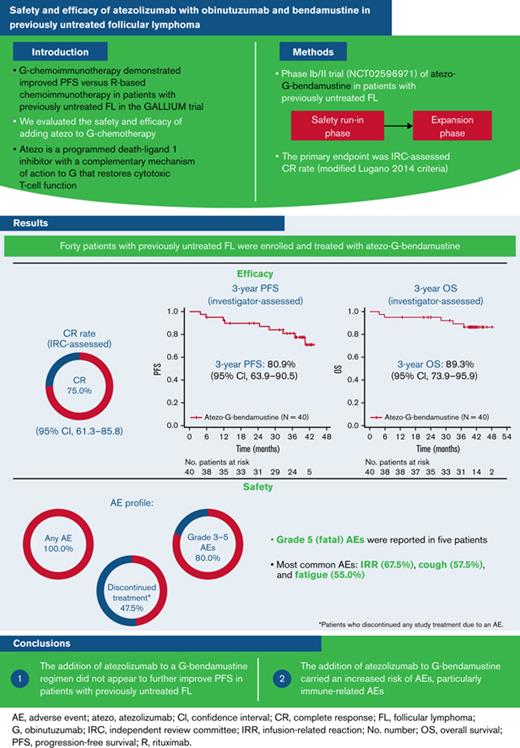
Key Points
The addition of atezolizumab to a G-bendamustine regimen does not appear to further improve PFS in patients with previously untreated FL.
The addition of atezolizumab to G-bendamustine carries an increased risk of AEs, particularly immune-related AEs.
Abstract
Obinutuzumab (G) chemoimmunotherapy demonstrated improved progression-free survival (PFS) vs rituximab-based chemoimmunotherapy in patients with previously untreated follicular lymphoma (FL) in the GALLIUM trial. Atezolizumab (atezo) is a programmed death-ligand 1 inhibitor with a complementary mechanism of action to G by restoring cytotoxic T-cell function. We evaluated the safety and efficacy of atezo-G-bendamustine in patients with previously untreated FL in a phase Ib/II trial (#NCT02596971). A safety run-in phase was followed by an expansion phase with atezo-G-bendamustine induction and atezo-G maintenance for ≤24 months. Forty patients with previously untreated FL were enrolled and treated with atezo-G-bendamustine. The primary endpoint, complete response (CR) rate, assessed by an independent review committee (IRC; modified Lugano 2014 criteria) was 75.0% (95% confidence interval [CI], 61.3% to 85.8%). Three-year investigator-assessed PFS and overall survival rates were 80.9% (95% CI, 63.9% to 90.5%) and 89.3% (95% CI, 73.9% to 95.9%), respectively. At baseline, 21/40 patients had circulating lymphoma-specific clonotypes and underwent repeat testing at end of induction; all were minimal residual disease negative (10−5 sensitivity), with 16 (76.2%) CRs, 3 (14.3%) partial responses, and 2 (9.5%) with stable disease (IRC assessed). Grade 5 (fatal) adverse events (AEs) were reported in 5 patients. The efficacy of atezo-G-bendamustine in previously untreated FL did not appear superior to G-bendamustine efficacy as seen in the GALLIUM trial, and the addition of atezo to G-bendamustine was associated with an increased risk of AEs. Particularly due to the unfavorable safety profile, this regimen cannot be recommended in patients with previously untreated FL. This trial was registered at www.clinicaltrials.gov as #NCT02596971.
Introduction
Follicular lymphoma (FL) is the most common indolent form of non-Hodgkin lymphoma.1 The anti-CD20 monoclonal antibody (mAb) rituximab (R), in combination with chemotherapy, has been the mainstay of treatment for advanced-stage FL for a number of years,2,3 significantly improving patient outcomes compared with chemotherapy alone.4,5 The addition of R to commonly used induction chemotherapy, including cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) and cyclophosphamide, vincristine, prednisone, was associated with superior overall survival (OS) compared with chemotherapy alone in patients with advanced-stage FL followed for up to approximately 4 years.4,5 The final analysis of data from the phase III PRIMA trial also confirmed the benefit of 2 years of R as maintenance therapy in patients responding to first-line R-containing immunochemotherapy, with a median progression-free survival (PFS) of 10.5 years compared with 4.1 years in the observation arm (P < .001); however, no significant difference in OS was seen in patients randomly assigned to R maintenance or observation.6
In the phase III GALLIUM trial in patients with previously untreated FL, chemoimmunotherapy with the more recently developed anti-CD20 mAb obinutuzumab (G) demonstrated a significant improvement in PFS when compared with R-based chemoimmunotherapy (hazard ratio [HR], 0.66; 3-year PFS: 80.0% vs 73.3%; P = .001),7 with the benefit of G over R observed for all 3 of the assessed chemotherapy backbones, including G-bendamustine (HR, 0.63; 3-year PFS: 84.0% [G-bendamustine] vs 76.0% [R-bendamustine]; P = .0062).8 Furthermore, in an exploratory analysis of GALLIUM, minimal residual disease (MRD) status in peripheral blood and bone marrow at end of induction (EOI) was reported to be prognostic for prolonged PFS, with a higher proportion of G-treated patients compared with R-treated patients achieving MRD-negative status.9
However, despite the long remission and disease-free periods that can be achieved with currently available therapies, FL is still considered incurable, and most patients eventually experience relapse.10 This unmet need has prompted research into new combination treatment regimens incorporating novel targeted or immunotherapeutic agents in an effort to improve outcomes for these patients.10
