Key Points
VITT plasma induces an FcγRIIa-dependent procoagulant response in donor platelets suppressible by heparin and IVIg.
Whole-blood procoagulant platelet flow cytometry has diagnostic potential to detect platelet-activating VITT antibodies.
Abstract
Vaccine-induced immune thrombotic thrombocytopenia (VITT) is a severe prothrombotic complication of adenoviral vaccines, including the ChAdOx1 nCoV-19 (Vaxzevria) vaccine. The putative mechanism involves formation of pathological anti–platelet factor 4 (PF4) antibodies that activate platelets via the low-affinity immunoglobulin G receptor FcγRIIa to drive thrombosis and thrombocytopenia. Functional assays are important for VITT diagnosis, as not all detectable anti-PF4 antibodies are pathogenic, and immunoassays have varying sensitivity. Combination of ligand binding of G protein–coupled receptors (protease-activated receptor-1) and immunoreceptor tyrosine–based activation motif–linked receptors (FcγRIIa) synergistically induce procoagulant platelet formation, which supports thrombin generation. Here, we describe a flow cytometry–based procoagulant platelet assay using cell death marker GSAO and P-selectin to diagnose VITT by exposing donor whole blood to patient plasma in the presence of a protease-activated receptor-1 agonist. Consecutive patients triaged for confirmatory functional VITT testing after screening using PF4/heparin ELISA were evaluated. In a development cohort of 47 patients with suspected VITT, plasma from ELISA-positive patients (n = 23), but not healthy donors (n = 32) or individuals exposed to the ChAdOx1 nCov-19 vaccine without VITT (n = 24), significantly increased the procoagulant platelet response. In a validation cohort of 99 VITT patients identified according to clinicopathologic adjudication, procoagulant flow cytometry identified 93% of VITT cases, including ELISA-negative and serotonin release assay–negative patients. The in vitro effect of intravenous immunoglobulin (IVIg) and fondaparinux trended with the clinical response seen in patients. Induction of FcγRIIa-dependent procoagulant response by patient plasma, suppressible by heparin and IVIg, is highly indicative of VITT, resulting in a sensitive and specific assay that has been adopted as part of a national diagnostic algorithm to identify vaccinated patients with platelet-activating antibodies.
Introduction
Vaccine-induced immune thrombotic thrombocytopenia (VITT) is a severe prothrombotic syndrome described in association with adenoviral coronavirus 2 (SARS-CoV-2) vaccination.1-3 In Australia, the ChAdOx1 nCoV-19 (Vaxzevria, AstraZeneca) vaccine is a key component of the national vaccination program. VITT can be severe and occasionally fatal.1,3 Mortality is significantly reduced with early recognition and institution of therapy, including infusion of intravenous immunoglobulin (IVIg) and commencement of nonheparin anticoagulants.4,5 The putative mechanism is analogous to heparin-induced thrombocytopenia (HIT), in which pathologic autoantibodies against platelet factor 4 (PF4) activate platelets via the low-affinity immunoglobulin G receptor FcγRIIa to drive thrombosis and associated thrombocytopenia.1,6,7 Not all immunologically detected anti-PF4 antibodies are pathologic, with 6.8% of anti-PF4 antibodies detectable by screening of well individuals after COVID-19 vaccination.8 The Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis suggests that diagnostic VITT samples be tested in one or more HIT functional assays.9
Procoagulant platelets (PPs) are a subpopulation of platelets that promote coagulation by providing procoagulant surfaces, enabling assembly of coagulation factors and thrombin generation.10,11 Detection of PPs by using a combination of P-selectin (platelet activation marker) and annexin V (phosphatidylserine marker) in the presence of PF4 has been proposed as a diagnostic assay for VITT.12 PPs are not typically generated in response to low-level agonist stimulation; however, the combination of ligand binding of G protein–coupled receptors (GPCRs) (eg, protease-activated receptor-1 [PAR1]) and immunoreceptor tyrosine–based activation motif (ITAM)-linked receptors, including FcγRIIa, synergistically induce formation of PPs.13,14
PPs are formed in response to VITT antibodies.12 Here, we describe a PP flow cytometric assay to diagnose VITT by using combinations of a cell death marker, GSAO [4-(N-(S-glutathionylacetyl)amino)phenylarsonous acid], and P-selectin. Platelets identified by GSAO uptake bind active factor X and support thrombin generation, whereas only a subset of platelets expressing phosphatidylserine bind active factor X.15,16 Thus, GSAO methodology yields more specific identification of functionally relevant PPs.17 We hypothesized that priming platelets from pedigree donors with a PAR1 agonist at levels sufficient to release PF4, but insufficient to generate significant procoagulant responses, would provide a platform in which measured procoagulant responses would reflect ITAM receptor ligand signaling by FcγRIIa-dependent procoagulant antibodies in patient plasma, without requiring additional PF4.
Methods
Study approval
Human studies were approved by Sydney Local Health District Human Research Ethics Committees (HREC/18/CRGH/294, X21-0160, 2021/ETH00945). Healthy donors gave written informed consent.
Study cohort
Citrated plasma from 47 Australian patients referred for confirmatory VITT testing after ChAdOx1 nCov-19 vaccination between 1 April 2021 and 11 August 2021 formed the development cohort. Data collected included time to onset of symptoms, platelet count, D-dimer level, site of thrombosis, and treatment initiation. All patients underwent anti-PF4 testing with PF4/heparin ELISA (Asserachrom HPIA IgG Assay, Stago Diagnostics) and VITT functional testing via standard (no exogenous PF4) serotonin release assay (SRA) and/or multiplate multiple electrode aggregometry (MEA). Patients with confirmed thrombosis were grouped as follows: (1) confirmed VITT if fulfilling all 5 criteria of (i) vaccine within 4 to 42 days of symptom onset, (ii) thrombocytopenia, (iii) D-dimer level >5 times the upper limit of normal, (iv) anti-PF4/polyanion ELISA positive and (v) SRA positive; (2) VITT negative if (i) ELISA negative, (ii) SRA negative, and (iii) clinically adjudicated not to be VITT; and (3) “ELISA false positive” if (i) ELISA positive, (ii) SRA negative or MEA negative, and (iii) normal platelet count. Comparison was made with plasma from 32 healthy individuals. The flow cytometry assay was assessed in a validation cohort of 99 VITT cases clinically adjudicated by members of the Thrombosis and Haemostasis Society of Australia and New Zealand (THANZ) advisory group based on clinicopathologic criteria.5,18
Healthy volunteers
Healthy donors recruited at Concord Hospital and ANZAC Research Institute (Sydney, Australia) were screened as suitable VITT donors in a 2-step process. First, FcγRIIa responders were identified by using light transmission aggregometry (LTA) in response to 1.5 µg/mL of anti-CD9, clone ALB6 (Santa Cruz Biotechnology), as described previously.19 Second, high responders exhibiting >80% aggregation with a time to initiation <180 seconds were subjected to a second screen and selected as suitable VITT donors if returning concordant results using a plasma panel of patients with known positive, negative, and low positive patterns (supplemental Figure 4). Eleven of 58 individuals were identified by using the LTA screen, and 9 of those 11 were suitable for VITT testing. Group O donors were preferred but not a limitation. Blood was collected from the antecubital fossa into 3.2% citrate tubes using a 21-gauge butterfly needle. The initial 3 mL of blood was discarded.
