Key Points
CIBMTR prognostic score predicts PFS and OS of patients with DLBCL receiving axicabtagene ciloleucel treatment after a prior autoHCT failure.
CIBMTR high/very high-risk score marks an adverse risk cohort where novel immunotherapy or relapse prevention approaches are warranted.
Abstract
Allogeneic transplant (alloHCT) and chimeric antigen receptor modified (CAR)-T cell therapy are potentially cuarative options of diffuse large B-cell lymphoma (DLBCL) relapsing after an autologous (auto)HCT. Although the Center for International Blood and Marrow Transplant Research (CIBMTR) prognostic model can predict outcomes of alloHCT in DLBCL after autoHCT failure, corresponding models of CAR-T treatment in similar patient populations are not available. In this noncomparative registry analysis, we report outcomes of patients with DLBCL (≥18 years) undergoing a reduced intensity alloHCT or CAR-T therapy with axicabtagene ciloleucel during 2012 to 2019 after a prior auto-HCT failure and apply the CIBMTR prognostic model to CAR-T recipients. A total of 584 patients were included. The 1-year relapse, nonrelapse mortality, overall survival (OS), and progression-free survival for CAR-T treatment after autoHCT failure were 39.5%, 4.8%, 73.4%, and 55.7%, respectively. The corresponding rates in the alloHCT cohort were 26.2%, 20.0%, 65.6%, and 53.8%, respectively. The 1-year OS of alloHCT recipients classified as low-, intermediate- and high/very high-risk groups according to the CIBMTR prognostic score was 73.3%, 59.9%, and 46.3%, respectively (P = .002). The corresponding rates for low-, intermediate-, and high/very high-risk CAR-T patients were 88.4%, 76.4%, and 52.8%, respectively (P < .001). This registry analysis shows that both CAR-T and alloHCT can provide durable remissions in a subset of patients with DLBCL relapsing after a prior autoHCT. The simple CIBMTR prognostic score can be used to identify patients at high risk of treatment failure after either procedure. Evaluation of novel relapse mitigations strategies after cellular immunotherapies are warranted in these high-risk patients.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is a curable disease, and approximately 50% to 60% of patients do not require further treatment after an initial anthracycline and rituximab-containing regimen.1 The current standard-of-care for fit patients with relapsed/refractory DLBCL, includes high-dose chemotherapy and autologous hematopoietic cell transplant (autoHCT) consolidation in those with disease responding to salvage therapies2-5 or chimeric antigen receptor modified T-cell (CAR-T) treatment of those with disease refractory to at least 2 prior therapy lines.6-10 The outcomes for patients with DLBCL relapsing after autoHCT have been historically poor, with median survival of generally less than 1 year.11
Depending on prior treatments, patients with DLBCL relapsing after an autoHCT, in the modern era, can potentially benefit from a number of approved agents (eg, polatuzumab, tafasitamab, selinexor, or loncastuximab tesirine),12-16 but these options are generally not expected to provide durable disease control. Cellular immunotherapies directed against defined lymphoma-specific antigens (eg, anti-CD19 CAR-T treatment) or against undefined tumor antigens after allogeneic (allo)HCT (ie, the graft-versus-lymphoma effect),17-24 are potentially curative in DLBCL, even after failure of high-dose therapy and autoHCT. Because of the favorable outcome data reported for CAR-T, acceptable toxicity profile, and feasibility in patients with refractory relapse, the application of alloHCT in patients with DLBCL with a failed prior autoHCT has witnessed a rapid decline.20 Although this shift in practice is also endorsed by recent expert consensus,25 large studies providing contemporary, supportive evidence for this change in patient management are lacking. In addition, although prognostic models able to predict outcomes of alloHCT in DLBCL after autoHCT failure have been developed,18 to our knowledge, corresponding models of CAR-T treatment in similar patient populations are not available. A single prognostic model predictive of outcomes of both CAR-T and alloHCT performed after autoHCT failure may help identify clinical settings where 1 type of cellular therapy may be preferrable over the other.
Using the Center for International Blood and Marrow Transplant Research (CIBMTR) registry, we retrospectively report alloHCT and CAR-T therapy outcomes in patients with DLBCL experiencing relapse after prior autoHCT. In addition, we applied the previously published CIBMTR prognostic score18 to the current patient cohorts to identify patients subsets at high risk for treatment failure with modern cell-based therapy approaches.
Methods
Data source
The CIBMTR is a collaborative working group of more than 380 international transplant and cellular immunotherapy centers managed by the Medical College of Wisconsin (MCW) and the National Marrow Donor Program. Detailed information on basic patient characteristics, demographics, clinical variables, and outcome data are contributed by members to the statistical center at the MCW. Participating centers are required to report and frequently update the information on all hematopoietic cell transplants. Cellular therapy data for any CAR-T recipients are collected longitudinally from 130 participating centers in the United States and Canada. Integrity and quality of data are monitored at different levels, including on-site audits and computerized checking for discrepancies. All studies conducted by the CIBMTR comply with applicable regulations to protect research participants.