The humanized immunoglobulin G1 mAb atezolizumab (atezo) is an immune checkpoint inhibitor that targets programmed death-ligand 1 (PD-L1) expressed on tumor-infiltrating lymphocytes, macrophages, peripheral blood T cells, and monocytes in patients with FL.11,12 Drugs that target PD-L1 and its interaction with its receptors, PD-1 and B7.1 (also known as CD80), have achieved considerable success in the treatment of solid tumors in recent years and are currently undergoing evaluation for hematologic malignancies.13 The ability of atezo to target the PD-L1 pathway confers it with a complementary mechanism of action to G, based on T-cell activation. By blocking the interaction of PD-L1 with its receptors PD-1 and B7.1, atezo prevents PD-L1/PD-1- and PD-L1/B7.1-mediated inhibition of the immune response, restoring the antitumor activity of cytotoxic T cells.14,15 Furthermore, nonclinical studies have shown that targeted therapies in combination with PD-1 inhibitors can lead to durable responses not achieved with either agent alone.16,17 Therefore, atezo is an appealing agent to attempt to improve patient outcomes when added to G-chemotherapy for the treatment of FL.18
To evaluate this hypothesis, we performed a phase Ib/II trial (NCT02596971) to assess the safety and efficacy of induction therapy with atezo-G-bendamustine followed by maintenance with atezo-G in patients with FL. A cohort of patients with diffuse large B-cell lymphoma treated with atezo-R-CHOP were also evaluated in the trial; the results of this analysis will be published separately.
Methods
Study design
This was a phase Ib/II, open-label, multicenter, nonrandomized trial that included an initial safety run-in phase with safety monitoring before the main enrollment (expansion phase). The first patient was enrolled on 28 December 2015, and the data cutoff date for the results presented here was 8 May 2020.
The trial was conducted in accordance with the declaration of Helsinki and Good Clinical Practice, and the protocol was approved by the appropriate institutional review board/independent ethics committee at each study center. All patients provided informed consent to participate.
Patients and treatment
Patients aged ≥18 years with previously untreated grade 1, 2, or 3a FL requiring therapy or relapsed/refractory (R/R) FL after treatment and an Eastern Cooperative Oncology Group performance status of 0 to 2 were eligible to participate. Patients with R/R disease were eligible only for the safety run-in. Previously untreated disease requiring therapy was defined as meeting at least one of the Groupe d’Etudes des Lymphomes Folliculaires (GELF) criteria.19 Exclusion criteria included central nervous system lymphoma or leptomeningeal infiltration. Full details of the patient inclusion and exclusion criteria are provided in the supplementary section.
Induction treatment comprised intravenous infusions of atezo 840 mg on days 1 and 15 of cycles 2 to 6; G 1000 mg on days 1, 8, and 15 of cycle 1 and day 1 of cycles 2 to 6; and bendamustine 90 mg/m2 on days 1 and 2 of cycles 1 to 6 (28-day cycles). Patients with CR or partial response (PR) at EOI (6-8 weeks after day 1 of cycle 6) received maintenance therapy comprising atezo 840 mg on days 1 and 2 of each month and G 1000 mg on day 1 of every other month for up to 24 months or until disease progression or unacceptable toxicity.
Assessments
The primary endpoint was CR rate on positron emission tomography-computed tomography (PET-CT) at EOI as determined by an independent review committee (IRC) using modified Lugano 2014 criteria. According to these modified criteria, designation of a PR requires the response to meet criteria for a PR by PET and a CR or PR by CT; in the case of bone marrow involvement at baseline, a CR must be confirmed by a negative bone marrow biopsy at EOI.20 Secondary endpoints included CR rate at EOI assessed by the investigator using Lugano 2014 criteria, CR rate at EOI by IRC and investigator using Cheson 2007 criteria,21 overall response rate (ORR) at EOI by IRC and investigator using Lugano 2014 and Cheson 2007 criteria, and OS and PFS by investigator. PFS was defined as the time from randomization to the first occurrence of progression or relapse, assessed using the Cheson 2007 criteria (PET-CT and CT)21, or death from any cause. MRD as an exploratory endpoint was centrally assessed by next-generation sequencing of the B-cell receptor VDJ region (Adaptive Biotechnologies Corp., Seattle, WA), as previously described.22 In brief for the MRD analyses, baseline tissue was used for clone identification using the ImmunoSEQ assay (Adaptive Technologies, version 2) at a sensitivity in the range of 10−5 to 10−6;23 patients who tested positive for circulating clones at baseline underwent repeat testing at EOI.
The relationship between EOI response and expression of PD-L1 or CD8 (median used as cutoff), as measured using immunohistochemistry (IHC), were evaluated post hoc as a further exploratory endpoint. IHC assays were performed on pretreatment formalin-fixed paraffin-embedded tissue samples for PD-L1 using clone SP142 and CD8 using clone SP57. PD-L1 was scored based on tissue area occupied by PD-L1–positive cells using the following algorithm: IHC 0 = <1%, IHC 1 = 1% to 5%, IHC 2 = 5% to 10%, and IHC 3 = >10%. CD8 staining was scored based on tumor area occupied by CD8-positive cells.
