Key Points
CARMEN regimen includes single doses of drugs delivered in 81 days (median), with good tolerability and lower risk of late toxicity.
A 5-year OS of ∼72% was recorded in MYC-translocated lymphomas, even in patients with HIV/AIDS, double-hit lymphoma, or meningeal disease.
Abstract
Patients with aggressive B-cell lymphoma and MYC rearrangement at fluorescence in situ hybridization exhibit poor outcome after R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone). In the last decade, 68 patients with Burkitt lymphoma ([BL] n = 46) or high-grade B-cell lymphoma ([HGBCL] single, double, or triple hit; n = 22) were treated with a dose-dense, short-term therapy termed “CARMEN regimen” at 5 Italian centers. Forty-six (68%) patients were HIV+. CARMEN included a 36-day induction with sequential, single weekly doses of cyclophosphamide, vincristine, rituximab, methotrexate, etoposide, and doxorubicin plus intrathecal chemotherapy, followed by high-dose-cytarabine–based consolidation. Patients who did not achieve complete remission (CR) after induction received BEAM (carmustina, etoposide, cytarabine, melfalan)-conditioned autologous stem cell transplantation (ASCT) after consolidation. Sixty-one (90%) patients completed induction, and 59 (87%) completed consolidation. Seventeen patients received ASCT. Grade 4 hematological toxicity was common but did not cause treatment discontinuation; grade 4 nonhematological toxicity was recorded in 11 (16%) patients, with grade 4 infections in 6 (9%). Six (9%) patients died of toxicity (sepsis in 4, COVID-19, acute respiratory distress syndrome). CR rate after the whole treatment was 73% (95% confidence interval [CI], 55% to 91%) for patients with HGBCL and 78% (95% CI, 66% to 90%) for patients with BL. At a median follow-up of 65 (interquartile range, 40-109) months, 48 patients remain event free, with a 5-year progression-free survival of 63% (95% CI, 58% to 68%) for HGBCL and 72% (95% CI, 71% to 73%) for BL, with a 5-year overall survival (OS) of 63% (95% CI, 58% to 68%) and 76% (95% CI, 75% to 77%), respectively. HIV seropositivity did not have a detrimental effect on outcome. This retrospective study shows that CARMEN is a safe and active regimen both in HIV-negative and -positive patients with MYC-rearranged lymphomas. Encouraging survival figures, attained with a single dose of doxorubicin and cyclophosphamide, deserve further investigation in HGBCL and other aggressive lymphomas.
Introduction
MYC translocations are a defining feature of Burkitt lymphoma (BL) and can be detected in a group of diffuse large B-cell lymphoma (DLBCL) and high-grade B-cell lymphoma (HGBCL). The term HGBCL was defined by the World Health Organization (WHO) 2017 classification1 and replaces the 2008 category of “unclassifiable B-cell lymphoma with features intermediate between DLBCL and BL.”2 A new category called HGBCL with MYC and BCL2 and/or BCL6 translocations has been established in the last WHO classification, which regards the cases of “double-/triple-hit” lymphomas. These MYC-driven lymphoma entities exhibit similar characteristics as those classically reported in patients with BL: that is, a more commonly disseminated disease, with involvement of multiple extranodal organs, increased lactate dehydrogenase (LDH) serum levels, high International Prognostic Index (IPI) score, high risk of central nervous system (CNS) dissemination, and inferior outcome when treated with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) chemoimmunotherapy.3,4 Available evidence suggests that intensified chemoimmunotherapy combinations are associated with high rates of disease control in HIV-negative and -positive patients with BL, whereas experience on the treatment of other MYC-translocated aggressive lymphomas is more limited. In the rituximab era, a few prospective trials, addressing modified GMALL, CODOX-M/IVAC (cyclophosphamide, vincristine, doxorubicin, methotrexate, ifosfamide, etoposide, cyatarbine), or DA-EPOCH (dose adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin) regimens, have been reported both in HIV-negative and -positive patients.5-7 Investigated regimens display high efficacy in patients with BL, with a 2-year overall survival (OS) of ∼70% but are delivered over 126 to 168 days and show important dose-limiting side-effects, prolonged hospitalization, and a treatment-related mortality of up to 16%. Related toxicity varied among the studies, which is in part explained by the different proportions of enrolled HIV+ patients. On average, one-third of patients did not complete treatment, often due to toxicity, such as severe mucositis, septic complications, and fungal infections, which were more common among HIV+ patients. Literature on the management of HIV/AIDS patients with HGBCL is even more limited; only a few cases are reported and usually analyzed together with patients with BL. Moreover, sometimes a central pathology review was not performed.
Some years ago, we performed a pilot retrospective study addressing efficacy and tolerability of a dose-dense, short-term chemotherapy program including 7 active drugs and intrathecal drug delivery in HIV/AIDS patients with BL, which was followed by a multicenter phase 2 trial called “CARMEN,” assessing the same combination in HIV/AIDS patients with BL, HGBCL not otherwise specified, and double-/triple-hit lymphomas.8,9 Both experiences have shown that CARMEN combination is safe and effective, with rare cases of grade 4 mucositis and opportunistic infections, a complete remission (CR) rate of 70% to 80%, and a 5-year OS of 75%. Following these encouraging survival and safety figures, Italian centers involved in these trials extended the use of the CARMEN program to HIV-negative and -positive patients with BL or HGBCL and MYC rearrangement.
Herein, we report the safety and efficacy of the CARMEN program in patients with MYC-driven aggressive lymphomas treated at 5 centers in the last 10 years. Results achieved after a long observation period prompted us to use this simple and cost-beneficial therapy in both HIV-negative and -positive patients with BL or HGBCL and to further investigate this strategy in aggressive non-Hodgkin lymphomas.
Patients and methods
Study population
Since 2010, a dose-dense, short-term sequential chemoimmunotherapy (CARMEN regimen) was offered as first-line treatment to patients with histologically proven diagnosis of BL, unclassifiable B-cell lymphoma with features intermediate between DLBCL and BL, or HGBCL at 5 Italian cancer centers. Feasibility, tolerability, efficacy, and late complications of treated patients were retrospectively analyzed. Both HIV− and HIV+ subjects and any stage of disease and ECOG (Eastern Cooperative Oncology Group) performance status (ECOG-PS) score were considered. Diagnostic histopathological material of treated patients was centrally reviewed by expert hemato-pathologists (F.F. and M.P.), fluorescence in situ hybridization (FISH) for MYC, BCL2, and BCL6 were centralized (L.P. and F.F.), and lymphoma entities were reclassified according to the WHO 2017 classification.1 DLBCL with MYC translocation and HGBCL without MYC translocation were excluded from this analysis. Patients with brain lesions were excluded, whereas patients with meningeal disease were considered. Hepatitis B virus (HBV) or hepatitis C virus (HCV) infections did not constitute an exclusion criterion. Due to the retrospective nature of this study and anonymized clinical data, ad hoc informed consent was waived. This study conformed to the Declaration of Helsinki and was approved by the institutional review boards of the involved institutions.