Patients
Adult patients with DLBCL (age ≥ 18 years), undergoing a first alloHCT or CAR-T therapy during 2012 and 2019, with history of a failed prior autoHCT, were included in this analysis. The CAR-T cohort was limited to treatment with axicabtagene ciloleucel (axi-cel). The alloHCT cohort was limited to patients receiving reduced-intensity (RIC) or nonmyeloablative (NMA) conditioning platforms. Eligible donors for the alloHCT cohort included matched sibling, 8/8 matched unrelated (allele-level match at HLA-A, -B, -C, and -DRB1), or related haploidentical donors. Graft-versus-host disease (GVHD) prophylaxis was limited to conventional calcineurin inhibitor–based or posttransplant cyclophosphamide-based (±calcineurin inhibitor and mycophenolate mofetil) approaches. Patients receiving ex vivo graft manipulation (eg, CD34 selection), or those with history of DLBCL transforming from indolent histologies were excluded.
Definitions and end points
The intensity of alloHCT conditioning regimens was categorized as RIC/NMA using the consensus criteria.26 Disease response at the time of HCT was determined using the International Working Group criteria in use during the era of this analysis.27,28 Progression-free survival (PFS), was defined as the time from either alloHCT or CAR-T treatment to relapse/progression or death from any cause. Overall survival (OS) was defined as the time from treatment to death from any cause, the cumulative incidence of nonrelapse mortality (NRM) was defined as death without preceding disease progression, and the cumulative incidence of relapse/progression was defined as the time from treatment to relapse or disease progression.
Acute and chronic GVHD were graded using established clinical criteria.29,30 Neutrophil recovery was defined as the first of 3 successive days with an absolute neutrophil count ≥ 500/µL after post-HCT or CAR-T nadir. Platelet recovery was defined as the first of 3 consecutive days with a platelet count of 20 000/µL or higher in the absence of platelet transfusion for 7 consecutive days. For neutrophil and platelet recovery, death without the event was considered a competing risk. For patients receiving CAR-T, the cumulative incidence of cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS) were calculated. CRS and ICANS were graded using the American Society of Transplant and Cellular Therapy grading criteria.31
Statistical analysis
Baseline patient, alloHCT, and CAR-T characteristics were assessed using the Pearson χ2 test for categorical variables and the Kruskal-Wallis test for continuous variables. Because there were notable differences in the characteristics of patients who received alloHCT compared with patients who received CAR-T (ie, alloHCT patients were younger, had better performance status, and more frequently had chemosensitive disease) and because of the potential for selection bias in using 1 approach vs the other, we did not compare outcomes between the alloHCT and CAR-T cohorts. The Kaplan-Meier estimator was used to analyze OS and PFS. The cumulative incidence rates of hematopoietic recovery, NRM, and relapse/progression rates were calculated while accounting for competing events. Four patients after alloHCT relapse underwent CAR-T therapy, and 5 relapsing patients after CAR-T received alloHCT. For OS analyses, these 9 subjects receiving alternative cellular therapy were not censored. In addition, we aimed to apply the previously published CIBMTR prognostic score (shown to predict PFS and OS for DLBCL undergoing alloHCT after a failed prior autoHCT)18 to the current cohort of a more contemporary population of patients with DLBCL undergoing either a RIC/NMA alloHCT or CAR-T treatment after a failed autoHCT. The details of CIBMTR prognostic score were previously published.18 Briefly the model consists of 3 adverse prognostic factors, including Karnofsky performance score (KPS) < 80 (4 points), autoHCT to alloHCT or CAR-T interval < 1 year (2 points), and chemoresistant disease at alloHCT or CAR-T (5 points). This CIBMTR prognostic model classifies patients into 4 groups: low risk (0 points), intermediate risk (2-5 points), high risk (6-9 points), or very high risk (11 points). Because of the small sample size of the very high-risk group (alloHCT = 9; CAR-T = 5), it was combined with the high-risk group for this analysis.
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Baseline characteristics
We identified 584 patients with DLBCL who received either CAR-T treatment (n = 181) or an alloHCT (n = 403) after prior autoHCT failure. Baseline characteristics are summarized in Table 1. The median age of CAR-T recipients was 61 years, 79 patients (43.6%) had a KPS of 90 to 100, and most had refractory disease (n = 122; 67.4%). Fludarabine and cyclophosphamide were used for lymphodepletion in 180 patients. Thirty-five patients received bridging treatment between the apheresis session(s) and start of lymphodepletion. The alloHCT cohort was significantly younger (median age, 56.7 years) and had a significantly greater proportion of patients with better performance status (KPS 90-100 = 261 [60.3%]) and chemosensitive disease (80.2%). Peripheral blood and matched siblings were the most common graft (90.1%) and donor (46.9%) source, respectively.