Safety and tolerability assessments included documentation of adverse events (AEs), serious AEs (SAEs), and AEs of special interest (AESIs; related to atezo). AESIs related to atezo included pneumonitis, colitis, endocrinopathies, hepatitis, systemic lupus erythematosus, neurological disorders, hypersensitivity reactions, nephritis, ocular toxicities, myositis, myopathies, vasculitis, grade ≥2 cardiac disorders, and severe cutaneous reactions.
Statistical methods
Up to 46 patients with FL (40 with previously untreated disease) were planned to be enrolled in the atezo-G-bendamustine treatment cohort: 6 patients with previously untreated or R/R FL in the safety run-in phase and 34 to 40 patients with previously untreated FL in the expansion phase. Assuming an observed PET-CT–defined CR rate of 55%, the sample size was deemed to be sufficient to provide adequate precision for the CR rate and for the lower limit of the 90% CI to rule out a clinically uninteresting rate of <40%, a value selected based on previous studies of first-line treatment for FL.24,25 The previously untreated FL population was analyzed for efficacy and safety. The primary efficacy analysis included patients who received at least 1 dose of atezo.
The proportion of patients who achieved a CR and the 2-sided 90% Clopper-Pearson exact CI were calculated. Patients without a post-baseline tumor assessment were considered nonresponders. Kaplan-Meier analysis was used to assess PFS and OS, with a Cox proportional hazards model used to calculate HRs and 95% CIs. As part of a later exploratory analysis, PFS was also compared for patients with MRD above the third quartile (Q3) and those with MRD ≤Q3.
Results
Patients
In total, 40 patients with previously untreated FL were enrolled and treated; 2 patients with R/R FL were enrolled in the safety run-in and were not included in the current analysis. Most patients were aged <65 years (85.0%) and of White race (87.5%), and the ratio of males to females was 1:1 (Table 1). A large majority of patients had advanced-stage disease (Ann Arbor stage III-IV: 92.5%).
Patient demographics and disease characteristics at baseline
| Characteristic . | Previously untreated FL population (N = 40) . |
|---|---|
| Median age (range) at baseline, y | 56.5 (29-75) |
| Age category, n (%) | |
| ≥65 y | 6 (15.0) |
| <65 y | 34 (85.0) |
| Sex, n (%) | |
| Male | 20 (50.0) |
| Female | 20 (50.0) |
| FL grade at diagnosis, n (%) | |
| 1 | 10 (25.0) |
| 2 | 20 (50.0) |
| 3a | 9 (22.5) |
| Unclassified | 1 (2.5) |
| Ann Arbor stage at diagnosis, n (%) | |
| II | 3 (7.5) |
| III | 15 (37.5) |
| IV | 22 (55.0) |
| FLIPI risk group at diagnosis, n (%) | |
| Low (0-1) | 9 (22.5) |
| Intermediate (2) | 18 (45.0) |
| High (≥3) | 13 (32.5) |
| Eastern Cooperative Oncology Group performance status, n (%) | |
| 0 | 29 (72.5) |
| 1 | 11 (27.5) |
| Bone marrow involvement, n (%) | 20 (50.0) |
| LDH elevated, n (%) | 7 (17.5) |
| Bulky disease (≥7 cm), n (%) | 9 (22.5) |
| Median SPD (range) of indicator lesions, mm2 | 2821 (740-23 025) |
| Characteristic . | Previously untreated FL population (N = 40) . |
|---|---|
| Median age (range) at baseline, y | 56.5 (29-75) |
| Age category, n (%) | |
| ≥65 y | 6 (15.0) |
| <65 y | 34 (85.0) |
| Sex, n (%) | |
| Male | 20 (50.0) |
| Female | 20 (50.0) |
| FL grade at diagnosis, n (%) | |
| 1 | 10 (25.0) |
| 2 | 20 (50.0) |
| 3a | 9 (22.5) |
| Unclassified | 1 (2.5) |
| Ann Arbor stage at diagnosis, n (%) | |
| II | 3 (7.5) |
| III | 15 (37.5) |
| IV | 22 (55.0) |
| FLIPI risk group at diagnosis, n (%) | |
| Low (0-1) | 9 (22.5) |
| Intermediate (2) | 18 (45.0) |
| High (≥3) | 13 (32.5) |
| Eastern Cooperative Oncology Group performance status, n (%) | |
| 0 | 29 (72.5) |
| 1 | 11 (27.5) |
| Bone marrow involvement, n (%) | 20 (50.0) |
| LDH elevated, n (%) | 7 (17.5) |
| Bulky disease (≥7 cm), n (%) | 9 (22.5) |
| Median SPD (range) of indicator lesions, mm2 | 2821 (740-23 025) |
FLIPI, Follicular Lymphoma International Prognostic Index; LDH, lactate dehydrogenase; SPD, sum of the products of the diameters.
As shown in supplemental Figure 1, 3 patients (7.5%) discontinued all study treatments during the induction phase (all due to an AE), and 36 patients entered the maintenance phase. At the time of data cutoff, the median duration of survival follow-up in the whole study population was 40.4 months (range, 3.8-48.1 months). The median length of time on treatment was 26.8 months (range, 1.0-31.3 months).