Procoagulant platelet assay
The GSAO-based whole-blood PP flow cytometry assay15 was modified to incorporate exogenous plasma.20 Serum samples were found to be unsuitable for this assay. Citrated whole blood (13 µL) was incubated with and without 5 µM SFLLRN (Auspep), unfractionated heparin (0.5-100 U/mL, Pfizer), and ChAdOx1 nCoV-19 (1:2000 [vol/vol], AstraZeneca). In some experiments, blood was pretreated with 10 µg/mL IV.3 antibody against FcγRIIa (Stemcell Technologies) or 10 mg/mL IVIg (Privigen, CSL Behring) for 15 minutes before testing. Where indicated, fondaparinux (1.2-100 µg/mL, Arixtra [GlaxoSmithKline]), SARS-CoV-2 spike protein (20 µg/mL HexaPro), or 25 µg/mL native human PF4 purified from expired platelet concentrate21 was added. A total of 5 µL citrated plasma or buffer was added simultaneously to the reaction mix for 10 minutes, with 2.5 mM Gly-Pro-Arg-Pro peptide (Sigma Aldrich) and 2.5 mM calcium chloride in Hanks balanced salt solution (pH 7.35) in a 50 µL reaction. The reaction was stopped by further dilution with Hanks balanced salt solution, followed by staining with antibodies to CD45 (HI30) (Stemcell Technologies), CD41a (HIP8) (BD Biosciences), CD62P (Psel.KO2.3) (eBioscience), or isotype control (eBioscience), and GSAO or control compound GSCA [4-(N-(S-glutathionylacetyl)amino)benzoic acid]. Samples were fixed with PAMFix (Platelet Solutions Ltd), centrifuged, and resuspended, before analysis on a BD LSRFortessa X-20 or BD FACSCanto II cytometer with acquisition of 3000 to 7000 platelet events (all steps performed at room temperature). A positive (confirmed VITT patients) and negative (healthy individuals) control plasma was incorporated with each run. Reagents are available on request.
SRA and multiplate MEA
Statistical analyses
Statistical analyses were performed by using GraphPad Prism 9.2 (GraphPad Software), with significance set at P < .05. Experimental data are presented as mean ± standard deviation.
Results
Characteristics of patients suspected of having VITT: development cohort
Forty-seven patients (19 female subjects, 28 male subjects) with a median age of 70 years (interquartile range: 58-81 years) referred for confirmatory VITT testing with thrombosis after ChAdOx1 nCoV-19 vaccination were analyzed for the development cohort. Demographic data are summarized in Table 1. Patients with confirmed VITT were significantly younger (median age, 65 years vs 74 years; P = .017) with lower platelet count (median, 45 × 109/L vs 130 × 109/L; P < .0001) and higher D-dimer levels (median, 40-fold vs 14.4-fold increase above the upper limit of normal; P = .006) compared with VITT-negative patients. No significant differences in sex, timing of presentation relative to vaccination, or incidence of thrombosis was observed between VITT-confirmed vs VITT-negative patients. All 23 confirmed VITT patients had thrombosis, including cerebral venous sinus thrombosis (n = 4), splanchnic vein thrombosis (n = 4), pulmonary embolism (n = 12), and deep vein thrombosis (n = 10). Most VITT-positive patients received IVIg. Forty-six of 47 samples were collected before treatment.
Laboratory and clinical characteristics of patients suspected of VITT in the development cohort
| Variable . | All (N = 47) . | VITT positive (n = 23) . | VITT negative (n = 20) . | ELISA false positive (n = 4) . | P* . |
|---|---|---|---|---|---|
| Sex | .350 | ||||
| Female | 19 | 11 | 6 | 2 | |
| Male | 28 | 12 | 14 | 2 | |
| Age, y | 70 (58-81) | 65 (52-75) | 74 (63-83) | 69 (50-84) | .017 |
| Days’ postvaccine | 10 (7-17) | 9 (8-14) | 12 (5-20) | 8 (7-15) | .912 |
| Platelet count (×109/L) | 85 (39-135) | 45 (30-65) | 130 (114-143) | 232 (209-255) | <.0001 |
| D-dimer (fold-change over ULN) | 33.8 (9.4-40) | 40 (30-70) | 14.4 (5.1-28.3) | <5× ULN in 3 patients | .006 |
| Thrombosis | 45 | 23 | 19 | 3 | .465 |
| Variable . | All (N = 47) . | VITT positive (n = 23) . | VITT negative (n = 20) . | ELISA false positive (n = 4) . | P* . |
|---|---|---|---|---|---|
| Sex | .350 | ||||
| Female | 19 | 11 | 6 | 2 | |
| Male | 28 | 12 | 14 | 2 | |
| Age, y | 70 (58-81) | 65 (52-75) | 74 (63-83) | 69 (50-84) | .017 |
| Days’ postvaccine | 10 (7-17) | 9 (8-14) | 12 (5-20) | 8 (7-15) | .912 |
| Platelet count (×109/L) | 85 (39-135) | 45 (30-65) | 130 (114-143) | 232 (209-255) | <.0001 |
| D-dimer (fold-change over ULN) | 33.8 (9.4-40) | 40 (30-70) | 14.4 (5.1-28.3) | <5× ULN in 3 patients | .006 |
| Thrombosis | 45 | 23 | 19 | 3 | .465 |
Continuous variables are expressed as median (interquartile range).
ULN, upper limit of normal.
Fisher’s exact test (categorical variables) or Mann-Whitney U test (continuous variables) between VITT-positive and VITT-negative groups. P < .05 are in bold.
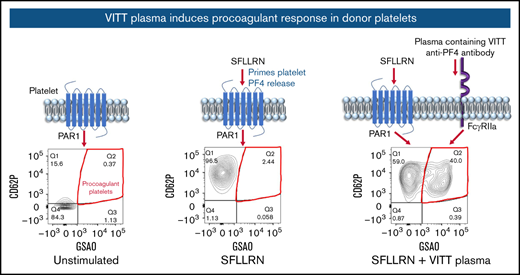
VITT plasma sensitizes healthy platelets to become procoagulant
PPs in whole-blood reactions were detected by flow cytometry and defined as CD41a-positive (platelet-specific marker) events that are GSAO- and P-selectin–positive. In proof-of-principle experiments, donor blood treated with the PAR1 agonist SFLLRN caused release of platelet α-granules represented by P-selectin expression, with minimal PPs formed (Figure 1A-B). Exposing healthy blood to both SFLLRN and plasma containing VITT anti-PF4 antibodies, but not plasma from a patient with sepsis, resulted in a synergistic effect, whereby a dramatic increase in PP proportion was observed by flow cytometry and evidence of ballooning with GSAO uptake by microscopy. In the development cohort (47 patients), minimal PPs (3.9% ± 1.0%) were generated by donor blood treated with SFLLRN (Figure 1C). Addition of plasma from healthy individuals (3.6% ± 1.4%), or ChAdOx-1 nCoV-19 vaccinated patients with thrombocytopenia and thrombosis but without detectable anti-PF4 antibodies (VITT-negative, 4.5% ± 2.1%), or ELISA false-positive patients (3.9% ± 0.8%), did not increase the procoagulant response in donor platelets. However, addition of plasma from confirmed VITT patients significantly increased PP response (25.7% ± 17.7%) compared with no plasma (P < .0001), healthy (P < .0001), VITT-negative (P < .0001), or ELISA false-positive (P = .0381) samples. The PP proportions induced by VITT plasma varied between patients (range, 5.7%-63.0%).