CARMEN treatment
Staging work-up and pretreatment tests included physical examination; peripheral blood cell counts; biochemical profile; HIV, HBV, and HCV serological evaluation; contrast-enhanced total-body computed tomography scan; 18 F-fluorodeoxyglucose-positron emission tomography (18 FDG-PET); gadolinium-enhanced whole-brain magnetic resonance imaging; bone marrow biopsy and aspirate; cerebrospinal fluid examination (cell count, physic-chemical exams, cytological examination, flow cytometry); and echocardiography. A pregnancy test was performed in each eligible female patient. In HIV+ patients, pretreatment tests also included CD4/CD8 T-cell quantification, HIV-RNA viral load, HIV resistance testing, citomegalovirus (CMV), Epstein-Barr virus and Toxoplasma immunoglobulin G and immunoglobulin M, HHV6-8, and parvovirus markers.
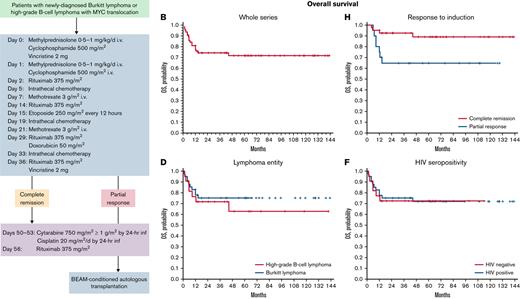
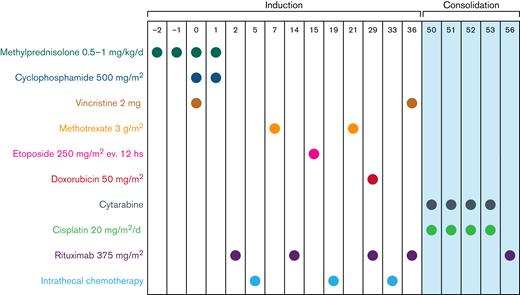
The CARMEN program consisted of a 36-day induction course of sequential doses of fractionated cyclophosphamide, vincristine, rituximab, methotrexate, etoposide, and doxorubicin, plus conventional triple-drug intrathecal chemotherapy (methotrexate 12 mg plus cytarabine 50 mg plus hydrocortisone 40 mg), delivered every 14 days by lumbar puncture (Table 1; Figure 1). In the case of meningeal involvement, intrathecal therapy consisted of 6 weekly doses in order to maintain dose intensity also on meninges/cerebrospinal fluid (CSF) as some used drugs (ie, cyclophosphamide, vincristine, rituximab, doxorubicin) do not achieve therapeutic concentrations in the CNS when delivered by IV route. Liposomal cytarabine 50 mg in alternative to conventional triple-drug scheme was permitted. Ommaya reservoir was not used.
Induction and consolidation phases
| Day . | Drug/dose/administration schedule . |
|---|---|
| Induction | |
| −2 | Methylprednisolone 0.5-1 mg/kg per d IV |
| −1 | Methylprednisolone 0.5-1 mg/kg per d IV |
| 0 | Methylprednisolone 0.5-1 mg/kg per d IVCyclophosphamide 500 mg/m2 over 1-h infusionVincristine 2 mg total dose IV bolus |
| 1 | Methylprednisolone 0.5-1 mg/kg per d IVCyclophosphamide 500 mg/m2 over 1-h infusion |
| 2 | Rituximab 375 mg/m2 |
| 5 | Methotrexate 12 mg + cytarabine 50 mg + steroids by IT route |
| 7 | Methotrexate 3 g/m2 IV over 6 h with leucovorin rescue therapy∗ |
| 14 | Rituximab 375 mg/m2 |
| 15 | Etoposide 250 mg/m2 every 12 h |
| 19 | Methotrexate 12 mg + cytarabine 50 mg + steroids by IT route |
| 21 | Methotrexate 3 g/m2 IV over 6 h with leucovorin rescue therapy# |
| 29 | Rituximab 375 mg/m2Doxorubicin 50 mg/m2 IV bolus |
| 33 | Methotrexate 12 mg + cytarabine 50 mg + steroids by IT route |
| 36 | Rituximab 375 mg/m2Vincristine 2 mg total dose IV bolus |
| Consolidation† | |
| HIV− | |
| 50-53 | Cytarabine 750 mg/m2 followed 3 h later by 1 g/m2 by 24-h infusionCisplatin 20 mg/m2 per d by 24-h infusion |
| 56 | Rituximab 375 mg/m2 |
| HIV+ | |
| 50-51 | Cytarabine 2 g/m2 in a 3-h infusion twice a day (every 12 h) |
| 52 | Rituximab 375 mg/m2 |
| 60 | Rituximab 375 mg/m2 |
| Day . | Drug/dose/administration schedule . |
|---|---|
| Induction | |
| −2 | Methylprednisolone 0.5-1 mg/kg per d IV |
| −1 | Methylprednisolone 0.5-1 mg/kg per d IV |
| 0 | Methylprednisolone 0.5-1 mg/kg per d IVCyclophosphamide 500 mg/m2 over 1-h infusionVincristine 2 mg total dose IV bolus |
| 1 | Methylprednisolone 0.5-1 mg/kg per d IVCyclophosphamide 500 mg/m2 over 1-h infusion |
| 2 | Rituximab 375 mg/m2 |
| 5 | Methotrexate 12 mg + cytarabine 50 mg + steroids by IT route |
| 7 | Methotrexate 3 g/m2 IV over 6 h with leucovorin rescue therapy∗ |
| 14 | Rituximab 375 mg/m2 |
| 15 | Etoposide 250 mg/m2 every 12 h |
| 19 | Methotrexate 12 mg + cytarabine 50 mg + steroids by IT route |
| 21 | Methotrexate 3 g/m2 IV over 6 h with leucovorin rescue therapy# |
| 29 | Rituximab 375 mg/m2Doxorubicin 50 mg/m2 IV bolus |
| 33 | Methotrexate 12 mg + cytarabine 50 mg + steroids by IT route |
| 36 | Rituximab 375 mg/m2Vincristine 2 mg total dose IV bolus |
| Consolidation† | |
| HIV− | |
| 50-53 | Cytarabine 750 mg/m2 followed 3 h later by 1 g/m2 by 24-h infusionCisplatin 20 mg/m2 per d by 24-h infusion |
| 56 | Rituximab 375 mg/m2 |
| HIV+ | |
| 50-51 | Cytarabine 2 g/m2 in a 3-h infusion twice a day (every 12 h) |
| 52 | Rituximab 375 mg/m2 |
| 60 | Rituximab 375 mg/m2 |
Dose intensity was maintained using granulocyte colony-stimulating factor whenever neutrophil count was ≤ 1.5 × 109/L. Antimicrobial prophylaxis (acyclovir 400 mg twice per day, fluconazole 100-200 mg once a day, and trimethoprim 160 mg/sulfamethoxazole 800 mg 3 times per week) was used. Levofloxacin 500 mg/d was added in patients with grade 4 neutropenia. Prophylaxis and treatment of HBV infection followed national guidelines. In brief, carriers of active infection (hepatitis B surface antigen (HbsAg) positivity and HBV DNA >2.000 IU) were treated with entecavir; carriers of inactive infection (HBV DNA undetectable levels) and carriers of occult infection (negative HbsAg, negative HBV DNA, and positive HBcAb) were treated with lamivudine (entecavir 0.5 mg/d for selected patients). Duration of prophylaxis was 12 months in HBsAg+ patients and 18 months in occult infection carriers. HBV reactivation was treated with tenofovir (entecavir 1 mg/d in case of renal dysfunction). HCV infection was monitored with frequent assessment of hepatic transaminases and HCV RNA level.