Efficacy: clinical data
Responses at EOI are summarized in Table 2. The IRC-assessed CR rate on PET-CT at EOI according to modified Lugano 2014 criteria (primary endpoint) was 75.0% (95% CI, 61.3% to 85.8%) (Table 2). As a secondary endpoint, the IRC-assessed CR rate at EOI was 75.0% when either Lugano 2014 or Cheson 2007 criteria were used. When assessed by the investigator, the CR rate was 80.0% using Cheson 2007 criteria, 85.0% by modified Lugano 2014 criteria, and 87.5% by Lugano 2014 criteria. The corresponding ORR at EOI ranged from 85.0% to 95.0% (Table 2). As an additional endpoint, the CR rate at the end of maintenance was reported to be 50.0% (based on Cheson 2007 criteria, investigator assessed).
Responses at EOI (N = 40)
| Response . | Modified Lugano 2014 (PET-CT) . | Lugano 2014 (PET) . | Cheson 2007 (PET-CT) . | |||
|---|---|---|---|---|---|---|
| IRC . | INV . | IRC . | INV . | IRC . | INV . | |
| ORR, n (%) | 34 (85.0) | 38 (95.0) | 36 (90.0) | 38 (95.0) | 36 (90.0) | 38 (95.0) |
| CR, n (%) | 30 (75.0) | 34 (85.0) | 30 (75.0) | 35 (87.5) | 30 (75.0) | 32 (80.0) |
| PR, n (%) | 4 (10.0) | 4 (10.0) | 6 (15.0) | 3 (7.5) | 6 (15.0) | 6 (15.0) |
| SD, n (%) | 4 (10.0) | 0 | 2 (5.0) | 0 | 2 (5.0) | 0 |
| NA, n (%)∗ | 2 (5.0) | 2 (5.0) | 2 (5.0) | 2 (5.0) | 2 (5.0) | 2 (5.0) |
| Response . | Modified Lugano 2014 (PET-CT) . | Lugano 2014 (PET) . | Cheson 2007 (PET-CT) . | |||
|---|---|---|---|---|---|---|
| IRC . | INV . | IRC . | INV . | IRC . | INV . | |
| ORR, n (%) | 34 (85.0) | 38 (95.0) | 36 (90.0) | 38 (95.0) | 36 (90.0) | 38 (95.0) |
| CR, n (%) | 30 (75.0) | 34 (85.0) | 30 (75.0) | 35 (87.5) | 30 (75.0) | 32 (80.0) |
| PR, n (%) | 4 (10.0) | 4 (10.0) | 6 (15.0) | 3 (7.5) | 6 (15.0) | 6 (15.0) |
| SD, n (%) | 4 (10.0) | 0 | 2 (5.0) | 0 | 2 (5.0) | 0 |
| NA, n (%)∗ | 2 (5.0) | 2 (5.0) | 2 (5.0) | 2 (5.0) | 2 (5.0) | 2 (5.0) |
INV, investigator; NA, not available.
Patients were not evaluable because of death due to an AE prior to response assessment.
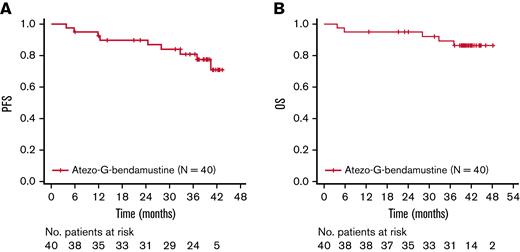
Investigator-assessed PFS and OS rates at 3 years were 80.9% (95% CI, 63.9% to 90.5%) and 89.3% (95% CI, 73.9% to 95.9%), respectively (Figure 1). A total of 5 patients died, with all fatalities due to an AE.
Kaplan-Meier assessments. (A) PFS. (B) OS. Atezo, atezolizumab; G, obinutuzumab; No., number; OS, overall survival; PFS, progression-free survival.
Kaplan-Meier assessments. (A) PFS. (B) OS. Atezo, atezolizumab; G, obinutuzumab; No., number; OS, overall survival; PFS, progression-free survival.
Efficacy: MRD analysis
Identification of lymphoma-specific clonotypes for MRD analysis was performed at baseline for 32/40 patients (n = 8, not assessed; supplemental Figure 2). Circulating clones were identified in 26/32 patients at baseline; 21 patients underwent repeat testing at EOI, all of whom were MRD negative (10−5 sensitivity; 5 patients did not have a sample for MRD analysis at EOI).
Of the 21 patients who became MRD negative at EOI after a positive test result at baseline, 16 (76.2%) were assessed to have a CR, 3 (14.3%) had a PR, and 2 (9.5%) had stable disease (SD; IRC assessment) (Table 3). These response rates were similar to those of the total efficacy-evaluable population. One of the patients with a PR and 1 patient with SD progressed approximately 1 year after EOI (12.4 months and 11.9 months, respectively); 1 of the patients with SD died at 36.9 months (progressive multifocal leukoencephalopathy), and the other 2 patients with a PR did not progress during the period of follow-up (43.3 months and 39.3 months).