Characteristics of adult patients with DLBCL who received CAR-T or alloHCT
| Characteristic . | CAR-T (N = 181) . | alloHCT (N = 403) . | P . |
|---|---|---|---|
| Median age, y (range) | 61.0 (21.9-80.0) | 56.7 (18.5-72.9) | <.01* |
| Male sex, no. (%) | 117 (64.6) | 261 (64.8) | 1.00† |
| Karnofsky performance score 90-100 | 79 (43.6) | 243 (60.3) | <.01† |
| HCT Comorbidity Index ≥ 3, no. (%) | 73 (40.3) | 189 (46.9) | .04‡ |
| Missing | 9 (5.0) | 7 (1.7) | |
| Median time from diagnosis to CAR-T or alloHCT (mo) | 34.1 (2.7-282.3) | 40.6 (3.2-340.5) | .12* |
| >12 mo | 175 (96.7) | 384 (95.3) | |
| Median time from prior autoHCT to CAR-T or alloHCT (mo) | 14.6 (3.2-268.7) | 15.5 (1.2-196.9) | .58* |
| >12 mo | 97 (53.6) | 208 (51.6) | |
| Graft source, no. (%) | N/A | ||
| Bone marrow | N/A | 40 (9.9) | |
| Peripheral blood | N/A | 363 (90.1) | |
| Donor type, no. (%) | N/A | ||
| HLA-identical sibling | N/A | 189 (46.9) | |
| Matched unrelated donor | N/A | 153 (38.0) | |
| Haploidentical related | N/A | 61 (15.1) | |
| Year of CAR-T or alloHCT, no. (%) | <.01‡ | ||
| 2012-2014 | 0 (0.0) | 201 (49.9) | |
| 2015-2017 | 2 (1.1) | 171 (42.4) | |
| 2018-2019 | 179 (98.9) | 31 (7.7) | |
| Disease status at CAR-T/alloHCT, no. (%) | <.01‡ | ||
| Complete remission | 9 (5.0) | 220 (54.6) | |
| Partial remission | 39 (21.5) | 103 (25.6) | |
| Resistant/untreated relapse | 122 (67.4) | 50 (12.4) | |
| Unknown | 11 (6.1) | 30 (7.4) | |
| Lymphodepleting chemotherapy, no. (%) | N/A | ||
| Cyclophosphamide | 1 (0.6) | N/A | |
| Cyclophosphamide + fludarabine | 180 (99.4) | N/A | |
| Bridging therapy use, no. (%) | 35 (19.3) | N/A | N/A |
| CIBMTR prognostic score, no. (%) | <.01 | ||
| Low (score 0) | 54 (29.8) | 212 (52.6) | |
| Intermediate (score 2,4,5) | 78 (43.1) | 148 (36.7) | |
| High/very high (score 6,7,9,11) | 49 (27.1) | 43 (10.7) | |
| Follow-up, median (range) (mo) | 13.0 (1.0-27.7) | 51.8 (0.2-98.6) |
| Characteristic . | CAR-T (N = 181) . | alloHCT (N = 403) . | P . |
|---|---|---|---|
| Median age, y (range) | 61.0 (21.9-80.0) | 56.7 (18.5-72.9) | <.01* |
| Male sex, no. (%) | 117 (64.6) | 261 (64.8) | 1.00† |
| Karnofsky performance score 90-100 | 79 (43.6) | 243 (60.3) | <.01† |
| HCT Comorbidity Index ≥ 3, no. (%) | 73 (40.3) | 189 (46.9) | .04‡ |
| Missing | 9 (5.0) | 7 (1.7) | |
| Median time from diagnosis to CAR-T or alloHCT (mo) | 34.1 (2.7-282.3) | 40.6 (3.2-340.5) | .12* |
| >12 mo | 175 (96.7) | 384 (95.3) | |
| Median time from prior autoHCT to CAR-T or alloHCT (mo) | 14.6 (3.2-268.7) | 15.5 (1.2-196.9) | .58* |
| >12 mo | 97 (53.6) | 208 (51.6) | |
| Graft source, no. (%) | N/A | ||
| Bone marrow | N/A | 40 (9.9) | |
| Peripheral blood | N/A | 363 (90.1) | |
| Donor type, no. (%) | N/A | ||
| HLA-identical sibling | N/A | 189 (46.9) | |
| Matched unrelated donor | N/A | 153 (38.0) | |
| Haploidentical related | N/A | 61 (15.1) | |
| Year of CAR-T or alloHCT, no. (%) | <.01‡ | ||
| 2012-2014 | 0 (0.0) | 201 (49.9) | |
| 2015-2017 | 2 (1.1) | 171 (42.4) | |
| 2018-2019 | 179 (98.9) | 31 (7.7) | |
| Disease status at CAR-T/alloHCT, no. (%) | <.01‡ | ||
| Complete remission | 9 (5.0) | 220 (54.6) | |
| Partial remission | 39 (21.5) | 103 (25.6) | |
| Resistant/untreated relapse | 122 (67.4) | 50 (12.4) | |
| Unknown | 11 (6.1) | 30 (7.4) | |
| Lymphodepleting chemotherapy, no. (%) | N/A | ||
| Cyclophosphamide | 1 (0.6) | N/A | |
| Cyclophosphamide + fludarabine | 180 (99.4) | N/A | |
| Bridging therapy use, no. (%) | 35 (19.3) | N/A | N/A |
| CIBMTR prognostic score, no. (%) | <.01 | ||
| Low (score 0) | 54 (29.8) | 212 (52.6) | |
| Intermediate (score 2,4,5) | 78 (43.1) | 148 (36.7) | |
| High/very high (score 6,7,9,11) | 49 (27.1) | 43 (10.7) | |
| Follow-up, median (range) (mo) | 13.0 (1.0-27.7) | 51.8 (0.2-98.6) |
N/A, not applicable.