IT, intrathecal route.
IV alkalinization was used to promote excretion of methotrexate according to institutional guidelines. Calcium leucovorin was administered at a dose of 15 mg/m2 IV starting 24 hours after completing methotrexate infusion and continued every 6 hours for 12 doses or, in excess, until methotrexate blood levels were <0·2 μmol/L. Methotrexate serum levels were monitored at 48, 72, and 96 hours from methotrexate infusion, and leucovorin dose was adjusted according to methotrexate serum levels.
Methotrexate dose was 5 g/m2 in day 21 in HIV-negative patients.
Leukapheresis to collect autologous peripheral-blood stem cells was performed after consolidation, starting granulocyte colony-stimulating factor 24 hours after the last dose of cytarabine.
Treatment schedule. Drugs, doses, and plan of induction and consolidation phases. Intrathecal chemotherapy was delivered weekly in patients with meningeal disease. Methotrexate dose on day 21 was 5 g/m2 in HIV-positive patients. Graphic represents consolidation phase for HIV− patients. Consolidation phase for HIV+ patients is reported in Table 1.
Treatment schedule. Drugs, doses, and plan of induction and consolidation phases. Intrathecal chemotherapy was delivered weekly in patients with meningeal disease. Methotrexate dose on day 21 was 5 g/m2 in HIV-positive patients. Graphic represents consolidation phase for HIV− patients. Consolidation phase for HIV+ patients is reported in Table 1.
Subsequent treatment was tailored according to the objective tumor response to induction phase: patients in CR received high-dose-cytarabine–based consolidation, which differs between HIV− and HIV+ patients (Table 1; Figure 1); patients in partial response (PR) after induction received consolidation plus BEAM (carmustine, etoposide, cytarabine, melfalan)/FEAM (fotemustine, etoposide, cytarabine, melfalan)-conditioned autologous stem cell transplantation (ASCT) (Table 2); patients with stable or progressive disease after/during induction received high-dose sequential intensification (Table 2). Involved-field irradiation (36-Gy) was performed according to institutional guidelines.
Intensification phase and myeloablative chemotherapy
| . | Drug/dose/administration schedule . |
|---|---|
| Intensification | |
| 1° and 4° week | One or 2 courses of R-IVAC or R-ICE (debulking chemotherapy) |
| 7° to 8° week | Cyclophosphamide 4 g/m2 |
| Rituximab 375 mg/m2 on days 3 and 10 | |
| In vivo–purged PBPC collection (day 11-13) | |
| 11° to 12° week | Cytarabine 2 g/m2 every 12 h for 4 days (days −5 to −2) |
| Rituximab 375 mg/m2 (day −1 and +11) | |
| Second in vivo–purged PBPC collection (only if needed) | |
| BEAM regimen | |
| Day 1 | Carmustine∗ 300 mg/m2 |
| Days 2-5 | Etoposide 100 mg/m2 every 12 h |
| Cytarabine 200 mg/m2 every 12 h | |
| Day 6 | Melphalan 140 mg/m2 |
| Day 8 | Reinfusion of ≥ 5 × 106 CD34+ cells per kg body weight |
| . | Drug/dose/administration schedule . |
|---|---|
| Intensification | |
| 1° and 4° week | One or 2 courses of R-IVAC or R-ICE (debulking chemotherapy) |
| 7° to 8° week | Cyclophosphamide 4 g/m2 |
| Rituximab 375 mg/m2 on days 3 and 10 | |
| In vivo–purged PBPC collection (day 11-13) | |
| 11° to 12° week | Cytarabine 2 g/m2 every 12 h for 4 days (days −5 to −2) |
| Rituximab 375 mg/m2 (day −1 and +11) | |
| Second in vivo–purged PBPC collection (only if needed) | |
| BEAM regimen | |
| Day 1 | Carmustine∗ 300 mg/m2 |
| Days 2-5 | Etoposide 100 mg/m2 every 12 h |
| Cytarabine 200 mg/m2 every 12 h | |
| Day 6 | Melphalan 140 mg/m2 |
| Day 8 | Reinfusion of ≥ 5 × 106 CD34+ cells per kg body weight |
BEAM, XXX; R-ICE, rituximab, ifosfamide, carboplatin, etoposide; R-IVAC, rituximab, ifosfamide, etoposide, cytarabine, cyclophosphamide; PBPC, peripheral blood progenitor cells.
When it was not available, carmustine was replaced by fotemustine 150 mg/m2 per day days 1 and 2 (FEAM [fotemustine, etoposide, cytaraine, melfalan] regimen).
Duration of the whole program (days) was estimated from the first day of induction course to the last day of drug delivery of consolidation or to the date of autologous stem cell reinfusion; duration data are expressed in median and interquartile range (IQR). Patients at high risk of tumor lysis syndrome were hospitalized until day 24 (Table 1); patients without this risk received the first week of induction in outpatient setting and were hospitalized before day 7. Induction from day 24 to day 36 was delivered in outpatient setting if clinically indicated. Consolidation required hospitalization for 5 to 7 days in every case.