Plasma from patients with VITT sensitizes healthy donor platelets to become procoagulant. (A) Schematic diagram and representative flow cytometry plots depicting platelet P-selectin expression (CD62P) and GSAO uptake on (i) unstimulated donor platelets and upon stimulation with (ii) 5 µM SFLLRN alone or (iii) a synergistic combination of SFLLRN and plasma containing VITT anti-PF4 antibodies or (iv) plasma from a patient with bacterial sepsis. PPs are defined as GSAO+/CD62P+ platelet events (red quadrant). (B) Confocal imaging of healthy donor platelet-rich plasma exposed to 5 µM SFLLRN alone or SFLLRN and VITT plasma. Platelets are identified by CD41a antibody (cyan, top panel), whereas P-selectin (CD62P in yellow, middle panel) marks activated platelets. GSAO uptake is shown in yellow (bottom panel). PPs are characterized by ballooning morphology and GSAO uptake (bottom right panel). (C) PP flow cytometry was performed by using healthy donor whole blood treated with platelet agonist 5 µM SFLLRN (n = 43) and incubated with plasma from healthy individuals (n = 32), ChAdOx1 nCoV-19–vaccinated patients with thrombocytopenia and thrombosis but without detectable anti-PF4 antibodies (VITT neg, n = 20), vaccinated patients who were not thrombocytopenic with detectable anti-PF4 antibodies (ELISA false pos, n = 4), or clinically confirmed SRA-positive VITT patients with thrombocytopenia, thrombosis, and detectable anti-PF4 antibodies (VITT pos, n = 23). PP percentages were defined by the proportion of GSAO+/CD62P+ platelet events. Kruskal-Wallis test with Dunn’s correction for multiple comparisons was performed. Error bars indicate mean ± standard deviation. *P < .05, ****P < .0001.
Plasma from patients with VITT sensitizes healthy donor platelets to become procoagulant. (A) Schematic diagram and representative flow cytometry plots depicting platelet P-selectin expression (CD62P) and GSAO uptake on (i) unstimulated donor platelets and upon stimulation with (ii) 5 µM SFLLRN alone or (iii) a synergistic combination of SFLLRN and plasma containing VITT anti-PF4 antibodies or (iv) plasma from a patient with bacterial sepsis. PPs are defined as GSAO+/CD62P+ platelet events (red quadrant). (B) Confocal imaging of healthy donor platelet-rich plasma exposed to 5 µM SFLLRN alone or SFLLRN and VITT plasma. Platelets are identified by CD41a antibody (cyan, top panel), whereas P-selectin (CD62P in yellow, middle panel) marks activated platelets. GSAO uptake is shown in yellow (bottom panel). PPs are characterized by ballooning morphology and GSAO uptake (bottom right panel). (C) PP flow cytometry was performed by using healthy donor whole blood treated with platelet agonist 5 µM SFLLRN (n = 43) and incubated with plasma from healthy individuals (n = 32), ChAdOx1 nCoV-19–vaccinated patients with thrombocytopenia and thrombosis but without detectable anti-PF4 antibodies (VITT neg, n = 20), vaccinated patients who were not thrombocytopenic with detectable anti-PF4 antibodies (ELISA false pos, n = 4), or clinically confirmed SRA-positive VITT patients with thrombocytopenia, thrombosis, and detectable anti-PF4 antibodies (VITT pos, n = 23). PP percentages were defined by the proportion of GSAO+/CD62P+ platelet events. Kruskal-Wallis test with Dunn’s correction for multiple comparisons was performed. Error bars indicate mean ± standard deviation. *P < .05, ****P < .0001.
VITT-induced PP response is suppressed by heparin
To explore whether charged molecules affected VITT-induced PP response, low-dose (0.5 U/mL) and high-dose (100 U/mL) heparin was added to the reaction. Low-dose heparin significantly reduced PP response induced by most VITT plasmas (12.5% ± 12.8% vs 25.7% ± 17.7%; P = .0096) (Figure 2A). Inhibition by low-dose heparin on VITT plasma-induced PP response was variable, with 0.1- to 1.0-fold (0.4 ± 0.2-fold) change from plasma alone. Interestingly, 3 of 23 patients exhibited an enhanced PP response, with low-dose heparin similar to the procoagulant response induced by HIT plasma (Figure 2B). In all cases, PP response was completely abolished with high-dose heparin (VITT, 3.5% ± 1.4% vs 25.7% ± 17.7% [P < .0001]; HIT, 3.5% ± 1.0% vs 42.8% ± 12.1% [P < .0001]).
PP response induced by VITT plasma is suppressed by heparin, is antibody mediated, and corresponded with clinical response to therapy. Healthy donor whole blood was treated with platelet agonist 5 µM SFLLRN and plasma from patients with VITT (n = 23) (A) or HIT (n = 8) (B) in the presence of low-dose (0.5 U/mL) or high-dose (100 U/mL) unfractionated heparin and assessed for PP formation by using flow cytometry. Blue lines represent patients with VITT who generated a heparin-enhancing PP response at low-dose heparin. Friedman test with Dunn’s correction for multiple comparisons was performed. Donor blood was pretreated with the FcγRIIa-blocking antibody IV.3 (10 µg/mL) (C) or IVIg (10 mg/mL) (D) for 15 minutes before exposure to VITT plasma and 5 µM SFLLRN. Wilcoxon matched-pairs signed rank test was performed. (E) PP response induced by VITT plasma (n = 4) collected pre-IVIg treatment was compared with the procoagulant response within 5 days’ post-IVIg therapy. The reduction in the PP response corresponded to the suppressive effect of exogenous IVIg (10 mg/mL) on the pre-IVIg sample. Results are presented as fold-change in PP proportion relative to no plasma control. Each line represents a unique patient. (F) Plasma samples from 6 patients with VITT collected before initiation of fondaparinux treatment was tested in the presence of fondaparinux (1.2 µg/mL) in vitro. PP response is normalized to no fondaparinux control. Four patients were subsequently found to be clinically responsive to fondaparinux, and 2 patients were fondaparinux resistant. Healthy donor whole blood was treated with platelet agonist 5 µM SFLLRN and VITT plasma in the presence of the ChAdOx1 nCoV-19 vaccine (AZD1222, 1:2000 [vol/vol], n = 23) (G) or recombinant SARS-CoV-2 spike protein (HexaPro 20 µg/mL, n = 10) (H) before flow cytometric assessment for PP formation. Wilcoxon matched-pairs signed rank test was performed. *P < .05, **P < .01, ***P < .001,****P <.0001. ns, not significant.
PP response induced by VITT plasma is suppressed by heparin, is antibody mediated, and corresponded with clinical response to therapy. Healthy donor whole blood was treated with platelet agonist 5 µM SFLLRN and plasma from patients with VITT (n = 23) (A) or HIT (n = 8) (B) in the presence of low-dose (0.5 U/mL) or high-dose (100 U/mL) unfractionated heparin and assessed for PP formation by using flow cytometry. Blue lines represent patients with VITT who generated a heparin-enhancing PP response at low-dose heparin. Friedman test with Dunn’s correction for multiple comparisons was performed. Donor blood was pretreated with the FcγRIIa-blocking antibody IV.3 (10 µg/mL) (C) or IVIg (10 mg/mL) (D) for 15 minutes before exposure to VITT plasma and 5 µM SFLLRN. Wilcoxon matched-pairs signed rank test was performed. (E) PP response induced by VITT plasma (n = 4) collected pre-IVIg treatment was compared with the procoagulant response within 5 days’ post-IVIg therapy. The reduction in the PP response corresponded to the suppressive effect of exogenous IVIg (10 mg/mL) on the pre-IVIg sample. Results are presented as fold-change in PP proportion relative to no plasma control. Each line represents a unique patient. (F) Plasma samples from 6 patients with VITT collected before initiation of fondaparinux treatment was tested in the presence of fondaparinux (1.2 µg/mL) in vitro. PP response is normalized to no fondaparinux control. Four patients were subsequently found to be clinically responsive to fondaparinux, and 2 patients were fondaparinux resistant. Healthy donor whole blood was treated with platelet agonist 5 µM SFLLRN and VITT plasma in the presence of the ChAdOx1 nCoV-19 vaccine (AZD1222, 1:2000 [vol/vol], n = 23) (G) or recombinant SARS-CoV-2 spike protein (HexaPro 20 µg/mL, n = 10) (H) before flow cytometric assessment for PP formation. Wilcoxon matched-pairs signed rank test was performed. *P < .05, **P < .01, ***P < .001,****P <.0001. ns, not significant.