Clinical response at EOI for MRD-negative patients
| Clinical response at EOI∗ . | MRD-evaluable patients† (N = 21) . |
|---|---|
| IRC-assessed, n (%) | |
| CR | 16 (76.2) |
| PR | 3 (14.3) |
| SD | 2 (9.5) |
| NA | 0 |
| INV-assessed, n (%) | |
| CR | 18 (85.7) |
| PR | 3 (14.3) |
| SD | 0 |
| NA | 0 |
| Clinical response at EOI∗ . | MRD-evaluable patients† (N = 21) . |
|---|---|
| IRC-assessed, n (%) | |
| CR | 16 (76.2) |
| PR | 3 (14.3) |
| SD | 2 (9.5) |
| NA | 0 |
| INV-assessed, n (%) | |
| CR | 18 (85.7) |
| PR | 3 (14.3) |
| SD | 0 |
| NA | 0 |
NA, not available.
Assessed according to modified Lugano 2014 criteria.
Patients with circulating clones at baseline were all MRD negative at EOI (see supplemental Figure 2 for patient flow).
MRD-evaluable patients with lymphoma-specific clones at or below Q3 at baseline appeared to have longer PFS than those with >Q3 (HR, 0.22 [95% CI, 0.04-1.14]); however, the number of events in this exploratory analysis was small (supplemental Figure 3). The association between MRD at EOI and survival could not be determined due to the lack of MRD-positive patients at EOI.
Efficacy: biomarker analysis
The analysis of the relationship between EOI response and expression of PD-L1 or CD8 measured using IHC showed no statistically significant association; however, a slightly higher CR rate was observed with higher PD-L1 expression in response to atezo-G-bendamustine (supplemental Figure 4).
Safety
All patients had at least 1 AE, and 32 (80.0%) patients had grade 3 to 5 AEs, which included 5 patients who had grade 5 (fatal) AEs (Pneumocystis pneumonia [n = 1], sudden death [n = 1], cardiac arrest [n = 1, following myocarditis and bronchiolitis obliterans], adenocarcinoma [likely primary site: gastrointestinal tract/biliary origin; n = 1], and progressive multifocal leukoencephalopathy [based on a positive John Cunningham virus test; n = 1]) (Table 4). The most common AEs were infusion-related reactions (67.5% of patients), cough (57.5%), and fatigue (55.0%) (Table 5). Neutropenia was the most frequent hematologic toxicity, reported in 14 patients (35.0%), followed by anemia, febrile neutropenia, and thrombocytopenia (each reported in 4 patients [10%]). The most common grade 3 to 5 AEs were neutropenia, lipase increase, and pneumonia. AEs led to discontinuation of any study treatment in 19 patients (47.5%); of these patients, in the induction phase, 3 patients discontinued atezo (7.5%), 3 patients discontinued G (7.5%), and 3 patients discontinued benda (7.5%), and in the maintenance phase, 16 patients discontinued atezo (40.0%) and 11 patients discontinued G (27.5%).
Summary of AEs in patients with previously untreated FL (N = 40)
| . | Induction phase n (%) . | Maintenance phase n (%) . | Follow-up phase n (%) . | Overall n (%) . |
|---|---|---|---|---|
| Patients with any AE | 40 (100.0) | 37 (92.5) | 14 (35.0) | 40 (100.0) |
| Grade 3-5 AE | 21 (52.5) | 24 (60.0) | 9 (22.5) | 32 (80.0) |
| Grade 5 AE | 1 (2.5) | 1 (2.5) | 3 (7.5) | 5 (12.5)∗ |
| SAE | 9 (22.5) | 12 (30.0) | 6 (15.0) | 18 (45.0) |
| SAE related to atezo | 3 (7.5) | 4 (10.0) | 3 (7.5) | 8 (20.0) |
| SAE related to G | 2 (5.0) | 5 (12.5) | 2 (5.0) | 8 (20.0) |
| SAE related to bendamustine | 2 (5.0) | 1 (2.5) | 0 (0.0) | 3 (7.5) |
| AE leading to any study treatment discontinuation | 4 (10.0) | 13 (32.5) | 2 (5.0) | 19 (47.5) |
| AESI related to atezolizumab | 12 (30.0) | 15 (37.5) | 3 (7.5) | 20 (50.0) |
| . | Induction phase n (%) . | Maintenance phase n (%) . | Follow-up phase n (%) . | Overall n (%) . |
|---|---|---|---|---|
| Patients with any AE | 40 (100.0) | 37 (92.5) | 14 (35.0) | 40 (100.0) |
| Grade 3-5 AE | 21 (52.5) | 24 (60.0) | 9 (22.5) | 32 (80.0) |
| Grade 5 AE | 1 (2.5) | 1 (2.5) | 3 (7.5) | 5 (12.5)∗ |
| SAE | 9 (22.5) | 12 (30.0) | 6 (15.0) | 18 (45.0) |
| SAE related to atezo | 3 (7.5) | 4 (10.0) | 3 (7.5) | 8 (20.0) |
| SAE related to G | 2 (5.0) | 5 (12.5) | 2 (5.0) | 8 (20.0) |
| SAE related to bendamustine | 2 (5.0) | 1 (2.5) | 0 (0.0) | 3 (7.5) |
| AE leading to any study treatment discontinuation | 4 (10.0) | 13 (32.5) | 2 (5.0) | 19 (47.5) |
| AESI related to atezolizumab | 12 (30.0) | 15 (37.5) | 3 (7.5) | 20 (50.0) |
atezo, atezolizumab; G, obinutuzumab.