Kruskal-Wallis test.
Fisher exact test.
Fisher exact test via Mon.
CAR-T outcomes
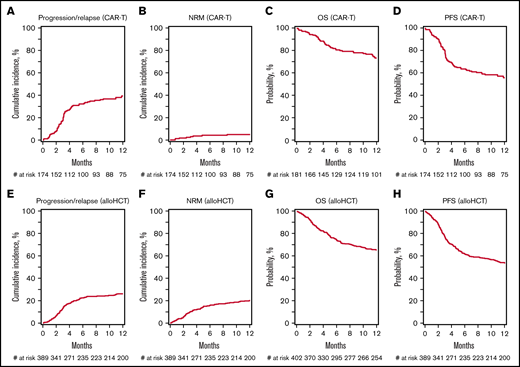
The median follow-up of CAR-T survivors was 13 months (range, 1.0-27.7; Table 2). The rates of grade ≥ 3 CRS and ICANS were 9.9% and 20.9%, respectively. The day 28 cumulative incidence of neutrophil recovery and day 100 incidence of platelet recovery was 89.7% (95% confidence interval [CI] = 84.7-93.8) and 86.7% (95% CI = 81.2-91.4), respectively. At 1 year, the cumulative incidence rates of relapse/progression and NRM were 39.5% (95% CI = 32.1-47.2) and 4.8% (95% CI = 2.1-8.6), respectively (Figure 1A-B). The probability of 1-year OS and PFS was 73.4% (95% CI = 66.4-79.9) and 55.7% (95% CI = 48.0-63.2), respectively (Figure 1C-D).
Outcomes of CAR-T and alloHCT
| Characteristic . | CAR-T . | alloHCT . |
|---|---|---|
| CRS grade 1-5, no. (%) | 149 (82.3) | N/A |
| CRS grade 3-5, no. (%) | 18 (9.9) | N/A |
| Neurotoxicity grade 1-4, no. (%) | 112 (61.9) | N/A |
| Neurotoxicity grade 3-4, no. (%) | 38 (20.9) | N/A |
| Neutrophil recovery, % (95% CI) | ||
| Day 28 | 89.7 (84.7-93.8) | 96.8 (94.8-98.3) |
| Platelet recovery, % (95% CI) | ||
| Day 100 | 86.7 (81.2-91.4) | 92.1 (89.2-94.6) |
| Grade 2-4 acute GVHD, % (95% CI) | ||
| Day 180 | N/A | 28.7 (18.9-39.7) |
| Grade 3-4 acute GVHD, % (95% CI) | ||
| Day 180 | N/A | 8.2 (3.0-15.6) |
| Chronic GVHD, % (95% CI) | ||
| 1 y | N/A | 35.6 (30.9-40.4) |
| OS, % (95% CI) | ||
| 1 y | 73.4 (66.4-79.9) | 65.6 (60.9-70.2) |
| PFS, % (95% CI) | ||
| 1 y | 55.7 (48.0-63.2) | 53.8 (48.8-58.7) |
| Relapse/progression, % (95% CI) | ||
| 1 y | 39.5 (32.1-47.2) | 26.2 (21.9-30.7) |
| NRM, % (95% CI) | ||
| 1 y | 4.8 (2.1-8.6) | 20.0 (16.2-24.2) |
| Characteristic . | CAR-T . | alloHCT . |
|---|---|---|
| CRS grade 1-5, no. (%) | 149 (82.3) | N/A |
| CRS grade 3-5, no. (%) | 18 (9.9) | N/A |
| Neurotoxicity grade 1-4, no. (%) | 112 (61.9) | N/A |
| Neurotoxicity grade 3-4, no. (%) | 38 (20.9) | N/A |
| Neutrophil recovery, % (95% CI) | ||
| Day 28 | 89.7 (84.7-93.8) | 96.8 (94.8-98.3) |
| Platelet recovery, % (95% CI) | ||
| Day 100 | 86.7 (81.2-91.4) | 92.1 (89.2-94.6) |
| Grade 2-4 acute GVHD, % (95% CI) | ||
| Day 180 | N/A | 28.7 (18.9-39.7) |
| Grade 3-4 acute GVHD, % (95% CI) | ||
| Day 180 | N/A | 8.2 (3.0-15.6) |
| Chronic GVHD, % (95% CI) | ||
| 1 y | N/A | 35.6 (30.9-40.4) |
| OS, % (95% CI) | ||
| 1 y | 73.4 (66.4-79.9) | 65.6 (60.9-70.2) |
| PFS, % (95% CI) | ||
| 1 y | 55.7 (48.0-63.2) | 53.8 (48.8-58.7) |
| Relapse/progression, % (95% CI) | ||
| 1 y | 39.5 (32.1-47.2) | 26.2 (21.9-30.7) |
| NRM, % (95% CI) | ||
| 1 y | 4.8 (2.1-8.6) | 20.0 (16.2-24.2) |
N/A, not applicable.