VITT-induced PP response requires FcγRIIa and can be inhibited by IVIg
Donor platelets pretreated with FcγRIIa function-blocking antibody IV.3 or IVIg before exposure to patient plasma showed that PP response is mediated through FcγRIIa and induced by antibodies. Regardless of extent of the PP response, the PP proportion was significantly reduced to baseline levels with IV.3 (4.5 ± 1.7 vs 25.8 ± 17.8; P < .0001) (Figure 2C). Although IVIg significantly decreased PP proportions induced by VITT plasma (6.4 ± 5.2 vs 25.8 ± 17.8; P < .0001) (Figure 2D), the extent of inhibition varied whereby partial inhibition was observed in some patients.
In vitro PP response to clinical therapies
Given that IVIg is recommended for treating VITT,4,5 we evaluated whether in vitro responses to IVIg could inform in vivo clinical response to IVIg therapy using confirmed cases collected both before and within 5 days of IVIg infusion (Figure 2E). Post-IVIg samples exhibited a procoagulant response similar to that of the pre-IVIg sample with exogenous IVIg. Nonheparin anticoagulants such as the synthetic factor Xa inhibitor (modeled on heparin pentasaccharide sequence) fondaparinux are used in VITT therapy.4,5 Treatment of SRA-confirmed HIT with fondaparinux resulted in mixed outcomes,23 and similar “resistance” may also occur in VITT. We compared the effect of exogenous fondaparinux on PP response induced by pretreatment plasma from 6 fondaparinux-treated patients with VITT. Inhibition of the PP response was reported at control concentrations aimed to disrupt charge-related interactions (100 µg/mL fondaparinux). At the therapeutic fondaparinux concentration (1.2 µg/mL), we observed a decreasing trend in PP response induced by plasma from 4 patients who clinically responded to fondaparinux therapy (Figure 2F). In contrast, no change in PP response was seen with fondaparinux when tested with plasma from patients who did not respond clinically. No relationship between response and ELISA optical density was detected (supplemental Figure 1).
Addition of ChAdOx1 nCoV-19 and SARS-CoV-2 spike protein resulted in an inconsistent effect on procoagulant response
Exogenous ChAdOx1 nCoV-19 did not induce a procoagulant response in the absence of VITT plasma (supplemental Figure 2). Addition of ChAdOx1 nCoV-19 enhanced PP response in some, but not all, patients with VITT (Figure 2G). The procoagulant response generated by 3 patients with heparin-enhancing VITT was reduced in the presence of vaccine. Overall, there was no significant change in PP response induced by 23 VITT plasmas in the presence of vaccine (27.1 ± 19.1 vs 25.8 ± 17.8; P = .6273).
Inclusion of HexaPro, a recombinant form of SARS-CoV-2 spike protein containing 6 amino acid substitutions for stable expression,24 did not significantly change the PP response generated by 10 VITT patient plasmas (25.5 ± 14.4 vs 23.6 ± 14.8; P = .3223) (Figure 2H). A positive, but nonsignificant, trend (Pearson r = 0.538; P = .1090) (supplemental Figure 3) was observed between the effect of ChAdOx1 nCoV-19 and HexaPro on VITT plasma–induced PP response.
Diagnostic utility of PP assay
To evaluate the diagnostic potential, a receiver-operating characteristic curve was generated using plasma from patients with confirmed VITT (n = 23) who tested positive on both ELISA and SRA, and VITT-negative patients (n = 24) comprising ELISA-negative and SRA-negative patients, and ELISA false-positive patients who were non-thrombocytopenic and SRA negative. The area under the curve was 0.97 ± 0.02 (P < .0001) with a cutoff of 1.7-fold increase in plasma-induced PP formation demonstrating 100% sensitivity and 91.7% specificity (Figure 3A; supplemental Table 1). Suppression of PP response by IV.3-mediated blockade of FcγRIIa may further discriminate between antibody-mediated (VITT) and non–antibody-mediated (non-VITT) procoagulant response. A <0.80-fold change with IV.3 achieved 100% sensitivity and 91.3% specificity, with an area under the curve of 0.99 ± 0.01 (P < .0001) (Figure 3B; supplemental Table 2) and identified individuals with non–antibody-mediated PP enhancement.
Diagnostic potential of PP flow cytometry assay in identifying VITT plasma. (A) Receiver-operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic potential of fold-increase compared with no plasma baseline in PP formation in healthy donors induced by plasma from patients with confirmed VITT (n = 23) who tested positive on both ELISA and SRA, and VITT-negative patients (n = 24) who tested negative on both ELISA and SRA, and ELISA false-positive patients who are non-thrombocytopenic and SRA-negative. (B) ROC analysis of the fold-change in PP proportion in the presence of the FcγRIIa-blocking antibody IV.3 (10 µg/mL) relative to SFLLRN alone. Supplemental Tables 1 and 2 provide lists of sensitivity and specificity at various cutoff values. Representative patterns of PP response demonstrating classical VITT (C), heparin-enhancing VITT (D), and negative profile (E). PP response of individual patients in the development cohort are shown in panels F, G, and H. Dotted horizontal line represents no plasma baseline. AUC, area under the curve.
Diagnostic potential of PP flow cytometry assay in identifying VITT plasma. (A) Receiver-operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic potential of fold-increase compared with no plasma baseline in PP formation in healthy donors induced by plasma from patients with confirmed VITT (n = 23) who tested positive on both ELISA and SRA, and VITT-negative patients (n = 24) who tested negative on both ELISA and SRA, and ELISA false-positive patients who are non-thrombocytopenic and SRA-negative. (B) ROC analysis of the fold-change in PP proportion in the presence of the FcγRIIa-blocking antibody IV.3 (10 µg/mL) relative to SFLLRN alone. Supplemental Tables 1 and 2 provide lists of sensitivity and specificity at various cutoff values. Representative patterns of PP response demonstrating classical VITT (C), heparin-enhancing VITT (D), and negative profile (E). PP response of individual patients in the development cohort are shown in panels F, G, and H. Dotted horizontal line represents no plasma baseline. AUC, area under the curve.
Pattern of PP response in VITT
Figure 3C-H presents the patterns of PP response used in reporting. A pattern we termed “classical VITT” shows the increase in PP compared with baseline with reduction in the presence of 0.5 U/mL heparin, abrogation in the presence of 100 U/mL heparin, and at least a 0.80-fold decrease with IV.3. The pattern we termed “heparin-enhancing VITT” exhibits the same as classical VITT but with an increased PP response in the presence of 0.5 U/mL heparin. An equivocal (non–antibody-mediated procoagulant) response shows an increase in PP but without reduction with IV.3 regardless of other reactivity.