Included Pneumocystis pneumonia (n = 1), sudden death (n = 1), cardiac arrest (n = 1), adenocarcinoma (n = 1), and progressive multifocal leukoencephalopathy (n = 1). The case of Pneumocystis pneumonia was considered to be possibly related to the use of prednisone used to treat elevated lipase (atezo-related) in the same patient.
All-grade AEs reported in >10% of patients and grade 3 to 5 AEs occurring in ≥5% of patients (N = 40)
| All-grade AE . | n (%) . | Grade 3-5 AE . | n (%) . |
|---|---|---|---|
| Hematologic toxicity | Hematologic toxicity | ||
| Neutropenia | 14 (35.0) | Neutropenia | 13 (32.5) |
| Febrile neutropenia | 4 (10.0) | ||
| Thrombocytopenia | 2 (5.0) | ||
| Anemia | 2 (5.0) | ||
| Nonhematologic toxicity | Nonhematologic toxicity | ||
| Infusion-related reaction | 27 (67.5) | Lipase increased | 12 (30.0) |
| Cough | 23 (57.5) | Pneumonia | 6 (15.0) |
| Fatigue | 22 (55.0) | Colitis | 2 (5.0) |
| Nausea | 20 (50.0) | Hepatitis | 2 (5.0) |
| Diarrhea | 19 (47.5) | Hyperglycemia | 2 (5.0) |
| Constipation | 17 (42.5) | Upper respiratory tract infection | 2 (5.0) |
| Headache | 17 (42.5) | ||
| Rash | 13 (32.5) | ||
| Upper respiratory tract infection | 13 (32.5) | ||
| Lipase increased | 12 (30.0) | ||
| Pruritus | 12 (30.0) | ||
| Pyrexia | 12 (30.0) | ||
| Back pain | 10 (25.0) | ||
| Chest pain | 10 (25.0) | ||
| Vomiting | 10 (25.0) | ||
| Arthralgia | 8 (20.0) | ||
| Oropharyngeal pain | 8 (20.0) | ||
| Pneumonia | 8 (20.0) | ||
| Abdominal pain | 7 (17.5) | ||
| Dyspepsia | 7 (17.5) | ||
| Dyspnea | 7 (17.5) | ||
| Influenza-like illness | 7 (17.5) | ||
| Nasal congestion | 7 (17.5) | ||
| Pain in extremity | 7 (17.5) | ||
| Sinusitis | 7 (17.5) | ||
| Urinary tract infection | 7 (17.5) | ||
| Upper abdominal pain | 6 (15.0) | ||
| Dizziness | 6 (15.0) | ||
| Hypertension | 6 (15.0) | ||
| Insomnia | 6 (15.0) | ||
| Anxiety | 5 (12.5) | ||
| Decreased appetite | 5 (12.5) | ||
| All-grade AE . | n (%) . | Grade 3-5 AE . | n (%) . |
|---|---|---|---|
| Hematologic toxicity | Hematologic toxicity | ||
| Neutropenia | 14 (35.0) | Neutropenia | 13 (32.5) |
| Febrile neutropenia | 4 (10.0) | ||
| Thrombocytopenia | 2 (5.0) | ||
| Anemia | 2 (5.0) | ||
| Nonhematologic toxicity | Nonhematologic toxicity | ||
| Infusion-related reaction | 27 (67.5) | Lipase increased | 12 (30.0) |
| Cough | 23 (57.5) | Pneumonia | 6 (15.0) |
| Fatigue | 22 (55.0) | Colitis | 2 (5.0) |
| Nausea | 20 (50.0) | Hepatitis | 2 (5.0) |
| Diarrhea | 19 (47.5) | Hyperglycemia | 2 (5.0) |
| Constipation | 17 (42.5) | Upper respiratory tract infection | 2 (5.0) |
| Headache | 17 (42.5) | ||
| Rash | 13 (32.5) | ||
| Upper respiratory tract infection | 13 (32.5) | ||
| Lipase increased | 12 (30.0) | ||
| Pruritus | 12 (30.0) | ||
| Pyrexia | 12 (30.0) | ||
| Back pain | 10 (25.0) | ||
| Chest pain | 10 (25.0) | ||
| Vomiting | 10 (25.0) | ||
| Arthralgia | 8 (20.0) | ||
| Oropharyngeal pain | 8 (20.0) | ||
| Pneumonia | 8 (20.0) | ||
| Abdominal pain | 7 (17.5) | ||
| Dyspepsia | 7 (17.5) | ||
| Dyspnea | 7 (17.5) | ||
| Influenza-like illness | 7 (17.5) | ||
| Nasal congestion | 7 (17.5) | ||
| Pain in extremity | 7 (17.5) | ||
| Sinusitis | 7 (17.5) | ||
| Urinary tract infection | 7 (17.5) | ||
| Upper abdominal pain | 6 (15.0) | ||
| Dizziness | 6 (15.0) | ||
| Hypertension | 6 (15.0) | ||
| Insomnia | 6 (15.0) | ||
| Anxiety | 5 (12.5) | ||
| Decreased appetite | 5 (12.5) | ||
AE, adverse event.