Outcome assessment and statistics
This was a retrospective, explorative study that did not follow a predetermined statistical plan. The analysis was aimed to define feasibility, tolerability, efficacy, and late effects of the CARMEN program in HIV-negative and -positive patients with BL or HGBCL and MYC translocation. Every patient who received at least the first day of treatment were considered assessable for toxicity and response. Treatment side effects were assessed separately for each chemotherapy phase and graded according to the National Cancer Institute-National Cancer Institute of Canada Common Toxicity Criteria (NCI-NCIC CTC) version 3.0.10 The worst toxicity per organ per patient was considered. CR rate according to investigator assessment was used as supportive evidence. Response was assessed after induction, after consolidation, and at the end of the whole program by whole-body computed tomography scan, 18FDG-PET, and other exams that were positive at baseline. Response definition followed the Revised Response Criteria for Malignant Lymphoma.11 In case of positive CSF, cytology examination was performed before every intrathecal chemotherapy dose; a reduction of >50% of cell number was considered PR, whereas a lower reduction was considered stable disease. The presence of tumor cells in CSF was confirmed by flow cytometry in all samples with ≥4 nucleated cells per μL.
Progression-free survival (PFS) and OS curves were generated using the Kaplan-Meier method and were estimated according to Revised Response Criteria for Malignant Lymphoma.11 Complementary radiotherapy for residual disease was not considered an event. The small number of events did not allow us to perform a reliable multivariable analysis. All analyses were carried out using the Statistica 10.0 statistical package for Windows (Statsoft Inc, 2011, Tulsa, OK).
Results
Study population
Sixty-eight patients (median age, 48; range, 21-77; 52 males) were treated at 5 centers between 2010 and 2020. The database lock for the present analysis was 31 December 2021. The analyzed series include 18 patients considered in previous reports.8,9 FISH analysis demonstrated MYC translocation in all cases. After central pathology review, 46 patients had a BL, and 22 patients had a HGBCL with MYC translocation: in detail, 12 patients had HGBCL not otherwise specified with single-hit MYC translocation, and 10 had HGBCL with MYC and BCL-2 and/or BCL-6 translocations (double-hit or triple-hit lymphoma). The main patient characteristics are summarized in Table 3. Most patients had high-risk disease, with large prevalence of advanced stage, extranodal disease, increased LDH serum levels, and age-adjusted IPI score ≥2. Forty-six (68%) patients had concomitant HIV infection, prevalently among patients with BL. Notably, the high prevalence of HIV+ patients in the present series does not reflect epidemiologic figures in the general lymphoma population, but it is due to the fact that participating institutions are referral centers for the treatment of patients with HIV-related lymphomas. Diagnoses of lymphoma and HIV infection were synchronous in 16 (35%) patients; the median interval between these diagnoses was 28 months (0-357 months) for the whole series. All the 30 patients with a prior diagnosis of HIV seropositivity received antiretroviral therapy at the time of lymphoma diagnosis: 6 were at first line, 6 at second line, 16 at third line, and 2 at fourth line. All patients had CD4+ counts under 600 cells per μL, with a median of 229 cells per μL (range, 16-560). Nine (13%) patients had CSF/meningeal disease, and 13 (19%) had bone marrow infiltration. Nineteen (28%) patients had HBV and/or HCV infection.
Patient characteristics
| . | HGBCL (n = 22) . | BL (n = 46) . |
|---|---|---|
| Median age (range) | 55 (26-77) | 45 (21-69) |
| Gender - males | 15 (68%) | 37 (80%) |
| ECOG-PS >1 | 8 (36%) | 18 (39%) |
| HIV seropositivity | 9 (41%) | 37 (80%) |
| HBV or HCV seropositivity | 4 (18%) | 15 (33%) |
| B symptoms | 8 (36%) | 20 (43%) |
| IPI ≥2 | 18 (82%) | 38 (83%) |
| Burkitt lymphoma IPI score ≥2∗ | NA | 25 (54%) |
| High LDH serum level | 18 (82%) | 37 (80%) |
| Stage (Ann Arbor) III-IV | 20 (91%) | 40 (87%) |
| Extranodal disease | 18 (82%) | 32 (70%) |
| CNS involvement† | 1 (4%) | 8 (17%) |
| Bone marrow infiltration | 2 (9%) | 11 (24%) |
| Bulky disease‡ | 11 (50%) | 22 (48%) |
| Single hit (FISH; MYC) | 12 (54%) | 46 (100%) |
| Double hit (MYC + BCL2 or BCL6) | 9 (41%) | — |
| Triple hit (MYC + BCL2 + BCL6) | 1 (4%) | — |
| . | HGBCL (n = 22) . | BL (n = 46) . |
|---|---|---|
| Median age (range) | 55 (26-77) | 45 (21-69) |
| Gender - males | 15 (68%) | 37 (80%) |
| ECOG-PS >1 | 8 (36%) | 18 (39%) |
| HIV seropositivity | 9 (41%) | 37 (80%) |
| HBV or HCV seropositivity | 4 (18%) | 15 (33%) |
| B symptoms | 8 (36%) | 20 (43%) |
| IPI ≥2 | 18 (82%) | 38 (83%) |
| Burkitt lymphoma IPI score ≥2∗ | NA | 25 (54%) |
| High LDH serum level | 18 (82%) | 37 (80%) |
| Stage (Ann Arbor) III-IV | 20 (91%) | 40 (87%) |
| Extranodal disease | 18 (82%) | 32 (70%) |
| CNS involvement† | 1 (4%) | 8 (17%) |
| Bone marrow infiltration | 2 (9%) | 11 (24%) |
| Bulky disease‡ | 11 (50%) | 22 (48%) |
| Single hit (FISH; MYC) | 12 (54%) | 46 (100%) |
| Double hit (MYC + BCL2 or BCL6) | 9 (41%) | — |
| Triple hit (MYC + BCL2 + BCL6) | 1 (4%) | — |
ECOG-PS, ECOG performance status score; NA, not applicable.
Burkitt Lymphoma IPI (BL-IPI) includes age ≥40 years old, ECOG-PS >1, serum LDH >3 times upper limit of normal, and CNS involvement.21
Only patients with meningeal disease were considered, whereas patients with intraparenchymal brain lesions were excluded.
Bulky disease was defined by a lesion with the largest diameter of 10 cm or more.