Evaluation of utility of standard flow cytometry assay in case ascertainment
Analysis of 108 cases referred for functional testing within the first 2.5 months of VITT in Australia (Table 2) found 59 cases consistent with VITT. No “VITT-negative” patients had a positive flow cytometry assay. Of the 59 patients with VITT, results of 41 cases were concordant between ELISA, standard SRA, and flow cytometry. Four patients who tested positive on both standard SRA and flow cytometry were ELISA negative but classified as VITT by the THANZ VITT advisory group who would have been missed using ELISA criteria alone. Fourteen patients were positive by standard flow cytometry who were missed by initial standard SRA testing. Thirteen of 14 were VITT serologically supported by either ELISA, PF4-enhanced SRA, or MEA as functional assays indicating likely true positive findings. One of 14 was clinically adjudicated to be VITT without additional serologic support. This standard flow cytometry assay enabled 7.4% cases in this cohort to be classified as VITT that would otherwise be missed by ELISA and 14.8% cases missed by standard SRA.
Laboratory and clinical data on serologically confirmed VITT patients with discrepant results on VITT testing platforms
| Sex . | Age (y) . | Platelet count (×109/L) . | D-dimer (fold-change) . | Thrombosis . | ELISA . | ELISA OD . | Flow cytometry . | SRA . | Multiplate . | Serologically confirmed VITT . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard . | PF4 enhanced . | Standard . | PF4 enhanced . | |||||||||
| Serologically confirmed VITT, standard flow cytometry negative/equivocal (n = 9) | ||||||||||||
| M | 75 | 41 | 25 | Renal artery occlusion | Positive | 2.4 | Negative | Classical VITT | Positive | NT | Positive | Yes |
| M | 79 | 249 | 7.2 | PE | Positive | 1.8 | Negative | Heparin-enhancing VITT | Positive | NT | Negative | Yes |
| M | 76 | 53 | 40 | CVST, bilateral DVT | Negative | 0.11 | Negative | Negative | Positive | NT | Negative | Yes |
| M | 73 | 124 | 14.5 | PE, DVT | Negative | 0.064 | Negative | Negative | Weak positive | Weak positive | NT | Yes |
| F | 53 | 45 | 17 | Carotid artery, CVST, PE, LL arterial | Positive | 0.36 | Negative | Classical VITT | Positive | NT | Negative | Yes |
| F | 35 | 153 | 5 | CVST | Positive | 1.34 | Equivocal | Equivocal | Weak positive | Equivocal | Inconclusive | Yes |
| F | 54 | 108 | 5.8 | MCA stroke | Positive | 0.88 | Equivocal | Classical VITT | Negative | NT | Negative | Yes |
| M | 63 | 110 | 26.6 | PE | Positive | 0.87 | Inconclusive | Classical VITT | Negative | Equivocal (negative) | NT | Yes |
| M | 70 | 100 | 22 | DVT | Positive | 0.74 | Negative | Classical VITT | NT | Inconclusive | NT | Yes |
| Serologically confirmed VITT, standard flow cytometry positive (n = 18) | ||||||||||||
| M | 55 | 198 | NT | PE | Negative | 0.14 | Classical VITT | NT | Positive | NT | Positive | Yes |
| M | 74 | 89 | 28.75 | PE, DVT | Negative | 0.16 | Classical VITT | Classical VITT | Positive | NT | Negative | Yes |
| M | 78 | 16 | 44 | ICA | Negative | 0.15 | Classical VITT | NT | Positive | NT | NT | Yes |
| M | 79 | 46 | 31 | Popliteal | Negative | 0.18 | Classical VITT | NT | Positive | NT | NT | Yes |
| M | 49 | 125 | 16 | DVT | Positive | 0.6 | Classical VITT | NT | Negative | Positive | Positive | Yes |
| M | 77 | 220 | 32.5 | PE, DVT | Weak positive | 0.38 | Classical VITT | NT | Negative | Negative | Positive | Yes |
| M | 77 | 138 | 16 | MCA stroke | Positive | 0.215 | Classical VITT | NT | Negative | Negative | Negative | Yes |
| F | 19 | 42 | 119.8 | PE | Positive | 2.56 | Classical VITT | NT | Negative | Negative | NT | Yes |
| M | 53 | 38 | 40 | Portal vein thrombosis | Positive | 2.1 | Classical VITT | NT | Negative | Positive | NT | Yes |
| F | 51 | 14 | 40 | CVST, bilateral ICA | Positive | 1.21 | Classical VITT | NT | Negative | Positive | NT | Yes |
| F | 82 | 161 | 62 | Proximal DVT | Weak positive | 0.29 | Classical VITT | NT | Negative | Negative | Negative | Yes |
| F | 40 | 25 | 40 | CVST, bilateral ICA, PE, portal vein thrombosis | Positive | 1.46 | Classical VITT | NT | Negative | Negative | Positive | Yes |
| M | 69 | 86 | 120 | Splanchnic vein thrombosis | Positive | 1.73 | Classical VITT | NT | Negative | NT | NT | Yes |
| F | 61 | 95 | 40 | Soleal vein thrombosis | Positive | 1.24 | Classical VITT | NT | Negative | Positive | Positive | Yes |
| M | 54 | 128 | 15.2 | Proximal DVT | Negative | NA | Classical VITT | NT | Negative | Negative | Negative | Yes |
| F | 74 | 62 | 19.8 | PE | Positive | 2.91 | Classical VITT | NT | Negative | Positive | Negative | Yes |
| M | 71 | 77 | 14.8 | Bilateral PE | Positive | 2.39 | Classical VITT | NT | Negative | NT | NT | Yes |
| F | 59 | 125 | 66 | PE | Negative | 0.07 | Classical VITT | NT | Negative | Positive | NT | Yes |
| Sex . | Age (y) . | Platelet count (×109/L) . | D-dimer (fold-change) . | Thrombosis . | ELISA . | ELISA OD . | Flow cytometry . | SRA . | Multiplate . | Serologically confirmed VITT . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard . | PF4 enhanced . | Standard . | PF4 enhanced . | |||||||||
| Serologically confirmed VITT, standard flow cytometry negative/equivocal (n = 9) | ||||||||||||
| M | 75 | 41 | 25 | Renal artery occlusion | Positive | 2.4 | Negative | Classical VITT | Positive | NT | Positive | Yes |
| M | 79 | 249 | 7.2 | PE | Positive | 1.8 | Negative | Heparin-enhancing VITT | Positive | NT | Negative | Yes |
| M | 76 | 53 | 40 | CVST, bilateral DVT | Negative | 0.11 | Negative | Negative | Positive | NT | Negative | Yes |
| M | 73 | 124 | 14.5 | PE, DVT | Negative | 0.064 | Negative | Negative | Weak positive | Weak positive | NT | Yes |
| F | 53 | 45 | 17 | Carotid artery, CVST, PE, LL arterial | Positive | 0.36 | Negative | Classical VITT | Positive | NT | Negative | Yes |
| F | 35 | 153 | 5 | CVST | Positive | 1.34 | Equivocal | Equivocal | Weak positive | Equivocal | Inconclusive | Yes |
| F | 54 | 108 | 5.8 | MCA stroke | Positive | 0.88 | Equivocal | Classical VITT | Negative | NT | Negative | Yes |
| M | 63 | 110 | 26.6 | PE | Positive | 0.87 | Inconclusive | Classical VITT | Negative | Equivocal (negative) | NT | Yes |
| M | 70 | 100 | 22 | DVT | Positive | 0.74 | Negative | Classical VITT | NT | Inconclusive | NT | Yes |
| Serologically confirmed VITT, standard flow cytometry positive (n = 18) | ||||||||||||
| M | 55 | 198 | NT | PE | Negative | 0.14 | Classical VITT | NT | Positive | NT | Positive | Yes |