AESIs related to atezo were observed in 20 patients (50.0%), with most events occurring during maintenance and follow-up (36 events in 18 patients [45.0%]) (Table 4). The most frequent AESIs related to atezo were lipase increase (10 patients [25.0%]) and rash (8 patients [20.0%]) (supplementary Table 1). The lipase-increase events were generally manageable with corticosteroids and discontinuation of atezo. One patient experienced a case of grade 4 myocarditis, the onset of which began approximately 12 days after the first dose of atezo during cycle 2. Study treatment was discontinued, and the patient partially recovered after admission to the intensive care unit and had intensive management, which included intubation, immune suppression, and extracorporeal membrane oxygenation. Approximately 3 months after treatment discontinuation, the patient experienced bronchiolitis obliterans, which was followed by severe respiratory failure and then death due to cardiac arrest 2 months later. The event of cardiac arrest was considered by the investigator to be related to atezo treatment.
The most common SAEs were pneumonia (reported in 5 patients [12.5%]), febrile neutropenia (reported in 4 patients [10.0%]), and pyrexia (reported in 3 patients [7.5%]). The most frequent treatment-related SAEs were febrile neutropenia (3 events) and pneumonia (2 events).
Overall, 29 patients received a colony-stimulating factor treatment (72.5%). Of these patients, 13 received a colony-stimulating factor treatment due to an AE (32.5%; neutropenia, neutrophil count decrease, and febrile neutropenia).
Discussion
In patients with previously untreated FL, combination chemoimmunotherapy with atezo-G-bendamustine resulted in a high CR rate of 75.0% at EOI. The safety profile was consistent with the known profiles of the individual drugs. However, although many AEs were manageable, almost half of the patients discontinued at least 1 study treatment because of AEs, and there were 5 fatal AEs, namely, pneumonia, sudden death, cardiac arrest (following a severe immune-mediated myocarditis and bronchiolitis obliterans), adenocarcinoma (likely primary site: gastrointestinal tract/biliary origin), and progressive multifocal leukoencephalopathy.
Although still in clinical development for hematologic malignancies, atezo is an approved treatment for urothelial carcinoma, non–small cell lung cancer (NSCLC), small cell lung cancer, triple-negative breast cancer, hepatocellular carcinoma, and melanoma.26-28 A recent meta-analysis encompassing these and other cancer types indicated that atezo can provide durable efficacy, with superior OS rates and improved tolerability vs chemotherapy.29 Atezo combined with G was also shown to demonstrate efficacy in a phase Ib trial in patients with R/R non-Hodgkin lymphoma, achieving an ORR of 57% in a group of 26 patients with FL.30 As an mAb that directly binds to PD-L1, atezo has a distinct mechanism of action from that of G based on T-cell activation.14,15 In the present study, atezo was added to G during cycle 2 in an attempt to mitigate the risk of increased infusion-related reactions during the first infusion of G because the incidence and severity of G-related infusion-related reactions were known to decrease substantially with the second and subsequent infusions.7
For the first-line treatment of FL, 3-year PFS with G-bendamustine in the GALLIUM study was 84% (95% CI, 79% to 88%) compared with 80.9% (95% CI, 63.9% to 90.5%) with atezo-G-bendamustine in the present study, suggesting that the 2 therapies provide similar efficacy.8 A potential contributing factor to the lack of added benefit observed with the addition of atezo to G-bendamustine for the treatment of FL compared with G-bendamustine is that targeting PDL-1/PD1 alone may not be sufficient, and inhibiting T-cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain/poliovirus receptor signaling (checkpoint molecules) may also be needed for optimal T-cell engagement.8,31,32 In addition, it is theoretically possible that the T-cell depletion associated with bendamustine33 negated any potential immune stimulatory benefit provided by atezo.
Baseline circulating clone levels in MRD-evaluable patients were prognostic for survival, such that patients with lower levels (≤Q3) achieved longer PFS compared with patients who had higher levels (>Q3); however, it was not possible to evaluate the association between EOI MRD and survival because all patients with available measurements were MRD negative at EOI. The observation of high MRD negativity at EOI in patients who did not achieve a CR suggests that there is a need for a more sensitive platform to measure MRD. A limitation of the ImmunoSEQ assay used to assess MRD was that the small panel of genes resulted in a large fraction of patients whose clones were not identified, and MRD could not be monitored in longitudinal blood samples.
An association between the expression of biomarkers (eg, PD-L1 and CD8 measured by IHC) and response to PDL-1/PD1 inhibitor therapy has been reported in patients with solid cancers, including those with NSCLC and melanoma.34,35 In patients with NSCLC, PD-L1 expression on tumor cells and tumor-infiltrating immune cells independently predicted improved OS with atezo.34 In contrast, in the present study, any association was solely limited to a trend toward a higher CR rate with a higher level of PD-L1 expression. The small number of biomarker-evaluable patients (CR, n = 23 and non-CR, n = 8) may have limited our ability to establish a strong association between PD-L1 expression and response. Furthermore, the lack of association could also be partially explained by expression of other checkpoint molecules, including T-cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain on exhausted T cells, and the immunosuppressive tumor microenvironment in FL.31,36
In agreement with the present study, hematologic toxicities were the most common grade 3 to 5 AEs documented with G-bendamustine in the GALLIUM trial.8 SAEs were experienced by 45.0% of patients in the present study, with a higher proportion of these being related to atezo and G than to bendamustine. Furthermore, occurrence of AESIs related to atezo was high at 20 patients (50.0%). Immune-related AEs are a documented side effect of immune checkpoint inhibitors,37,38 and the potential for the occurrence of adverse reactions with an immune-related cause, including pneumonitis, hepatitis, colitis, endocrinopathies, and infections, is highlighted in the current atezo prescribing information for solid malignancies.26,27 Not unexpectedly, AESIs related to atezo and suggestive of an immune-related cause, including lipase increase, colitis, hepatitis, pneumonitis, and myocarditis, were reported in the present study. The incidence of increased lipase, the most common AESI, was considerably higher than expected based on the overall safety profile of atezo (grade 3-4, 5.9% incidence).39 The reason for this increased incidence may have been due to the requirement for regular mandatory assessment of lipase in the current phase Ib/II trial and not in other studies. Most of the lipase AESIs were reversible and nonserious. Notably, a larger proportion of patients discontinued atezo due to an AE that occurred in the maintenance phase (40.0%) compared with the induction phase (7.5%); similarly, a greater number of AESIs related to atezo occurred during the maintenance phase (37.5%) than in the induction phase (30.0%).