Feasibility and toxicity
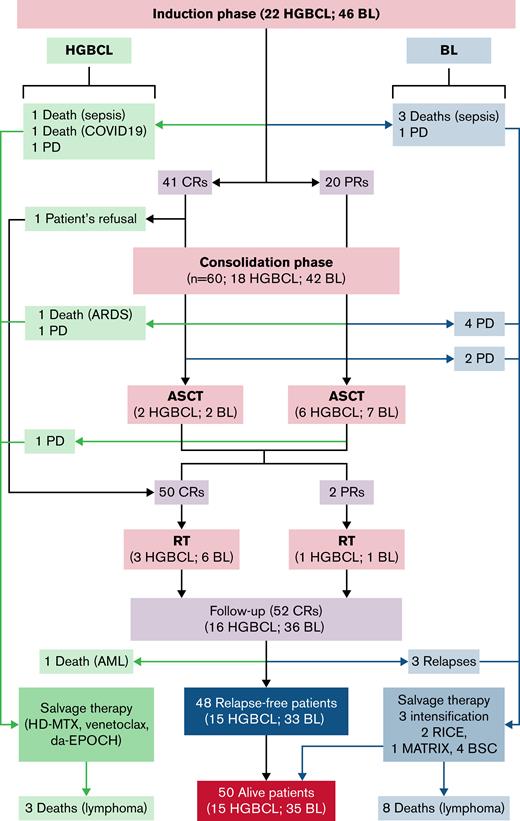
Sixty-one (90%) patients completed the induction (Figure 2): 19 (86%) HGBCL and 42 (91%) BL; induction interruptions were due to progressive lymphoma in 2 patients and fatal infective complications in 5 (6%): 3 patients due to septic shock, 1 due to multiple infections (Stenotrophomonas maltophilia, Staphylococcus epidermidis, S. hominis, CMV, Pneumocystis jirovecii, Aspergillus), and 1 due to COVID-19 complications; 3 of them were HIV+. Drug doses were reduced by 25% or more in 8 (12%) patients, and methotrexate and etoposide occasionally required delivery delay due to transaminase increase or neutropenia. Hematological toxicity during induction was common but manageable (Table 4): grade 4 neutropenia and thrombocytopenia occurred in 53 (78%) and 23 (34%) patients, respectively; grade 4 bacterial infections occurred in 3 (7%) patients associated with systemic fungal infection in 1 of them. Fifty-three (78%) patients needed recombinant human granulocyte colony-stimulating factor, mostly after etoposide (day 15) and/or methotrexate (day 21) delivery. Only 5 (7%) episodes of grade 4 nonhematological toxicity (hepatotoxicity and mucositis) were recorded during induction (Table 4). Tumor lysis syndrome occurred in 4 (6%) patients, followed early by normalization of biochemical exams. Six (10%) of the 61 patients who completed induction treatment received only 2 intrathecal chemotherapy doses due to deliquoration; none of these patients experienced lymphoma relapse. Lumbar punctures were well tolerated, with 11 episodes of grade 1 to 2 headache in 7 patients and 2 episodes of vomiting.
Consolidated Standards of Reporting Trials diagram. See Table 2 for “intensification” as salvage therapy. AML, acute myeloid leukemia; BSC, best supportive care; CRs, complete responders; HD-MTX, high-dose methotrexate; MATRix, methotrexate, cytarabine, thiotepa, and rituximab; PD, progressive disease; PR, partial responders; RICE, rituximab, ifosfamide, cyclophosphamide, and etoposide; RT, radiation therapy.
Consolidated Standards of Reporting Trials diagram. See Table 2 for “intensification” as salvage therapy. AML, acute myeloid leukemia; BSC, best supportive care; CRs, complete responders; HD-MTX, high-dose methotrexate; MATRix, methotrexate, cytarabine, thiotepa, and rituximab; PD, progressive disease; PR, partial responders; RICE, rituximab, ifosfamide, cyclophosphamide, and etoposide; RT, radiation therapy.
Grade 3 to 5 toxicities following induction and consolidation phases
| Type of toxicity . | Induction (n = 68) . | Consolidation (n = 60) . | ||||
|---|---|---|---|---|---|---|
| Grade 3 . | Grade 4 . | Grade 5 . | Grade 3 . | Grade 4 . | Grade 5 . | |
| Neutropenia∗ | 8 (12%) | 53 (78%) | — | 8 (13%) | 37 (62%) | — |
| Anemia | 26 (38%) | 8 (12%) | — | 18 (30%) | 1 (2%) | — |
| Thrombocytopenia | 10 (15%) | 23 (34%) | — | 4 (7%) | 40 (67%) | — |
| FN/bacterial infections | 20 (29%) | 3 (4%) | 5 (7%)† | 17 (28%) | 0 (0%) | 1 (2%)†,‡ |
| CMV reactivation | 3 (4%) | 0 (0%) | — | 1 (2%) | 0 (0%) | — |
| Hepatotoxicity | 8 (12%) | 1 (1%) | — | 1 (2%) | 0 (0%) | — |
| Mucositis | 7 (10%) | 4 (6%) | — | 1 (2%) | 0 (0%) | — |
| Diarrhea | 1 (1%) | 0 (0%) | — | 3 (5%) | 1 (2%) | — |
| Bowel obstruction | 0 (0%) | 0 (0%) | — | 0 (0%) | 1 (2%) | — |
| Cardiotoxicity | 0 (0%) | 0 (0%) | — | 0 (0%) | 2 (3%)§ | — |
| Stroke | 0 (0%) | 0 (0%) | — | 0 (0%) | 1 (2%) | — |
| Type of toxicity . | Induction (n = 68) . | Consolidation (n = 60) . | ||||
|---|---|---|---|---|---|---|
| Grade 3 . | Grade 4 . | Grade 5 . | Grade 3 . | Grade 4 . | Grade 5 . | |
| Neutropenia∗ | 8 (12%) | 53 (78%) | — | 8 (13%) | 37 (62%) | — |
| Anemia | 26 (38%) | 8 (12%) | — | 18 (30%) | 1 (2%) | — |
| Thrombocytopenia | 10 (15%) | 23 (34%) | — | 4 (7%) | 40 (67%) | — |
| FN/bacterial infections | 20 (29%) | 3 (4%) | 5 (7%)† | 17 (28%) | 0 (0%) | 1 (2%)†,‡ |
| CMV reactivation | 3 (4%) | 0 (0%) | — | 1 (2%) | 0 (0%) | — |
| Hepatotoxicity | 8 (12%) | 1 (1%) | — | 1 (2%) | 0 (0%) | — |
| Mucositis | 7 (10%) | 4 (6%) | — | 1 (2%) | 0 (0%) | — |
| Diarrhea | 1 (1%) | 0 (0%) | — | 3 (5%) | 1 (2%) | — |
| Bowel obstruction | 0 (0%) | 0 (0%) | — | 0 (0%) | 1 (2%) | — |
| Cardiotoxicity | 0 (0%) | 0 (0%) | — | 0 (0%) | 2 (3%)§ | — |
| Stroke | 0 (0%) | 0 (0%) | — | 0 (0%) | 1 (2%) | — |
FN, febbrile neutropenia; CMV, citomegalovirus.