| M | 74 | 89 | 28.75 | PE, DVT | Negative | 0.16 | Classical VITT | Classical VITT | Positive | NT | Negative | Yes |
| M | 78 | 16 | 44 | ICA | Negative | 0.15 | Classical VITT | NT | Positive | NT | NT | Yes |
| M | 79 | 46 | 31 | Popliteal | Negative | 0.18 | Classical VITT | NT | Positive | NT | NT | Yes |
| M | 49 | 125 | 16 | DVT | Positive | 0.6 | Classical VITT | NT | Negative | Positive | Positive | Yes |
| M | 77 | 220 | 32.5 | PE, DVT | Weak positive | 0.38 | Classical VITT | NT | Negative | Negative | Positive | Yes |
| M | 77 | 138 | 16 | MCA stroke | Positive | 0.215 | Classical VITT | NT | Negative | Negative | Negative | Yes |
| F | 19 | 42 | 119.8 | PE | Positive | 2.56 | Classical VITT | NT | Negative | Negative | NT | Yes |
| M | 53 | 38 | 40 | Portal vein thrombosis | Positive | 2.1 | Classical VITT | NT | Negative | Positive | NT | Yes |
| F | 51 | 14 | 40 | CVST, bilateral ICA | Positive | 1.21 | Classical VITT | NT | Negative | Positive | NT | Yes |
| F | 82 | 161 | 62 | Proximal DVT | Weak positive | 0.29 | Classical VITT | NT | Negative | Negative | Negative | Yes |
| F | 40 | 25 | 40 | CVST, bilateral ICA, PE, portal vein thrombosis | Positive | 1.46 | Classical VITT | NT | Negative | Negative | Positive | Yes |
| M | 69 | 86 | 120 | Splanchnic vein thrombosis | Positive | 1.73 | Classical VITT | NT | Negative | NT | NT | Yes |
| F | 61 | 95 | 40 | Soleal vein thrombosis | Positive | 1.24 | Classical VITT | NT | Negative | Positive | Positive | Yes |
| M | 54 | 128 | 15.2 | Proximal DVT | Negative | NA | Classical VITT | NT | Negative | Negative | Negative | Yes |
| F | 74 | 62 | 19.8 | PE | Positive | 2.91 | Classical VITT | NT | Negative | Positive | Negative | Yes |
| M | 71 | 77 | 14.8 | Bilateral PE | Positive | 2.39 | Classical VITT | NT | Negative | NT | NT | Yes |
| F | 59 | 125 | 66 | PE | Negative | 0.07 | Classical VITT | NT | Negative | Positive | NT | Yes |
CVST, cerebral venous sinus thrombosis; DVT, deep vein thrombosis; F, female; ICA, internal carotid artery; LL, lower limb; M, male; MCA, middle cerebral artery; NA, not available; NT, not tested; OD, optical density; PE, pulmonary embolism.
Addition of PF4 in clinically high probability cases that are negative in standard flow cytometry
Addition of exogenous PF4 has shown improved sensitivity of functional platforms to detect platelet-activating antibodies in VITT.1,12,25,26 We investigated the effect of purified human PF4 on performance of our flow cytometry assay using plasma from confirmed VITT-positive and VITT-negative cases. Exogenous PF4 did not alter results of VITT-positive (n = 6) or VITT-negative (n = 8) cases reported by standard flow cytometry assay without PF4 (Figure 4A). Plasma samples that were inconclusive or negative in our standard assay, but positive via SRA or PF4-SRA, were tested with exogenous PF4 addition to flow cytometry. Two of 3 patients who were equivocal or inconclusive according to standard flow cytometry became positive, whereas one remained equivocal after PF4 addition (Table 2). Three of 5 patients who were thought to be false negative on standard flow cytometry (SRA positive) were labeled positive with PF4-enhanced flow cytometry.
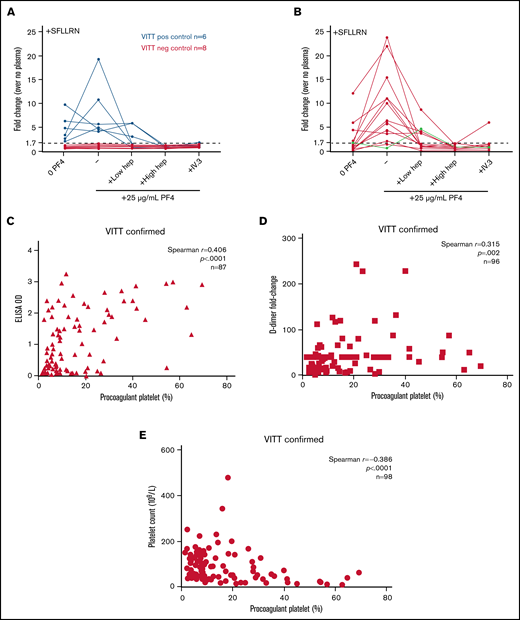
PF4 enhanced PP flow cytometry. Plasma samples from 6 VITT-positive patients and 8 VITT-negative patients previously tested on the standard flow cytometry assay (A) or 15 individuals classified as negative, inconclusive, or equivocal on the standard assay were retested on the PF4 enhanced assay (B). Green line represents a patient with heparin-enhancing response. Donor whole blood was treated with platelet agonist 5 µM SFLLRN and patient plasma in the presence of 25 µg/mL purified native human PF4, unfractionated heparin (0.5 U/mL or 100 U/mL), and/or FcγRIIa-blocking antibody IV.3 (10 µg/mL). Dotted horizontal line represents 1.7-fold increase above no plasma baseline determined in Figure 3 as the optimal cutoff for VITT. The PP response induced by VITT-positive plasma was correlated with anti-PF4 antibody titer represented by ELISA optical density (OD) values (n = 87) (C), patient platelet count at the time of testing (n = 98) (D), and D-dimer levels represented by fold-change above upper limit of normal (n = 96) (E). All patients recorded a platelet count nadir of <150 × 109/L. Spearman correlation analysis was performed.
PF4 enhanced PP flow cytometry. Plasma samples from 6 VITT-positive patients and 8 VITT-negative patients previously tested on the standard flow cytometry assay (A) or 15 individuals classified as negative, inconclusive, or equivocal on the standard assay were retested on the PF4 enhanced assay (B). Green line represents a patient with heparin-enhancing response. Donor whole blood was treated with platelet agonist 5 µM SFLLRN and patient plasma in the presence of 25 µg/mL purified native human PF4, unfractionated heparin (0.5 U/mL or 100 U/mL), and/or FcγRIIa-blocking antibody IV.3 (10 µg/mL). Dotted horizontal line represents 1.7-fold increase above no plasma baseline determined in Figure 3 as the optimal cutoff for VITT. The PP response induced by VITT-positive plasma was correlated with anti-PF4 antibody titer represented by ELISA optical density (OD) values (n = 87) (C), patient platelet count at the time of testing (n = 98) (D), and D-dimer levels represented by fold-change above upper limit of normal (n = 96) (E). All patients recorded a platelet count nadir of <150 × 109/L. Spearman correlation analysis was performed.
Performance of flow cytometry assay in cases referred for VITT testing
Of 99 cases adjudicated as VITT by the THANZ VITT advisory group from 1 April 2021 to 7 September 2021 (Table 3), 98 underwent flow cytometry testing. Four patients had no serologic support by either ELISA or any functional test platform. Seventy-six of the remaining 94 were positive on flow cytometry without PF4 enhancement. Fifteen of 18 individuals classified as negative, inconclusive, or equivocal returned a positive result with PF4 enhancement (Figure 4B). Three remained equivocal (increased procoagulant response to plasma without IV.3 suppression). Overall, 93% of patients with clinicopathologic adjudication of VITT were detectable by flow cytometry assay with sequential testing of negative and equivocal samples with addition of exogenous PF4. PP formation showed a positive correlation with anti-PF4 antibody (r = 0.406; P < .0001) (Figure 4C) and D-dimer (r = 0.315; P = .002) (Figure 4D) levels, and negative correlation against platelet count (r = –0.386; P < .0001) (Figure 4E).