Outcomes of CAR-T cell therapy after autologous transplant failure. (A) Progression/relapse. (B) NRM. (C) OS. (D) PFS. Outcomes of alloHCT after autoHCT failure. (E) Progression/relapse. (F) NRM. (G) OS. (H) PFS.
Outcomes of CAR-T cell therapy after autologous transplant failure. (A) Progression/relapse. (B) NRM. (C) OS. (D) PFS. Outcomes of alloHCT after autoHCT failure. (E) Progression/relapse. (F) NRM. (G) OS. (H) PFS.
Allogeneic HCT outcomes
The median follow-up of alloHCT survivors was 51.8 months (range, 0.2-98.6; Table 2). The day 180 cumulative incidence rates of grade 2 to 4 and 3 to 4 acute GVHD were 28.7% (95% CI = 18.9-39.7) and 8.2% (95% CI = 3.0-15.6), respectively. The cumulative incidence of chronic GVHD at 1 year was 35.6% (95% CI = 30.9-40.4). The day 28 cumulative incidence of neutrophil recovery and day 100 incidence of platelet recovery was 96.8 (95% CI = 94.8-98.3) and 92.1 (95% CI = 89.2-94.6), respectively. At 1 year, the cumulative incidence rates of relapse/progression and NRM were 26.2 (95% CI = 21.9-30.7) and 20.0 (95% CI = 16.2-24.2), respectively (Figure 1E-F). The probability of 1-year OS and PFS was 65.6 (95% CI = 60.9-70.2) and 53.8 (95% CI = 48.8-58.7), respectively (Figure 1G-H).
CIBMTR prognostic score
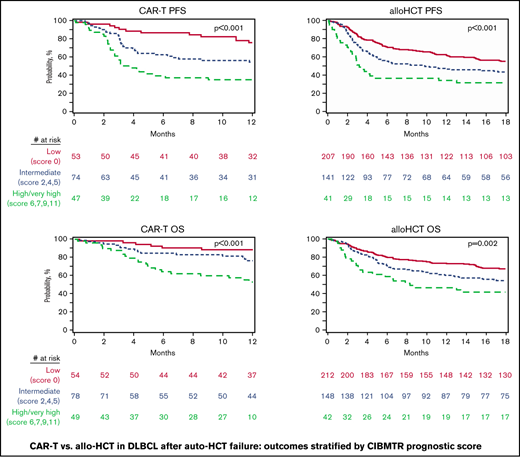
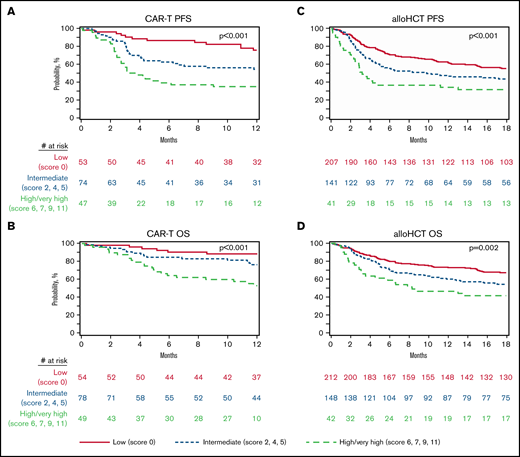
In the CAR-T cohort 54 (29.8%), 78 (43.1%) and 40 (27.1%) patients were classified into low-, intermediate- and high/very high-risk groups according to the CIBMTR prognostic score, respectively (Table 1). The 1-year PFS probabilities for the low-, intermediate-, and high/very high-risk groups were 75.8% (95% CI = 62.9-86.6), 54.3% (95% CI = 42.3-66.1), and 34.9% (95% CI = 21.9-49.1), respectively (P < .001; Figure 2A; Table 3). The 1-year OS probabilities in similar order were 88.4% (95% CI = 78.4-95.6), 76.4% (95% CI = 65.4-85.8), and 52.8% (95% CI = 38.5-66.9), respectively (P < .001; Figure 2B; Table 3).