All patients received Granulocyte-stimulating factor after methotrexate (day 21) and etoposide (day 15) delivery.
These 6 grade 5 infections were only treatment discontinuations secondary to toxicity.
One patient died of acute respiratory distress syndrome without documented infection.
Grade 4 cardiotoxicity consisted of heart failure and atrial fibrillation.
Sixty (98%) of the 61 patients who achieved an objective response (CR or PR) after induction received consolidation (Figure 1): 18 (82%) with HGBCL and 42 (91%) with BL. Toxicity of consolidation phase was mild and manageable (Table 4); the most common grade 4 toxicities were neutropenia (37 patients; 62%) and thrombocytopenia (40; 67%). Only 5 (8%) patients experienced grade 4 nonhematological toxicity: diarrhea, heart failure, bowel obstruction, atrial fibrillation, and stroke. One patient died of acute respiratory distress syndrome (ARDS) without documented infection; this was the single death due to toxicity after consolidation. Overall, there were 6 treatment interruptions due to toxicity; these were the 6 above-mentioned grade 5 infections occurred during induction (n = 5) and after consolidation (n = 1) (Figure 2).
Seventeen patients (8 HGBCL and 9 BL) were referred to autologous stem cells collection, followed by ASCT. Engraftment was successful in the expected times; usual toxicities of myeloablative chemotherapy were recorded.
Toxicity in the 19 patients with HBV or HCV infections was similar to those recorded in the other patients, without cases of viral reactivation, and with only 3 cases of grade 3 hepatotoxicity. The median duration of the whole program of the 56 (89%) patients who received the planned treatment was 81 days (IQR, 67-120). There was a single case of second cancer: an HIV+ 47-year-old gentleman with HGBCL developed a myelodysplastic syndrome followed by secondary acute myeloid leukemia after 37 months from CARMEN treatment and died of leukemia a few months later.
Activity and efficacy
After induction (Figure 2), 61 patients achieved an objective tumor response (overall response rate, 90%; 95% CI, 80% to 94%), and 2 patients experienced progressive disease (Table 5). All but 1 of the responders received consolidation, and 17 of them received ASCT. Eleven (4 HGBCL and 7 BL) patients received complementary involved-field radiotherapy; irradiated volume included PET+ residual lymphadenopathies in 3, PET− prior bulky lesions in 6, and prophylactic irradiation of residual testis in 2. No cases of unexpected postactinic toxicity were recorded. At the end of the whole therapy, 52 patients achieved a CR (76%; 95% CI, 65% to 84%). CR rate at the end of the CARMEN program was similar in HGBCL (73%; 95% CI, 55% to 91%) and BL (78%; 95% CI, 66% to 90%) patients (P = .63). CR rate was 76% (95% CI, 64% to 87%) in HIV+ patients and 78% (95% CI, 61% to 95%) in HIV− patients (P = .56).
Responses after induction, consolidation, and whole program in patients with HGBCL and BL
| . | HGBCL (n = 22) . | BL (n = 46) . |
|---|---|---|
| Response to induction | ||
| Complete remission | 11 (50%) | 30 (65%) |
| Partial response | 8 (36%) | 12 (26%) |
| Progressive disease | 1 (4%) | 1 (2%) |
| Toxic deaths | 2 (9%) | 2 (4%) |
| Response to consolidation | ||
| Complete remission | 13 (59%) | 32 (70%) |
| Partial response | 3 (14%) | 4 (9%) |
| Progressive disease | 1 (4%) | 6 (13%) |
| Toxic deaths | 1 (4%) | 0 (0%) |
| Not performed | 5 (23%) | 3 (7%) |
| Response to the whole program | ||
| Complete remission | 16 (73%) | 36 (78%) |
| Partial response | 0 (0%) | 0 (0%) |
| Progressive disease | 3 (14%) | 7 (15%) |
| Toxic deaths | 3 (14%) | 2 (4%) |
| . | HGBCL (n = 22) . | BL (n = 46) . |
|---|---|---|
| Response to induction | ||
| Complete remission | 11 (50%) | 30 (65%) |
| Partial response | 8 (36%) | 12 (26%) |
| Progressive disease | 1 (4%) | 1 (2%) |
| Toxic deaths | 2 (9%) | 2 (4%) |
| Response to consolidation | ||
| Complete remission | 13 (59%) | 32 (70%) |
| Partial response | 3 (14%) | 4 (9%) |
| Progressive disease | 1 (4%) | 6 (13%) |
| Toxic deaths | 1 (4%) | 0 (0%) |
| Not performed | 5 (23%) | 3 (7%) |
| Response to the whole program | ||
| Complete remission | 16 (73%) | 36 (78%) |
| Partial response | 0 (0%) | 0 (0%) |
| Progressive disease | 3 (14%) | 7 (15%) |
| Toxic deaths | 3 (14%) | 2 (4%) |
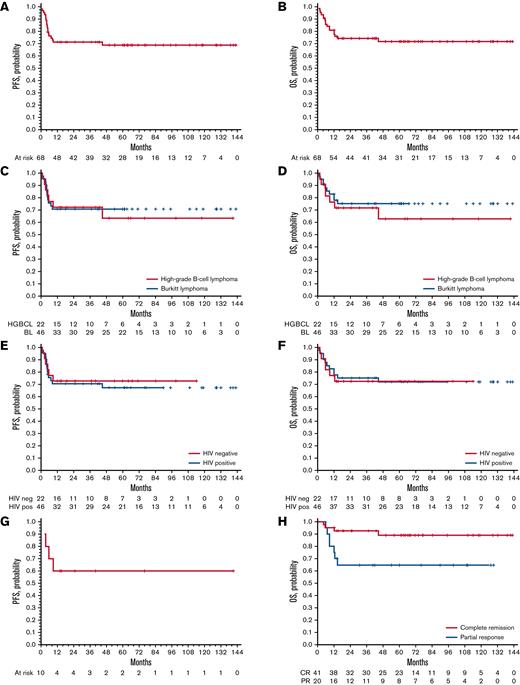
At a median follow-up of 65 (IQR, 40-109) months, there were 20 events: 7 in the HGBCL group and 13 among the patients with BL (Table 5; Figure 2); 6 patients died of toxicity, 10 experienced progressive disease during treatment, 3 (BL) experienced early relapse after initial CR, and 1 died of secondary acute leukemia while relapse free. Overall, 48 (71%; 15 HGBCL and 33 BL) patients remain event free, with a 5-year PFS of 70% (95% CI, 69% to 71%) for the whole series, 63% (95% CI, 58% to 68%) for patients with HGBCL, and 72% (95% CI, 71% to 73%) for patients with BL (Figure 3A,C). At disease progression or relapse (n = 13), lymphoma was disseminated involving primary and secondary sites in 10 patients and was limited to the CNS in 3; the latter were HIV+ patients with BL, with meningeal infiltration at initial lymphoma diagnosis in 1 of them. Salvage therapy (Figure 2) consisted of planned intensification (Table 2) in 5 patients, high-dose-methotrexate–based chemotherapy in 2, venetoclax in 1, (dose adjusted etoposide, vincristine, cyclophosphamide, doxorubicin, prednisone) in 1, and supportive care in 4; only 2 patients achieved a second remission and are relapse free at 74 and 99 months. Among the 9 patients with CSF/meningeal disease at presentation, 6 achieved a CR, and 5 of them are relapse free at 18, 56, 66, 77, and 90 months, whereas the sixth one experienced relapse with meningeal disease and died early; 2 died of bacterial infection during induction, and 1 experienced lymphoma progression outside the CNS and died early due to lymphoma progression.
