLO combination studies (40 mg/m2) with cytarabine or cyclophosphamide with or without etoposide.18-22 This might have affected efficacy, but it has to be taken into account that cumulative toxicity leading to more “off-protocol treatment” (as described in this study) suggests that intensive chemotherapy administered in successive courses might not allow the introduction of a higher dose of CLO. Lastly, the results with respect to MRD appeared encouraging, with a better MRD-response in CLO-treated patients. It, however, did not translate into better outcome, probably due to toxicity.
In conclusion, CLO added to induction and consolidation chemotherapy in adult patients with ALL does not improve EFS and OS, whereas CLO appeared associated with more toxicity and more patients going off protocol not due to completion, relapse, or death. Although CLO appeared associated with a higher incidence of MRD negativity in a subset of patients, relapse in either study arm appeared similar, which might possibly be due to increased toxicity and more patients going off protocol not due to completion, relapse, or death.
Acknowledgments
The authors would like to thank all laboratory technicians at Erasmus MC in Rotterdam, Sanquin in Amsterdam, VUB in Brussels, and SKION in Utrecht, all in the Netherlands. They would also like to thank the local institutional data managers as well as the HOVON Data Center Trial team for their support. The authors would also like to thank the members of the Data Safety and Monitoring Board: R. Pieters (Utrecht, The Netherlands), N. Gökbuget (Frankfurt, Germany), and V. Levy (Paris, France).
The authors thank The Dutch Cancer Foundation for financial support (grant 2008-4330). This investigator-sponsored trial was financially supported by Sanofi-Genzyme, and the drug clofarabine was provided free of charge.
Authorship
Contribution: This trial was conceived and designed by the Leukemia Working Group of the Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON), which included all authors; M.B., C.H.E.H., V.d.H., and V.H.J.v.d.V. performed immunodiagnostics and MRD analyses; B.J.B. reviewed cytogenetic analyses; A.W.R., O.d.W., B.J.B., A.A.v.d.L., L.E.v.d.W., M.B., M.v.G., W.J.F.M.v.d.V., D.S., D.v.L.-V., C.J.M.H., R.F., V.H., G.L.v.S., M.-C.L., D.D., A.G., H.A.M.S., D.A.B., A.J., O.L., W.E.T., R.S.B., D.M., A.T., L.W.T., K.B., J.A.M., and J.J.C. recruited patients; B.v.d.H. conducted statistical analyses; A.W.R., B.v.d.H., and J.J.C. reviewed the data analyses, including their interpretation, and produced the first version of the manuscript, which was circulated for comments to all authors; and all authors approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anita W. Rijneveld, Department of Hematology (NA-810), Erasmus University Medical Center, PO Box 2040, 3000 CA Rotterdam, The Netherlands; e-mail: a.rijneveld@erasmusmc.nl.
References
Author notes
Requests for data sharing may be submitted to Anita W. Rijneveld (a.rijneveld@erasmusmc.nl).
The full-text version of this article contains a data supplement.