Outcomes of CAR T-cell therapy. (A) OS.PFS. alloHCT (B), OS (C) and PFS (D), stratified according to the CIBMTR prognostic score.
Outcomes of CAR T-cell therapy. (A) OS.PFS. alloHCT (B), OS (C) and PFS (D), stratified according to the CIBMTR prognostic score.
PFS and OS after CAR-T cell or alloHCT according to the CIBMTR prognostic score
| . | Low risk (score 0) . | Intermediate risk (score 2,4,5) . | High/very high risk (score 6,7,9,11) . | Overall P . | |
|---|---|---|---|---|---|
| CAR-T cohort | 1-y OS (95% CI) | 88.4 (78.4-95.6) | 76.4 (65.4-85.8) | 52.8 (38.5-66.9) | <.001 |
| 1-y PFS (95% CI) | 75.8 (62.9-86.6) | 54.3 (42.3-66.1) | 34.9 (21.9-49.1) | <.001 | |
| alloHCT cohort | 1-y OS (95% CI) | 73.3 (67.1-79.1) | 59.9 (51.9-67.7) | 46.3 (31.5-61.5) | .002 |
| 1-y PFS (95% CI) | 62.6 (55.9-69.1) | 46.6 (38.4-54.9) | 34.1 (20.6-49.2) | <.001 | |
| . | Low risk (score 0) . | Intermediate risk (score 2,4,5) . | High/very high risk (score 6,7,9,11) . | Overall P . | |
|---|---|---|---|---|---|
| CAR-T cohort | 1-y OS (95% CI) | 88.4 (78.4-95.6) | 76.4 (65.4-85.8) | 52.8 (38.5-66.9) | <.001 |
| 1-y PFS (95% CI) | 75.8 (62.9-86.6) | 54.3 (42.3-66.1) | 34.9 (21.9-49.1) | <.001 | |
| alloHCT cohort | 1-y OS (95% CI) | 73.3 (67.1-79.1) | 59.9 (51.9-67.7) | 46.3 (31.5-61.5) | .002 |
| 1-y PFS (95% CI) | 62.6 (55.9-69.1) | 46.6 (38.4-54.9) | 34.1 (20.6-49.2) | <.001 | |
The CIBMTR prognostic score classified 212 (52.6%), 148 (36.7%), and 43 (10.7%) of alloHCT patients into low-, intermediate- and high/very high-risk groups, respectively (Table 1). The 1-year PFS probabilities for the low-, intermediate-, and high/very high-risk groups were 62.6% (95% CI = 55.9-69.1), 46.6% (95% CI = 38.4-54.9), and 34.1% (95% CI = 20.6-49.2), respectively (P < .001; Figure 2C; Table 3). The 1-year OS probabilities in similar order were 73.3% (95% CI = 67.1-79.1), 59.9% (95% CI = 51.9-67.7), and 46.3% (95% CI = 31.5-61.5), respectively (P = .002; Figure 2D; Table 3).
Subset analysis
In an exploratory subset analysis, we looked at the outcomes of patients with refractory or untreated relapse (n = 172). The 1-year PFS and OS of patients receiving CAR-T with refractory or untreated relapse (n = 122) was 51.5% and 71%, respectively. The corresponding estimates for alloHCT patients (n = 50) were 37.6 and 49%, respectively.
Cause of death
During the follow-up, 199 patients (49.3%) from the alloHCT group and 55 (30.3%) from the CAR-T group died. The primary disease was the most common cause of mortality in both groups, accounting for 40% (n = 79) and 73% (n = 40) of deaths in the alloHCT and CAR-T groups, respectively. Other common causes were infections (11%; n = 22) and organ failure (7.5%; n = 15) in the alloHCT group and infections (9.1%; n = 5) and second cancers (5.5%; n = 3 new myeloid malignancies) in the CAR-T group.
Discussion
In this retrospective analysis using the CIBMTR registry, we describe the outcomes of patients with DLBCL relapsing after an autoHCT and subsequently undergoing either CAR-T therapy or alloHCT. We also applied the CIBMTR prognostic score18 to these 2 patient cohorts to identify patients at high risk of treatment failure. Our noncomparative cohort analysis shows the following after autoHCT failure: (a) CAR-T treatment in a cohort of mostly refractory patients provides 1-year PFS of ∼55% with a ∼5% NRM risk, (b) alloHCT in a group of predominantly chemosensitive patients produces a 1-year PFS of ∼54% with a 20% NRM risk, and (c) the CIBMTR prognostic score group of high/very high-risk disease identifies a cohort of patients at particularly high risk of therapy failure after either CAR-T therapy or alloHCT.