PFS and OS survival curves. PFS curves are represented in the left graphics and OS curves in right ones. PFS (A) and OS (B) curves of the whole series and of analyzed subgroups according to lymphoma entity (C-D) and to HIV sieropositivity (E-F). (G) PFS curves of the subgroup of patients with double-/triple-hit lymphoma; PFS and OS curves were superimposable in patients with double-/triple-hit lymphoma. OS (H) curves of patients according to the response to the induction (P = .02).
PFS and OS survival curves. PFS curves are represented in the left graphics and OS curves in right ones. PFS (A) and OS (B) curves of the whole series and of analyzed subgroups according to lymphoma entity (C-D) and to HIV sieropositivity (E-F). (G) PFS curves of the subgroup of patients with double-/triple-hit lymphoma; PFS and OS curves were superimposable in patients with double-/triple-hit lymphoma. OS (H) curves of patients according to the response to the induction (P = .02).
Fifty (74%; 15 HGBCL and 35 BL) patients are alive, with a 5-year OS of 72% (95% CI, 71% to 73%) (Figure 3B). Cause of death was lymphoma in 11 patients, septic complications in 4, COVID-19–related complications in 1, ARDS in 1, and secondary acute leukemia in 1. The 5-year OS was 63% (95% CI, 58% to 68%) for patients with HGBCL and 76% (95% CI, 75% to 77%) for patients with BL (P = .49) (Figure 3D). The 5-year OS was 72% (95% CI, 70% to 74%) for HIV− patients and 73% (95% CI, 72% to 74%) for HIV+ patients (P = .89) (Figure 3E-F). Among the 10 patients with double-/triple-hit lymphoma, 6 are alive at 14, 15, 25, 40, 76, and 121 months, respectively; 2 died of lymphoma, 1 died of ARDS after consolidation, and 1 died of COVID-19 complications, with a 5-year PFS and OS of 60% (95% CI, 52% to 67%) (Figure 3G). According to the response achieved after induction, the 41 patients with a CR had a significantly better OS than the 20 patients with PR (5-year, 89%; 95% CI, 89% to 89% vs 65%; 95% CI, 61% to 69%; P = .02) (Figure 3H).
Discussion
In this multicenter series of patients with BL or HGBCL and MYC translocation, the CARMEN program was associated with encouraging safety and efficacy profiles, both in HIV-positive and -negative patients. Efficacy of the CARMEN regimen in patients with BL is reproducible; in fact, the 2-year OS with this combination was 77% in the pioneering retrospective study on 22 HIV− patients,12 73% in the pilot study on 15 HIV+ patients,9 75% in the 16 HIV+ patients enrolled in the phase 2 trial,8 and 76% in the 46 patients considered in the present study. Consistency of results among these prior studies suggests that three-fourths of patients with BL can be cured with this short-term, dose-dense regimen. In line with prior studies, feasibility and efficacy of CARMEN treatment is similar in HIV+ and HIV− patients with BL, which is presently shown also among patients with HGBCL. Importantly, survival curves of patients with HGBCL treated with the CARMEN program are the same as those attained in patients with BL, a curable aggressive neoplasm. Although explored on a small number of patients, this study provides 2 important novel and promising evidences. The first one regards the fact that none of the 9 HIV+ patients with HGBCL with MYC rearrangement experienced relapse or died of lymphoma after a follow-up ranging between 31 and 141 months, which is an encouraging finding in this lymphoma entity poorly investigated in HIV+ subjects. The second evidence is that only 2 of the 10 patients with double-/triple-hit lymphoma experienced tumor relapse, attaining an encouraging survival plateau close to 60% at 5 years, which contrast with the poor results reported with R-CHOP13 and deserves to be addressed in a larger, prospective series.
This study exhibits a few limitations. First, due to the retrospective nature of this study, data on potentially eligible patients treated with other strategies were not collected. For instance, some old and unfit patients could have been addressed to less intensified treatments, whereas, more importantly, FISH was not routinely used to diagnose HGBCL (formerly “unclassifiable lymphoma with features intermediate between DLBCL and BL”), double-/triple-hit lymphoma, and DLBCL before 2017. Thus, some potential candidates received a different diagnosis and other treatments. However, the indication of the CARMEN regimen in a multicenter setting could be suitable to assess the feasibility and efficacy of this treatment in patients aged 18 to 77 years with ECOG ≤3. This is confirmed by the above-mentioned consistency of safety and activity of CARMEN treatment in patients with BL considered in the present and prior studies.8,9,12 Moreover, almost all considered patients had findings associated with high-risk disease, such as HIV infection, increased LDH serum levels, disseminated, and extranodal disease, which excludes bona fide a favorable patient selection. Second, any conclusion on survival figures of patients with HGBCL, particularly of DHL, should be considered with caution as this subgroup of patients is small, limiting statistical power of analyses. Third, as in any retrospective study, some forms of toxicity could be underreported. In particular, the more subjective side effects or toxicities that are assessed through patient interview, such as neuropathy, nausea, and mucositis, cannot be evaluated clearly in a retrospective study. However, bacterial and opportunistic infections, treatment-related mortality, and dose intensity in BL are similar to those reported in prior prospective studies, suggesting that putative underestimation should not affect safety conclusions. On the other hand, this study exhibits some strengths related to the central pathology review and the fact that diagnostic tissue samples of every considered patient were assessed by FISH for MYC, BCL-2, and BCL-6, which allowed us to define lymphoma entities according to modern diagnostic criteria.