Our study is noteworthy because it explored a novel treatment approach. However, the relatively modest number of patients with untreated FL enrolled (n = 40), the lack of a control group, and the limited duration of follow-up (<3 years) should be taken into consideration when interpreting the results.
In conclusion, although effective in the first-line treatment of FL, the combination of atezo-G-bendamustine does not appear to offer a significant clinical benefit over that achievable with G-bendamustine alone. Furthermore, the addition of atezo to G-bendamustine appears to carry an increased risk of clinically significant AEs, particularly immune-related AEs. Therefore, due to the unfavorable safety profile, atezo-G-bendamustine should not be recommended in patients with previously untreated FL. Further clinical studies focusing on new therapies for FL are needed.
Acknowledgments
Third-party medical writing assistance, under the direction of Anas Younes and Gila Sellam, was provided by Katie Smith and Zoe Toland of Ashfield MedComms, an Ashfield Health company.
This study was funded by F. Hoffmann-La Roche Ltd.
Authorship
Contribution: A.Y. and J.P.S. designed the study; J.M.B., C.D., C.K., J.P.S., M.T., G.S., S.Y., and M.G. conducted the study; J.M.B., C.D., S.F., C.K., J.P.S., M.T., C.U., U.V., S.Y., and M.G. provided recruitment and follow-up of patients; S.F., C.K., C.U., A.R., G.S., and M.G. collected data; J.P.S., A.R., M.S., G.S., and M.G. analyzed the data; and J.M.B., C.D., S.F., J.P.S., U.V., A.R., M.S., T.G.N., G.S., and M.G. interpreted the data.
A.Y. is employed by and has stock and other ownership interests in AstraZeneca; has received honoraria from Merck, F. Hoffmann-La Roche, Takeda, Janssen, AbbVie, Curis, and Epizyme; reports a consulting or advisory role with Bio-Path Holdings, Inc., Xynomic Pharma, Epizyme, F. Hoffmann-La Roche, Celgene, and HCM; has received research funding from Janssen, Curis, F. Hoffmann-La Roche, Genentech, Inc., Merck, Bristol-Myers Squibb, Syndax; and has other relationship with AstraZeneca. J.M.B. reports an advisory board role for Gilead, Bristol Myers Squibb, F. Hoffmann-La Roche, Bayer, AstraZeneca, AbbVie, Verastem, MorphoSys, Adaptive Biotechnologies, Epizyme, Kura, and Seattle Genetics; and has served on speakers’ bureaus for Seattle Genetics and Beigene. C.D. reports an advisory board/consultancy role for Celgene, F. Hoffmann-La Roche, Genentech, Inc., Bristol Myers Squibb, Merck, and Seattle Genetics. C.K. has served on speakers’ bureaus for Genentech, Inc., AstraZeneca, AbbVie, Beigene, Incyte, Kite, ADC Therapeutics, Karyopharm, and Seattle Genetics. J.P.S. reports a consultancy role with AbbVie, AstraZeneca, Beigene, Bristol-Myers Squibb, Pfizer, and Genentech, Inc. C.U. reports a consultancy role for Genentech, Inc. U.V. reports an advisory board role for Janssen, Gilead, Celgene, Juno Therapeutics, Incyte, and Genmab; and receives lecture fees from F. Hoffmann-La Roche, Janssen, Gilead, and AbbVie. S.Y. has received travel assistance from Seattle Genetics. A.R. reports previous employment with Genentech, Inc./F. Hoffmann-La Roche. M.S. is employed by F. Hoffmann-La Roche. T.G.N. is employed by and has stock ownership in F. Hoffmann-La Roche. G.S. is employed by and owns non-voting shares in F. Hoffmann-La Roche. The remaining authors declare no competing financial interests.
The current affiliation for A.Y. is AstraZeneca, Gaithersburg MD.
Correspondence: Anas Younes, 40 E 78 St, New York, NY 10075; e-mail: anas_younes@mac.com.
References
Author notes
For eligible studies, qualified researchers may request access to individual patient-level clinical data through a data request platform. At the time of writing, this request platform is Vivli (https://vivli.org/ourmember/roche/). Up-to-date details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents are available at https://go.roche.com/data_sharing. Anonymized records for individual patients across more than one data source external to Roche cannot, and should not, be linked due to a potential increase of patient reidentification.
The full-text version of this article contains a data supplement.
final version published online 19 October 2022