At the onset, it is important to acknowledge that the cohort of patients getting CAR-T treatment and alloHCT in our study are fundamentally different. For example, patients receiving CAR-T were older, less fit, and mostly had refractory disease. Patients in the alloHCT cohort were younger, had a better performance score, and most had disease responding to salvage treatments. Most CAR-T procedures were done during 2018 to 2019, whereas alloHCT spanned a wider time period (2012-2019). These important differences warrant caution while interpreting even an adjusted comparison across the 2 cohorts (as shown in supplemental Table 1). The short median follow-up of CAR-T–treated patients means that the long-term outcomes cannot be analyzed using the current dataset. Chemoresponse is not a prerequisite for offering CAR-T therapy, meaning a patient relapsing after autoHCT may be able to receive this treatment without salvage attempts. The large real-world analyses have also shown feasibility of this treatment in older individuals with comorbid conditions.7,32 In contrast, establishing chemosensitivity and careful patient selection are critical for alloHCT outcomes.17,33,34 To reduce heterogeneity, the CAR-T cohort in the current analysis was limited to axi-cel, as it represented the predominant commercial product reported to the CIBMTR registry during the analysis period.
There is a paucity of data directly comparing outcomes of alloHCT vs CAR-T treatment in DLBCL. Dreger et al35 compared outcomes of CAR-T and alloHCT in relapse/refractory DLBCL by retrospectively applying the intention-to-treat principle. The analysis (not limited to the post-autoHCT failure setting) included 41 and 60 patients for whom CAR-T cells and alloHCT were intended, respectively. In both cohorts, virtually all patients had active disease at the time of indication. The 1-year estimates for NRM, relapse, PFS, and OS for CAR-T cells vs alloHCT were 3% vs 21% (P = .04), 59% vs 44% (P = .12), 39% vs 33% (P = .97), and 68% vs 54% (P = .32), respectively.35
In our analysis, we demonstrated that the CIBMTR prognostic score can predict PFS and OS of both alloHCT and CAR-T in patients with DLBCL with a prior autoHCT failure. The main advantage of this model is the ease of its use, because it uses easily available clinical variables. We acknowledge that additional variables (eg, metabolic tumor volumes, CAR-T cell fitness, composition and expansion, bilirubin level, lactate dehydrogenase, Eastern Cooperative Oncology Group performance score)32,36,37 have been shown to predict outcomes of CAR-T therapy but are not generally available in the CIBMTR registry. It possible a that a prognostic model incorporating such variables may have better discriminatory ability in post-autoHCT CAR-T recipients. Of note, the 1-year OS of alloHCT patients in the low- (73%), intermediate- (60%), and high/very high-risk (46%) groups of the current analysis (era 2012-2019) compare favorably against the corresponding 1-year rates in the original publication (era 2000-2012: low risk, 63%; intermediate risk, 52%; high/very high risk, 17%-38%).18 This difference is possibly because of general improvements in HCT supportive care, advances in GVHD prevention and treatment,38 and the restriction of the current cohort to RIC regimens.18,33,39 The CIBMTR prognostic score of high/very high-risk disease identifies a cohort of patients at particularly high risk of therapy failure after both CAR-T therapy or alloHCT. Effective approaches to mitigate risk of therapy failure after alloHCT or CAR-T treatment of such patients is clearly an area of unmet need, and investigation of novel maintenance or consolidation approaches in this setting are warranted.14,16
In patients with DLBCL with an autoHCT failure, both alloHCT and CAR-T are available options. Although not a direct comparison, the current analysis and the prior report by Dreger et al35 do not suggest an inferiority of CAR-T treatment in this setting. Indirect comparison of the outcomes of various risk groups identified by the CIBMTR score also does not identify a subgroup where 1 treatment is clearly superior over the other. In the absence of such data, CAR-T treatment has now practically emerged as the preferred cell-based therapy option in post-autoHCT failures, likely because of its safety and documented efficacy in refractory disease.25 The CIBMTR and European Society for Blood and Marrow Transplantation consensus guidelines, however, identify rare circumstances where alloHCT may be considered as the first cellular immunotherapy in relapsed/refractory DLBCL (eg, in patients with refractory cytopenias with or without signs of therapy-related myelodysplasia, cases with CAR-T manufacturing failure, or in regions or centers where CAR-T treatment is not available). In addition, limited data suggest that alloHCT may salvage a subset of CAR-T recipients with evidence of persistent or recurrent disease.40 Because, in such patients, higher-intensity conditioning regimens are associated with higher NRM and inferior OS,40 it is important to use lower-intensity conditioning platforms to minimize the risk of procedure-related morbidity and mortality.20,33,34,39
In conclusion, this noncomparative, retrospective, registry analysis shows that both CAR-T and alloHCT can provide durable remissions in a subset of patients with DLBCL relapsing after prior autoHCT. The profiles of patients benefiting from these approaches are distinct. The simple-to-use CIBMTR prognostic score can be used to identify patients at high risk of treatment failure after either procedure in clinical practice or incorporated in the design of trials evaluating novel relapse mitigations strategies after cellular immunotherapies in patients with DLBCL with a prior autoHCT failure.