Laboratory and clinical characteristics of adjudication-confirmed VITT patients referred for VITT testing between 1 April 2021 and 7 September 2021
| Variable . | Adjudication-confirmed VITT . |
|---|---|
| n | 99 |
| Female | 55 |
| Male | 44 |
| Age, y | 63 (53-73) |
| Days’ postvaccine | 10 (8-15) |
| Platelet count at time of testing (×109/L) | 70 (37-128) |
| D-dimer (fold-change over ULN) | 40 (15-43) |
| Thrombosis | 99 |
| Cerebral venous sinus thrombosis | 28 |
| Splanchnic thrombosis | 19 |
| Pulmonary embolism | 40 |
| Deep vein thrombosis | 26 |
| Other | 21 |
| Mortality | 7 |
| Variable . | Adjudication-confirmed VITT . |
|---|---|
| n | 99 |
| Female | 55 |
| Male | 44 |
| Age, y | 63 (53-73) |
| Days’ postvaccine | 10 (8-15) |
| Platelet count at time of testing (×109/L) | 70 (37-128) |
| D-dimer (fold-change over ULN) | 40 (15-43) |
| Thrombosis | 99 |
| Cerebral venous sinus thrombosis | 28 |
| Splanchnic thrombosis | 19 |
| Pulmonary embolism | 40 |
| Deep vein thrombosis | 26 |
| Other | 21 |
| Mortality | 7 |
Continuous variables are expressed as median (interquartile range).
ULN, upper limit of normal.
Discussion
Mass vaccination is imperative to battle COVID-19, but rare thrombotic events associated with SARS-CoV-2 vaccination raise concerns regarding adenoviral vector vaccine safety. Early and accurate diagnosis of potentially fatal VITT has far-reaching implications for public health and clinical management, as early intervention significantly improves patient outcomes. We previously established a whole-blood flow cytometry–based PP assay for HIT diagnosis that exploited the synergistic procoagulant effect of activating platelet GPCR and ITAM signaling pathways.20 Considering the pathophysiological similarities between HIT and VITT, we adapted our assay to ascertain diagnostic utility in a development cohort of 47 patients suspected of VITT and confirmed this within 290 sequential patients referred for VITT functional testing. This assay has been incorporated into the diagnostic strategy of VITT in Australia.
As hypothesized, the level of PP formation with low-dose SFLLRN stimulation was minimal in healthy donor whole blood, despite near complete α-granule release. There was no increase in FcγRIIa-dependent procoagulant response with the addition of negative control plasmas that lack ITAM receptor engagement, whereas plasma with HIT or VITT antibodies capable of activating ITAM signaling exhibited a procoagulant response consistent with the known synergistic effect of combined GPCR and ITAM signaling on formation of this platelet subpopulation.
Evaluation using plasma samples from ELISA-positive, SRA-positive VITT patients confirmed that our assay detected plasma-induced FcγRIIa-dependent PP responses in donor platelets suppressible by high-dose heparin and IVIg. The procoagulant response was not observed in vaccinated patients without anti-PF4 antibodies and SRA-negative, or those with detectable nonpathologic anti-PF4 antibodies (ELISA-positive, SRA-negative, non-thrombocytopenic patients), demonstrating specificity. Inclusion of criterion for suppression of plasma-induced procoagulant activity by IV.3 increased assay specificity. The heightened PP response generated by VITT plasma was positively associated with anti-PF4 antibody levels and trended with decreasing platelet count at time of diagnosis. Extent of thrombocytopenia has been associated with significant mortality risk whereby for every 50% reduction in baseline platelet count there is a 1.7-fold increased risk of VITT-related death.27
Two other flow cytometry assays have been described that detect VITT antibodies: PF4-induced flow cytometry–based platelet activation by Handtke et al,28 performed on hirudinated donor whole blood with addition of PF4 with P-selectin MFI as output; and a washed platelet PP assay, by Althaus et al,12 again supplemented with PF4, with combined annexin V binding and P-selectin expression as readout. Our assay of PP response induced by patient plasma in citrated whole blood from responsive donors represents an additional functional platform to heparin-induced platelet activation, SRA, PF4-induced flow cytometry–based platelet activation, and MEA.
Increasingly, data suggest that VITT is a spectrum disorder. There is variation in anti-PF4 antibody levels, and one quality assessment exercise revealed significant variability in functional assay VITT antibody detection, showing the need for ongoing refinement of VITT diagnostic assays.29 Our application of functional assays to a large cohort of patients referred for VITT functional testing in Australia showed heterogeneity. Overall, the standard procoagulant assay seems more sensitive than ELISA or SRA, and procoagulant assay with PF4 was more sensitive again. However, some patients with platelet-activating antibodies that were detectable by flow cytometry were not detected by SRA and vice versa. An in-depth comparison of assays would aid understanding (manuscript in preparation E.J.F., C.S.M.L., T.A.B., L.J.C., D.D., M.K., I.T.-E., F.H.P., H.T., and V.M.C.).
Unlike HIT, inclusion of low-dose heparin diminished PP response induced by VITT plasma except for 3 patients with heparin-dependent exacerbation of procoagulant response. Huynh et al6 reported that VITT antibodies occupy binding sites similar to heparin on PF4. Addition of heparin presumably competes with VITT antibodies for PF4 binding, nullifying any platelet-activating effect with complete abrogation at high-dose heparin. The variability in the effect of low-dose heparin, whereby partial suppression was observed in some patients but full suppression was achieved in others, is consistent with published reports. For instance, 5 of 241 and 3 of 812 VITT sera remained positive in platelet-activation assays in the presence of 0.2 U/mL heparin.
Through molecular simulations, Baker et al30 found that the negatively charged surface of the ChAdOx1 viral capsid interacts with positively charged PF4, presumably leading to exposure of neo-epitopes on PF4, thereby triggering immune responses against PF4. Consistent with this, Greinacher et al31 reported interactions between PF4 and ChAdOx1 nCoV-19 using super-resolution microscopy. Akin to heparin in HIT, these observations suggest that vaccine components may be cofactors in altering PF4 conformation, causing exposure of neo-epitopes triggering an immune response. However, at the concentration tested here, we observed an inconsistent effect of ChAdOx1 nCoV-19 on the PP response induced by VITT plasma.
An ongoing goal is for assays to offer personalized predictors for patients. This assay can be used to monitor persistence or resolution of platelet-activating properties of VITT antibodies that may be discordant to ELISA results, to help guide duration of anticoagulation.32 Although in vitro responses cannot be fully correlated with clinical response, we speculate that individuals with “heparin-enhancing” responses would be at risk of thrombus formation/progression in the presence of heparin, similar to patients with HIT, and they should avoid future heparin exposure.33 The concordance observed between the in vitro effect of IVIg and fondaparinux on VITT-induced PP response, and the clinical response of these patients to IVIg and fondaparinux therapy, are intriguing. Increased numbers could determine utility in identifying individuals who would benefit from change in anticoagulant or additional immune-suppression therapy.
Our assay offers some advantages over other platelet-activating assays. Use of standard citrated whole blood is practical and provides a more physiological environment for assay reactions, and it requires minimal manipulation of donor platelets. Requiring only 5 µL of plasma per test condition and standard flow cytometers add to practicality. Notably, our standard assay detected the majority of VITT cases without requirement for exogenous PF4. This eliminated potential variability introduced by variable PF4 oligomerization34,35 and additional expense. This assay is used in association with other functional assays, within diagnostic pathways of VITT in Australia, demonstrating feasibility of translation to diagnostic settings. To date, >360 patient samples had been tested. The nature of PP being a subset of the whole platelet population rather than an “all-or-nothing” response characteristic of α-granule and dense granule release detection methods enables detection of heparin enhancement in plasma with strongly activating heparin-independent antibodies. In assays with 100% dense granule release with serum alone, detection of further increases in the presence of low-dose heparin is not possible.