The CARMEN program showed some strengths, like short duration, good acute tolerability, low rates of mucositis and opportunistic infections, and favorable cost-efficacy balance. Notably, 89% of patients received the planned CARMEN program, delivered in a median of 81 days (IQR, 67-120), which is remarkably shorter than the period needed to deliver whole doses of other intensified regimens. In fact, 8 courses of HyperC-VAD (cyclophosphamide, vincristine, doxorubicin, dexamethasone alternated with high doses of methotrexate and cytarabine) are delivered in a minimum of 168 days, 6 courses of DA-EPOCH are delivered in 126 days in patients with high-risk BL, and 2 courses of CODOX-M/IVAC are delivered in a minimum of 98 days. In line with our prior studies, the CARMEN regimen was associated with common, but manageable, hematological toxicity, with rare episodes of grade 4 nonhematological toxicity and infections and with a treatment-related mortality of 9%. Feasibility and tolerability in patients with HBV or HCV infections, 2 common high-risk infections in HIV+ patients, were similar than those recorded for the rest of the series. Overall, the safety profile of the CARMEN regimen is similar to those reported with other time- and resource-consuming regimens addressed in prior prospective and retrospective studies. In the largest prospective trial,14 113 patients with BL, 25% of them with HIV seropositivity, have been treated with 6 courses of risk-adapted DA-EPOCH-R. Eighty-two percent of patients have completed the planned treatment, which lasted close to 6 months, with a treatment-related mortality of 6%, 25% of serious infective events, and 19% of grade 3 to 4 mucositis.14 Recent real-life studies, respectively on 249 and 641 patients with BL, have shown that HyperC-VAD, DA-EPOCH, and CODOX-M/IVAC regimens are associated with a treatment-related mortality of 18%, 13%, and 7%, respectively, and confirmed that secondary cancers, mainly acute myeloid leukemia, affect ∼6% of patients with BL.15,16 An additional advantage of the CARMEN regimen is the potentially lower risk of late complications, particularly infertility and cardiac toxicity, which is an important issue considering that patients with BL are usually young subjects with high probabilities of cure. This potential advantage may be due to the fact that the CARMEN regimen includes only 2 doses of cyclophosphamide and a single dose of doxorubicin (Figure 1; Table 1), 2 drugs that are used in larger amounts in cyclic regimens like HyperC-VAD, DA-EPOCH, and CODOX-M/IVAC regimens. However, the benefit of the use of single doses of these anticancer drugs on long-term toxicity, particularly on infertility and second cancers, remains to be confirmed on a larger number of treated patients and after a longer follow-up period.
The efficacy of the CARMEN regimen in patients with BL (5-year OS, 76%) is similar to those reported with other intensive rituximab-containing regimens. In fact, DA-EPOCH combination has been associated with a 4-year OS of 85% in a prospectively selected series of 113 patients.14 However, the 2 above-mentioned recent retrospective studies,15,16 performed on hundreds of patients with BL, suggest that these results are hardly achievable in real life, where DA-EPOCH-R has been associated with a 3-year OS of 60% to 69%. Efficacy of the CARMEN regimen is of particular interest in the subgroup of patients with HGBCL with MYC translocation. Although only 22 patients with this neoplasm were considered, the 5-year OS of 63% seems to be better than the 5-year OS of 25% to 30% reported with R-CHOP,17,18 and the median OS of 15+ months achieved in double-hit lymphomas seems better than the previously reported 8 months.19 Interestingly, none of the 22 patients with HGBCL treated with CARMEN experienced CNS relapse, which contrasts with the 3-year cumulative risk of 13% previously reported for double-hit lymphomas.3 The present results are in line with a retrospective multicenter study that has suggested that intensive chemotherapy regimens are more effective than R-CHOP in patients with double-hit lymphoma.20 Results obtained with the CARMEN regimen are also similar to the 4-year OS of ∼70% reported in a single-arm phase 2 trial addressing DA-EPOCH-R in 30 patients with HGBCL and MYC translocation (24 double-hit lymphomas and 6 single-hit lymphomas).7 However, the inclusion of several cases of DLBCL and other lymphomas entities in some of the above-mentioned studies impedes to draw reliable conclusions on these comparisons.
In conclusion, the CARMEN program is associated with promising safety and efficacy profiles in HIV− and HIV+ patients with high-risk BL or HGBCL with MYC translocations. Overall results are similar to those reported with other intensified regimens. The CARMEN regimen was delivered in a short period, with uncommon cases of extrahematological toxicity, mucositis and infections, and low cumulative doses of cytostatics, which may suggest a lower risk of late complications, secondary cancers, and sequels. Consistency of the present results with previously reported retrospective and prospective studies lead us to consider the CARMEN treatment as a valid option for HIV− and HIV+ patients with BL. Promising results in HGBCL and MYC translocation and double-hit lymphoma prompt us to further explore this combination in aggressive B-cell lymphomas.
Acknowledgments
The authors are indebted to enrolled patients and their families for their generous commitment. The authors appreciate the excellent technical assistance and sustained scientific collaboration of hematologists, oncologists, pathologists, radiologists, research nurses, and data managers of the participating centers.
Authorship
Contribution: A.J.M.F., G.R., M.S., and A.R. designed the research study; P.A. and F.E. designed and completed database; F.F. and M.P. performed the central pathology review; L.P. contributed essential reagents and performed cytogenetic exams; C.C., L.V., A.L., B.A., C.P., M.F., M.S., S.S., and E.F. registered and treated patients and collected clinical data; and A.J.M.F., G.R., M.S., and A.R. analyzed the data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrés J. M. Ferreri, Lymphoma Unit, Department of Onco-Hematology, IRCCS (Istituto di Ricerca e Cura a Carattere Scientifico) San Raffaele Scientific Institute, Via Olgettina 60, 20132 Milan, Italy; e-mail: ferreri.andres@hsr.it.
References
Author notes
For original data, please contact ferreri.andres@hsr.it.