Acknowledgments
The CIBMTR is supported primarily by Public Health Service grant U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute, and the National Institute of Allergy and Infectious Diseases, grant U24CA233032 from the NCI, grant HHSH250201700006C from the Health Resources and Services Administration, and grants N00014-20-1-2705 and N00014-20-1-2832 from the Office of Naval Research. Support is also provided by Be the Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, and the following commercial entities: AbbVie, Accenture, Actinium Pharmaceuticals, Inc., Adaptive Biotechnologies Corporation, Adienne SA, Allovir, Inc., Amgen, Inc., Astellas Pharma US, bluebird bio, inc., Bristol Myers Squibb Co., CareDx, CSL Behring, CytoSen Therapeutics, Inc., Daiichi Sankyo Co., Ltd., Eurofins Viracor, ExcellThera, Fate Therapeutics, Gamida-Cell, Ltd., Genentech Inc, Gilead, GlaxoSmithKline, Incyte Corporation, Janssen/Johnson & Johnson, Jasper Therapeutics, Jazz Pharmaceuticals, Inc., Karyopharm Therapeutics, Kiadis Pharma, Kite, a Gilead Company, Kyowa Kirin, Magenta Therapeutics, Medac GmbH, Merck & Co., Millennium, the Takeda Oncology Co., Miltenyi Biotec, Inc., MorphoSys, Novartis Pharmaceuticals Corporation, Omeros Corporation, Oncopeptides, Inc., Orca Biosystems, Inc., Pfizer, Inc., Pharmacyclics, LLC, Sanofi Genzyme, Seagen, Inc., Stemcyte, Takeda Pharmaceuticals, Tscan, Vertex, Vor Biopharma, and Xenikos BV.
Authorship
Contribution: M.H., F.L.L., A.K.G., and M.P. conceived and designed the study; X.Q. collected and assembled the data; X.Q. and S.K. analyzed the data; M.H. prepared the first draft of the manuscript; and all authors interpreted the data and revised the manuscript.
Conflict-of-interest disclosure: M.H. received research support/funding from Takeda Pharmaceutical Company, Spectrum Pharmaceuticals, and Astellas Pharma; consulted for Janssen, Incyte Corporation, ADC Therapeutics, Celgene Corporation, Omeros, Verastem, and MorphoSys; and was on the speaker’s bureau for Sanofi Genzyme, AstraZeneca, and BeiGene. A.K.G. received research funding from Bristol Myers Squibb, Pharmacyclics, Gilead, Genentech, AstraZeneca, Pfizer, Teva, Takeda, Acrotec, IgM, I-Mab, Agios, and Merck and consulted for Abbvie, Genentech, Janssen, AstraZeneca, Pharmacyclics, Bristol Myers Squibb, Amgen, Morphosys, TG Therapeutics, Kite Pharma, Adaptive, SeaGen, Epizyme, Gilead, ADCT, Incyte, Karyopharm, Actinium, Asana bio, Nurix, and Aptevo. F.L.L. was a scientific advisor for Allogene, Amgen, Bluebird Bio, BMS/Celgene, Calibr, Cellular Biomedicine Group, GammaDelta Therapeutics, Iovance, Kite Pharma, Janssen, Legend Biotech, Novartis, Wugen, and Umoja; received research funding from Kite Pharma (institutional), Allogene (institutional), Novartis (institutional); and holds several patents (held by the institution in his name; unlicensed) in the field of cellular immunotherapy. S.C. was a scientific advisor for Allogene, Acrotech, Cellularity, CareDx, Kiadis Pharma, Milteniy Biotech, and Molmed and received research funding from Miltenyi and Kiadis Pharma. P.B.D. sat on an advisory board for Kite (Gilead). J.G. received institutional research support from CCSG and SCCA Swim Across America and received consulting fees from JMP, Eusapharma, Larvol, and Multerra Bio. The remaining authors declare no competing financial interests.
Correspondence: Mehdi Hamadani, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, 9200 W Wisconsin Ave, Suite C5500, Milwaukee, WI 53226; e-mail: mhamadani@mcw.edu.
References
Author notes
M.H. and A.K.G. contributed equally to this study.
CIBMTR supports accessibility of research in accord with the National Institutes of Health Data Sharing Policy and the National Cancer Institute Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases de-identified datasets that comply with all relevant global regulations regarding privacy and confidentiality.
The full-text version of this article contains a data supplement.