The requirement for suitable donor platelets remains a limitation of all functional platelet assays. Interestingly, although all donors with spontaneous aggregation by LTA in response to anti-CD9 antibody were responsive donors in a PP HIT assay, only a proportion of these donors were suitable for VITT assays (supplemental Figure 4). The contribution of FcγRIIa-expressing neutrophils to the procoagulant response observed in our assay remains to be addressed. This assay also does not evaluate the patient’s own platelet PP profile, only the effect of the plasma-containing antibody. The standard assay using SFLLRN to release PF4 did not detect all VITT cases. Sequential testing of initially negative or equivocal samples, with a modified assay in which PF4 was added, increased the VITT patients identified to 93%. In a diagnostic setting, we propose that testing negative samples with exogenous PF4 be performed sequentially to avoid the requirement for exogenous PF4 in >80% of referrals.
FcγRIIa polymorphisms alter receptor affinity for immunoglobulin G, which may account for variation in donor platelet reactivity36 and is associated with thrombosis in HIT37,38 and atherosclerosis.36 An intronic PAR1 gene polymorphism influences platelet PAR1 receptor density and reactivity to SFLLRN.39 Although platelet P-selectin expression is increased in homozygous individuals for the PAR1 intronic variant in response to SFLLRN,39 we observed minimal variation in PP responses in healthy donors (3.9 ± 1.0%) (Figure 1C). Nevertheless, genetic variants may determine the suitability of healthy individuals for VITT/HIT testing. Donor selection is an issue for all functional assays. Hence, we propose that healthy donors undergo initial screening by LTA for response to the anti-CD9 antibody ALB6, and that reactive donors undergo secondary screening by flow cytometry using VITT plasma samples that induce known responses (equivocal, intermediate, and strong PP responses) to confirm suitability. We also suggest use of a positive control VITT plasma with each run.
In conclusion, induction of FcγRIIa-dependent PP responses by patient plasma that were suppressible by heparin and IVIg is highly indicative of VITT in the correct clinical circumstance. Our assay modification of priming platelets from known FcγRIIa-responsive donors with a GPCR agonist to potentiate ITAM signaling from platelet-activating immune complexes results in a sensitive and specific assay. This represents a functional platform to identify patients with platelet-activating antibodies and potentially predict treatment responses, with proven translational capacity.
Acknowledgments
The authors thank NSW Health for supplying the ChAdOx1 nCoV-19 vaccine and Jamie Triccas and Claudio Counoupas for supplying the recombinant protein HexaPro. They greatly appreciate Diane Criminale and the staff at CRGH Blood Collection Unit for assistance in blood collection. They also thank the members of the THANZ VITT advisory group and the THANZ VITT ELISA group.
This study is partly funded by NSW Health Pathology. V.M.C. is funded by a NSW Ministry of Health Senior Clinician Scientist Cardiovascular Capacity Building Grant.
Authorship
Contribution: C.S.M.L. designed and performed experiments, analyzed data, and wrote the manuscript; H.P.H.L., D.E.C., A.D., H.C., S.W., and D.C. performed flow cytometry experiments and analyzed data; F.H.P. performed multiplate MEA; D.D., M.K., and T.A.B. provided SRA data; S.M.H., P.Y.-I.C., and E.E.G. provided purified human PF4; I.T.-E., L.J.C., H.T., and E.J.F. collected clinical and ELISA information; V.M.C. designed and supervised the study, collected clinical information, analyzed data, and wrote the manuscript; and all authors helped revise the manuscript and approved its submission.
Conflict-of-interest disclosure: V.M.C. holds a US patent (“Selective targeting of procoagulant platelets”; US15/521435). C.S.M.L. and V.M.C. hold International (PCT) Patent Application No. PCT/AU2021/051233. The remaining authors declare no competing financial interests.
Correspondence: Vivien M. Chen, Department of Haematology, Ground Floor, Concord Hospital, Hospital Rd, Concord, NSW 2139, Australia; e-mail: vivien.chen@sydney.edu.au.
References
Author notes
Prior version of this research appeared in abstract form in: https://ashpublications.org/blood/article/138/Supplement%201/3211/479440/Flow-Cytometric-Detection-of-Procoagulant.
Requests for original data should be submitted to the corresponding author (e-mail: vivien.chen@sydney.edu.au).
The full-text version of this article contains a data supplement.


![PP response induced by VITT plasma is suppressed by heparin, is antibody mediated, and corresponded with clinical response to therapy. Healthy donor whole blood was treated with platelet agonist 5 µM SFLLRN and plasma from patients with VITT (n = 23) (A) or HIT (n = 8) (B) in the presence of low-dose (0.5 U/mL) or high-dose (100 U/mL) unfractionated heparin and assessed for PP formation by using flow cytometry. Blue lines represent patients with VITT who generated a heparin-enhancing PP response at low-dose heparin. Friedman test with Dunn’s correction for multiple comparisons was performed. Donor blood was pretreated with the FcγRIIa-blocking antibody IV.3 (10 µg/mL) (C) or IVIg (10 mg/mL) (D) for 15 minutes before exposure to VITT plasma and 5 µM SFLLRN. Wilcoxon matched-pairs signed rank test was performed. (E) PP response induced by VITT plasma (n = 4) collected pre-IVIg treatment was compared with the procoagulant response within 5 days’ post-IVIg therapy. The reduction in the PP response corresponded to the suppressive effect of exogenous IVIg (10 mg/mL) on the pre-IVIg sample. Results are presented as fold-change in PP proportion relative to no plasma control. Each line represents a unique patient. (F) Plasma samples from 6 patients with VITT collected before initiation of fondaparinux treatment was tested in the presence of fondaparinux (1.2 µg/mL) in vitro. PP response is normalized to no fondaparinux control. Four patients were subsequently found to be clinically responsive to fondaparinux, and 2 patients were fondaparinux resistant. Healthy donor whole blood was treated with platelet agonist 5 µM SFLLRN and VITT plasma in the presence of the ChAdOx1 nCoV-19 vaccine (AZD1222, 1:2000 [vol/vol], n = 23) (G) or recombinant SARS-CoV-2 spike protein (HexaPro 20 µg/mL, n = 10) (H) before flow cytometric assessment for PP formation. Wilcoxon matched-pairs signed rank test was performed. *P < .05, **P < .01, ***P < .001,****P <.0001. ns, not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/6/11/10.1182_bloodadvances.2021006698/2/m_advancesadv2021006698f2.png?Expires=1767724248&Signature=J0Fqcra1kR6MWHNCDoDEmrKq6wtUY2BBheQmcGMhb1~QJIFtcciERkqGuklr74fN0DPFYQJqid7Z8Y6n1oI-BsEL2GbkoMUZxyHP7doaCokPeO9OQ6liAFfgOqdPIH1a~hf65XD5Y8j7oGZtYuQmXTCCc2z8SPwD3fo7dfNSyhWqMVu4decdOYzd1bfLBQDNY~NdsMrcDCHKoj10frIxAzq47ClRFcDJbQaVy1cXJc9vQ8NB3znhp5IxMRGjUU-rWE6Ce6bmvV8-tXdsfPfHSQOYGGbDkTCTpVzGkJyqpDw98U-A-TcgYXkxiJ-e7UZpCJ3YFO~DzAWXLjoi0BYlZQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)